Tuning Magnetic and Semiconducting Properties of Cr-Doped CaTiO3 Perovskites for Advanced Spintronic Applications
Abstract
:1. Introduction
2. Theoretical Calculation Method
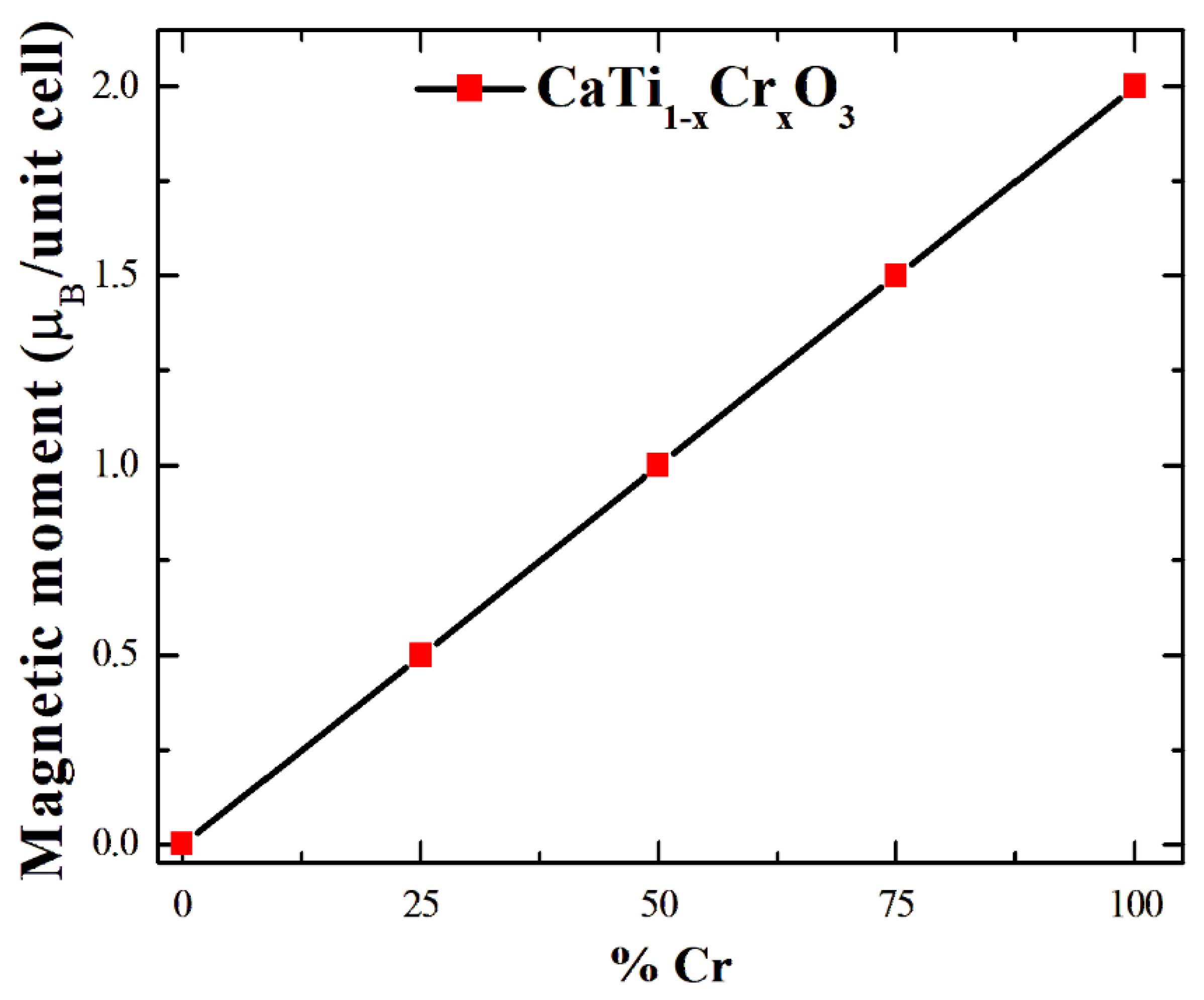
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Hu, C.; Wang, D. Ferromagnetism in (Cr, Mn)-co-doped 3C-SiC analyzed using density functional theory. AIP Adv. 2023, 13, 065330. [Google Scholar] [CrossRef]
- Souidi, A.; Bentata, S.; Benstaali, W.; Bouadjemi, B.; Abbad, A.; Lantri, T. First principle study of spintronic properties for double perovskites Ba2XMoO6 with X = V, Cr and Mn. Mater. Sci. Semicond. Process. 2016, 43, 196–208. [Google Scholar] [CrossRef]
- M’hid, A.A.; Boughrara, M.; Li, G.; Kerouad, M.; Wang, Q. First-principles investigations and Monte Carlo simulation of Ti and Cr-doped w-ZnO and (Ti,Cr) co-doped w-ZnO based magnetic semiconductors: Materials for spintronic applications. J. Magn. Magn. Mater. 2024, 589, 171540. [Google Scholar] [CrossRef]
- Dar, S.A.; Murtaza, G.; Zelai, T.; Nazir, G.; Alkhaldi, H.; Albalawi, H.; Kattan, N.A.; Irfan, M.; Mahmood, Q.; Mahmoud, Z. Study of structural, electronic, magnetic, and optical properties of A2FeMnO6 (A = Ba, La) double perovskites, experimental and DFT analysis. Colloids Surf. A Physicochem. Eng. Asp. 2023, 664, 131145. [Google Scholar] [CrossRef]
- Rehman, M.U.; Wang, Q.; Yu, Y. Electronic, Magnetic and Optical Properties of Double Perovskite Compounds: A First Principle Approach. Crystals 2022, 12, 1597. [Google Scholar] [CrossRef]
