Construction and Evaluation of Pepper Core Collection Based on Phenotypic Traits and SSR Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Accessions and Field Evaluation
2.2. Phenotypic Trait Determination
2.3. Construction and Evaluation of Pepper Core Germplasm Bank Based on Phenotype Data
2.4. Construction of Pepper Core Germplasm Bank Based on SSR Molecular Markers
2.5. Construction of Core Collection Based on SSR Markers
2.6. Evaluation, Validation, and Improvement of Core Collection Based on SSR Molecular Markers
2.7. Integrated Construction of Phenotypic and Molecular Core Collection
3. Results
3.1. Genetic Diversity Analysis of Pepper Germplasm
3.2. Determination of Overall Sample Retention Ratio
3.3. Determination of Intragroup Sampling Ratio Method and Construction of Core Collection
3.4. Evaluation of Pepper Core Collection Based on Phenotypic Data
3.4.1. Evaluation of Core Collection Based on Basic Genetic Parameters
3.4.2. Evaluation of Core Collection Based on Principal Component Analysis
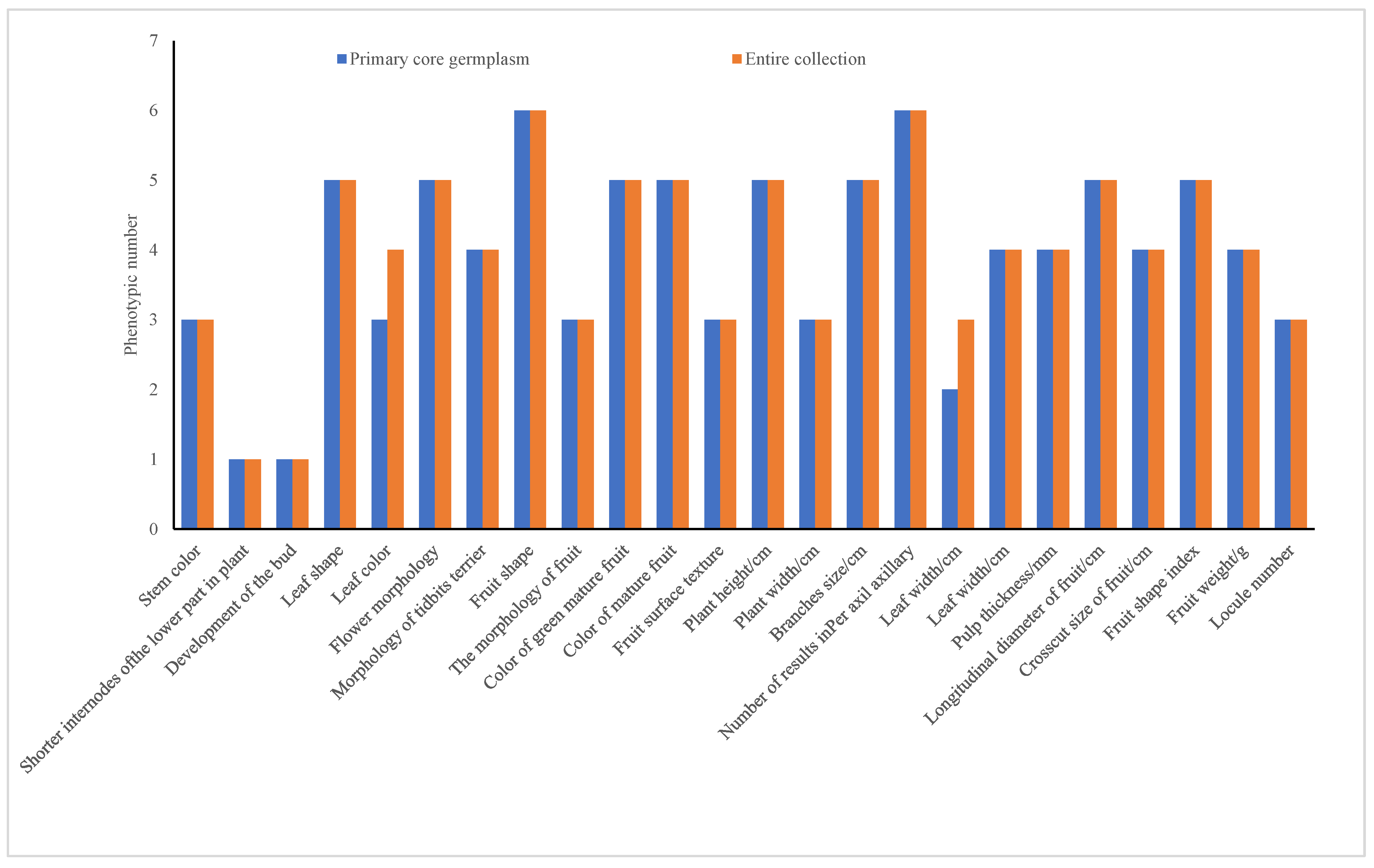
3.4.3. Evaluation of Pre-Selected Core Germplasm Based on Phenotypic Retention Number
3.5. Construction and Evaluation of Chili Core Collection Based on SSR Markers
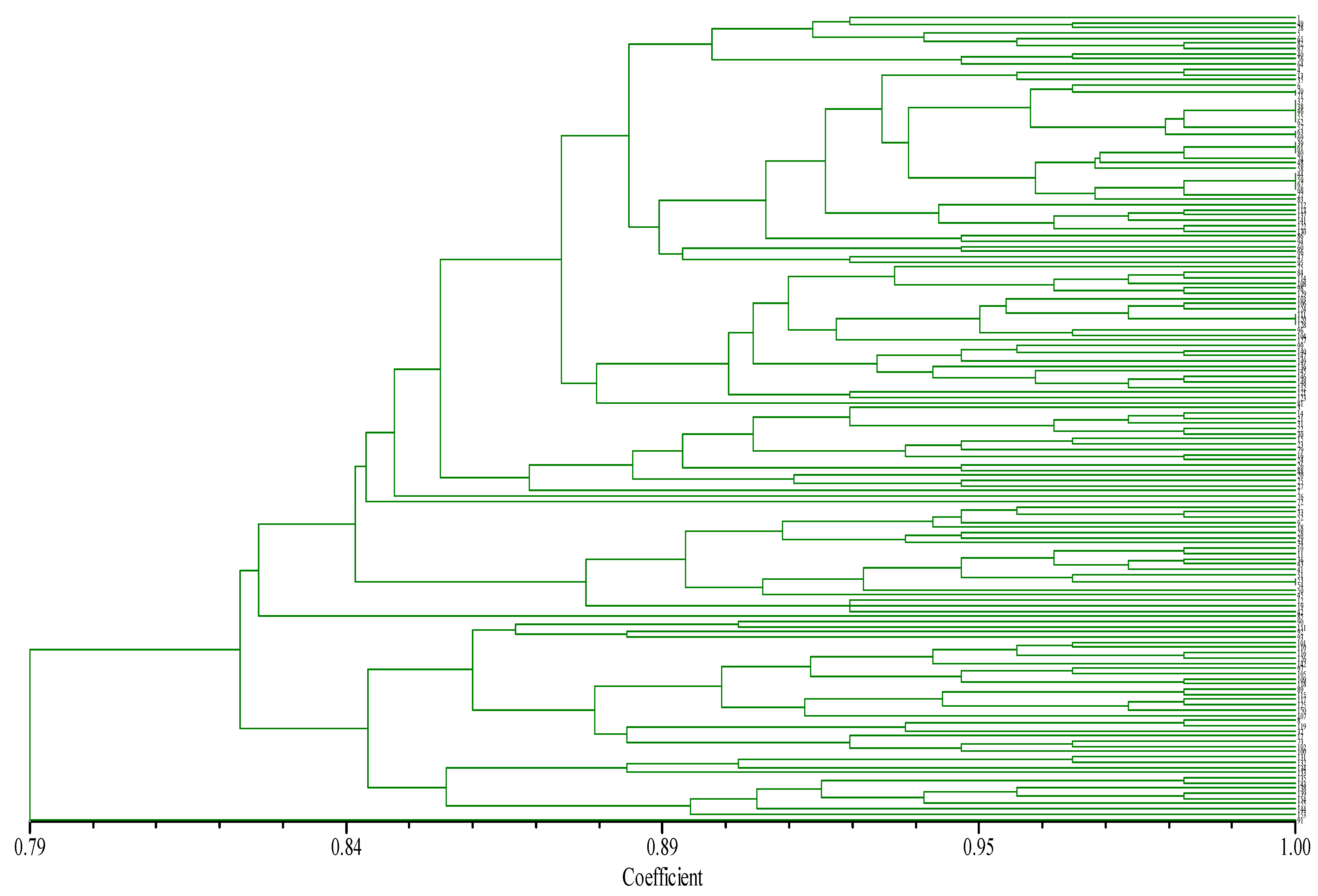
3.5.1. Cluster Analysis of Chili Germplasm Based on SSR Markers and Reserve Ratio Analysis of Pre-Selected Core Collection
3.5.2. Shannon-Weaver Index Analysis of Pre-Selected Core Collection of Peppers Obtained with Different Clustering Compression Ratios
3.5.3. Evaluation of Pre-Selected Core Collection by Molecular Markers
3.5.4. Improvement of Molecular Marker Pre-Selection of Core Collection
3.5.5. Integrating Phenotypic and Molecular Core Germplasm to Construct the Final Pepper Core Collection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ou, L.; Li, D.; Lv, J.; Chen, W.; Zhang, Z.; Li, X.; Yang, B.; Zhou, S.; Yang, S.; Li, W. Pan-genome of cultivated pepper (Capsicum) and its use in gene presence-absence variation analyses. New Phytol. 2018, 220, 360. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Singh, J. Cayenne/American pepper. In Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2006; pp. 12–54. [Google Scholar]
- Ramchiary, N.; Kole, C. The Capsicum Genome; Springer International Publishing: Cham, Switzerland, 2019; pp. 102–112. [Google Scholar]
- Kenyon, L.; Kumar, S.; Tsai, W.S.; Hughes, J.D.A. Virus diseases of peppers (Capsicum spp.) and their control. In Advances in Virus Research; Loebenstein, G., Katis, N., Eds.; Academic Press: Burlington, ON, Canada, 2014; Volume 90, pp. 5–43. [Google Scholar]
- Duranova, H.; Valkova, V.; Gabriny, L. Chili peppers (Capsicum spp.): The spice not only for cuisine purposes: An update on current knowledge. Phytochem. Rev. 2022, 21, 1379–1413. [Google Scholar] [CrossRef]
- Lee, H.Y.; Ro, N.Y.; Jeong, H.J.; Kwon, J.K.; Jo, J.; Ha, Y.; Jung, A.; Han, J.W.; Venkatesh, J.; Kang, B.C. Genetic diversity and population structure analysis to construct a core collection from a large Capsicum germplasm. BMC Genet. 2016, 17, 142. [Google Scholar] [CrossRef]
