Hydrothermal Manufacture of Zeolitic Lightweight Aggregates from Clay and Marine Plastic Litter
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation of Mixtures
- As it has already been proven that the kaolin has zeolitization capacity [21], it has been used as a main component in mixtures with different proportions of plastic, namely 0, 5, 10, and 20 wt.% of powdered MPF (Figure 1b), in order to check how it affects the manufacturing process of zeolitic lightweight aggregates (ZLAs).
- Secondly, knowing the results obtained in the first stage, the kaolin was mixed with the rejected Mg-clay in proportions 1:0, 2:1, 1:2, and 0:1, thus covering 100%, 33%, 67%, and 0% of each clay in the mixture. A fixed percentage of MPF (in this case 10 wt.%) was added to the resulting clay mixture based on the results of the first stage of the investigation, something that will be discussed later.
2.3. Granulation and Firing
2.4. Hydrothermal Treatment
2.5. Characterization of the Aggregates
3. Results and Discussion
3.1. Zeolitic Lightweight Aggregates Obtained from Kaolin and Different Proportions of Marine Plastic
3.1.1. Mineralogical Changes and Zeolitization
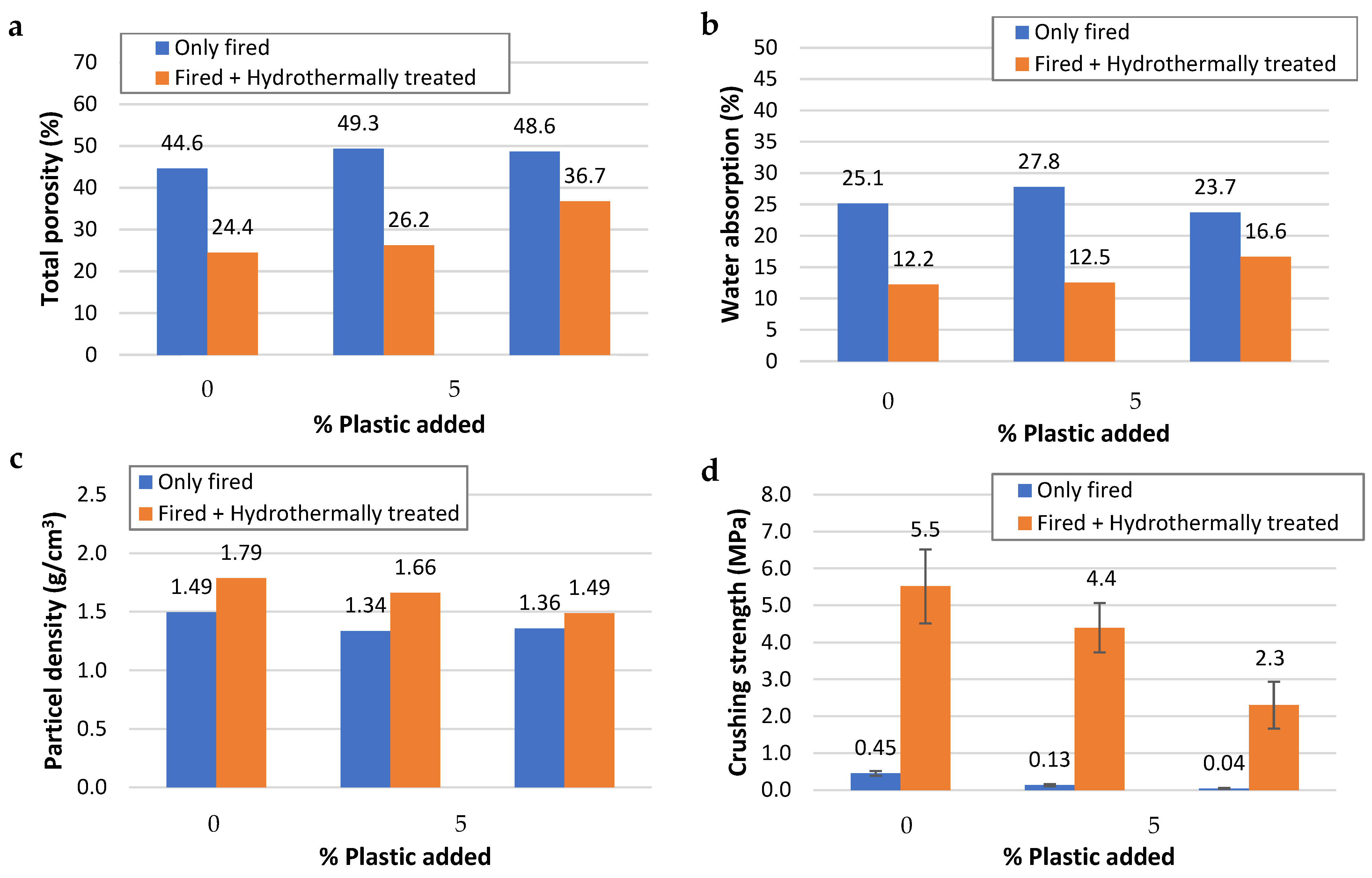
3.1.2. Technological Properties of the ZLAs
3.2. Effect of the Addition of the Rejected Mg-Clay (Rich in Smectite and Sepiolite) on the Zeolitization of Lightweight Aggregates
3.2.1. Mineralogical Changes and Zeolitization
3.2.2. Impact of the Rejected Mg-Clay on the Technological Properties of the ZLAs
4. Conclusions
- The incorporation of MPF in proportions not exceeding 10 wt.% would be positive since its combustion during firing favors the formation of open porosity, which makes the subsequent alkaline hydrothermal treatment more effective. Therefore, adjusting the percentage of MPF in the mixture could help control the final properties of the material obtained. Thus, although the mechanical strength decreases with the MPF, the lightness and degree of zeolitization improve.