- Yang, F.; Yang, L.; Ai, C.; Xie, P.; Lin, S.; Wang, C.Z.; Lu, X. Tailoring bandgap of perovskite BaTiO3 by transition metals co-doping for visible-light photoelectrical applications: A first-principles study. Nanomaterials 2018, 8, 455. [Google Scholar] [CrossRef]
- Rizwan, M.; Usman, Z.; Shakil, M.; Gillani, S.S.A.; Azeem, S.; Jin, H.B.; Cao, C.B.; Mehmood, R.F.; Nabi, G.; Asghar, M.A. Electronic and optical behaviour of lanthanum doped CaTiO3 perovskite. Mater. Res. Express 2020, 7, 015920. [Google Scholar] [CrossRef]
- Al-Qhtani, M.; Mustafa, G.M.; Mazhar, N.; Bouzgarrou, S.; Mahmood, Q.; Mera, A.; Zaki, Z.I.; Mostafa, N.Y.; Alotaibi, S.H.; Amin, M.A. Half Metallic Ferromagnetism and Transport Properties of Zinc Chalcogenides ZnX2Se4 (X = Ti, V, Cr) for Spintronic Applications. Materials 2022, 15, 55. [Google Scholar] [CrossRef]
- Saad H-E, M.M. Impact of 3d-transition metal [T = Sc, Ti, V, Cr, Mn, Fe, Co] on praseodymium perovskites PrTO3: Standard spin-polarized GGA and GGA+U investigations. Bull. Mater. Sci. 2022, 45, 1–18. [Google Scholar] [CrossRef]
- Ben Hassen, A.; Rhouma, F.I.H.; Dhahri, J.; Abdelmoula, N. Effect of the substitution of titanium by chrome on the structural, dielectric and optical properties in Ca0.67La0.22Ti(1-x)CrxO3 perovskites. J. Alloys Compd. 2016, 663, 436–443. [Google Scholar] [CrossRef]
- Alsobhi, B.O. First-principle calculations of the electronic, structural, optical, thermoelectric and elastic properties of CeXO3 (X = Ti, V and Cr) perovskites. J. Taibah Univ. Sci. 2021, 15, 1078–1093. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, J.; Liu, X.; Lin, L.; Tao, H. Electronic structure and high magnetic properties of (Cr, co)-codoped 4h–sic studied by first-principle calculations. Crystals 2020, 10, 634. [Google Scholar] [CrossRef]
