Residues from the Oil Pressing Process as a Substrate for the Production of Alternative Biochar Materials
Abstract
1. Introduction
2. Literature Review
3. Materials and Methods
3.1. Research Object
3.2. Pyrolysis Process
3.3. Combustion Process
3.4. Analysis of Samples
3.5. Names of Tests
3.6. Statistical Analysis
4. Results and Discussion
4.1. Elemental Analysis
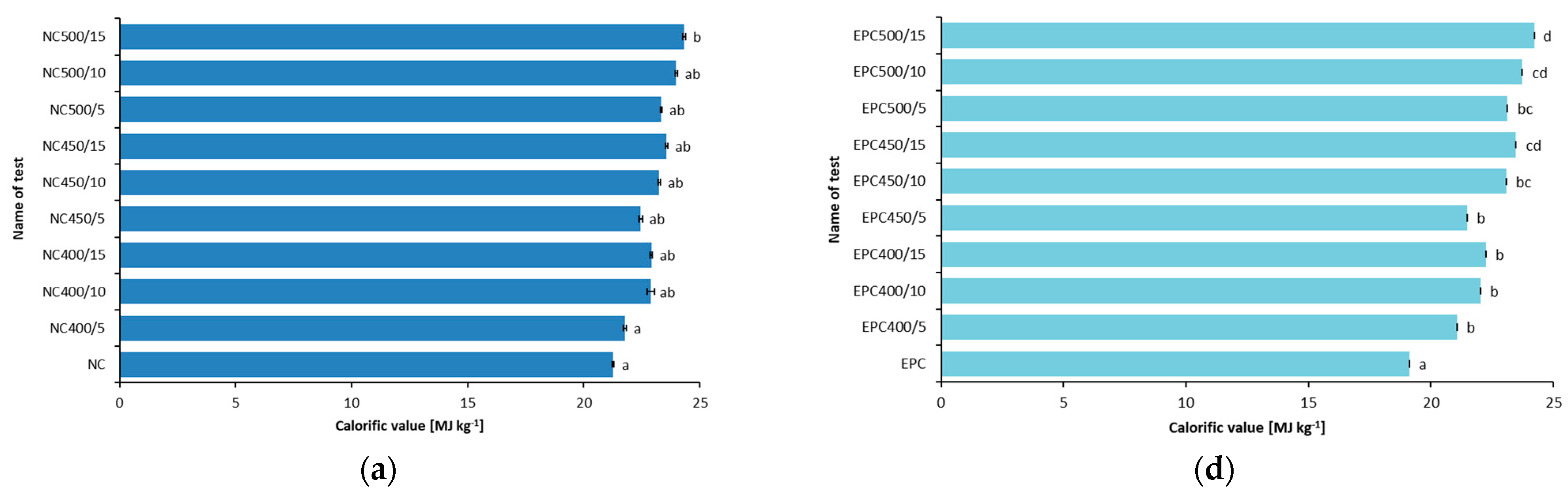
4.2. Calorific Value
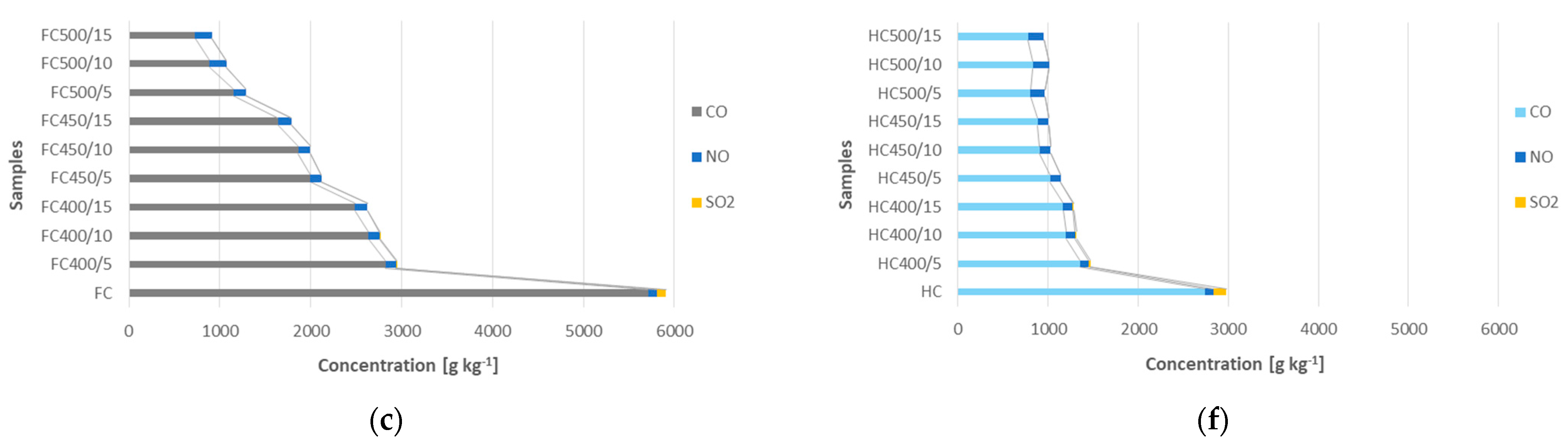
4.3. Emissions of CO, NOx and SO2
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rokicki, T.; Michalski, K.; Ratajczak, M.; Szczepaniuk, H.; Golonko, M. Use of renewable energy sources in European Union countries. Szkoła Główna Gospodarstwa Wiejskiego. Rocz. Ochr. Sr. 2018, 20, 1318–1334. [Google Scholar]
- Chen, S.T.; Kuo, H.I.; Chen, C.C. Modelling the Relationship between the Oil Price and Global Food Prices. Appl. Energy 2010, 87, 2517–2525. [Google Scholar] [CrossRef]
- Perea-Moreno, M.A.; Samerón-Manzano, E.; Perea-Moreno, A.J. Biomass as Renewable Energy: Worldwide Research Trends. Sustainability 2019, 11, 863. [Google Scholar] [CrossRef]
- Kilkis, S.; Krajacic, G.; Duic, N.; Rosen, M.A.; Al-Nimr, M.A. Advancements in sustainable development of energy, water and environment systems. Energy Convers. Manag. 2018, 176, 164–183. [Google Scholar] [CrossRef]
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B. New approaches to measuring biochar density and porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from cono-carpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Bochniak, M. Quality of Two-Stage Pressed Rapeseed Oil after Low-Temperature Bleaching Process. Ph.D. Thesis, Uniwersytet Ekonomiczny We Wrocławiu, Wrocław, Poland, 2020. [Google Scholar] [CrossRef]
- Grela, R.E.; Zaworska-Zakrzewska, A.; Milewski, S. Nutritional value and feed suitability of by-products from organic farms in animal nutrition. Wiadomości Zootech. 2023, R. LXI, 38–48. [Google Scholar]
- Babicz, M.; Kropiwiec-Domańska, K.; Szymanowska, U. Selected Issues in Respect to the Production of Raw Materials, Food and Cosmetics; Wydawnictwo Uniwersytetu Przyrodniczego W Lublinie: Lublin, Poland, 2023; ISBN 978-83-7259-342-9. [Google Scholar] [CrossRef]
- Kita, A.; Górnicka, E.; Wojdyło, A.; Drożdż, W. Effect of the addition of rapeseed pomace on the properties of fried potato chips. Rośliny Oleiste Oilseed Crops 2012, 33, 286–288. [Google Scholar]
- Karatas, H.; Olgun, H.; Akgun, F. Experimental results of gasification of waste tire with air in a bubbling fluidized bed gasifier. Fuel Process. Technol. 2012, 102, 166–174. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Gajek, M.; Zajemska, M.; Jayaraman, K.; Gokalp, I. Combustion and kinetics parameters estimation of torrefied pine, acacia and Miscanthus giganteus using experimental and modeling techniques. Bioresour. Technol. 2017, 243, 304–314. [Google Scholar] [CrossRef]
