Featured Application
Polystyrene recycling.
Abstract
This work proposes a conceptual design for recovering polystyrene (PS) using solvents of agro-industrial origin. The literature describes the dilution of expanded polystyrene (EPS) in limonene, followed by its insolubilization with alcohols for recovery. However, there is no information on the solubility limit for the PS + limonene + alcohol system, which is critical for the process design. To determine the solubility limit, we diluted the waste EPS in D-limonene, mixed it with ethanol to form a precipitate, and used a gravimetric method to measure the mass of the compounds. These results allowed for the conceptual design of an EPS recycling process using a chemical process simulator, which includes a separator, a distillation column, and auxiliary equipment such as heaters, coolers, and pumps. An empirical correlation was obtained for the solubility limit, which enabled the design of a process for the treatment of 52 kg/s of PS using 0.75 kg/s of ethanol and 2.4 kg/s of D-limonene once the stationary state had been reached. The distillation column is six-stage, with a reflux ratio of 1.5 and duties of 30,000 and −25 847 kW for the condenser and reboiler, respectively.
1. Introduction
Expanded polystyrene (EPS) is a foam-like material with low density and high malleability, making it ideal for creating a variety of shapes. It is microbe and impact-resistant, with low thermal and electrical conductivity, making it ideal for packaging and construction [1]. Free radical vinyl polymerization in a suspension is used to produce EPS, which is then foamed with n-pentane. Once the styrene has reached the desired conversion point, steam heating transforms it into boiling foam, resulting in an eightfold increase in volume. After the maturation period is complete, the molds are filled with polymer and heated further with steam. This causes the polymer to expand and form the final cellular foam structure. During this process, air replaces the foaming agent, accounting for approximately 98% of the EPS volume [2]. Because of its thermal and biological stability, this material is frequently used to make trays, cups, and disposable food packaging. Furthermore, due to its mechanical properties, it is used as packaging for the transportation of electronic and household appliances, as well as a structural component that provides thermal and acoustic insulation in the construction industry [3].
EPS is a waste that causes problems in water and land because it is not degradable by nature [4] and takes up to 500 years for degradation [5]. In addition to having a faulty disposition plan, EPS accumulates in ponds, seas, and other places. Furthermore, animals mistake EPS for food, leading them to consume it. The linear consumption market results in a shorter shelf life for EPS; however, the new trend is the Circular Economy (CE) model, where consumers are responsible for more than just the 3 Rs, Reduce, Recycle, and Reuse, and must add 4 more Rs, Repair, Refurbish, Recover, and Rethink [6]. PS is a low-cost plastic, so its inclusion in a CE is complicated. In fact, achieving Reduce, Reuse, Repair, Refurbish, and Recover is nearly impossible, as people tend to view PS cups and trays as commodities that can be thrown away. In this context, alternatives to fighting PS pollution are to rethink and recycle, focusing on processes based on green chemistry and energy efficiency.
On the other hand, transport and storage are the main challenges in recycling EPS. Storing EPS is difficult because it takes up a large amount of space. A metric ton of waste EPS can occupy up to 200 cubic meters. Traditional treatment methods are unsuitable for the final arrangement of EPS, such as use as a material in sanitary landfills, because the compacting process requires significant effort due to its configuration and mechanical strength.
Researchers have proposed various treatments for EPS waste recycling, including mechanical recycling, pyrolysis, and dissolution [7]. Mechanical recycling involves physical changes, such as size reduction by crushing, melting in the extruder, and pelletization for reuse in the elaboration of new products. Some companies now use thermal compacting, pellet formation, and re-foaming to create new EPS for non-food applications [8]. Pyrolysis is a thermal decomposition to obtain waxes, oils, char, and gases; the temperature must be high (between 600 and 1000 K), and the atmosphere must be oxygen-low. These products serve as fuels or feedstock for other processes. For EPS, styrene is the main product of pyrolysis [9], but the reaction has a high activation energy and is very endothermic because the polymer is thermally stable [10]. Finally, the dissolution/precipitation method uses solvents to recycle plastics while preserving the chemical structure of polymers. The traditional methods of dissolving treatment involve choosing a solvent that matches the desired polymer for recycling, incorporating the polymer into the solvent, and recovering the polymer through filtration or decantation. Subsequently, adding an antisolvent or lowering the temperature precipitates the polymer.
In the past, scientists suggested diluting EPS with fossil solvents like benzene or toluene [11,12,13]. However, it is important to note that these substances are hazardous to human health and the environment [14].
A pilot-scale study proposed recycling EPS using p-cymene as a solvent and precipitating it with heptane to reduce the use of fossil-derived solvents. Furthermore, a comparison between mechanical and dissolution treatments revealed that the first treatment reduced PS’s molecular weight by 30% after four cycles, whereas the dissolution treatment did not affect the molecular weight and offered the advantage of removing contaminants such as chlorine, calcium, and iron from wastes [15]. Another study discusses the economical evaluation of recovery PS using R-(+)-limonene as a solvent and carbon dioxide in a pilot-scale plant to obtain microcellular foams. At the time of analysis, the reduction of the carbon footprint of the process was examined. The findings indicate the need to process more than 10 tons of PS per day to achieve economic benefits. However, the authors emphasized the environmental importance of recycling PS and reducing the carbon footprint for virgin PS production [16]. Recovery PS has the same mechanical properties as virgin PS in a dissolution-precipitation process, making it suitable for use in composites [4].
Given the preceding information, it is recommended to choose a solvent with a low environmental impact. A viable alternative to fossil solvents is to use molecules derived from plants or microorganisms, such as ethyl acetate or the terpene family [17,18]. Researchers have investigated several commercial oils, including limonene, nard, violet, lavender, and orange blossom [19] and star anise, eucalyptus, thyme, and chamomile [20], as environmentally friendly solvents for EPS. Both studies successfully recovered PS by adding alcohol but did not quantify the solubility limit. Other authors used commercial omega-3 to dissolve PS and precipitate it by adding alcohol [21]. Nevertheless, the study did not provide a specific value for the solubility limit. The effect of temperature on the time it takes for EPS to dissolve in limonene and eucalyptus oil has been investigated [14]. However, we did not interpret these results as a quantification of solubility limits. The studies aimed to characterize the polyester produced during the dilution-precipitation process using various essential oils.
In this work, we propose the use of limonene as a solvent for EPS and ethanol for precipitation. Limonene has the advantage of acting as an antioxidant for PS and protecting the polymer chains. On the other hand, ethanol is considered a cost-effective raw material because of its availability and reasonable price [22] (0.6 $/kg [23]) compared to the price of naphtha (0.65 $/kg [24]). Furthermore, it permits operations to be done at atmospheric pressure and reduces explosion risk in chemical processes.
Limonene is the predominant component of citrus oils, giving them their distinct aroma. Limonene accounts for 98% of the essential oil extracted from orange skin or peel [25]. It has long been used in fragrances and flavorings, and it serves a variety of functions as a solvent and cleanser. It is classified as a terpene, specifically a limonoid, and is part of a large group of functional foods and phytonutrients known for their antioxidant properties, which protect polymer chains and facilitate PS recycling [26]. Citrus oils are industrially produced by cold pressing the peel, steam distilling the pulp and rinds after juice extraction, and removing terpenes from essential citrus oils [27,28].