- Luitel, B.P.; Ro, N.Y.; Ko, H.C.; Sung, J.S.; Rhee, J.H.; Hur, O.S. Phenotypic variation in a germplasm collection of pepper (Capsicum chinense Jacq.) from Korea. J. Crop Sci. Biotech. 2018, 21, 499–506. [Google Scholar] [CrossRef]
- Ramchiary, N.; Kehie, M.; Brahma, V.; Kumaria, S.; Tandon, P. Application of genetics and genomics towards Capsicum translational research. Plant Biotechnol. Rep. 2014, 8, 101–123. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Nelson, R.L.; Geraldi, I.; Cruz, C.D.; Toledo, J.F. Establishing a soybean germplasm core collection. Field Crops Res. 2010, 119, 277–289. [Google Scholar] [CrossRef]
- Frankel, O.H.; Brown, A.H.D. Plant genetic resources today: A critical appraisal. In Crop Genetic Resources: Conservation and Evaluation; Holden, J.H.W., Williams, J.T., Eds.; George Allen and Unwin: London, UK, 1984; pp. 249–257. [Google Scholar]
- Sandhu, N.; Singh, J.; Singh, G.; Sethi, M.; Singh, M.P.; Pruthi, G.; Raigar, O.P.; Kaur, R.; Singh Sarao, P.; Singh Lore, J.; et al. Development and validation of a novel core set of KASP markers for the traits improving grain yield and adaptability of rice under direct-seeded cultivation conditions. Genomics 2022, 114, 110269. [Google Scholar] [CrossRef]
- Ambati, D.; Phuke, R.M.; Vani, V.; Sai Prasad, S.V.; Singh, J.B.; Patidar, C.P.; Malviya, P.; Gautam, A.; Dubey, V.G. Assessment of genetic diversity and development of core germplasm in durum wheat using agronomic and grain quality traits. Cereal Res. Commun. 2020, 48, 375–382. [Google Scholar] [CrossRef]
- Yao, Q.; Fan, P.; Zou, S. Constructing a core collection for maize (Zea mays L.) landrace from Wuling Mountain region in China. Agric. Sci. China 2008, 7, 1423–1432. [Google Scholar] [CrossRef]
- Guo, Y.; Kuang, L.; Xu, Y.; Yan, T.; Jiang, L.; Dong, J.; Wu, D. Construction of a worldwide core collection of rapeseed and association analysis for waterlogging tolerance. Plant Growth Regul. 2022, 98, 321–328. [Google Scholar] [CrossRef]
- Lassois, L.; Denancé, C.; Ravon, E.; Guyader, A.; Guisnel, R.; Hibrand-Saint-Oyant, L.; Poncet, C.; Lasserre-Zuber, P.; Feugey, L.; Durel, C. Genetic diversity, population structure, parentage analysis, and construction of core collections in the French apple germplasm based on SSR Markers. Plant Mol. Biol. Rep. 2016, 34, 827–844. [Google Scholar] [CrossRef]
- Liang, W.; Dondini, L.; De Franceschi, P.; Paris, R.; Sansavini, S.; Tartarini, S. Genetic diversity, population structure and construction of a core collection of apple cultivars from Italian germplasm. Plant Mol. Biol. Rep. 2015, 33, 458–473. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Sun, H.; Ning, N.; Yang, L. Construction and evaluation of a primary core collection of apricot germplasm in China. Sci. Hortic. 2011, 128, 311–329. [Google Scholar] [CrossRef]
- Wang, R.; Zhong, Y.; Hong, W.; Luo, H.; Li, D.; Zhao, L.; Zhang, H.; Wang, J. Genetic diversity evaluation and core collection construction of pomegranate (Punica granatum L.) using genomic SSR markers. Sci. Hortic. 2023, 319, 112192. [Google Scholar] [CrossRef]