- Chang, F.; Zhang, N.; Yang, F.; Wang, S.; Song, G. Effect of Cr substitution on the structure and electrical properties of BiFeO3 ceramics. J. Phys. D Appl. Phys. 2007, 40, 7799–7803. [Google Scholar] [CrossRef]
- Gong, S.; Chen, P.; Liu, B.G. Structural, electronic, and magnetic properties of double perovskite Pb2CrMO6 (M=Mo, W and Re) from first-principles investigation. J. Magn. Magn. Mater. 2014, 349, 74–79. [Google Scholar] [CrossRef]
- Rasul, M.N.; Mehmood, M.; Hussain, A.; Rafiq, M.A.; Iqbal, F.; Khan, M.A.; Manzoor, A. Investigation of the Physical Properties of XCRh3 (X = Ti, V, Cr, Mn, Fe, Ni, Cu, Zn, Zr, Nb, Mo, Tc, Ru, Pd, Ag) Inverse Perovskites from First Principles. J. Electron. Mater. 2022, 51, 5880–5896. [Google Scholar] [CrossRef]
- Kishore, N.; Nagarajan, V.; Chandiramouli, R. High-pressure studies on electronic and mechanical properties of FeBO3 (B = Ti, Mn, Cr) ceramics–a first-principles study. Phase Transit. 2018, 91, 382–397. [Google Scholar] [CrossRef]
- Mahmood, Q.; Ali, S.A.; Hassan, M.; Laref, A. First principles study of ferromagnetism, optical and thermoelectric behaviours of AVO3 (A = Ca, Sr, Ba) perovskites. Mater. Chem. Phys. 2018, 211, 428–437. [Google Scholar] [CrossRef]
- Saxena, P.; Mishra, A. Structural and electrical properties of YMnO3 manganites: Influence of Cr ion doping. J. Solid State Chem. 2021, 301, 122364. [Google Scholar] [CrossRef]