Ethanol, on the other hand, is a clear, rapidly evaporating liquid with a distinct odor and flavor. Industrial applications include the production of acetaldehyde, vinegar, butadiene, ethyl chloride, and nitrocellulose. Ethanol is widely used as a solvent in the manufacture of pharmaceuticals, plastics, lacquers, perfumes, and cosmetics. Furthermore, it serves as a source of energy, a surgical disinfectant, and an essential component in chemical production. It is also useful in the preservation of both normal and abnormal biological samples.
Because of their biodegradability, these molecules are attractive for developing green chemistry processes, as reported in the literature [29,30,31]. The goal of this research is to feature a chemical process for recycling EPS by dissolving it in D-limonene and then recovering it in solid form by insolubilizing it with ethanol [20]. We calculated the maximum amount of PS that can dissolve in a solution of D-limonene and ethanol and expressed it as an empirical equation, allowing us to optimize the amount of solvents required. Finally, we proposed a preliminary scheme for recycling EPS, which includes determining the necessary solvents, energy requirements, and fundamental equipment design characteristics.
2. Materials and Methods
2.1. Materials Used
This study utilized waste PS derived from laboratory material packaging. The PS was thoroughly cleaned to eliminate any non-polystyrene particles, such as dust. The D-limonene employed was a commercially available product from the Econoclear brand, boasting a purity level of 99%, as stated by the manufacturer. Absolute ethanol from the Meyer brand, with a purity of 99.5%, was utilized without undergoing any further purification procedures.
To characterize PS, we utilized the Mark–Houwink–Sakurada correlation to determine its average molecular weight through viscosity measurements. Benzene was employed as the solvent, and a Cannon-Fenske capillary viscometer model 9721-F59 CFOF-100 from Cannon Instrument Company (State College, PA, USA), immersed in a thermostatic bath at 298.15 K, was utilized. Once the intrinsic viscosity was determined, we referred to the literature to obtain the necessary parameters [32].
2.2. Determining Solubility Limit
Various blends of EPS and D-limonene were created with precise mass ratios ranging from 5% to 40% (w/w). These mixtures were then allowed to settle at room temperature for a period of two days to ensure that complete dissolution occurred. In order to separate any solid particles that were evenly distributed in the solution, the mixtures underwent centrifugation at a speed of 4500 rpm for a duration of 10 min. A 3 mL sample was introduced into a test tube, and the weight of the PS solution in the tube was measured. Ethanol was then added gradually using a burette until the cloud point was reached [33,34]. At this moment, the weight of the added alcohol was measured. Finally, the sample was centrifuged at 4500 rpm for 10 min. We evaporated the solvents by placing one milliliter of the supernatant in a porcelain capsule. Finally, we quantified the amount of PS in the solution using a gravimeter. The masses were determined using a VeLab model VE-204 analytical balance (Mexico City, Mexico), while the centrifuge employed was a clinical type DM0412S from Science Med (Helsinki, Finland).
2.3. Modeling of Continuous Stirred Tank (CST)
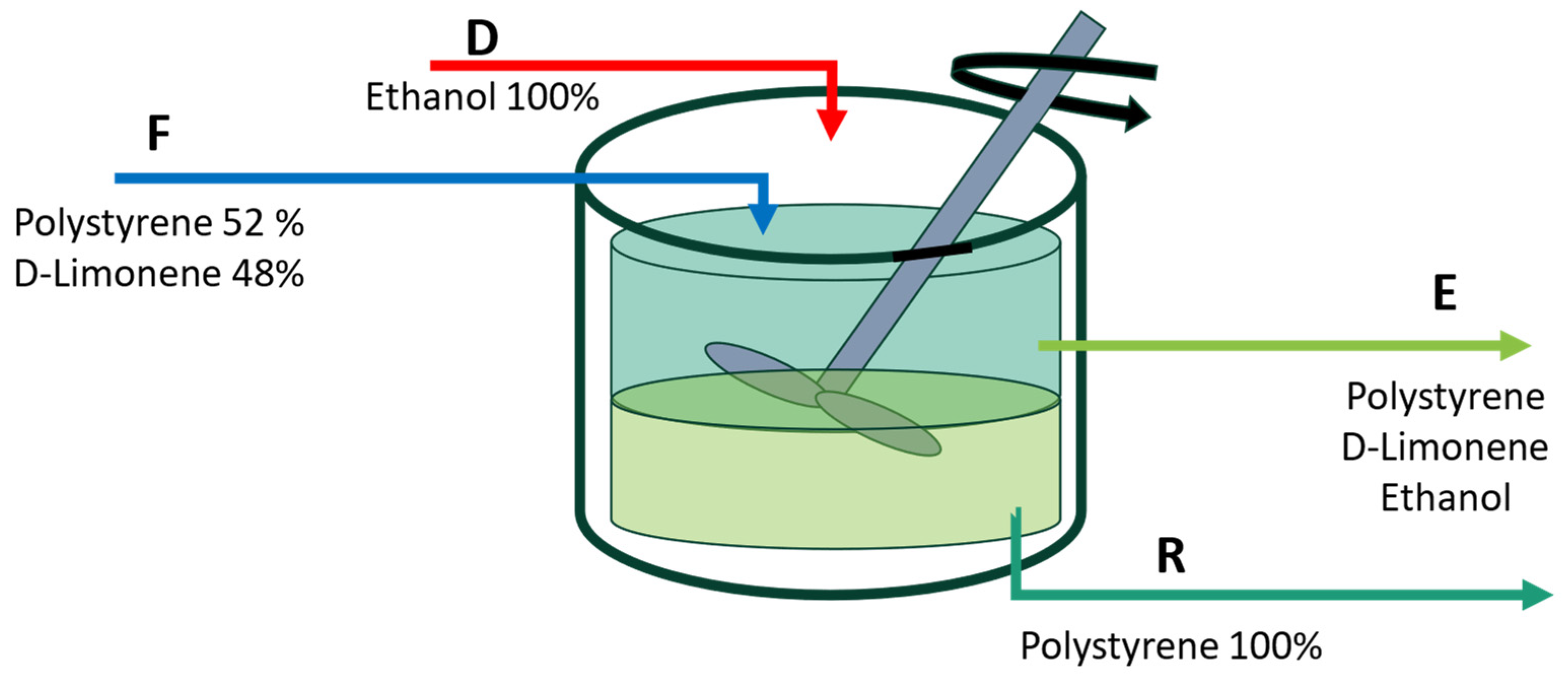
Figure 1 shows the intended application of a continuous stirred tank as a means of separation. Enough time is supposed to be allocated to achieve equilibrium in the solution.
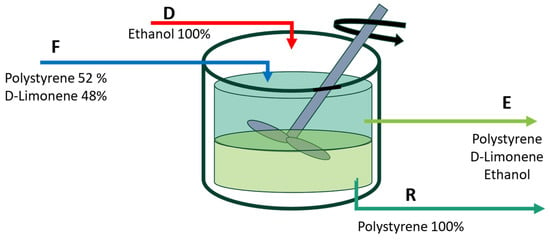
Figure 1.