- Nie, X.; Wang, Z.; Liu, N.; Song, L.; Yan, B.; Xing, Y.; Zhang, Q.; Fang, K.; Zhao, Y.; Chen, X.; et al. Fingerprinting 146 Chinese chestnut (Castanea mollissima Blume) accessions and selecting a core collection using SSR markers. J. Integr. Agric. 2021, 20, 1277–1286. [Google Scholar] [CrossRef]
- Sun, Q.; Bai, L.; Ke, L.; Xiang, X.; Zhao, J.; Ou, L. Developing a core collection of litchi (Litchi chinensis Sonn.) based on EST-SSRgenotype data and agronomic traits. Sci. Hortic. 2012, 146, 29–38. [Google Scholar] [CrossRef]
- Li, P.; Su, T.; Yu, S.; Wang, H.; Wang, W.; Yu, Y.; Zhang, D.; Zhao, X.; Wen, C.; Zhang, F. Identifcation and development of a core set of informative genic SNP markers for assaying genetic diversity in Chinese cabbage. Hortic. Environ. Biotechnol. 2019, 60, 411–425. [Google Scholar] [CrossRef]
- Gopal, J.; Kumar, V.; Kumar, R.; Mathur, P. Comparison of different approaches to establish a core collection of andigena (Solanum tuberosum Group Andigena) Potatoes. Potato Res. 2013, 56, 85–98. [Google Scholar] [CrossRef]
- Corradoa, G.; Caramantea, M.; Piffanelli, P.; Rao, R. Genetic diversity in Italian tomato landraces: Implications for the development of a core collection. Sci. Hortic. 2014, 168, 138–144. [Google Scholar] [CrossRef]
- Benke, A.P.; Krishna, R.; Mahajan, V.; Ansari, W.A.; Gupta, A.J.; Khar, A.; Shelke, P.; Thangasamy, A. Genetic diversity of Indian garlic core germplasm using agrobiochemical traits and SRAP markers. Saudi J. Biol. Sci. 2021, 28, 4833–4844. [Google Scholar] [CrossRef]
- Wang, W.; Miyatake, K.; Saito, T.; Harada, Y.; Yamaguchi, S.; Koyama, M.; Nakamura, K. Acetylcholine content in 100 accessions from the worldwide eggplant (Solanum melongena L.) core collection. J. Food Compos. Anal. 2023, 119, 105233. [Google Scholar] [CrossRef]
- Hoshikawa, K.; Lin, Y.P.; Schafleitner, R.; Shirasawa, K.; Isobe, S.; Nguyen, D.; Ohsawa, R.; Yoshioka, Y. Genetic diversity analysis and core collection construction for Amaranthus tricolor germplasm based on genome wide single nucleotide polymorphisms. Sci. Hortic. 2023, 307, 111428. [Google Scholar] [CrossRef]
- Zhuang, F.; Zhao, Z.; Li, X.; Hu, H.; Fang, Z. A core collection of chinese traditional carrot germplasm. Acta Hortic. Sin. 2006, 33, 46–51. [Google Scholar]
- Fan, M.; Chen, S.; Engle, L.M. The study on genetic diversity of the core collection for Capsicum using random amplified. J. Agric. Res. China 2004, 53, 165–178. [Google Scholar]
- Hanson, P.M.; Yang, R.-Y.; Lin, S.; Tsou, S.C.S.; Lee, T.-C.; Wu, J.; Shieh, J.; Gniffke, P.; Ledesma, D. Variation for antioxidant activity and antioxidants in a subset of AVRDC-the World Vegetable Center Capsicum core collection. Plant Genet. Resour. 2004, 2, 153–166. [Google Scholar] [CrossRef]
- Nicolaï, M.; Cantet, M.; Lefebvre, V.; Sage-Palloix, A.M.; Palloix, A. Genotyping a large collection of pepper (Capsicum spp.) with SSR loci brings new evidence for the wild origin of cultivated C. annuum and the structuring of genetic diversity by human selection of cultivar types. Genet. Resour. Crop Evol. 2013, 60, 2375–2390. [Google Scholar] [CrossRef]