- The type of clay used in manufacturing is a critical aspect. Kaolin is very prone to zeolitization, being a suitable raw material for the manufacture of ZLAs. On the contrary, the rejected Mg-clay investigated (rich in magnesium smectite and sepiolite) seems to negatively affect zeolitization when it is added in high proportions. However, in a moderate content (33% rejected Mg-clay over 67% kaolin), it can give rise to materials with properties similar, or even superior (e.g., mechanical strength), to those obtained with kaolin alone. This invites further research into this clay with different formulation and manufacturing conditions, including the application of acid pretreatments for Mg removal.
- The hydrothermal zeolitization method allows working at significantly lower firing temperatures (600–900 °C in this work) than those usual for lightweight aggregates (>1000 °C). This can provide an important economic and environmental advantage by reducing energy consumption during manufacturing. In any case, it will be necessary to investigate these aspects in more detail in the future (for example, through life cycle assessment), especially to check if the emissions linked to the combustion of the MPF are compensated by the energy savings during hydrothermal manufacturing.
- The high zeolite content in the new lightweight aggregates could give them advanced properties compared to conventional ones. In addition to a significant increase in mechanical strength (even >50 fold compared to the starting fired material), it is well known the adsorbent capacity of zeolites, thus paving the way to investigate the decontamination capacity of the new ZLAs.
- Despite the potential environmental advantages indicated above, the impact generated by the burning of plastic waste in the manufacturing process is an aspect to be evaluated (pending for another study). However, it should be noted that the most widely used way of disposing of plastic waste in Europe is energy recovery by incineration [42], which indicates that a viable and safe technology already exists, which could be adapted to the manufacturing method presented in this work.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fundación Conama. Decálogo Ciudadano Contra las Basuras Marinas; Ministerio para la Transición Ecológica, Ed.; Centro de Publicaciones: Madrid, Spain, 2016; Catálogo de Publicaciones de la Administración General del Estado, NIPO: 638-18-002-8. [Google Scholar]
- Zhang, Y.; Wu, P.; Xu, R.; Wang, X.; Lei, L.; Schartup, A.T.; Peng, Y.; Pang, Q.; Wang, X.; Mai, L.; et al. Plastic waste discharge to the global ocean constrained by seawater observations. Nat. Commun. 2023, 14, 1372. [Google Scholar] [CrossRef]
- Ministerio para la Transición Ecológica y el Reto Demográfico. Basuras Marinas. 2023. Available online: https://www.miteco.gob.es/es/costas/temas/proteccion-medio-marino/basuras-marinas.html (accessed on 11 October 2023).
- Cózar, A.; Sanz-Martín, M.; Martí, E.; González-Gordillo, J.I.; Ubeda, B.; Gálvez, J.A.; Irigoien, X.; Duarte, M. Plastic Accumulation in the Mediterranean Sea. PLoS ONE 2015, 10, e0121762. [Google Scholar] [CrossRef]
- Ministerio para la Transición Ecológica. Basuras Marinas; Catálogo Publicaciones de la Administración General de Estado: Madrid, Spain, 2016; NIPO: 013-18-097-2. [Google Scholar]
- Fu, Y.C.; Ho, M.L.; Wu, S.C.; Hsieh, H.S.; Wang, C.K. Porous bioceramic bead prepared by calcium phosphate with sodium alginate gel and PE powder. Mater. Sci. Eng. C 2008, 28, 1149–1158. [Google Scholar] [CrossRef]
- Vitale-Brovarone, C.; Verné, E.; Robiglio, L.; Martinasso, G.; Canuto, R.A.; Muzio, G. Biocompatible glass–ceramic materials for bone substitution. J. Mater. Sci. Mater. Med. 2008, 19, 471–478. [Google Scholar] [CrossRef]
- Kirschner, L.I. Lightweight Aggregate for Concrete and Method for Making Same. United States Patent No. 3992216, 16 November 1976. [Google Scholar]
- Malloy, R.; Kashi, M.G.; Swan, C.W. Fly Ash/Mixed Plastic Aggregate and Products Made Therefrom. United States Patent No. US6669773B2, 30 December 2003. [Google Scholar]