The suggested solubility/insolubility process’s modeling scheme. F is the feed stream containing a mixture of D-limonene + expanded polystyrene; D is the ethanol stream to precipitate the polystyrene; E is the extract current that contains D-limonene + ethanol + polystyrene; R is the raffinate current containing the recovered polystyrene.
The literature states that D-limonene can dissolve up to 55% (w/w) of PS at 323.15 K [35] and 47.1% (w/w) at room temperature [36]. We can estimate the maximum PS processing capacity by averaging these values, given a polymer concentration of 52% (w/w) for a feed mass flow of 100 kg/s. The ethanol supply is intended to be fresh, and the quantity of alcohol required to extract a minimum of 99% of the PS in the raffinate stream is computed. According to the information presented in Figure 1, the mass balances are as follows:
where represents the feed containing a mixture of PS and limonene, is the incoming ethanol stream, corresponds to the extract, indicates the raffinate, and is the mass composition. The number in the subindex corresponds to the following substances: (1) polystyrene, (2) D-limonene, and (3) ethanol. The symbol in Equation (4) denotes the solubility limit, which is dependent on the ethanol concentration in the solvent and represents a ratio between the mass of PS and the weight of the solvent mixture of ethanol and D-limonene.
2.4. Conceptual Design and Process Simulation Development
After calculating the specific amount of ethanol required to recover 99% of PS, we devised a conceptual design for a more complete procedure that encompassed the retrieval and reutilization of solvents. The proposal entails the combination of PS and D-limonene, the introduction of ethanol, the retrieval of PS in solid form, the preparation of the solvent stream for separation in a plate distillation column, and ultimately, the recycling of the solvents. The PS was simulated using the Compound Creator Wizard, which incorporated data regarding its melting point, normal boiling temperature, and solid density. The design of the distillation column incorporated heuristic rules [37], beginning with the simulation of a column using a short-cut method. Following that, a sensitivity analysis was conducted to ascertain the precise number of stages and the optimal feeding plate. Ultimately, a rigorous distillation column model was employed, utilizing the Naphtali-Sandholm simultaneous correction method. The process simulation was conducted using DWSIM [38].
3. Results
3.1. Polystyrene Molecular Weight
The intrinsic viscosity () of PS-benzene solutions was evaluated using the Wolf model [39]:
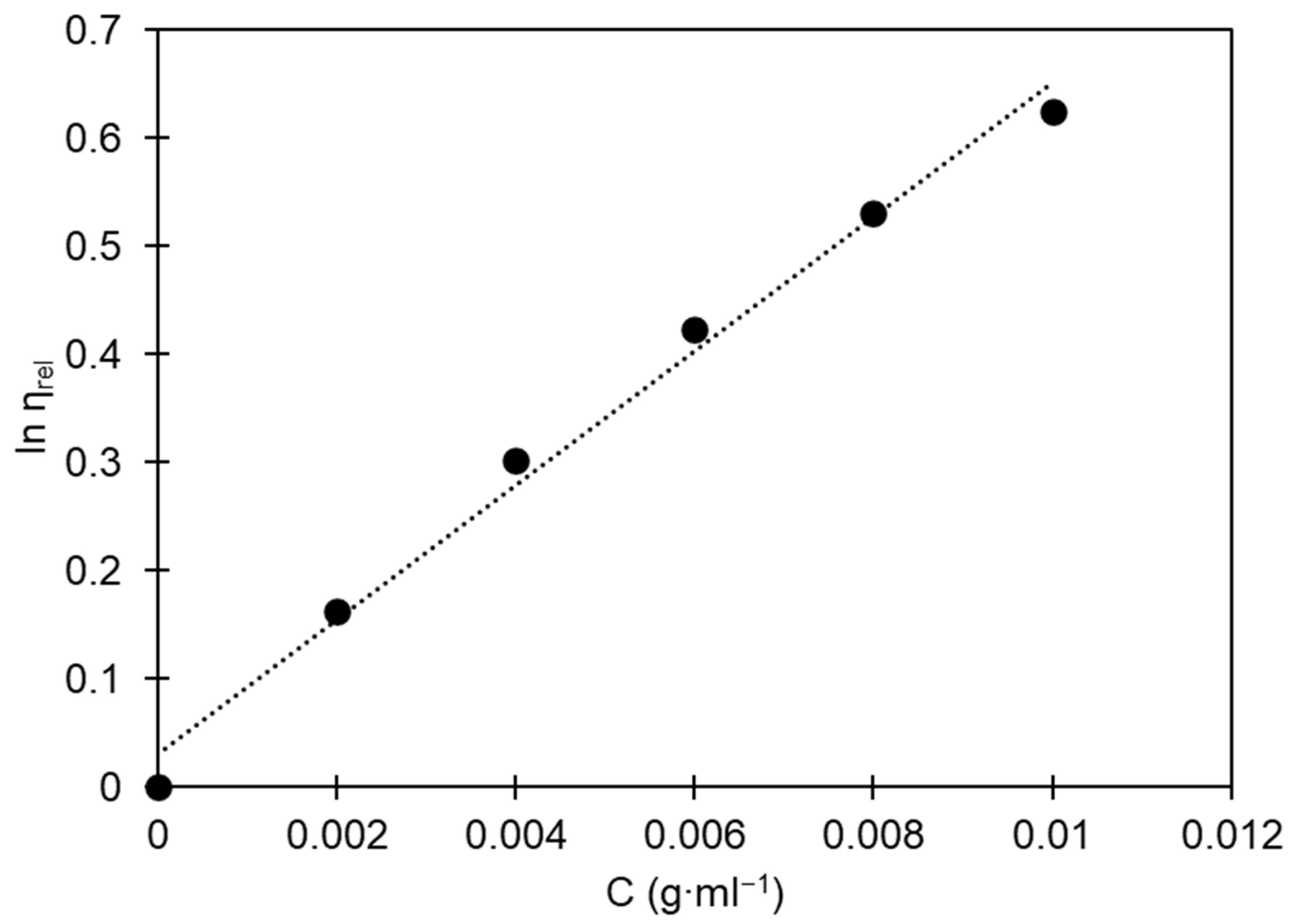
where is the relative viscosity, which is calculated as the ratio between the flow time of the polymer solution and the flow time of the pure solvent; is the concentration of polymer solutions, represents a specific system constant, and is the characteristic specific hydrodynamic volume. Figure 2 illustrates the relationship between the concentration of PS in benzene and the corresponding relative viscosity (). Using the least-squares method, we fit parameters (), , and to experimental data.
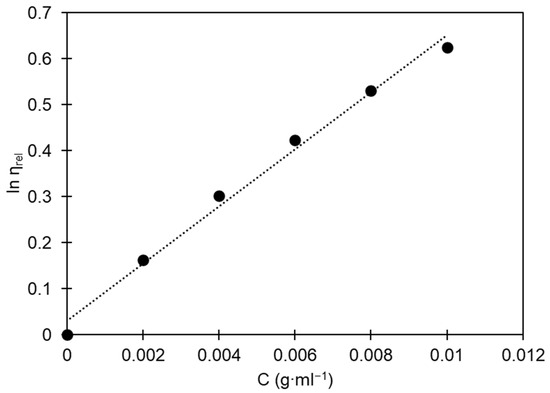
Figure 2.