- Quenouille, J.; Saint-Felix, L.; Moury, B.; Palloix, A. Diversity of genetic backgrounds modulating the durability of a major resistance gene. Analysis of a core collection of pepper landraces resistant to Potato virus Y. Mol. Plant Pathol. 2016, 17, 296–302. [Google Scholar] [CrossRef]
- Thies, J.A.; Fery, R.L. Evaluation of a core of the U.S. Capsicum germplasm collection for reaction to the northern root-knot nematode. HortScience 2002, 37, 805–810. [Google Scholar] [CrossRef]
- Zewdie, Y.; Tong, N.; Bosland, P. Establishing a core collection of Capsicum using a cluster analysis with enlightened selection of accessions. Genet. Resour. Crop Evol. 2004, 51, 147–151. [Google Scholar] [CrossRef]
- Jiang, D.F.; Li, J.D.; Hong, Y. Techniques for chili pepper cultivation in the open field in Hainan island, China. J. China Capsicum 2004, 2, 17–18. [Google Scholar]
- Li, X.; Zhang, B.X. Specification and Data Standards for Describing Chili Germplasm Resources; China Agricultural Publishing House: Beijing, China, 2006; pp. 47–98. [Google Scholar]
- Liu, M.X.; Liu, C.H.; Fan, X.C.; Guo, D.L.; Zhang, G.H.; Sun, H.S. Construction of primary core collection of grape genetic resources. J. Plant Genet. Resour. 2012, 13, 72–76. [Google Scholar]
- Shen, Z.J.; Ma, R.J.; Yu, M.L.; Cai, Z.X.; Xu, J.L. Establishment of peach primary core collection based on accessions conserved in national fruit germplasm repository of Nanjing. Acta Hortic. Sin. 2013, 40, 125–134. [Google Scholar]
- Zhang, W. SPSS Statistical Analysis Fundamentals Tutorial; Higher Education Press: Beijing, China, 2017; pp. 6–102. [Google Scholar]
- Wu, Z.; Liu, W.; Tang, X.; Cui, J.; Cheng, J.; Hu, K. Development and application of EST-SSR markers for chili peppers. J. South China Agric. Univ. 2012, 33, 171–174. [Google Scholar]
- Brown, A.H.D. The core collection at the crossroads. In Core Collection of Plant Genetic Resources; Hodgkin, T., Brwon, A.H., van Hintum, T.H.L., Eds.; IPGRI, A Wiley-Sayce Publication: Hoboken, NJ, USA, 1995; Volume 3–20, pp. 1163–1168. [Google Scholar]
- Yonezawa, K.; Nomura, T.; Morishima, H. Sampling strategies for use in stratified germplasm collections. In Core Collection of Plant Genetics Resources; Hodgkin, T., Brown, A.H.D., van Hintum, T.H.L., Eds.; IPGRI, A Wiley-Sayce Publication: Hoboken, NJ, USA, 1995; Volume 90, pp. 35–54. [Google Scholar]
- Frankel, O.H. Genetic perspectives of germplasm conservation. In Genetic Manipulation: Impact on Man and Society; Arber, W., Llimensee, K., Peacock, W.J., Starlinger, P., Eds.; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Bar-Hen, A.; Charcosset, A.; Bourgoin, M.; Guiard, J. Relationship between genetic markers and morphological traits in maize inbred lines collection. Euphytica 1995, 84, 145–154. [Google Scholar] [CrossRef]
- Liu, Z.J.; Sun, J.H.; Yang, Y.; Cao, Z.M. Comparative study on the construction of Capsicum chinense Jacquin Core Collection. Chin. J. Trop. Crops 2015, 36, 2155–2160. [Google Scholar]
- Gu, X.Z. The Research of Genetic Diversity of Pepper (Capsicum spp.) Germplasms of China and Core Collection Construction; Chinese Academy of Agricultural Sciences: Beijing, China, 2016; pp. 34–37. [Google Scholar]