- Moreno-Maroto, J.M.; González-Corrochano, B.; Alonso-Azcárate, J.; Martínez García, C. A study on the valorization of a metallic ore mining tailing and its combination with polymeric wastes for lightweight aggregates production. J. Clean. Prod. 2019, 212, 997–1007. [Google Scholar] [CrossRef]
- Moreno-Maroto, J.M.; González-Corrochano, B.; Alonso-Azcárate, J.; Martínez García, C. Sintering of sepiolite-rich by-products for the manufacture of lightweight aggregates: Technological properties, thermal behavior and mineralogical changes. Mater. Construcción 2021, 71, e241. [Google Scholar] [CrossRef]
- Ramamurthy, K.; Harikrishnan, K.I. Influence of binders on properties of sintered fly ash aggregate. Cem. Concr. Compos. 2006, 28, 33–38. [Google Scholar] [CrossRef]
- Güneyisi, E.; Gesoglu, M.; Altan, I.; Öz, H.O. Utilization of cold bonded fly ash lightweight fine aggregates as a partial substitution of natural fine aggregate in self-compacting mortars. Constr. Build. Mater. 2015, 74, 9–16. [Google Scholar] [CrossRef]
- Nor, A.M.; Yahya, Z.; Abdullah, M.M.A.B.; Razak, R.A.; Ekaputri, J.J.; Faris, M.A.; Hamzah, H.N. A Review on the Manufacturing of Lightweight Aggregates Using Industrial By-Product. In Proceedings of the 2nd International Conference on Green Design and Manufacture 2016 (IConGDM 2016) MATEC Web of Conferences, Phuket, Thailand, 1–2 May 2016; Volume 78, p. 01067. [Google Scholar] [CrossRef]
- Mackenzie, K.J.; Welter, M. Geopolymer (aluminosilicate) composites: Synthesis, properties and applications. In Advances in Ceramic Matrix Composites; Woodhead Publishing: Cambridge, UK, 2014; pp. 445–470. [Google Scholar] [CrossRef]
- Peyne, J.; Gautron, J.; Doudeau, J.; Rossignol, S. Development of low temperature lightweight geopolymer aggregate, from industrial Waste, in comparison with high temperature processed aggregates. J. Clean. Prod. 2018, 189, 47–58. [Google Scholar] [CrossRef]
- Ayati, B.; Ferrándiz-Mas, V.; Newport, D.; Cheeseman, C. Use of clay in the manufacture of lightweight aggregate. Constr. Build. Mater. 2018, 162, 124–131. [Google Scholar] [CrossRef]
- Fawer, M.; Concannon, M.; Rieber, W. Life cycle inventories for the production of sodium silicates. Int. J. Life Cycle Assess. 1999, 4, 207–212. [Google Scholar] [CrossRef]
- AMI2030. Materials 2030 Manifesto: Systemic Approach of Advanced Materials for Prosperity—A 2030 Perspective. Advanced Materials Initiative 2030. 2022. Available online: https://www.ami2030.eu/wp-content/uploads/2022/06/advanced-materials-2030-manifesto-Published-on-7-Feb-2022.pdf (accessed on 7 February 2022).
- AMI2030. Materials 2030 Roadmap. Advanced Materials Initiative 2030. 2022. Available online: https://www.ami2030.eu/wp-content/uploads/2022/12/2022-12-09_Materials_2030_RoadMap_VF4.pdf (accessed on 7 February 2022).
- Moreno-Maroto, J.M.; Alonso-Azcárate, J. Hydrothermal zeolitization: Towards a paradigm shift for producing stronger and more sustainable construction materials. Constr. Build. Mater. 2024, 427, 136269. [Google Scholar] [CrossRef]
- ASTM D4318-10e1; Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2017.
- Moreno-Maroto, J.M.; González-Corrochano, B.; Alonso-Azcárate, J.; Rodríguez, L.; Acosta, A. Development of lightweight aggregates from stone cutting sludge, plastic wastes and sepiolite rejections for agricultural and environmental purposes. J. Environ. Manag. 2017, 200, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Occasional Papers of the Geological Institute of Hungary; Geological Institute of Hungary: Budapest, Hungary, 2011; Volume 213, ISBN 978-963-671-288-4. [Google Scholar]
- Yashima, S.; Kanda, Y.; Sano, S. Relationship between particle size and fracture energy or impact velocity required to fracture as estimated from single particle crushing. Powder Technol. 1987, 51, 277–282. [Google Scholar] [CrossRef]
- Li, Y.; Wu, D.; Zhang, J.; Chang, L.; Fang, Z.; Shi, Y. Measurement and statistics of single pellet mechanical strength of differently shaped catalysts. Powder Technol. 2000, 113, 176–184. [Google Scholar] [CrossRef]
- EN 1097-6; Tests for Mechanical and Physical Properties of Aggregates—Part 6: Determination of Particle Density and Water Absorption. European Committee for Standardization: Brussels, Belgium, 2013.