Relationship between the natural logarithm of relative viscosity and the concentration of polystyrene solutions in benzene for determining intrinsic viscosity. Black dots are experimental data; the dotted line is a linear regression line.
The relationship between intrinsic viscosity and average molecular weight () for the PS + benzene system can be described by an empirical correlation [32]:
In this study, the intrinsic viscosity of the analyzed sample was found to be 87.55 mL∙g−1, indicating a molecular weight of 229,013 g∙mol−1. This value corresponds to the expected molecular weight for commercially available PS [2]. The Newton–Raphson method was employed to numerically solve Equation (6).
3.2. Solubility Limit
D-limonene is a natural solvent for PS and can be easily mixed with ethanol, regardless of the presence of the hydroxyl group in the alcohol’s structure. However, the lack of polarity in the polymer is unsuitable for the polarity of ethanol, resulting in the precipitation of the PS and its conversion into a solid form. This phenomenon is referred to as the solubility limit, which was assessed in this study using gravimetric analysis. The solubility limit was determined using the equation
where represents the remaining mass of PS after the solvent has been evaporated, and is the initial mass of the mixture consisting of PS, D-limonene, and ethanol, which was placed in the porcelain capsule before evaporation.
We establish a relationship between solubility and the ethanol composition in the solvent mixture free of PS, using the equation
where represents the mass of ethanol added during the insolubility experiment, represents the mass composition of PS in the mixture of PS + D-limonene before the precipitation experiment, and represents the mass of the PS + D-limonene mixture used in the precipitation experiment.
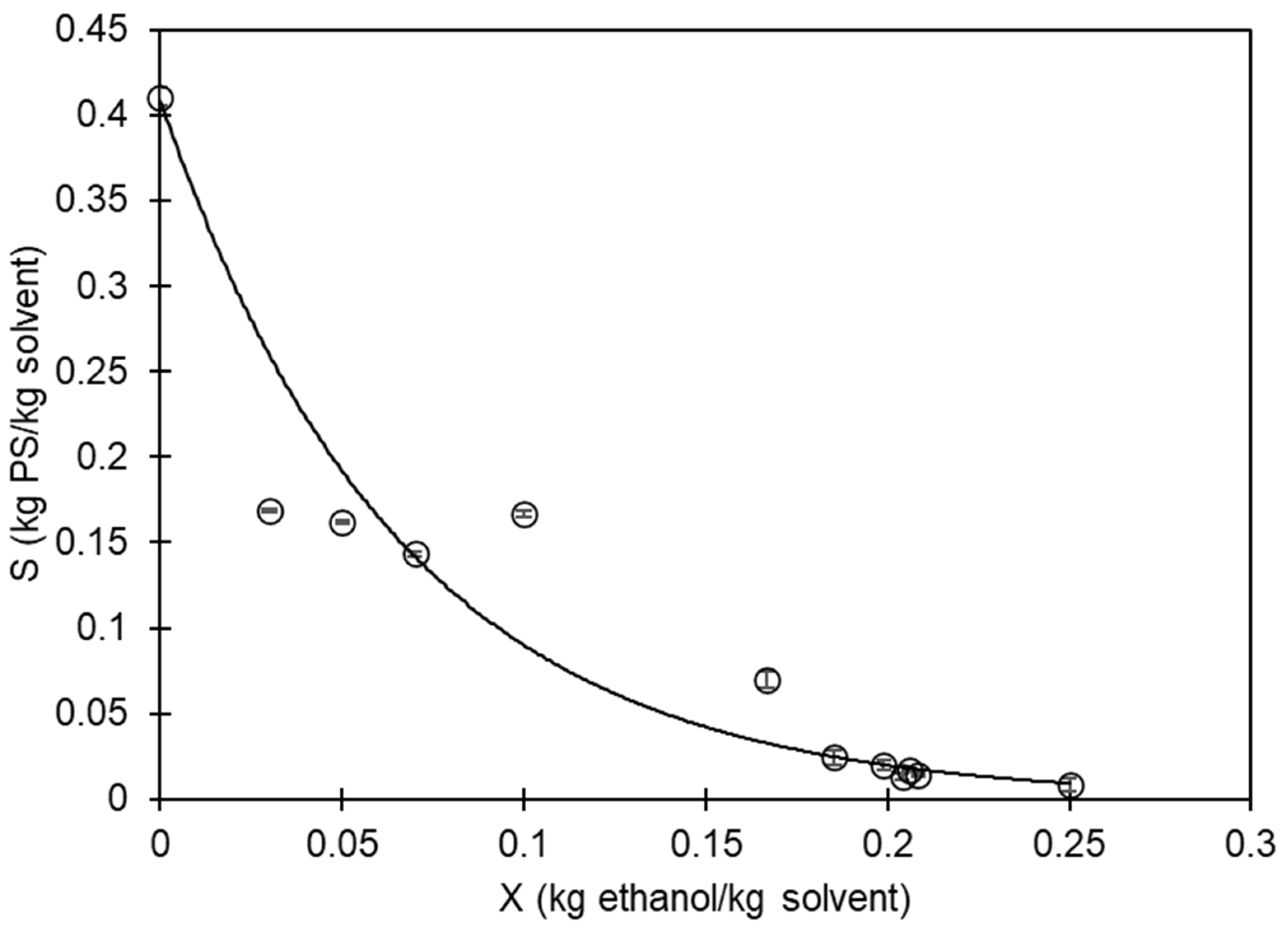
Figure 3 displays the empirical data regarding the maximum amount of PS that can dissolve in the mixture of D-limonene and ethanol. It is evident that as the concentration of ethanol in the mixture increases, the solubility of PS in D-limonene decreases. The data were adjusted to an exponential-type equation using the least square method:
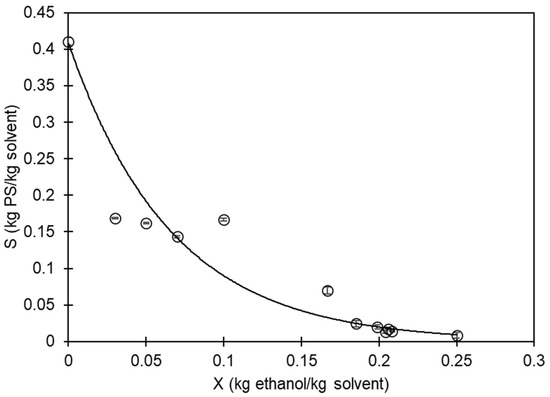
Figure 3.
Correlation between ethanol (X) and polystyrene (S) mass in the solvent mixture. White dots with error bars are experimental data; the solid line represents the solubility limit.