- Li, Y.X.; Gao, Q.J.; Li, T.H. Sampling strategy based on fruit characteristics for a primary core collection of peach cultivars. J. Fruit Sci. 2006, 3, 359–364. [Google Scholar]
- Miyatake, K.; Shinmura, Y.; Matsunaga, H.; Fukuoka, H.; Saito, T. Construction of a core collection of eggplant (Solanum melongena L.) based on genome-wide SNP and SSR genotypes. Breed. Sci. 2019, 69, 498–502. [Google Scholar] [CrossRef]
- Mongkolporn, O.; Hanyong, S.; Chunwongse, J.; Wasee, S. Establishment of a core collection of chilli germplasm using microsatellite analysis. Plant Genet. Resour. 2015, 13, 104–110. [Google Scholar] [CrossRef]
- He, J.W.; Han, S.Y. Construction of Core Capsicum Germplasm Bank Based on Different Distance Clustering and Sampling Method of SSR Marker. Southwest China J. Ayicultural Sci. 2015, 28, 2199–2204. [Google Scholar]
- Wu, Q. Genetic Diverstiy Analysis and Estalishment of the Core Collcetion of Pepper Germplasm Resources Based on SRAP, SSR Makers; Jiangxi Agriculture University: Nanchang, China, 2015; pp. 46–49. [Google Scholar]

| Primer Name | Sequence (5′ to 3′) |
|---|---|
| CIDAM-1F | CTTTCAGCGACACAACCAGG |
| CIDAM-1R | ACAACGTCTGAAATTAGTCCCAAA |
| CIDAM-2F | ACATGAATTGTGACCGAGGAAGT |
| CIDAM-2R | AGGGGTTGTTTGGTAGCTAGC |
| CIDAM-3F | TGTGAGACACAAGTGTTGCAG |
| CIDAM-3R | AGACGCTTTTTCCCTCTGGT |
| CIDAM-4F | GGCAGAGGAATAAATTGGCCA |
| CIDAM-4R | ACGTGACGCATTCTAAGACCT |
| CIDAM-5F | TGGAGGCCCAGAGTCAACAT |
| CIDAM-5R | GCTATTTGTGTGTGCCGCTC |
| CIDAM-6F | ACACATCATTGTCCAACCAAGT |
| CIDAM-6R | ACTGGATGATGGGTAGTTTGTGA |
| CIDAM-7F | TCCCATGGTTGTAACTTTGATTTCC |
| CIDAM-7R | TGCTAAAGGTGGTGAGCGTT |
| CIDAM-8F | GAGTACATAAGTCCCCGCCC |
| CIDAM-8R | TATCTTTGCGGCCTTCGTGT |
| Grouping | Number of Resource Shares | Simple Sample Retention Ratio (%) | Diversity Index (Hi) |
|---|---|---|---|
| var. fasciculatum Bailey | 9 | 5.81% | 0.6699 |
| var. grossum Bailey | 16 | 10.32% | 0.6905 |
| var. cerasiorme Bailey | 3 | 1.94% | 0.3833 |
| var. conoides Bailey | 11 | 7.10% | 0.8294 |
| var. longum Bailey | 116 | 74.84% | 0.7729 |
| Total | 155 | 100.00% | 0.7954 * |
| Intragroup Retention Ratio Method | Overall Sample Retention Ratio | Ratio of Phenotypic Retention (RPR) | Coincidence Rate of Range (CR) | Change Rate of Coefficient of Variation CVR | Genetic Diversity Index (Hi) |
|---|---|---|---|---|---|
| Simple sample retention | 5% | 79.80% | 75.76% | 122.82% | 0.9466 |
| 10% | 82.83% | 77.64% | 122.42% | 0.9118 | |
| 15% | 88.89% | 82.15% | 129.47% | 0.9521 | |
| 20% | 92.93% | 85.07% | 130.53% | 0.9589 | |
| 25% | 94.95% | 86.11% | 133.15% | 0.9582 | |
| 30% | 94.95% | 86.11% | 134.62% | 0.9284 | |
| Logarithmic retention sample | 5% | 74.75% | 74.51% | 116.81% | 0.8728 |
| 10% | 88.89% | 84.03% | 148.98% | 0.9364 | |
| 15% | 91.92% | 85.07% | 151.50% | 0.9545 | |
| 20% | 94.95% | 86.11% | 149.65% | 0.9378 | |
| 25% | 95.96% | 87.15% | 152.83% | 0.9383 | |
| 30% | 96.97% | 89.24% | 156.15% | 0.9397 | |
| Square root retention sample | 5% | 76.77% | 74.51% | 171.23% | 0.8793 |
| 10% | 86.87% | 82.99% | 173.85% | 0.9286 | |
| 15% | 91.92% | 85.07% | 175.95% | 0.9491 | |
| 20% | 93.94% | 86.11% | 177.62% | 0.9537 | |
| 25% | 96.97% | 87.15% | 181.75% | 0.9517 | |