- IZA. Database of Zeolite Structures. International Zeolite Association. 2024. Available online: https://europe.iza-structure.org/IZA-SC/ftc_table.php (accessed on 1 July 2024).
- Hansen, S.; Fälth, L. X-ray study of the nepheline hydrate I structure. Zeolites 1982, 2, 162–166. [Google Scholar] [CrossRef]
- Lin, D.-C.; Xu, X.-W.; Zuo, F.; Long, Y.-C. Crystallization of JBW, CAN, SOD and ABW type zeolite from transformation of meta-kaolin. Microporous Mesoporous Mater. 2004, 70, 63–70. [Google Scholar] [CrossRef]
- Deng, Y.; Flury, M.; Harsh, J.B.; Felmy, A.R.; Qafoku, O. Cancrinite and sodalite formation in the presence of cesium, potassium, magnesium, calcium and strontium in Hanford tank waste simulants. Appl. Geochem. 2006, 21, 2049–2063. [Google Scholar] [CrossRef]
- Selim, A.Q.; Mohamed, E.A.; Seliem, M.K.; Zayed, A.M. Synthesis of sole cancrinite phase from raw muscovite: Characterization and optimization. J. Alloys Compd. 2018, 762, 653–667. [Google Scholar] [CrossRef]
- Chen, X.; Qiao, M.; Xie, S.; Fan, K.; Zhou, W.; He, H. Self-Construction of Core−Shell and Hollow Zeolite Analcime Icositetrahedra: A Reversed Crystal Growth Process via Oriented Aggregation of Nanocrystallites and Recrystallization from Surface to Core. J. Am. Chem. Soc. 2007, 129, 13305–13312. [Google Scholar] [CrossRef]
- Ma, X.; Yang, J.; Ma, H.; Liu, C.; Zhang, P. Synthesis and characterization of analcime using quartz syenite powder by alkali-hydrothermal treatment. Microporous Mesoporous Mater. 2015, 201, 134–140. [Google Scholar] [CrossRef]
- Jacas-Rodríguez, A.; Rodríguez-Pascual, P.; Franco-Manzano, D.; Contreras, L.; Polop, C.; Rodriguez, M.A. Mixed Matrix Membranes prepared from polysulfone and Linde Type A zeolite. Sci. Eng. Compos. Mater. 2020, 27, 236–244. [Google Scholar] [CrossRef]
- Campoverde, J.; Guaya, D. From Waste to Added-Value Product: Synthesis of Highly Crystalline LTA Zeolite from Ore Mining Tailings. Nanomaterials 2023, 13, 1295. [Google Scholar] [CrossRef]
- Zgureva, D.; Boycheva, S. Synthetic Zeolitic Ion-Exchangers from Coal Ash for Decontamination of Nuclear Wastewaters. Bulg. Nucl. Soc. Trans. 2015, 20, 132–136. [Google Scholar]
- Tang, Q.; Ge, Y.Y.; Wang, K.T.; He, Y.; Cui, X.M. Preparation of porous P-type zeolite spheres with suspension solidification method. Mater. Lett. 2015, 161, 558–560. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, X.; Qiu, Q.; Zhao, Y.; Long, L. Synthesis of high-quality NaP1 zeolite from municipal solid waste incineration fly ash by microwave-assisted hydrothermal method and its adsorption capacity. Sci. Total Environ. 2023, 855, 158741. [Google Scholar] [CrossRef] [PubMed]
- Khodabandeh, S.; Davis, M.E. Zeolites P1 and L as precursors for the preparation of alkaline-earth zeolites. Microporous Mater. 1997, 12, 347–359. [Google Scholar] [CrossRef]
- EN 13055-1; Lightweight Aggregates. Part 1: Lightweight Aggregates for Concrete, Mortar and Grout. European Committee for Standardization: Brussels, Belgium, 2002.
- European Parliament. Plastic Waste and Recycling in the EU: Facts and Figures. 2024. Available online: https://www.europarl.europa.eu/topics/en/article/20181212STO21610/plastic-waste-and-recycling-in-the-eu-facts-and-figures (accessed on 11 August 2024).