Figure 3 depicts the solubility limit, which can be utilized to recover and utilize EPS waste. By dissolving it in D-limonene, the trapped air that makes it voluminous is eliminated. Furthermore, by adding ethanol, the PS can be recovered as a solid. The D-limonene + ethanol mixture can then be separated through distillation to recover the solvents.
3.3. The Continuous Stirred Tank
To obtain an initial estimate of process variables, we proceed as follows: First, suppose a mass flow feed with a mass composition of PS ; we vary the mass flow of fresh ethanol () from 0 to 50 kg/s. After that, we compute using the definition from Equation (8) as follows:
and calculate from Equation (9). To find the mass composition of PS () and the mass flow of extract (), we solve Equations (1), (2) and (4) simultaneously using algebraic methods, obtaining
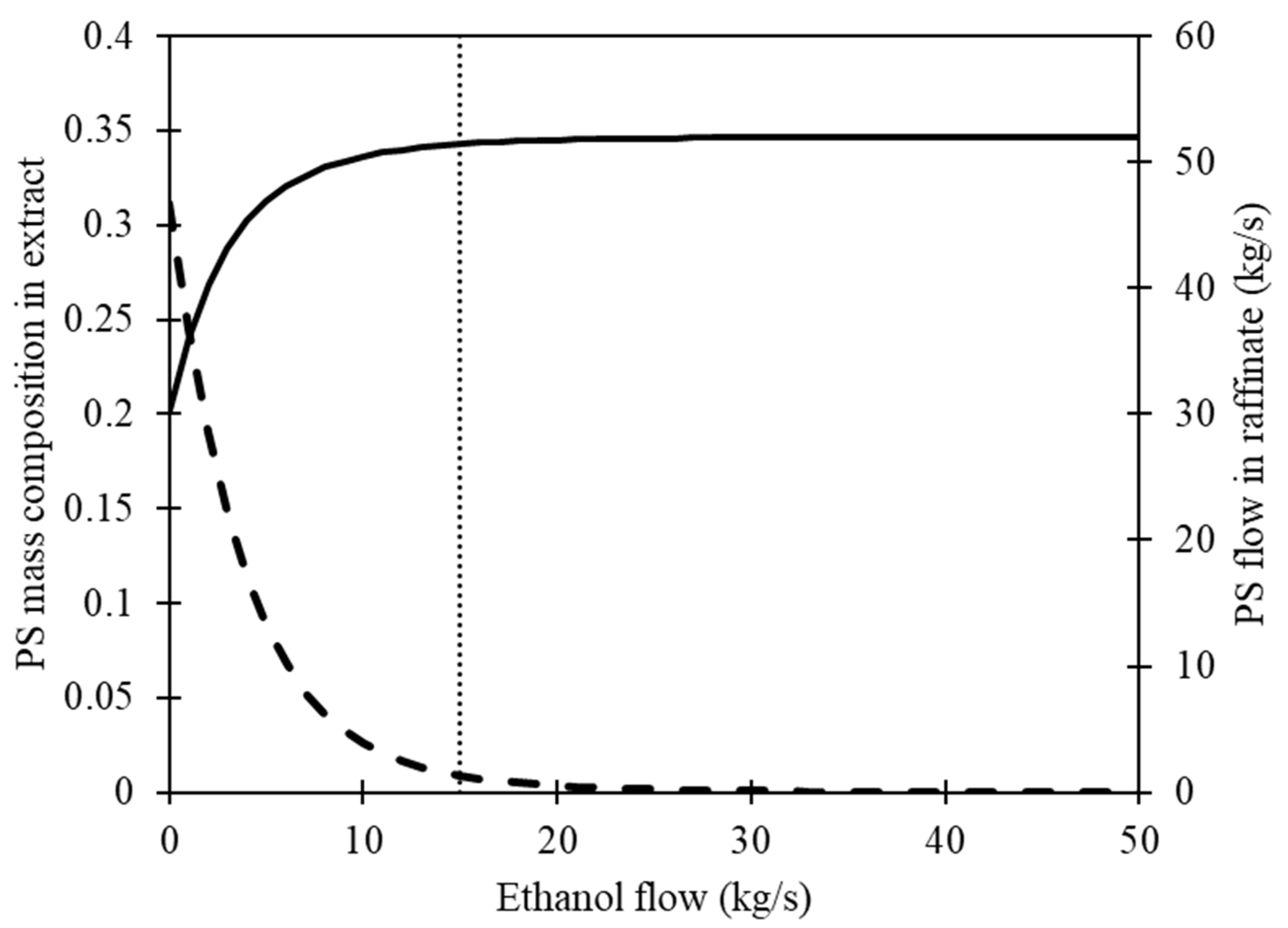
Finally, we use Equation (3) to compute the raffinate mass flow, and Figure 4 presents the results. As the ethanol flow to the stirred tank increases, the concentration of PS in the extract decreases, resulting in an increase in the amount of PS recovered in the raffinate. The objective of recovering 99% of the fed PS can be accomplished by using 15 kg/s of ethanol flow. However, it is important to note that regardless of the amount of ethanol added to the polymer mixture, there will still be some PS residue present in the extract stream due to the inherent characteristics of the process. The obtained results were utilized as an initial step in the development of the conceptual design for the PS recovery process.
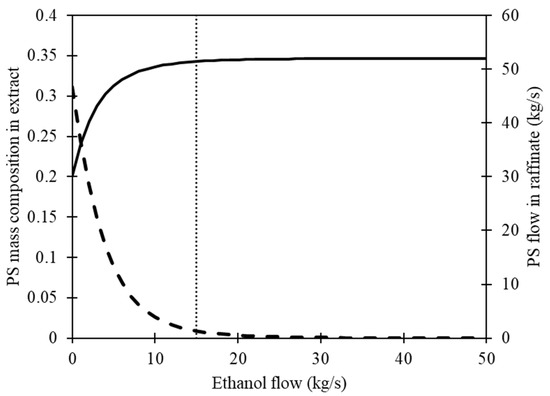
Figure 4.
The influence of ethanol in a stirred tank on the behavior of a polystyrene (PS) composition in extract and mass flow in raffinate. The solid line represents the flow of PS in the raffinate, while the dashed line represents the mass composition of PS in the extract. The dotted line indicates a flow rate of 15 kg/s of ethanol, with a recovery efficiency of 99% for solid PS in the raffinate.
3.4. Process Simulation Results
The thermodynamic model used for this design was the Non-Random Two Liquids (NRTL) model. The binary interaction parameters for the ethanol and D-limonene mixture were adjusted based on experimental data using the regression analysis tool included in the process simulator. The Interior Point Optimizer (IPOPT) method was employed, and the Objective Function (OF) was as follows [40]:
Two isobaric databases for the ethanol + D-limonene mixture have been found in the literature, one at a pressure of 40 kPa [25] and another at a pressure of 101.3 kPa [41]. The parameter values are presented in Table 1. The Root-Mean-Square Deviation (RMSD) for the vapor composition was 0.017 at 40 kPa and 0.038 at 101.3 kPa. The RMSD for temperature was 2.3 K at 40 kPa and 3.7 K at 101.3 kPa.

Table 1.