- Ghebouli, B.; Ghebouli, M.A.; Choutri, H.; Fatmi, M.; Chihi, T.; Louail, L.; Bouhemadou, A.; Bin-Omran, S.; Khenata, R.; Khachai, H. An ab initio study of the structural, elastic, electronic, optical properties and phonons of the double perovskite oxides Sr2AlXO6 (X = Ta, Nb, V). Mater. Sci. Semicond. Process. 2016, 42, 405–412. [Google Scholar] [CrossRef]
- Uto, O.T.; Adebambo, P.O.; Akinlami, J.O.; Kenmoe, S.; Adebayo, G.A. Electronic, Structural, Mechanical, and Thermodynamic Properties of CoYSb (Y = Cr, Mo, W) Half-Heusler Compounds as Potential Spintronic Materials. Solids 2022, 3, 22–33. [Google Scholar] [CrossRef]
- Saeed, M.; Haq, I.U.; Saleemi, A.S.; Rehman, S.U.; Haq, B.U.; Chaudhry, A.R.; Khan, I. First-principles prediction of the ground-state crystal structure of double-perovskite halides Cs2AgCrX6 (X = Cl, Br, and I). J. Phys. Chem. Solids 2022, 160, 110302. [Google Scholar] [CrossRef]
- El Amine Monir, M.; Dahou, F.Z. Structural, thermal, elastic, electronic and magnetic properties of cubic lanthanide based perovskites type oxides PrXO3 (X = V, Cr, Mn, Fe): Insights from ab initio study. SN Appl. Sci. 2020, 2, 465. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Jia, Y.; Yan, W.; Li, Q.; Zhou, J.; Wu, K. Engineering the Electronic Structure towards Visible Lights Photocatalysis of CaTiO3 Perovskites by Cation (La/Ce)-Anion (N/S) Co-Doping: A First-Principles Study. Molecules 2023, 28, 7134. [Google Scholar] [CrossRef]
- Bukhari, S.; Giorgi, J. Redox stability of Sm0.95Ce0.05Fe1−xCrxO3−δ perovskite materials. J. Electrochem. Soc. 2011, 158, H1027. [Google Scholar] [CrossRef]