- Erdei, B.; Frankó, B.; Galbe, M.; Zacchi, G. Separate hydrolysis and co fermentation for improved xylose utilization in integrated ethanol production from wheat meal and wheat straw. Biotechnol. Biofuels 2012, 5, 12. [Google Scholar] [CrossRef]
- Beims, R.F.; Hu, Y.; Shui, H.; Xu, C. Hydrothermal liquefaction of biomass to fuels and value-added chemicals: Product applications and challenges to develop largescale operations. Biomass Bioenergy 2020, 135, 105510. [Google Scholar] [CrossRef]
- Yang, F.; Li, G.; Shi, H.; Wang, Y. Effects of phosphogypsum and superphosphate on compost maturity and gaseous emis-sions during kitchen waste composting. Waste Manag. 2015, 36, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Biller, P.; Madsen, R.B.; Klemmer, M.; Becker, J.; Iversen, B.B.; Glasius, M. Effect of hydrothermal liquefaction aqueous phase recycling on bio-crude yields and composition. Bioresour. Technol. 2016, 220, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Kobyłecki, R.; Bis, Z. Autothermal thermolysis as an efficient technology to produce clean and high energy fuels. Archiwum Spalania 2006, 6, 114–119. [Google Scholar]
- Saletnik, B.; Zaguła, G.; Bajcar, M.; Tarapatskyy, M.; Bobula, G.; Puchalski, C. Biochar as a Multifunctional Component of the Environment—A Review. Appl. Sci. 2019, 9, 1139. [Google Scholar] [CrossRef]
- Saletnik, B.; Zaguła, G.; Saletnik, A.; Bajcar, M.; Puchalski, C. Biochar and Ash Fertilization Alter the Chemical Properties of Basket Willow (Salix viminalis L.) and Giant Miscanthus (Miscanthus x giganteus). Agronomy 2020, 10, 660. [Google Scholar] [CrossRef]
- Retajczyk, M.; Wróblewska, A. Biomass pyrolysis as an energy source. Wiadomości Chemiczne 2018, 72, 127–146. [Google Scholar]
- Liu, Q.; Chmely, S.C.; Abdoulmoumine, N. Biomass Treatment Strategies for Thermochemical Conversion. Energy Fuels 2017, 31, 3525–3536. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels production through biomass pyrolysis—A technological review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Chhiti, Y.; Kemiha, M. Thermal Conversion of Biomass, Pyrolysis and Gasification: A Review. Int. J. Eng Sci. 2013, 2, 75–85. [Google Scholar]
- Varma, A.; Shankar, R.; Mondal, P. A Review on Pyrolysis of Biomass and the Impacts of Operating Conditions on Product Yield, Quality, and Upgradation. Recent Adv. Biofuels Bioenergy Util. 2018, 227–259. [Google Scholar]
- Rhodes, A.H.; Carlin, N.A.; Semple, O.R. Impact of black carbon in the extraction and mineralization of phenanthrene in soil. Environ. Sci. Technol. 2008, 42, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, W.M.; Radziemska, E.; Ryms, M.; Ostrowski, P. Modern methods of thermochemical biomass conversion into gas, liquid and solid fuels. Proc. ECOpole 2010, 4, 2. [Google Scholar]
- Bis, Z. Biowęgiel–powrót do przeszłości, szansa dla przyszłości. Czysta Energia 2012, 6. [Google Scholar]