Binary interaction parameters of the NRTL equation of state for ethanol (1) + D-limonene (2) mixture.
The DWSIM process simulator does not have specific features for polymer processes, but it is still possible to utilize the Compound Creator wizard for this purpose. The PS was defined by incorporating data on the molecular weight determined in this study, and the standard boiling point was supposed to be 673.15 K, considering that polymers start to boil before decomposition [42], the melting temperature (513.15 K) [43], and the density of the solid phase (0.92 g/cm3) [42].
The conceptual design of the process considered the results from the previous section, which included a flow of a mixture consisting of 100 kg/s of PS (52% w/w) and D-limonene (48% w/v), as well as a fresh ethanol feed of 15 kg/s.
The initial scheme examined consisted of a mixer to merge the feed streams and a compound separator. The design variable for the separator was set to achieve a recovery rate of 99%. The resulting solvent mixture consists of 75.6% w of D-limonene, 23.6% w of ethanol, and 0.8% w of PS. A distillation column was designed using the shortcut method module, with ethanol selected as the light key component and D-limonene as the heavy key component. A proposal was made to achieve a recovery rate of 99.99% for the key components located in both the dome and bottom. The specified pressure for the condenser was 206.8 kPa (30 psia), while for the reboiler it was 241.3 kPa (35 psia), with the utilization of a total condenser. A sensitivity analysis was conducted to determine the minimum reflux ratio (Rmin. The reflux ratio was varied from 1.2 to 20 times Rmin. It was found that a reflux ratio of 1.5 reduces the number of stages without impacting the quality of the distillate. The findings are displayed in Table 2.

Table 2.
Results for the separation of ethanol + D-limonene using the shortcut method for simulation of a distillation column.
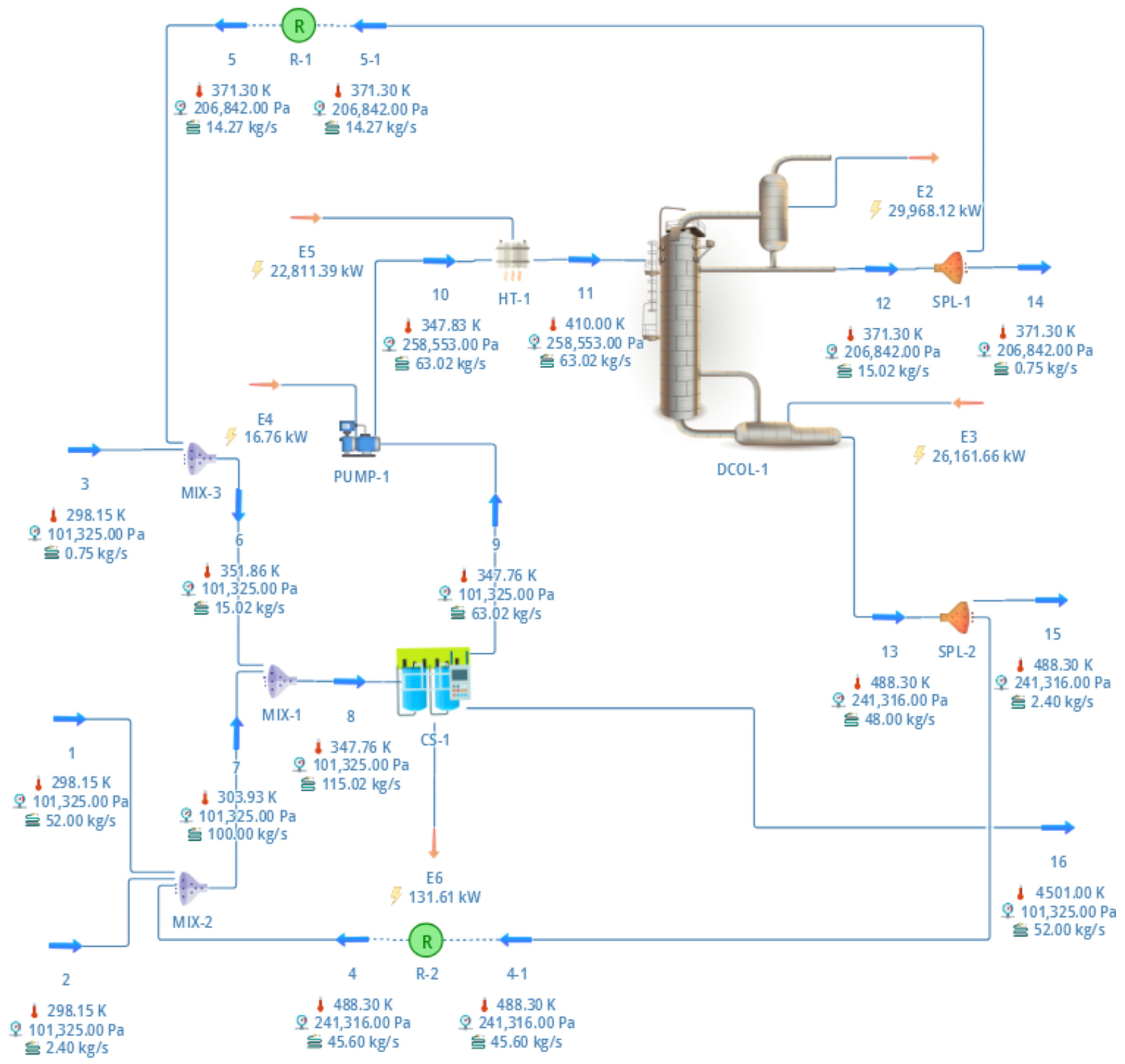
The final design of the distillation column was determined using the rigorous method, which involved six equilibrium stages and feeding on plate four. After achieving convergence, the proposal for the recirculation of solvents was presented, as depicted in Figure 5. The temperature in Stream 16 is extremely high. This is because the property dataset for PS is not sufficient. Because the amount of PS in other currents is insignificant, this does not affect the thermodynamic properties of other streams.
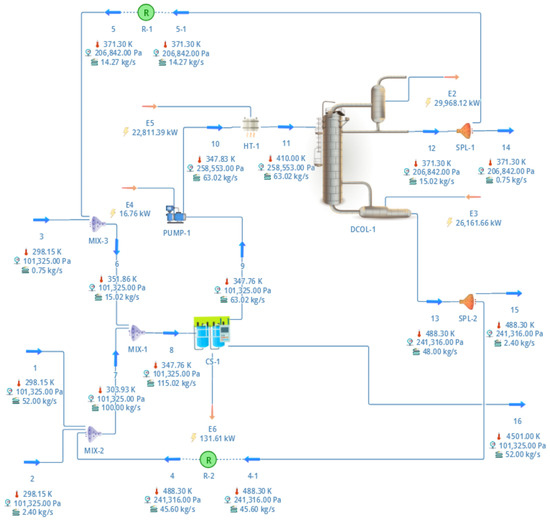
Figure 5.