- Sato, N.; Haruta, M.; Sasagawa, K.; Ohta, J.; Jongprateep, O. Fe and Co-doped (Ba, Ca)TiO3 perovskite as potential electrocatalysts for glutamate sensing. Eng. J. 2019, 23, 265–278. [Google Scholar] [CrossRef]
- Wen, H.; Luo, X. Tuning Bandgaps of Mixed Halide and Oxide Perovskites CsSnX3 (X=Cl, I), and SrBO3 (B=Rh, Ti). Appl. Sci. 2021, 11, 6862. [Google Scholar] [CrossRef]
- Lewis, C.; Liu, H.; Han, J.; Wang, L.; Yue, S.; Brennan, N.; Wong, S. Probing charge transfer in a novel class of luminescent perovskite-based heterostructures composed of quantum dots bound to re-activated CaTiO3 phosphors. Nanoscale 2016, 8, 2129–2142. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Okazaki, Y.; Matsuda, M.; Miyake, M. Photocatalytic H2 evolution by layered perovskite Ca3Ti2O7 codoped with Rh and Ln (Ln = La, Pr, Nd, Eu, Gd, Yb, and Y) under visible light irradiation. J. Ceram. Soc. Jpn. 2009, 117, 1175–1179. [Google Scholar] [CrossRef]
- Dunyushkina, L.; Gorbunov, V. Crystal structure and electrical conductivity correlation in CaTi1−xFexO3−δ system. Ionics 2002, 8, 256–261. [Google Scholar] [CrossRef]
- Riaz, A.; Witte, K.; Bodnar, W.; Seitz, H.; Schell, N.; Springer, A.; Burkel, E. Tunable pseudo-piezoelectric effect in doped calcium titanate for bone tissue engineering. Materials 2021, 14, 1495. [Google Scholar] [CrossRef]
- Novianti, D.; Haikal, F.; Rouf, U.; Hardian, A.; Prasetyo, A. Synthesis and characterization of Fe-doped CaTiO3 polyhedra prepared by molten NaCl salt. Sci. Technol. Indones. 2022, 7, 17–21. [Google Scholar] [CrossRef]