- Matovic, D. Biochar as a viable carbon sequestration option: Global and Canadian perspective. Energy 2011, 36, 2011–2016. [Google Scholar] [CrossRef]
- Bednik, M.; Medyńska–Juraszek, A.; Dudek, M.; Kloc, S.; Kręt, A.; Łabaz, B.; Waroszewski, J. Wheat Straw Biochar and NPK Fertilization Efficiency in Sandy Soil Reclamation. Agronomy 2020, 10, 496. [Google Scholar] [CrossRef]
- Malińska, K. Prawne i jakościowe aspekty dotyczące wymagań dla biowęgla. Inżynieria I Ochr. Sr. 2015, 18, 359–371. [Google Scholar]
- Pereira, R.C.; Muetzel, S.; Arbestain, M.C.; Bishop, P.; Hina, K.; Hedley, M. Assessment of the influence of biochar on rumen and silage fermentation: A laboratory-scale experiment. Anim. Feed Sci. Technol. 2014, 196, 22–31. [Google Scholar] [CrossRef]
- Belluati, M.; Tabasso, S.; Gaudino, E.C.; Cravotto, G.; Manzoli, M. Biomass-derived carbon-based catalysts for lignocellulosic biomass and waste valorisation: A circular approach. Green Chem. 2024, 26, 8642–8668. [Google Scholar] [CrossRef]
- Garcia, B.; Alves, O.; Rijo, B.; Lourinho, G.; Nobre, C. Biochar: Production, Applications, and Market Prospects in Portugal. Environments 2022, 9, 95. [Google Scholar] [CrossRef]
- Tang, J.; Zhy, W.; Kookana, R.; Katayama, A. Characteristics of biochar and its application in remediation of contaminated soil. J. Biosci. Bioeng. 2013, 116, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Sarswat, A.; Ok, S.Y.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.; Melear, N.; Harris, K.; Das, K.C. Biochar as bulking agent for poultry litter composting. Carbon Manag. 2011, 2, 227–230. [Google Scholar] [CrossRef]
- Malińska, K.; Zabochnicka–Świątek, M.; Dach, J. Effects of biochar amendment on ammonia emission during composting of sewage sludge. Ecol. Eng. 2014, 71, 474–478. [Google Scholar] [CrossRef]
- Wu–Jun, L.; Hong, J.; Han–Qing, Y. Emerging applications of biochar-based materials for energy storage and conversion. Energy Environ. Sci. 2019, 12, 1751–1779. [Google Scholar]
- Kwapinski, W.; Byrne, C.M.P.; Kryachko, E.; Wolfram, P.; Adley, C.; Leahy, J.J.; Novotny, E.H.; Hayes, M.H.B. Biochar from Biomass and Waste. Waste Biomass Valorization 2010, 1, 177–189. [Google Scholar] [CrossRef]
- Sànchez, M.E.; Lindao, E.; Margaleff, D.; Martínez, O.; Morán, A. Pyrolysis of agricultural residues from rape and sunflower: Production and characterization of biofuels and biochar soil management. J. Anal. Appl. Pyrolysis 2019, 85, 142–144. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk-and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Gao, B.; Chen, H.; Jiang, L.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Yang, L.; Xue, Y.; Li, H. Adsorption of sulfamethoxazole on biochar and its impact on reclaimed water irrigation. J. Hazard. Mater 2012, 209, 408–413. [Google Scholar] [CrossRef]