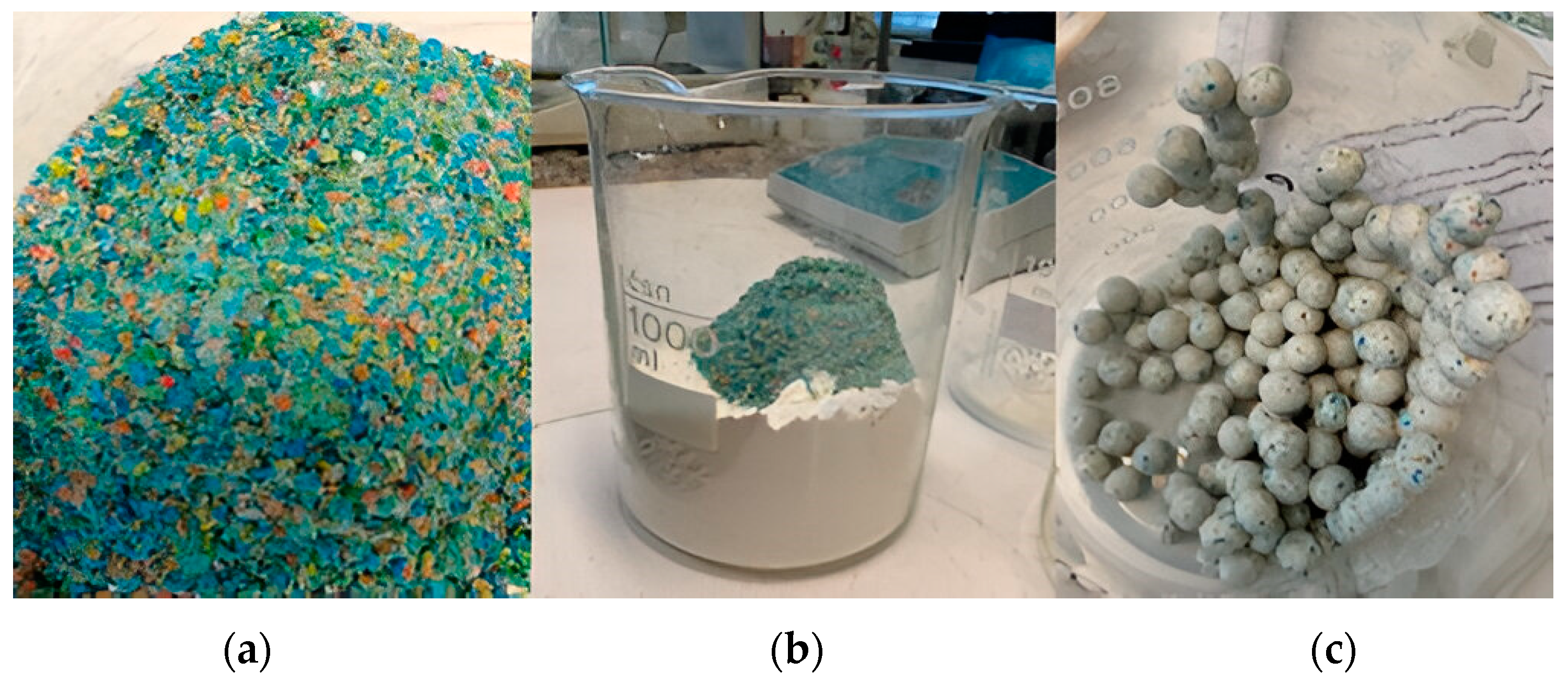
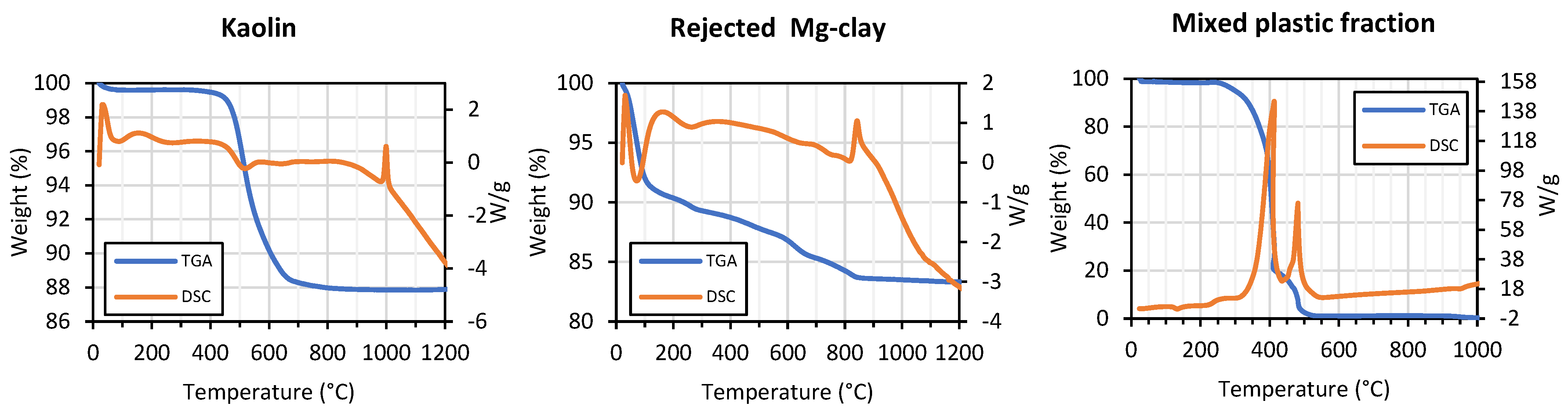


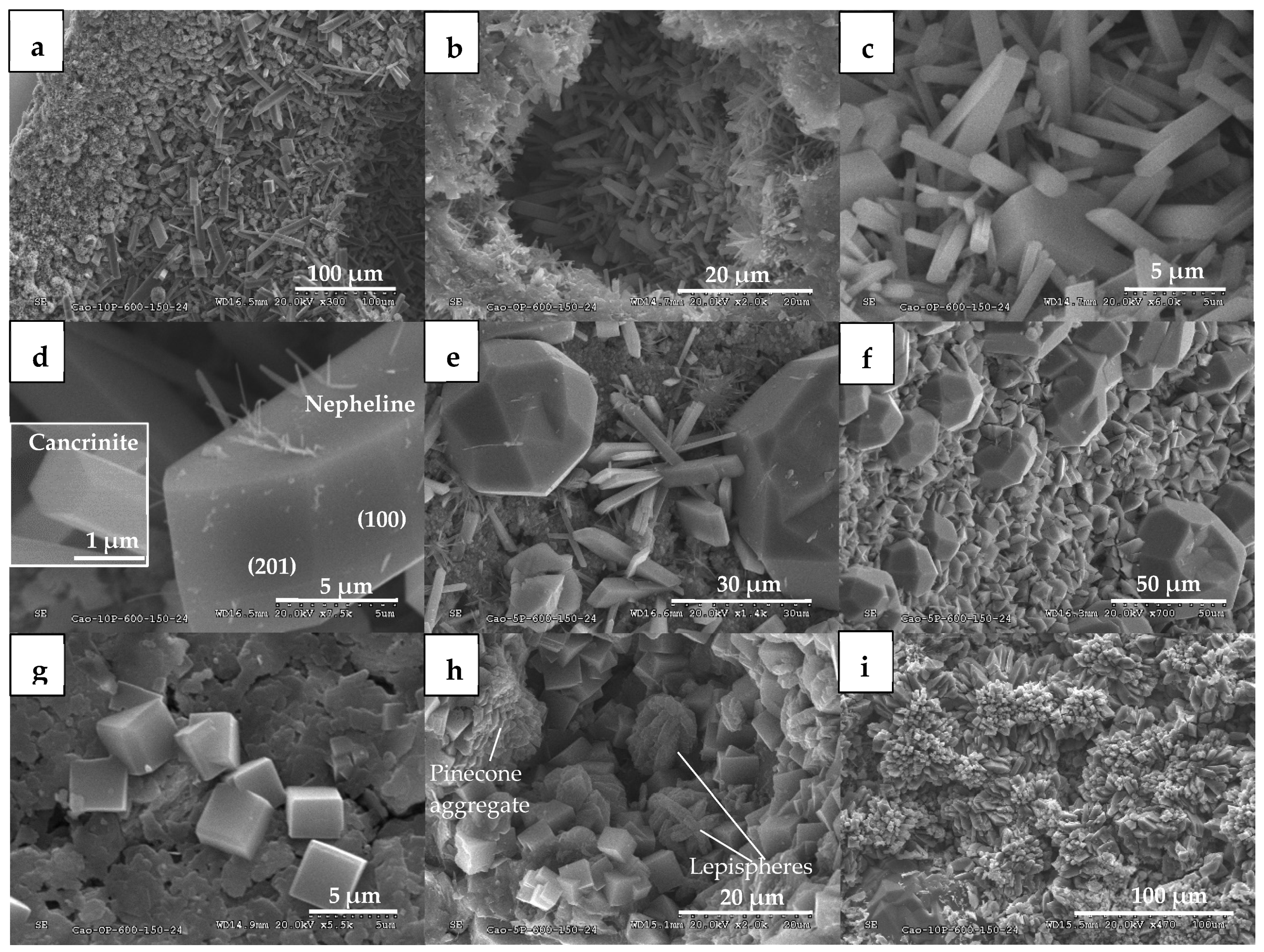
| Clay | Particle Size (µm) a | Atterberg Limits b | Carbon (%) c | Chemical Composition (%) d | Mineralogy (%) e | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d50 | Mean | LL | PL | Total (Org.) | SiO2 | Al2O3 | Fe2O3 | K2O | CaO | MgO | Others | LOI | Kao | Sm | Sp | Il | Q | Pg | Fp | Cal | Dol | |
| Kaolin | 4.6 | 8.3 | 42.1 | 27.0 | ̶ | 50.9 | 35 | 0.4 | 0.4 | 0.1 | 0.1 | 0.5 | 12.6 | 84.9 | ̶̶̶̶ | ̶̶̶̶ | 2.5 | 12.6 | ̶ | ̶ | ̶ | ̶ |
| Rejected Mg-clay | 18.5 | 16.4 | 171.1 | 74.8 | 0.85 (0.44) | 54.2 | 7.2 | 2.0 | 1.6 | 2.0 | 21.9 | 1.3 | 9.8 | ̶ | 37.8 | 30 | 3.9 | 11.1 | 4.3 | 7.9 | 2.6 | 2.4 |
| Sample | Mineralogical Composition (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kao | Ill | Q | Zeo A a | Can ab | Hysod ab | Zeo P1 a | Neph ab | Ana a | Am | Σ Zeolite ab | Σ Other Minerals | |
| Kaolin (unfired) | 84.9 | 2.5 | 12.6 | 100 | ||||||||
| K-0P-600 | 6.0 | 2.6 | 9.2 | 82.2 | 17.8 | |||||||
| K-5P-600 | 2.2 | 1.9 | 9.2 | 86.7 | 13.3 | |||||||
| K-10P-600 | 2.1 | 1.9 | 9.8 | 86.2 | 13.8 | |||||||
| K-0P-600-H | 0.7 | 5.0 | 9.6 | 20.1 | 3.9 | 0.2 | 16.9 | 1.0 | 42.6 | 51.7 | 5.7 | |