Process flow diagram for the conceptual design proposed in this work. Temperatures, pressures, and flows for each stream are shown. Streams descriptions: 1. Expanded polystyrene; 2. Fresh D-limonene; 3. Fresh ethanol; 4. Recycled D-limonene; 5. Recycled ethanol; 8. Mixture polystyrene + D-limonene + ethanol; 9. Extract current containing D-limonene + ethanol + polystyrene; 11. Feed to distillation column; 12. Distillate product; 13. Bottom product; 14. Ethanol purge; 15. D-limonene purge; 16. Recovered polystyrene.
A 5% purge is suggested for the distillation column’s product streams to recover materials like residual PS in the solvents. Subsequent equipment, including pumps, heaters, and coolers, was installed to condition the streams before each treatment. Table 3 presents a concise overview of the main results.

Table 3.
Summary of principal results for the process simulated.
4. Conclusions
In this work, we present experimental data and empirical correlation for the solubility limit for the mixture PS + D-limonene + ethanol. We discovered that increasing the amount of polar molecules in the solvent resulted in the insolubilization of diluted PS. We used the experimental data from this work on the solubility limit to conceptually design a process that treats EPS and recovers it as a solid, ready for market reintroduction. The process for recycling PS from solution and precipitation can be performed at atmospheric pressure and temperatures near ambient conditions; for the distillation column, the pressure is near two atmospheres, and temperatures range from 378 to 480 K for distillate and bottom products, respectively. The conceptual design incorporates two recycling processes for limonene and ethanol, thereby reducing the consumption of fresh solvents to 5%. We use D-limonene and ethanol instead of fossil solvents like benzene or toluene because we can obtain them from agro-waste. It is necessary to supplement experimental information on PS properties in order to improve the results for this material.
Author Contributions
Á.I.H.G.: Investigation, Methodology, Validation, Formal analysis, Software, and Writing—original draft; S.A.-H.: Investigation, Methodology, Validation, Formal analysis, Software, Supervision, Visualization, Writing—original draft, and Writing—review and editing; Z.B.C.-C.: Conceptualization, Investigation, Supervision, Writing—original draft, and Writing—review and editing; J.A.G.-P.: Conceptualization, Investigation, Supervision, Writing—original draft, and Writing—review and editing; J.M.V.-R.: Conceptualization, Methodology, Investigation, Supervision, Writing—original draft, and Writing—review and editing; D.G.-Z.: Conceptualization, Funding acquisition, Project administration, Methodology, Formal analysis, Validation, Visualization, Software, Writing—original draft, and Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Consejo de Ciencia y Tecnología del Estado de Tabasco (CCYTET), project number PRODECTI-2022-01/84.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request to the corresponding author.
Acknowledgments
The authors would like to thank the Juárez Autonomous University of Tabasco for the facilities granted for the development of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- White, D.; Winchester, N. The Plastic Intensity of Industries in the USA: The Devil Wears Plastic. Environ. Model. Assess. 2023, 28, 15–28. [Google Scholar] [CrossRef]
- Gausepohl, H.; Nießner, N. Polystyrene and Styrene Copolymers. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 7735–7741. [Google Scholar] [CrossRef]
- Lafond, C.; Blanchet, P. Technical Performance Overview of Bio-Based Insulation Materials Compared to Expanded Polystyrene. Buildings 2020, 10, 81. [Google Scholar] [CrossRef]
- Kan, A.; Demirboğa, R. A new technique of processing for waste-expanded polystyrene foams as aggregates. J. Mater. Process. Technol. 2009, 209, 2994–3000. [Google Scholar] [CrossRef]
- Al-Odaini, N.A.; Kannan, N. Sequestration and Redistribution of Emerging and Classical POPS by Polystyrene: An Aspect Overlooked? In Persistent Organic Chemicals in the Environment: Status and Trends in the Pacific Basin Countries I Contamination Status; Bommanna, G.L., Jong Seong, K., Prasada Rao, S.K., Shigeki, M., Eds.; American Chemical Society: Washington, DC, USA, 2016; Chapter 10; pp. 219–236. [Google Scholar] [CrossRef]
- Enarevba, D.R.; Haapala, K.R. A Comparative Life Cycle Assessment of Expanded Polystyrene and Mycelium Packaging Box Inserts. Procedia CIRP 2023, 116, 654–659. [Google Scholar] [CrossRef]
- Li, H.; Aguirre-Villegas, H.A.; Allen, R.D.; Bai, X.; Benson, C.H.; Beckham, G.T.; Bradshaw, S.L.; Brown, J.L.; Brown, R.C.; Cecon, V.S.; et al. Expanding plastics recycling technologies: Chemical aspects, technology status and challenges. Green Chem. 2022, 24, 8899–9002. [Google Scholar] [CrossRef]
- Samper, M.D.; Garcia-Sanoguera, D.; Parres, F.; López, J. Recycling of Expanded Polystyrene from Packaging. Prog. Rubber Plast. Recycl. Technol. 2010, 26, 83–92. [Google Scholar] [CrossRef]
- Guyot, A. Recent developments in the thermal degradation of polystyrene—A review. Polym. Degrad. Stab. 1986, 15, 219–235. [Google Scholar] [CrossRef]
- Peterson, J.D.; Vyazovkin, S.; Wight, C.A. Kinetics of the Thermal and Thermo-Oxidative Degradation of Polystyrene, Polyethylene and Poly(propylene). Macromol. Chem. Phys. 2001, 202, 775–784. [Google Scholar] [CrossRef]
- Achilias, D.S.; Kanellopoulou, I.; Megalokonomos, P.; Lappas, A.; Antonakou, E. Chemical Recycling of Polystyrene. In Proceedings of the International Conference Protection and Restoration of the Environment, Crete, Greece, 3–7 July 2006; pp. 3–7. Available online: https://www.researchgate.net/profile/Dimitris-Achilias/publication/266371180_CHEMICAL_RECYCLING_OF_POLYSTYRENE/links/54af9ac40cf2b48e8ed675f0/CHEMICAL-RECYCLING-OF-POLYSTYRENE.pdf (accessed on 19 July 2024).