- Ibarrola, R.; Shackely, S.; Hammond, J. Pyrolysis biochar systems for recovering biodegradable materials: A life cycle carbon assessment. Waste Manag. 2012, 32, 859–868. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Quality variations of poultry litter biochar generated at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- PN-EN ISO 16948:2015-07; Solid Biofuels—Determination of Total Carbon, Hydrogen and Nitrogen Content. ISO: Geneva, Switzerland, 2015.
- PN-EN ISO 18122:2023-05; Solid Biofuels—Determination of Ash Content. ISO: Geneva, Switzerland, 2023.
- PN-EN ISO 18123:2023; Solid Biofuels—Determination of Volatile Matter. ISO: Geneva, Switzerland, 2023.
- PN-EN ISO 18125:2017-07; Solid Biofuels—Determination of Calorific Value. ISO: Geneva, Switzerland, 2017.
- Ferens, W. Analysis of the change in the composition of solid products of biomass pyrolysis. Zesz. Energetyczne 2017, IV, 63–71. [Google Scholar]
- Hmid, A.; Mondelli, D.; Fiore, S.; Fanizzi, F.P.; Al Chami, Z.; Dumontet, S. Production and characterization of biochar from three-phase olive mill waste through slow pyrolysis. Biomass Bioenergy 2014, 71, 330–339. [Google Scholar] [CrossRef]
- Martínez-Gómez, A.; Fe Andrés, M.; Barón-Sola, Á.; Díaz-Manzano, F.E.; Yousef, I.; Mena, I.F.; Díaz, E.; Gómez-Torres, Ó.; González-Coloma, A.; Hernández, L.E.; et al. Biochar from grape pomace, a waste of vitivinicultural origin, is effective for root-knot nematode control. Biochar 2023, 3, 30. [Google Scholar] [CrossRef]
- Mazurek, K.; Drużyński, S.; Kiełkowska, U.; Wróbel-Kaszanek, A.; Igliński, B.; Cichosz, M. The Application of Pyrolysis Biochar Obtained from Waste Rapeseed Cake to Remove Copper from Industrial Wastewater: An Overview. Energies 2024, 17, 498. [Google Scholar] [CrossRef]
- Sładeczek, F.; Głodek-Bucyk, E. Study on the use of low-temperature pyrolysis to convert waste biomass into biochar in a test plant. Pr. Inst. Ceram. I Mater. Bud. 2017, 10, 50–61. [Google Scholar]
- Li, Y.; Grupta, R.; Zhang, Q.; You, S. Review of biochar production via crop residue pyrolysis: Development and perspectives. Bioresour. Technol. 2023, 369, 128423. [Google Scholar] [CrossRef]
- Crombie, K.; Mašek, O.; Sohi, S.P.; Brownsort, P.; Cross, A. The effect of pyrolysis conditions on biochar stability as determined by three methods. Gcb Bioenergy 2013, 5, 122–131. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.F.; Ribau, J.P.; Costa, M. A decision support method for biochars characterization from carbonization of grape pomace. Biomass Bioenergy 2021, 145, 105946. [Google Scholar] [CrossRef]
- Angın, D.; Köse, T.E.; Selengil, U. Production and characterization of activated carbon prepared from safflower seed cake biochar and its ability to absorb reactive dyestuff. Appl. Surf. Sci. 2013, 280, 705–710. [Google Scholar] [CrossRef]
- Sun, Y.; Li, C.; Li, Q.; Zhang, S.; Hu, X. Pyrolysis of flaxseed residue: Exploration of characteristics of the biochar and bio-oil products. J. Energy Inst. 2021, 97, 1–12. [Google Scholar] [CrossRef]