| K-5P-600-H | 0.4 | 3.1 | 20.5 | 5.9 | 8.4 | 5.2 | 16.8 | 1.5 | 38.3 | 58.3 | 3.5 | |
| K-10P-600-H | 33.5 | 2.3 | 0.8 | 33.2 | 10.1 | 20.2 | 79.9 | |||||
| Aggregate | Diameter (mm) | Crushing Strength (MPa) | Density (g/cm3) | Water Absorption (%) | Porosity (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | St. Dv. | Δ (%) a | Mean | St. Dv. | Δ Ratio a | ρrd | ρR | Δ ρrd (%) a | WA24 | Δ (%) a | PT | Δ PT (%) a | PO | PC | |
| K-0P-600 | 10.6 | 0.7 | 0.45 | 0.07 | 1.49 | 2.695 | 25.1 | 44.6 | 37.6 | 7.0 | |||||
| K-5P-600 | 10.7 | 0.7 | 0.13 | 0.03 | 1.34 | 2.635 | 27.8 | 49.3 | 37.2 | 12.2 | |||||
| K-10P-600 | 10.5 | 0.6 | 0.04 | 0.02 | 1.36 | 2.641 | 23.7 | 48.6 | 32.2 | 16.4 | |||||
| K-0P-600-H | 10.7 | 0.6 | 0.9 | 5.5 | 1.0 | 12.3 | 1.79 | 2.365 | 19.7 | 12.2 | −51.4 | 24.4 | −45.2 | 21.9 | 2.6 |
| K-5P-600-H | 11.2 | 0.8 | 4.7 | 4.4 | 0.7 | 33.8 | 1.66 | 2.249 | 24.3 | 12.5 | −54.9 | 26.2 | −46.9 | 20.8 | 5.4 |
| K-10P-600-H | 11.0 | 0.6 | 4.8 | 2.3 | 0.6 | 57.5 | 1.49 | 2.348 | 9.5 | 16.6 | −29.8 | 36.7 | −24.5 | 24.8 | 11.9 |
| Sample | Mineralogical Composition (%) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kao | Ill | Sm | Sp | Q | Plag | Fp | Cal | Dol | Pig | Mu | Zeo A a | Can ab | Sod ab | Phi a | Neph ab | Am | Σ Zeolite | Σ Other Minerals | |