- Sharma, P.; Pramanik, M.; Limaye, M.; Singh, S. Magnetic field-enhanced oxygen evolution in YMn1–xCrxO3 (x = 0, 0.05, and 0.1) perovskite oxides. J. Phys. Chem. C 2023, 127, 16259–16266. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Methfessel, M.; Paxton, A.T. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 1989, 40, 3616–3621. [Google Scholar] [CrossRef]
- Wendari, T.P.; Akbar, M.A.; Izzati, A.F.; Haidar, H.; Rizki, A.; Zulhadjri; Arief, S.; Mufti, N.; Blake, G.R. Structure, Dielectric, and Energy Storage Properties of Perovskite CaTiO3 Ceramic Synthesized Using the Natural Calcium from Pensi Shell (Corbicula moltkiana) Waste. J. Mol. Struct. 2024, 1307, 137949. [Google Scholar] [CrossRef]
- Yan-Qing, T.; Meng, Y.; Yong-Mei, H. Structure and colossal dielectric permittivity of Ca2TiCrO6 ceramics. J. Phys. D Appl. Phys. 2013, 46, 015303. [Google Scholar] [CrossRef]
- Zhou, J.-S.; Jin, C.-Q.; Long, Y.-W.; Yang, L.-X.; Goodenough, J.B. Anomalous Electronic State in CaCrO3 and SrCrO3. Phys. Rev. Lett. 2006, 96, 046408. [Google Scholar] [CrossRef]
- Sasaki, S.; Prewitt, C.T.; Bass, J.D.; Schulze, W.A. Orthorhombic perovskite CaTiO3 and CdTiO3: Structure and space group. Acta Cryst. 1987, C43, 1668–1674. [Google Scholar] [CrossRef]
- A stand out family. Nat. Mater. 2021, 20, 1303. [CrossRef] [PubMed]
- Streltsov, S.V.; Khomskii, D.I. Jahn-Teller Effect and Spin-Orbit Coupling: Friends or Foes? Phys. Rev. X 2020, 10, 031043. [Google Scholar] [CrossRef]