- Mukhambet, Y.; Shah, D.; Tatkeyeva, G.; Sarbassov, Y. Slow pyrolysis of flax straw biomass produced in Kazakhstan: Characterization of enhanced tar and high-quality biochar. Fuel 2022, 324, 124676. [Google Scholar] [CrossRef]
- Tag, A.T.; Duman, G.; Ucar, S.; Yanik, J. Effects of feedstock type and pyrolysis temperature on potential applications of biochar. J. Anal. Appl. Pyrolisis 2016, 120, 200–206. [Google Scholar] [CrossRef]
- Mulimani, H.V.; Navindgi, M.C. Production of solid fuel biochar from de-oiled seed cake by pyrolysis. J. Mech. Civ. Eng. 2017, 14, 57–61. [Google Scholar] [CrossRef]
- Kazimierski, P.; Januszewicz, K.; Godlewski, W.; Fijuk, A.; Suchocki, T.; Chaja, P.; Barczak, B.; Kardaś, D. The Course and the Effects of Agricultural Biomass Pyrolysis in the Production of High-Calorific Biochar. Materials 2022, 15, 1038. [Google Scholar] [CrossRef]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Ozcimen, D.; Karaosmanoglu, F. Production and characterization of bio-oil and biochar from rapeseed cake. Renew. Energy 2004, 29, 779–787. [Google Scholar] [CrossRef]
- Durak, H.; Genel, S. Characterization of bio-oil and bio-char obtained from black cumin seed by hydrothermal liquefaction: Investigation of potential as an energy source. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 45, 3205–3215. [Google Scholar] [CrossRef]
- Prade, T.; Svensson, S.E.; Mattsson, J.E. Energy balances for biogas and solid biofuel production from industrial hemp. Biomass Bioenergy 2012, 40, 36–52. [Google Scholar] [CrossRef]
- Prade, T.; Finell, M.; Svensson, S.E.; Mattsson, J.E. Effect of harvest date on combustion related fuel properties ofindustrial hemp (Cannabis sativa L.). Fuel 2012, 102, 592–604. [Google Scholar] [CrossRef]
- Parvez, A.M.; Lewis, J.D.; Afzal, M.T. Potential of industrial hemp (Cannabis sativa L.) for bio-energy production in Canada: Status, challenges and outlook. Renew. Sustain. Energy Rev. 2021, 141, 110784. [Google Scholar] [CrossRef]
- Zouari, M.; Devallance, D.B.; Marrot, L. Effect of Biochar Addition on Mechanical Properties, Thermal Stability, and Water Resistance of Hemp-Polylactic Acid (PLA) Composites. Materials 2022, 15, 2271. [Google Scholar] [CrossRef] [PubMed]
- Marrot, L.; Candelier, K.; Valette, J.; Lanvin, C.; Horvat, B.; Legan, L.; David, B.; DeVallance, D.B. Valorization of hemp stalkwaste through thermochemical conversion for energy and electrical applications. Waste Biomass Valorization 2022, 13, 2267–2285. [Google Scholar] [CrossRef]
- Di Maro, M.; Faga, M.G.; Pedraza, R.; Malucelli, G.; Bartoli, M.; Gomez d’Ayala, G.; Duraccio, D. Effect of Hemp Hurd Biocharand Humic Acid on the Flame Retardant and Mechanical Properties of Ethylene Vinyl Acetate. Polymers 2023, 15, 1411. [Google Scholar] [CrossRef]