| 30% | 96.97% | 88.19% | 183.05% | 0.9336 | |
| Diversity retention sample | 5% | 67.68% | 72.43% | 196.68% | 0.8121 |
| 10% | 87.88% | 82.99% | 197.19% | 0.9291 | |
| 15% | 90.91% | 85.07% | 197.92% | 0.9316 | |
| 20% | 92.93% | 85.07% | 198.26% | 0.9257 | |
| 25% | 92.93% | 85.07% | 201.20% | 0.9217 | |
| 30% | 94.95% | 89.24% | 204.81% | 0.9286 | |
| original sample | 100% | 100% | 100% | 100% | 0.7954 |
| Overall Sample Retention Ratio | Germplasm Category | Intragroup Retention Ratio Method | |||
|---|---|---|---|---|---|
| Simple Sample Retention | Logarithmic Retention Sample | Square Root Retention Sample | Diversity Retention Sample | ||
| 5% | var. fasciculatum Bailey | 1 | 2 | 2 | 2 |
| var. grossum Bailey | 1 | 2 | 2 | 2 | |
| var. cerasiforme Bailey | 1 | 1 | 1 | 1 | |
| var. conoides Bailey | 1 | 2 | 2 | 2 | |
| var. longum Bailey | 6 | 3 | 4 | 2 | |
| Number of pre-selected core germplasm | 10 | 10 | 11 | 9 | |
| 10% | var. fasciculatum Bailey | 1 | 3 | 3 | 4 |
| var. grossum Bailey | 2 | 4 | 3 | 4 | |
| var. cerasiforme Bailey | 1 | 2 | 2 | 2 | |
| var. conoides Bailey | 2 | 3 | 3 | 4 | |
| var. longum Bailey | 12 | 6 | 8 | 4 | |
| Number of pre-selected core germplasm | 18 | 18 | 19 | 18 | |
| 15% | var. fasciculatum Bailey | 2 | 4 | 4 | 5 |
| var. grossum Bailey | 3 | 5 | 4 | 5 | |
| var. cerasiforme Bailey | 1 | 2 | 2 | 3 | |
| var. conoides Bailey | 2 | 5 | 4 | 6 | |
| var. longum Bailey | 18 | 9 | 11 | 6 | |
| Number of pre-selected core germplasm | 26 | 25 | 25 | 25 | |
| 20% | var. fasciculatum Bailey | 2 | 6 | 5 | 7 |
| var. grossum Bailey | 4 | 7 | 6 | 7 | |
| var. cerasiforme Bailey | 1 | 3 | 3 | 3 | |
| var. conoides Bailey | 3 | 6 | 5 | 8 | |
| var. longum Bailey | 24 | 12 | 15 | 8 | |
| Number of pre-selected core germplasm | 34 | 34 | 34 | 33 | |
| 25% | var. fasciculatum Bailey | 3 | 7 | 6 | 8 |
| var. grossum Bailey | 4 | 9 | 7 | 8 | |
| var. cerasiforme Bailey | 1 | 3 | 3 | 3 | |
| var. conoides Bailey | 3 | 8 | 6 | 10 | |
| var. longum Bailey | 29 | 14 | 19 | 9 | |
| Number of pre-selected core germplasm | 40 | 41 | 41 | 38 | |
| 30% | var. fasciculatum Bailey | 3 | 8 | 7 | 9 |
| var. grossum Bailey | 5 | 10 | 9 | 10 | |
| var. cerasiforme Bailey | 1 | 3 | 3 | 3 | |
| var. conoides Bailey | 4 | 9 | 7 | 11 | |
| var. longum Bailey | 35 | 17 | 22 | 11 | |
| Number of pre-selected core germplasm | 48 | 47 | 48 | 44 | |
| Traits | Min | Max | Range | Mean | SD | CV/% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stem color | 1 | 1 | 3 | 3 | 2 | 2 | 1.51 | 1.25 | 0.84 | 0.64 | 55.57% | 51.18% |
| Shorter internodes of the lower part in plant lower part in plant | 9 | 9 | 9 | 9 | 0 | 0 | 9.00 | 9.00 | 0.00 | 0.00 | 0.00% | 0.00% |
| Development of the bud | 5 | 5 | 5 | 5 | 0 | 0 | 5.00 | 5.00 | 0.00 | 0.00 | 0.00% | 0.00% |
| Leaf shape | 1 | 1 | 3 | 3 | 2 | 2 | 1.38 | 1.35 | 0.53 | 0.45 | 38.72% | 33.51% |
| Leaf color | 1 | 1 | 5 | 7 | 4 | 6 | 2.95 | 3.18 | 1.26 | 1.09 | 42.83% | 34.27% |
| Flower blooming form | 3 | 3 | 7 | 7 | 4 | 4 | 3.37 | 3.17 | 0.97 | 0.66 | 28.77% | 20.67% |
| The attached morphology of flower stalks | 3 | 3 | 9 | 9 | 6 | 6 | 5.24 | 4.59 | 2.01 | 2.00 | 38.33% | 43.51% |