- Zhang, H.; Li, N.; Li, K.; Xue, D. Structural Stability and Formability of ABO3-Type Perovskite Compounds. Acta Cryst. 2007, B63, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Enterkin, J.A.; Poeppelmeier, K.R. Bonding at Oxide Surfaces. In Bond Valences; Brown, I., Poeppelmeier, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 158, pp. 154–196. [Google Scholar] [CrossRef]
- Lufaso, M.W.; Barnes, P.W.; Woodward, P.M. Structure Prediction of Ordered and Disordered Multiple Octahedral Cation Perovskites Using SPuDS. Acta Crystallogr. Sect. B Struct. Sci. 2006, 62, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Lisco, F.; Kaminski, P.M.; Abbas, A.; Bass, K.; Bowers, J.W.; Claudio, G.; Losurdo, M.; Walls, J.M. The structural properties of CdS deposited by chemical bath deposition and pulsed direct current magnetron sputtering. Thin Solid Film. 2015, 582, 323–327. [Google Scholar] [CrossRef]
- Zdorovets, M.V.; Borgekov, D.B.; Zhumatayeva, I.Z.; Kenzhina, I.E.; Kozlovskiy, A.L. Synthesis, Properties, and Photocatalytic Activity of CaTiO3-Based Ceramics Doped with Lanthanum. Nanomaterials 2022, 12, 2241. [Google Scholar] [CrossRef]
- Roa-Rojas, J.; Toro, C.E.D.; Rebaza, A.V.G.; Moya, X.A.V.; Téllez, D.A.L. Spintronic Properties in Complex Perovskites: A Concordance between Experiments and Ab-Initio Calculations. In Research Topics in Bioactivity, Environment and Energy; Taft, C.A., de Lazaro, S.R., Eds.; Engineering Materials; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Alouani, M.; Baadji, N.; Abdelouahed, S.; Bengone, O.; Dreyssé, H. Effect of Spin-Orbit Coupling on the Magnetic Properties of Materials: Results. In Advances in the Atomic-Scale Modeling of Nanosystems and Nanostructured Materials; Massobrio, C., Bulou, H., Goyhenex, C., Eds.; Lecture Notes in Physics; Springer: Berlin/Heidelberg, Germany, 2010; Volume 795. [Google Scholar] [CrossRef]