- Visković, J.; Zheljazkov, V.D.; Sikora, V.; Noller, J.; Latković, D.; Ocamb, C.M.; Koren, A. Industrial Hemp (Cannabis sativa L.) Agronomy and Utilization: A Review. Agronomy 2023, 13, 931. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, W.; Hu, D.; Ou, L.; Tong, Y.; Shen, G.; Shen, H.; Wang, X. Emissions of carbon monoxide and carbon dioxide from uncompressed and pelletized biomass fuel burning in typical household stoves in China. Atmos. Environ. 2012, 56, 136–142. [Google Scholar] [CrossRef]
- Jelonek, Z.; Drobniak, A.; Mastalerz, M.; Jelonek, I. Emissions during grilling with wood pellets and chips. Atmos. Environ. X 2021, 12, 100140. [Google Scholar] [CrossRef]
- Vicente, E.D.; Duarte, M.A.; Calvo, A.I.; Nunes, T.F.; Tarelho, L.; Alves, C.A. Emission of carbon monoxide, total hydrocarbons and particulate matter during wood combustion in a stove operating under distinct conditions. Fuel Process. Technol. 2015, 131, 182–192. [Google Scholar] [CrossRef]
- Sher, F.; Yaqoob, A.; Saeed, F.; Zhang, S.; Jahan, Z.; Klemeš, J.J. Torrefied biomass fuels as a renewable alternative to coal in co-firing for power generation. Energy 2020, 209, 118444. [Google Scholar] [CrossRef]
- Lasek, J.; Kopczyński, M.; Janusz, M.; Iluk, A.; Zuwała, J. Combustion properties of torrefied biomass obtained from flue gas-enhanced reactor. Energy 2017, 119, 362–368. [Google Scholar] [CrossRef]
- Xue, J.; Chellappa, T.; Ceylan, S.; Goldfarb, J.L. Enhancing biomass + coal Co-firing scenarios via biomass torrefaction and carbonization: Case study of avocado pit biomass and Illinois No. 6 coal. Renew. Energy 2018, 122, 152–162. [Google Scholar] [CrossRef]
- Mun, T.Y.; Tumsa, T.Z.; Lee, U.D.; Yang, W. Performance evaluation of co-firing various kinds of biomass with low rank coals in a 500 MWe coal-fired power plant. Energy 2016, 115, 954–962. [Google Scholar] [CrossRef]
- Rentizelas, A.A.; Li, J. Techno-economic and carbon emissions analysis of biomass torrefaction downstream in international bioenergy supply chains for co-firing. Energy 2016, 114, 129–142. [Google Scholar] [CrossRef]
- Cucek, L.; Klemes, J.J.; Varbanov, P.S.; Kravanja, Z. Significance of environmental footprints for evaluating sustainability and security of development. Clean Technol. Environ. Policy 2015, 17, 2125–2141. [Google Scholar] [CrossRef]




| Calculated Parameters | Method of Calculation |
|---|---|
| The amount of carbon monoxide (CO) | SCO = 0.5 × (|Xn−1 − Xn|) × (tn−1 − tn) |
| Amount of gas mixture taken | Sgases = tc × 100 |
| Percentage of carbon dioxide (CO) in the analyzed gas mixture | %CO = SCO/Sgases |
| Amount of gas mixture taken during the test | AL. = tc × 0.025 [L/s] |
| Amount of carbon monoxide (CO) taken during the test | ACO = AL. × %CO |
| Carbon monoxide (CO) density at room temperature (20 °C) [mg L−1] | 1250 |
| Amount of carbon monoxide (CO) taken during measurement [mg] | ACO × 1250 |
| Gases Analyzed | Gas Density [mg L−1] |
|---|---|
| Carbon monoxide (CO) | 1250 |
| Nitrogen oxides (NOx) | 1340 |
| Sulfur dioxide (SO2) | 2830 |
| Parameter | Research Method | |