| Kaolin (unfired) | 84.9 | 2.5 | 12.6 | 100 | |||||||||||||||
| Rejected Mg-clay (unfired) | 3.9 | 37.8 | 30 | 11.1 | 4.3 | 7.9 | 2.6 | 2.4 | 100 | ||||||||||
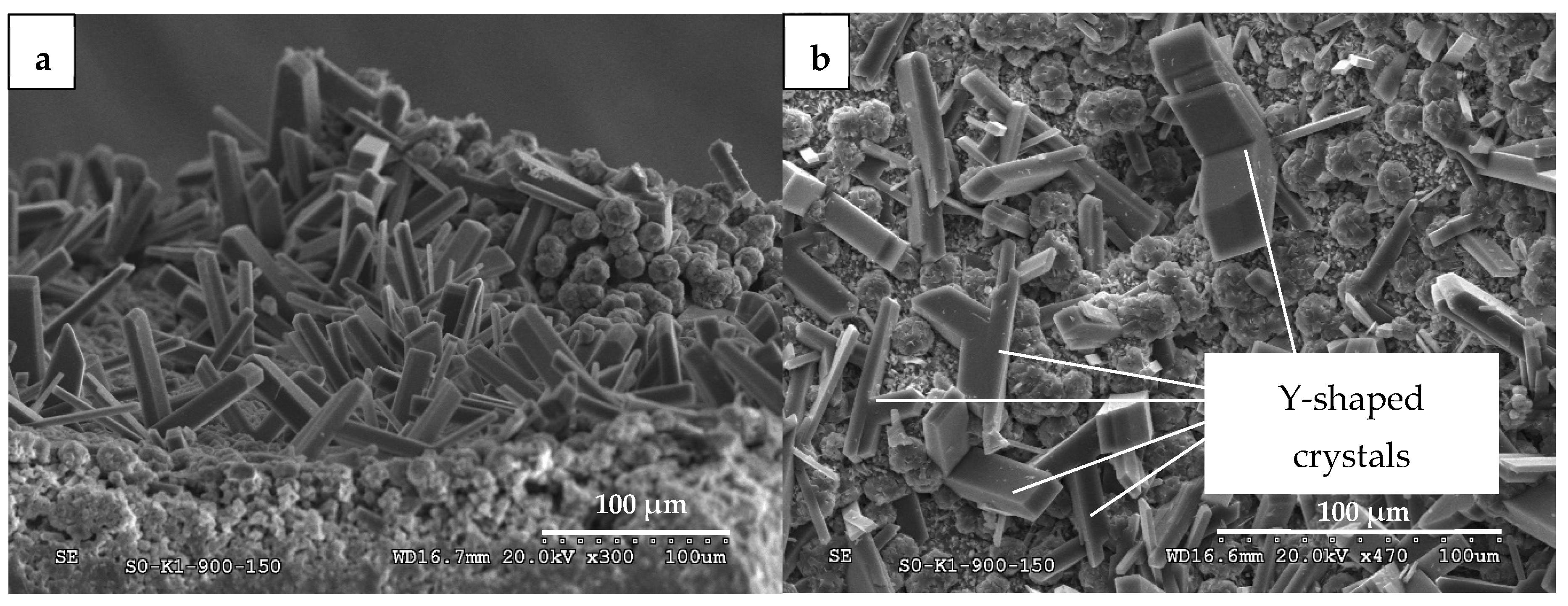
| S0K1-10P-900 c | 3.2 | 12.2 | 84.7 | 15.4 | |||||||||||||||
| S1K2-10P-900 c | 1.7 | 9.1 | 1.4 | 0.7 | 3.8 | 13 | 70.3 | 29.7 | |||||||||||
| S0K1-10P-900-H c | 0.1 | 1.7 | 45.4 | 5 | 13.1 | 11.8 | 22.8 | 77 | 0.1 | ||||||||||
| S1K2-10P-900-H c | 0.2 | 1.5 | 40.9 | 1.4 | 4.2 | 51.8 | 48 | 0.2 | |||||||||||
| Aggregate | Diameter (mm) | Crushing Strength (MPa) | Density (g/cm3) | Water Absorption (%) | Porosity (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | St. Dv. | Δ (%) a | Mean | St. Dv. | Δ Ratio a | ρrd | ρR | Δ ρrd (%) a | WA24 | Δ (%) a | PT | Δ PT (%) a | PO | PC | |
| S0K1-10P-900 | 10.0 | 0.9 | 0.2 | 0.1 | 1.39 | 2.722 | 25.8 | 48.8 | 36.0 | 12.7 | |||||
| S1K2-10P-900 | 9.5 | 0.6 | 1.7 | 0.4 | 1.44 | 2.859 | 24.1 | 49.7 | 34.8 | 15.0 | |||||
| S0K1-10P-900-H | 10.5 | 0.7 | 5.0 | 3.1 | 0.6 | 20.7 | 1.65 | 2.312 | 18.1 | 11.8 | −54.1 | 28.8 | −41.0 | 19.5 | 9.2 |