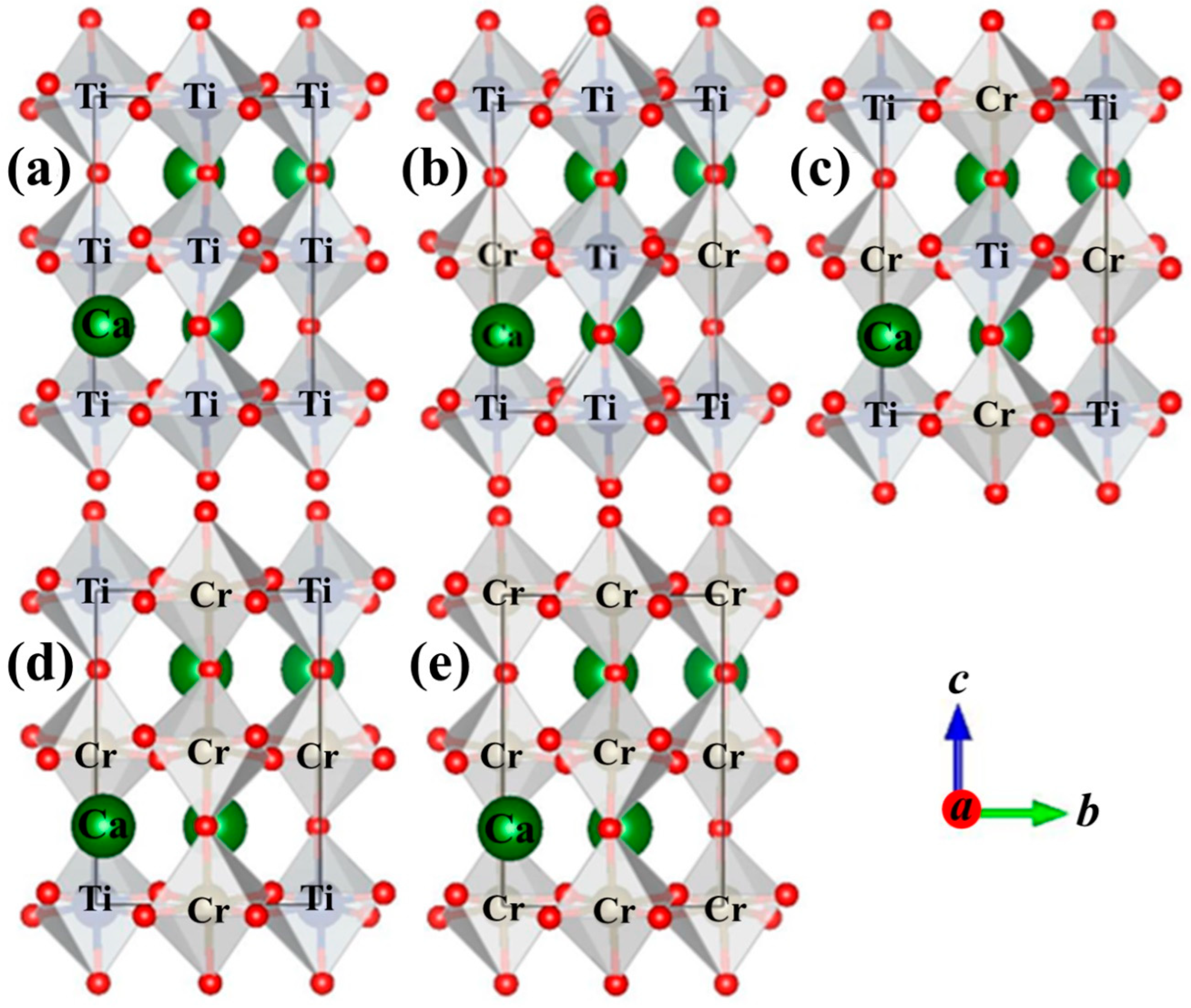

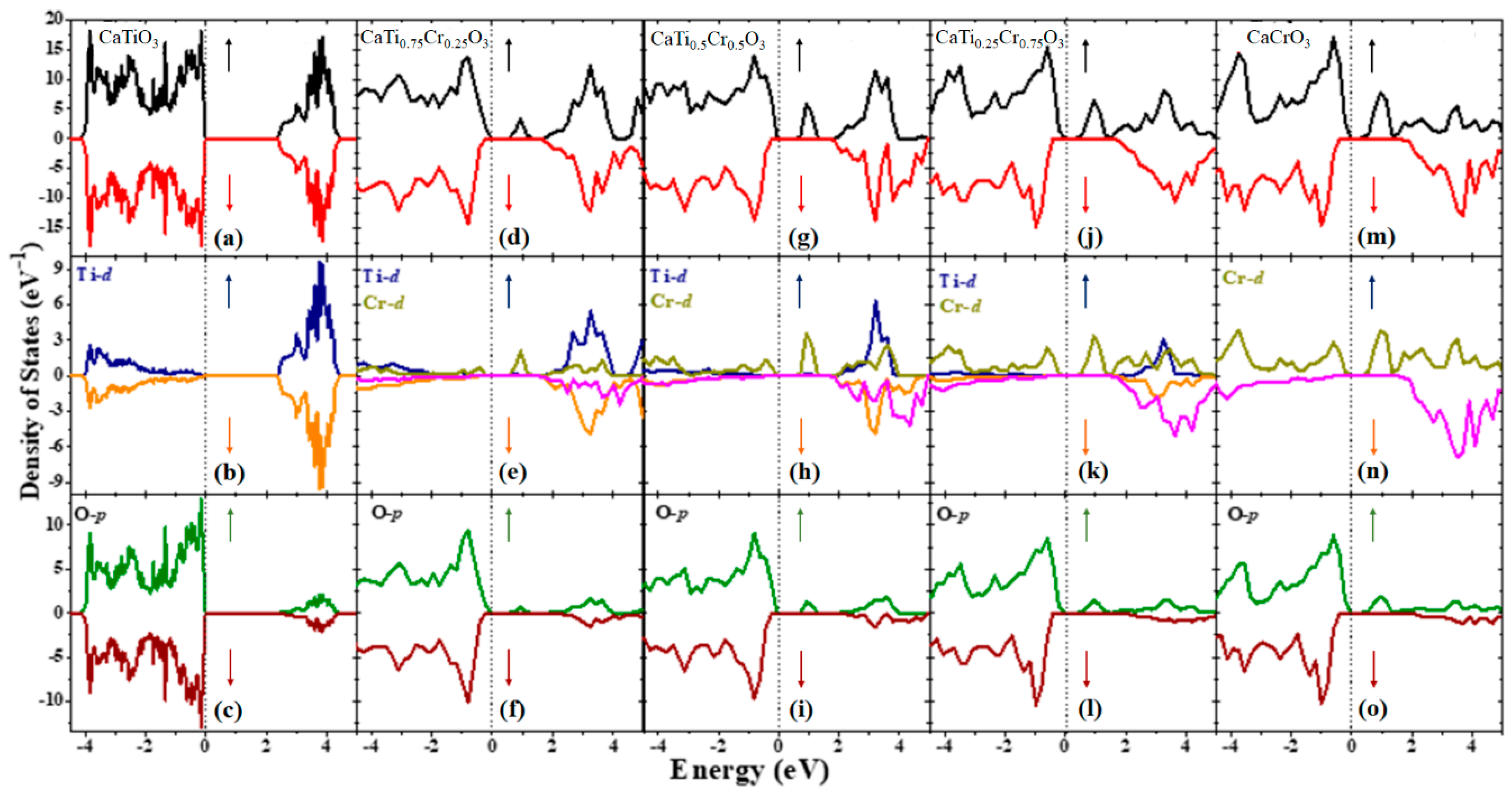
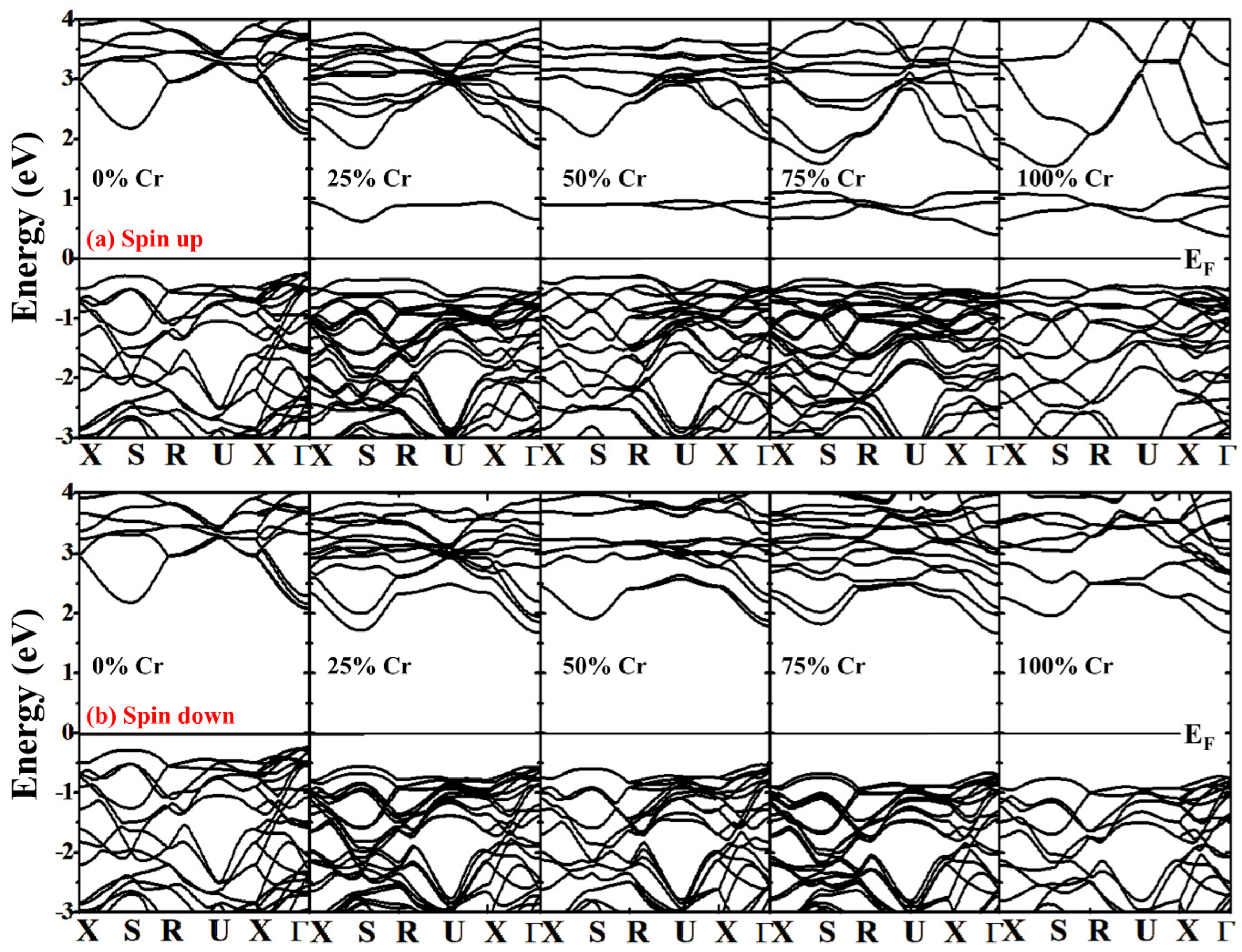
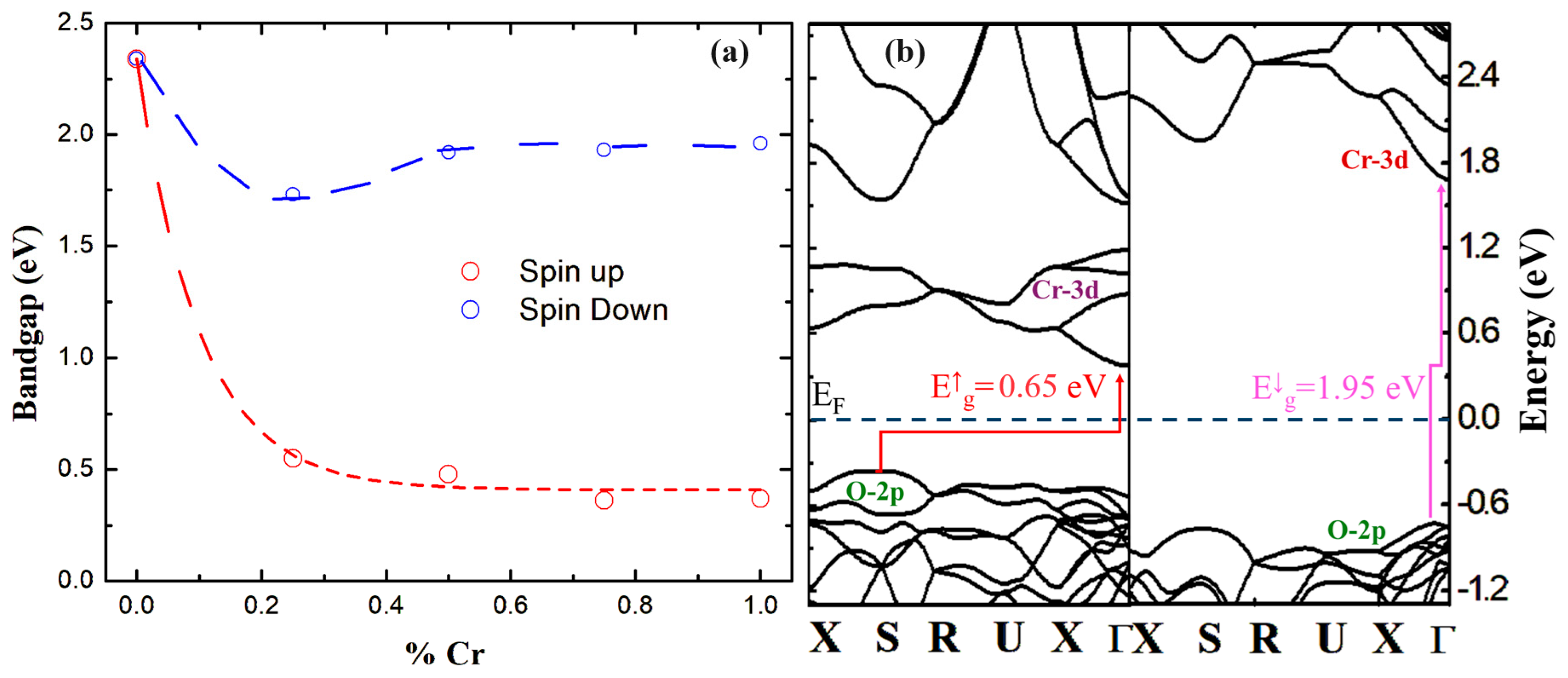
| Structural Parameters | 0% | 25% | 50% | 75% | 100% |
|---|---|---|---|---|---|
| a (Å) | 5.4067 5.3928 [39] | 5.3878 | 5.3929 5.3511 [40] | 5.3476 | 5.3221 5.2886 [41] |
| b (Å) | 5.5056 5.4494 [39] | 5.4935 | 5.4596 5.3936 [40] | 5.4748 | 5.4668 5.3172 [41] |
| c (Å) | 7.6949 7.6582 [39] | 7.6552 | 7.5989 7.5874 [40] | 7.5720 | 7.5341 7.4844 [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deluque-Toro, C.E.; Ariza-Echeverri, E.A.; Landínez-Téllez, D.A.; Vergara, D.; Roa-Rojas, J. Tuning Magnetic and Semiconducting Properties of Cr-Doped CaTiO3 Perovskites for Advanced Spintronic Applications. Appl. Sci. 2024, 14, 7326. https://doi.org/10.3390/app14167326
Deluque-Toro CE, Ariza-Echeverri EA, Landínez-Téllez DA, Vergara D, Roa-Rojas J. Tuning Magnetic and Semiconducting Properties of Cr-Doped CaTiO3 Perovskites for Advanced Spintronic Applications. Applied Sciences. 2024; 14(16):7326. https://doi.org/10.3390/app14167326
Chicago/Turabian StyleDeluque-Toro, C. E., E. A. Ariza-Echeverri, D. A. Landínez-Téllez, D. Vergara, and J. Roa-Rojas. 2024. "Tuning Magnetic and Semiconducting Properties of Cr-Doped CaTiO3 Perovskites for Advanced Spintronic Applications" Applied Sciences 14, no. 16: 7326. https://doi.org/10.3390/app14167326