| Fruit shape | 1 | 1 | 7 | 7 | 6 | 6 | 5.10 | 5.63 | 2.30 | 1.93 | 45.12% | 34.22% |
| Fruit growth state | 3 | 3 | 7 | 7 | 4 | 4 | 6.32 | 6.64 | 1.15 | 0.87 | 18.20% | 13.06% |
| Color of green mature fruit | 1 | 1 | 6 | 6 | 5 | 5 | 4.10 | 4.15 | 1.04 | 0.84 | 25.48% | 20.15% |
| Color of mature fruit | 1 | 1 | 5 | 5 | 4 | 4 | 3.78 | 3.89 | 0.69 | 0.46 | 18.24% | 11.92% |
| Fruit surface texture | 1 | 1 | 3 | 3 | 2 | 2 | 1.39 | 1.12 | 0.74 | 0.43 | 53.05% | 38.14% |
| Plant height/cm | 1 | 1 | 9 | 9 | 8 | 8 | 4.71 | 3.81 | 2.18 | 1.60 | 46.36% | 42.14% |
| Plant width/cm | 3 | 3 | 7 | 7 | 4 | 4 | 4.41 | 4.38 | 1.28 | 1.08 | 29.09% | 24.72% |
| Branches size/cm | 1 | 1 | 5 | 5 | 4 | 4 | 2.17 | 1.92 | 1.05 | 0.89 | 48.21% | 46.12% |
| Number of results in Per axil axillary | 1 | 1 | 4 | 4 | 3 | 3 | 1.38 | 1.15 | 0.73 | 0.51 | 52.93% | 44.02% |
| Leaf width/cm | 3 | 3 | 5 | 7 | 2 | 4 | 3.93 | 4.12 | 1.01 | 1.05 | 25.71% | 25.39% |
| Leaf width/cm | 3 | 3 | 7 | 7 | 4 | 4 | 3.71 | 3.70 | 1.15 | 1.01 | 30.90% | 27.21% |
| Pulp thickness/mm | 3 | 3 | 9 | 9 | 6 | 6 | 5.44 | 5.77 | 1.98 | 1.62 | 36.32% | 28.02% |
| Longitudinal diameter of fruit/cm | 1 | 1 | 9 | 9 | 8 | 8 | 5.24 | 5.59 | 1.80 | 1.29 | 34.32% | 23.11% |
| Crosscut size of fruit/cm | 3 | 3 | 9 | 9 | 6 | 6 | 5.15 | 5.13 | 1.64 | 1.52 | 31.80% | 29.70% |
| Fruit shape index | 1 | 1 | 9 | 9 | 8 | 8 | 4.02 | 4.25 | 1.96 | 1.66 | 48.59% | 39.08% |
| Fruit weight/g | 3 | 3 | 9 | 9 | 6 | 6 | 5.34 | 5.61 | 1.73 | 1.50 | 32.32% | 26.77% |
| Locule number | 1 | 1 | 3 | 3 | 2 | 2 | 2.02 | 1.94 | 0.57 | 0.55 | 28.13% | 28.65% |
| Component | Entire Collection | Pre-Core Collection | Difference Between Entire Collection and Pre-Core Collection | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Eigenvalues | Proportion | Cumulative | Eigenvalues | Proportion | Cumulative | Difference of Eigenvalues | Difference of Proportion | Difference of Cumulative | |
| PC1 | 3.8869 | 17.67% | 17.67% | 4.2723 | 19.42% | 19.42% | 0.3854 | 1.75% | 1.75% |
| PC2 | 3.1616 | 14.37% | 32.04% | 3.841 | 17.46% | 36.88% | 0.6794 | 3.09% | 4.84% |
| PC3 | 2.1318 | 9.69% | 41.73% | 2.1479 | 9.76% | 46.64% | 0.0161 | 0.07% | 4.91% |
| PC4 | 1.6503 | 7.50% | 49.23% | 1.9645 | 8.93% | 55.57% | 0.3142 | 1.43% | 6.34% |
| PC5 | 1.2407 | 5.64% | 54.87% | 1.4793 | 6.72% | 62.30% | 0.2386 | 1.08% | 7.43% |
| PC6 | 1.146 | 5.21% | 60.08% | 1.2538 | 5.70% | 68.00% | 0.1078 | 0.49% | 7.92% |
| PC7 | 1.0439 | 4.74% | 64.82% | 1.1717 | 5.33% | 73.32% | 0.1278 | 0.59% | 8.50% |
| Primer Name | Total Amplified Bands | Polymorphic Bands | Proportion of Polymorphic Bands |
|---|---|---|---|
| CIDAM-1 | 4 | 4 | 100.00% |
| CIDAM-2 | 11 | 11 | 100.00% |
| CIDAM-3 | 5 | 46 | 100.00% |
| CIDAM-4 | 4 | 47 | 100.00% |
| CIDAM-5 | 3 | 51 | 100.00% |
| CIDAM-6 | 6 | 52 | 100.00% |
| CIDAM-7 | 14 | 54 | 100.00% |
| CIDAM-8 | 17 | 54 | 100.00% |
| Compression Ratio | Number of Pre-Selected Core Germplasm | Number of Polymorphic Loci | Allelic Variation Retention Ratio | Number of Phenotypic Reservations | Phenotypic Retention Ratio |
|---|---|---|---|---|---|
| 10.97% | 17 | 43 | 79.63% | 71 | 76.34% |