| S1K2-10P-900-H | 9.6 | 0.6 | 1.1 | 3.4 | 0.8 | 2.0 | 1.74 | 2.395 | 21.1 | 11.5 | −52.4 | 27.4 | −45.0 | 20.0 | 7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Maroto, J.M.; Govea, J.M.; Poza, P.; Regadío, M.; Cuevas, J.; Ruiz, A.I.; Fernández, R.; Alonso-Azcárate, J. Hydrothermal Manufacture of Zeolitic Lightweight Aggregates from Clay and Marine Plastic Litter. Appl. Sci. 2024, 14, 7674. https://doi.org/10.3390/app14177674
Moreno-Maroto JM, Govea JM, Poza P, Regadío M, Cuevas J, Ruiz AI, Fernández R, Alonso-Azcárate J. Hydrothermal Manufacture of Zeolitic Lightweight Aggregates from Clay and Marine Plastic Litter. Applied Sciences. 2024; 14(17):7674. https://doi.org/10.3390/app14177674
Chicago/Turabian StyleMoreno-Maroto, José Manuel, Julia M. Govea, Pablo Poza, Mercedes Regadío, Jaime Cuevas, Ana Isabel Ruiz, Raúl Fernández, and Jacinto Alonso-Azcárate. 2024. "Hydrothermal Manufacture of Zeolitic Lightweight Aggregates from Clay and Marine Plastic Litter" Applied Sciences 14, no. 17: 7674. https://doi.org/10.3390/app14177674
APA StyleMoreno-Maroto, J. M., Govea, J. M., Poza, P., Regadío, M., Cuevas, J., Ruiz, A. I., Fernández, R., & Alonso-Azcárate, J. (2024). Hydrothermal Manufacture of Zeolitic Lightweight Aggregates from Clay and Marine Plastic Litter. Applied Sciences, 14(17), 7674. https://doi.org/10.3390/app14177674