|---|---|---|
| Content of carbon, nitrogen and hydrogen | PN-EN ISO 16948:2015-07 [45] | Solid biofuels—determination of total carbon, hydrogen and nitrogen content |
| Ash content | PN-EN ISO 18122:2023-05 [46] | Solid biofuels—determination of ash content |
| Volatile matter | PN-EN ISO 18123:2023 [47] | Solid biofuels—determination of ash content |
| Calorific value | PN-EN ISO 18125:2017-07 [48] | Solid biofuels—determination of ash content |
| Number | Symbol | Type of Material | Pyrolysis Temperature [°C] | Duration of the Pyrolysis [min.] |
|---|---|---|---|---|
| 1. | NC | fennel flower cakes | - | - |
| 2. | NC400/5 | 400 | 5 | |
| 3. | NC400/10 | 10 | ||
| 4. | NC400/15 | 15 | ||
| 5. | NC450/5 | 450 | 5 | |
| 6. | NC450/10 | 10 | ||
| 7. | NC450/15 | 15 | ||
| 8. | NC500/5 | 500 | 5 | |
| 9. | NC500/10 | 10 | ||
| 10. | NC500/15 | 15 | ||
| 11. | RC | rapeseed cakes | - | - |
| 12. | RC400/5 | 400 | 5 | |
| 13. | RC400/10 | 10 | ||
| 14. | RC400/15 | 15 | ||
| 15. | RC450/5 | 450 | 5 | |
| 16. | RC450/10 | 10 | ||
| 17. | RC450/15 | 15 | ||
| 18. | RC500/5 | 500 | 5 | |
| 19. | RC500/10 | 10 | ||
| 20. | RC500/15 | 15 | ||
| 21. | FC | flax cakes | - | - |
| 22. | FC400/5 | 400 | 5 | |
| 23. | FC400/10 | 10 | ||
| 24. | FC400/15 | 15 | ||
| 25. | FC450/5 | 450 | 5 | |
| 26. | FC450/10 | 10 | ||
| 27. | FC450/15 | 15 | ||
| 28. | FC500/5 | 500 | 5 | |
| 29. | FC500/10 | 10 | ||
| 30. | FC500/15 | 15 | ||
| 31. | EPC | evening primrose cakes | - | - |
| 32. | EPC400/5 | 400 | 5 | |
| 33. | EPC400/10 | 10 | ||
| 34. | EPC400/15 | 15 | ||
| 35. | EPC450/5 | 450 | 5 | |
| 36. | EPC450/10 | 10 | ||
| 37. | EPC450/15 | 15 | ||
| 38. | EPC500/5 | 500 | 5 | |
| 39. | EPC500/10 | 10 | ||
| 40. | EPC500/15 | 15 | ||
| 41. | MTC | milk thistle cakes | - | - |
| 42. | MTC400/5 | 400 | 5 | |
| 43. | MTC400/10 | 10 | ||
| 44. | MTC400/15 | 15 | ||
| 45. | MTC450/5 | 450 | 5 | |
| 46. | MTC450/10 | 10 | ||
| 47. | MTC450/15 | 15 | ||
| 48. | MTC500/5 | 500 | 5 | |
| 49. | MTC500/10 | 10 | ||
| 50. | MTC500/15 | 15 | ||
| 51. | HC | hemp cakes | - | - |
| 52. | HC400/5 | 400 | 5 | |
| 53. | HC400/10 | 10 | ||
| 54. | HC400/15 | 15 | ||
| 55. | HC450/5 | 450 | 5 | |
| 56. | HC450/10 | 10 | ||
| 57. | HC450/15 | 15 | ||
| 58. | HC500/5 | 500 | 5 | |
| 59. | HC500/10 | 10 | ||
| 60. | HC500/15 | 15 |
| Research Material | General Nitrogen | Total Carbon | Hydrogen | Ash | Volatile Substances |
|---|---|---|---|---|---|
| % | |||||
| NC | 4.77 a ± 0.04 | 49.55 a ± 0.11 | 7.43 d ± 0.02 | 6.15 a ± 0.02 | 73.41 f ± 0.11 |
| NC400/5 | 4.84 a ± 0.02 | 57.66 b ± 0.10 | 4.94 c ± 0.01 | 12.86 b ± 0.06 | 27.13 e ± 0.05 |
| NC400/10 | 4.90 a ± 0.03 | 61.61 bc ± 0.07 | 4.91 c ± 0.01 | 13.96 b ± 0.11 | 25.19 e ± 0.07 |
| NC400/15 | 5.57 b ± 0.07 | 61.76 bc ± 0.08 | 4.92 c ± 0.02 | 15.62 c ± 0.09 | 24.48 de ± 0.08 |
| NC450/5 | 6.64 c ± 0.07 | 61.49 bc ± 0.13 | 4.53 b ± 0.01 | 16.81 c ± 0.10 | 23.25 cd ± 0.07 |