| 14.19% | 22 | 44 | 83.33% | 78 | 83.87% |
| 18.06% | 28 | 46 | 85.19% | 80 | 86.02% |
| 24.52% | 38 | 47 | 87.04% | 85 | 91.40% |
| 30.32% | 47 | 51 | 94.44% | 87 | 93.55% |
| 40.65% | 63 | 52 | 96.30% | 91 | 97.85% |
| 52.90% | 82 | 54 | 100.00% | 91 | 97.85% |
| 58.71% | 91 | 54 | 100.00% | 91 | 97.85% |
| 69.68% | 108 | 54 | 100.00% | 91 | 97.85% |
| 74.19% | 114 | 54 | 100.00% | 93 | 100.00% |
| 90.32% | 140 | 54 | 100.00% | 93 | 100.00% |
| Compression Ratio | Number of Pre-Selected Core Germplasm | Number of Missing Alleles | Shannon–Weaver Diversity Index | Phenotypic Shannon Index |
|---|---|---|---|---|
| 10.97% | 17 | 11 | 0.2956 | 77.99% |
| 14.19% | 22 | 10 | 0.2909 | 81.27% |
| 18.06% | 28 | 8 | 0.2840 | 81.09% |
| 24.52% | 38 | 7 | 0.2837 | 81.46% |
| 30.32% | 47 | 3 | 0.2759 | 82.25% |
| 40.65% | 63 | 2 | 0.2737 | 81.08% |
| 52.90% | 82 | 0 | 0.2667 | 80.09% |
| 58.71% | 91 | 0 | 0.2638 | 80.29% |
| 69.68% | 108 | 0 | 0.2546 | 78.36% |
| 74.19% | 115 | 0 | 0.2523 | 79.62% |
| 90.32% | 140 | 0 | 0.2449 | 79.52% |
| 100.00% | 155 | 0 | 0.2391 | 79.54% |
| Germplasm Group | Number of Germplasms | Number of Polymorphic Loci | Percentage of Polymorphic Loci | Shannon Weaver Diversity Index | Average Number of Alleles |
|---|---|---|---|---|---|
| core collection | 22 | 45 | 83.33% | 121.66% | 2.0455 |
| Primitive germplasm | 155 | 54 | 100.00% | 100.00% | 0.3484 |
| Germplasm Group | Number of Germplasm | Number of polymorphic Loci | Percentage of Polymorphic Loci | Shannon Weaver Diversity Index | Shannon Weaver Index Change Rate | Average Number of Alleles |
|---|---|---|---|---|---|---|
| core collection | 32 | 54 | 100.00% | 0.3005 | 125.68% | 1.6875 |
| Primitive germplasm | 155 | 54 | 100.00% | 0.2391 | 100% | 0.3484 |
| Compression Ratio | Germplasm Number | Number of Phenotypic Reservations | Phenotypic Retention Ratio | Number of Polymorphic Loci Retained | Polymorphic Site Retention Ratio |
|---|---|---|---|---|---|
| 40.00% | 26 | 83 | 88.30% | 38 | 70.37% |
| 50.00% | 32 | 83 | 88.30% | 42 | 77.78% |
| 60.00% | 38 | 84 | 89.36% | 45 | 83.33% |
| 70.00% | 45 | 86 | 91.49% | 46 | 85.19% |
| 80.00% | 51 | 86 | 91.49% | 50 | 92.59% |
| 90.00% | 57 | 87 | 92.55% | 54 | 100.00% |
| 100.00% | 63 | 87 | 92.55% | 54 | 100.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Q.; Zhao, S.; Guan, C.; Fu, G.; Yu, S.; Lin, S.; Wang, Z.; Fu, H.; Lu, X.; Cheng, S. Construction and Evaluation of Pepper Core Collection Based on Phenotypic Traits and SSR Markers. Appl. Sci. 2024, 14, 7473. https://doi.org/10.3390/app14177473
Deng Q, Zhao S, Guan C, Fu G, Yu S, Lin S, Wang Z, Fu H, Lu X, Cheng S. Construction and Evaluation of Pepper Core Collection Based on Phenotypic Traits and SSR Markers. Applied Sciences. 2024; 14(17):7473. https://doi.org/10.3390/app14177473
Chicago/Turabian StyleDeng, Qin, Shuniu Zhao, Congcong Guan, Genying Fu, Shuang Yu, Shisen Lin, Zhiwei Wang, Huizhen Fu, Xu Lu, and Shanhan Cheng. 2024. "Construction and Evaluation of Pepper Core Collection Based on Phenotypic Traits and SSR Markers" Applied Sciences 14, no. 17: 7473. https://doi.org/10.3390/app14177473