- García, M.T.; Gracia, I.; Duque, G.; de Lucas, A.; Rodríguez, J.F. Study of the solubility and stability of polystyrene wastes in a dissolution recycling process. Waste Manag. 2009, 29, 1814–1818. [Google Scholar] [CrossRef]
- Eskander, S.B.; Tawfik, M.E. Polymer–cement composite based on recycled expanded polystyrene foam waste. Polym. Compos. 2011, 32, 1430–1438. [Google Scholar] [CrossRef]
- Pardo Mendoza, I.D.; León Pulido, J. Efecto de la concentración y temperatura en la disolución de poliestireno expandido usando solventes naturales. Av. Investig. Ing. 2021, 18, 1–14. [Google Scholar] [CrossRef]
- Pin, J.-M.; Soltani, I.; Negrier, K.; Lee, P.C. Recyclability of Post-Consumer Polystyrene at Pilot Scale: Comparison of Mechanical and Solvent-Based Recycling Approaches. Polymers 2023, 15, 4714. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, C.; Rodríguez, J.F.; Gracia, I.; de Lucas, A.; García, M.T. Reduction of the carbon footprint through polystyrene recycling: Economical evaluation. Process Saf. Environ. Prot. 2016, 101, 144–151. [Google Scholar] [CrossRef]
- Shin, C.; Chase, G.G. Nanofibers from recycle waste expanded polystyrene using natural solvent. Polym. Bull. 2005, 55, 209–215. [Google Scholar] [CrossRef]
- Hattori, K. Recycling of Expanded Polystyrene Using Natural Solvents. In Recycling Materials Based on Environmentally Friendly Techniques; InTech: Rijeka, Croatia, 2015. [Google Scholar] [CrossRef]
- Gil-Jasso, N.D.; Giles-Mazón, E.A.; Soriano-Giles, G.; Reinheimer, E.W.; Varela-Guerrero, V.; Ballesteros-Rivas, M.F. A methodology for recycling waste expanded polystyrene using flower essential oils. Fuel 2022, 307, 121835. [Google Scholar] [CrossRef]
- Gil-Jasso, N.D.; Segura-González, M.A.; Soriano-Giles, G.; Neri-Hipolito, J.; López, N.; Mas-Hernández, E.; Barrera-Díaz, C.E.; Varela-Guerrero, V.; Ballesteros-Rivas, M.F. Dissolution and recovery of waste expanded polystyrene using alternative essential oils. Fuel 2019, 239, 611–616. [Google Scholar] [CrossRef]
- García-Barrera, L.V.; Ortega-Solís, D.L.; Soriano-Giles, G.; Lopez, N.; Romero-Romero, F.; Reinheimer, E.; Varela-Guerrero, V.; Ballesteros-Rivas, M.F. A Recycling Alternative for Expanded Polystyrene Residues Using Natural Esters. J. Polym. Environ. 2022, 30, 3832–3839. [Google Scholar] [CrossRef]
- Dagle, R.A.; Winkelman, A.D.; Ramasamy, K.K.; Lebarbier Dagle, V.; Weber, R.S. Ethanol as a Renewable Building Block for Fuels and Chemicals. Ind. Eng. Chem. Res. 2020, 59, 4843–4853. [Google Scholar] [CrossRef]
- Trading Economics, “Ethanol”. Available online: https://tradingeconomics.com/commodity/ethanol (accessed on 21 July 2024).
- Trading Economics, “Naphtha”. Available online: https://tradingeconomics.com/commodity/naphtha (accessed on 21 July 2024).
- Ngema, P.T.; Matkowska, D.; Naidoo, P.; Hofman, T.; Ramjugernath, D. Vapor-liquid equilibrium data for binary systems of 1-methyl-4-(1-methylethenyl)-cyclohexene + {ethanol, propan-1-ol, propan-2-ol, butan-1-ol, pentan-1-ol, or hexan-1-ol} at 40 kPa. J. Chem. Eng. Data 2012, 57, 2053–2058. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef]
- Ravichandran, C.; Badgujar, P.C.; Gundev, P.; Upadhyay, A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. 2018, 120, 668–680. [Google Scholar] [CrossRef]
- Sun, C.; Theodoropoulos, C.; Scrutton, N.S. Techno-economic assessment of microbial limonene production. Bioresour. Technol. 2020, 300, 122666. [Google Scholar] [CrossRef]
- Zapata-Boada, S.; Gonzalez-Miquel, M.; Jobson, M.; Cuéllar-Franca, R.M. Life Cycle Environmental Evaluation of Alternative Solvents Used in Lipid Extraction─The Case of Algae Biodiesel. ACS Sustain. Chem. Eng. 2023, 11, 11934–11946. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, H.; Qi, Z. Experimental and Molecular Insights into Efficient Deterpenation of Essential Oil Intensified by Extraction with Deep Eutectic Solvent. Ind. Eng. Chem. Res. 2024, 63, 10761–10772. [Google Scholar] [CrossRef]
- Zein, S.H.; Hussain, A.A.; Yansaneh, O.Y.; Jalil, A.A. Modelling and Simulation of Dissolution/Reprecipitation Technique for Low-Density Polyethene Using Solvent/Non-Solvent System. Processes 2022, 10, 2387. [Google Scholar] [CrossRef]
- Wagner, H.L. The Mark–Houwink–Sakurada Equation for the Viscosity of Atactic Polystyrene. J. Phys. Chem. Ref. Data 1985, 14, 1101–1106. [Google Scholar] [CrossRef]
- Bernardo, G.; Vesely, D. Equilibrium solubility of alcohols in polystyrene attained by controlled diffusion. Eur. Polym. J. 2007, 43, 938–948. [Google Scholar] [CrossRef]
- de Oliveira, L.H.; Pinto, R.R.; Monteiro Filho, E.S.; Aznar, M. Density, Refractive Index, pH, and Cloud Point Temperature Measurements and Thermal Expansion Coefficient Calculation for PPG 400, PE62, L64, L35, PEG 400, PEG 600, or PEG 1000 + Water Systems. J. Chem. Eng. Data 2021, 66, 2959–2975. [Google Scholar] [CrossRef]
- Hattori, K.; Naito, S.; Yamauchi, K.; Nakatani, H.; Yoshida, T.; Saito, S.; Aoyama, M.; Miyakoshi, T. Solubilization of polystyrene into monoterpenes. Adv. Polym. Technol. 2008, 27, 35–39. [Google Scholar] [CrossRef]
- Kol, R.; Nachtergaele, P.; De Somer, T.; D’hooge, D.R.; Achilias, D.S.; De Meester, S. Toward More Universal Prediction of Polymer Solution Viscosity for Solvent-Based Recycling. Ind. Eng. Chem. Res. 2022, 61, 10999–11011. [Google Scholar] [CrossRef]
- Henley, E.J.; Seader, J.D. Equilibrium Stage Separation Operations in Chemical Engineering, 1st ed.; Wiley: New York, NY, USA, 1981. [Google Scholar]
- Medeiros, D. DWSIM—Open Source Process Simulation, Modeling and Optimization Version 8.8.0 2024. Available online: https://dwsim.org (accessed on 15 July 2024).
- Wolf, B.A. Polyelectrolytes Revisited: Reliable Determination of Intrinsic Viscosities. Macromol. Rapid Commun. 2007, 28, 164–170. [Google Scholar] [CrossRef]
- López, J.A.; Trejos, V.M.; Cardona, C.A. Objective functions analysis in the minimization of binary VLE data for asymmetric mixtures at high pressures. Fluid Phase Equilibria 2006, 248, 147–157. [Google Scholar] [CrossRef]
- Kodama, D.; Shinobu, Y.; Miyakoshi, Y.; Kato, M. Vapor-Liquid Equilibria for Ethanol + Limonene and 1-Propanol + Limonene. Netsu Bussei 2003, 17, 266–269. [Google Scholar] [CrossRef][Green Version]
- Prathiba, R.; Shruthi, M.; Miranda, L.R. Pyrolysis of polystyrene waste in the presence of activated carbon in conventional and microwave heating using modified thermocouple. Waste Manag. 2018, 76, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Pasztor, A.J.; Landes, B.G.; Karjala, P.J. Thermal properties of syndiotactic polystyrene. Thermochim. Acta 1991, 177, 187–195. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).