| NC450/10 | 7.40 d ± 0.05 | 62.01 bc ± 0.07 | 4.53 b ± 0.01 | 18.92 d ± 0.11 | 22.41 cd ± 0.06 |
| NC450/15 | 7.68 d ± 0.05 | 62.53 bc ± 0.09 | 4.53 b ± 0.01 | 20.09 de ± 0.10 | 21.63 c ± 0.10 |
| NC500/5 | 6.84 cd ± 0.04 | 62.95 bc ± 0.13 | 4.10 a ± 0.01 | 21.24 de ± 0.16 | 19.44 b ± 0.10 |
| NC500/10 | 6.96 cd ± 0.01 | 63.57 c ± 0.08 | 4.09 a ± 0.01 | 22.77 e ± 0.08 | 17.66 ab ± 0.04 |
| NC500/15 | 7.30 d ± 0.03 | 64.06 c ± 0.09 | 3.93 a ± 0.00 | 23.22 e ± 0.09 | 16.50 a ± 0.06 |
| RC | 4.34 a ± 0.06 | 51.97 a ± 0.06 | 7.25 c ± 0.00 | 5.32 a ± 0.02 | 79.33 f ± 0.07 |
| RC400/5 | 4.65 ab ± 0.05 | 60.33 b ± 0.16 | 4.74 b ± 0.00 | 11.21 b ± 0.06 | 28.16 e ± 0.09 |
| RC400/10 | 4.89 b ± 0.04 | 62.32 b ± 0.06 | 4.66 b ± 0.00 | 12.48 b ± 0.05 | 27.14 de ± 0.11 |
| RC400/15 | 5.40 c ± 0.04 | 62.80 b ± 0.03 | 4.66 b ± 0.01 | 13.89 c ± 0.05 | 24.84 d ± 0.02 |
| RC450/5 | 5.60 c ± 0.05 | 63.65 b ± 0.10 | 4.34 b ± 0.01 | 14.72 c ± 0.05 | 21.46 c ± 0.09 |
| RC450/10 | 6.01 d ± 0.02 | 64.32 b ± 0.06 | 4.26 ab ± 0.04 | 17.03 d ± 0.14 | 20.28 c ± 0.10 |
| RC450/15 | 6.22 d ± 0.05 | 65.06 b ± 0.05 | 4.25 ab ± 0.01 | 18.57 d ± 0.07 | 18.62 bc ± 0.07 |
| RC500/5 | 5.77 cd ± 0.04 | 65.89 b ± 0.08 | 4.06 ab ± 0.15 | 19.86 de ± 0.06 | 17.53 ab ± 0.05 |
| RC500/10 | 5.95 cd ± 0.02 | 66.45 b ± 0.05 | 3.89 a ± 0.05 | 21.13 e ± 0.03 | 17.07 ab ± 0.05 |
| RC500/15 | 6.50 d ± 0.02 | 66.43 b ± 0.14 | 3.81 a ± 0.00 | 22.09 e ± 0.02 | 16.33 a ± 0.08 |
| FC | 4.92 a ± 0.03 | 49.29 a ± 0.05 | 7.16 c ± 0.01 | 6.35 a ± 0.02 | 75.69 f ± 0.08 |
| FC400/5 | 5.37 ab ± 0.06 | 59.47 b ± 0.05 | 4.89 b ± 0.01 | 11.06 b ± 0.05 | 27.36 e ± 0.02 |
| FC400/10 | 5.75 b ± 0.06 | 62.43 b ± 0.05 | 4.84 b ± 0.01 | 12.25 b ± 0.08 | 25.87 de ± 0.08 |
| FC400/15 | 6.10 b ± 0.03 | 62.75 b ± 0.05 | 4.84 b ± 0.01 | 13.80 c ± 0.07 | 24.37 d ± 0.03 |
| FC450/5 | 5.91 b ± 0.05 | 63.04 b ± 0.11 | 4.49 ab ± 0.01 | 14.80 c ± 0.04 | 21.07 c ± 0.02 |
| FC450/10 | 5.96 b ± 0.02 | 63.63 b ± 0.04 | 4.44 ab ± 0.02 | 17.01 d ± 0.12 | 20.05 bc ± 0.06 |
| FC450/15 | 6.49 cd ± 0.04 | 64.27 b ± 0.03 | 4.44 ab ± 0.01 | 18.36 d ± 0.08 | 18.84 b ± 0.08 |
| FC500/5 | 6.43 cd ± 0.04 | 64.89 b ± 0.08 | 4.11 a ± 0.09 | 19.59 de ± 0.05 | 15.50 a ± 0.04 |
| FC500/10 | 6.63 cd ± 0.03 | 65.48 b ± 0.07 | 4.01 a ± 0.05 | 20.98 e ± 0.04 | 14.38 a ± 0.04 |
| FC500/15 | 6.92 d ± 0.01 | 65.71 b ± 0.04 | 3.94 a ± 0.03 | 21.91 e ± 0.03 | 13.93 a ± 0.05 |
| EPC | 3.49 a ± 0.01 | 49.23 a ± 0.05 | 5.71 d ± 0.02 | 7.12 a ± 0.02 | 70.32 e ± 0.04 |
| EPC400/5 | 3.60 ab ± 0.02 | 58.45 b ± 0.37 | 4.81 c ± 0.02 | 16.79 b ± 0.02 | 30.15 d ± 0.07 |
| EPC400/10 | 3.65 ab ± 0.03 | 60.97 b ± 0.04 | 4.64 c ± 0.02 | 16.85 b ± 0.03 | 29.22 d ± 0.08 |
| EPC400/15 | 3.76 ab ± 0.03 | 61.06 b ± 0.07 | 4.75 c ± 0.01 | 17.52 b ± 0.07 | 28.45 d ± 0.10 |
| EPC450/5 | 3.90 bc ± 0.05 | 58.69 b ± 0.03 | 4.31 bc ± 0.02 | 18.6 bc ± 0.05 | 27.92 cd ± 0.19 |
| EPC450/10 | 4.49 c ± 0.05 | 61.64 b ± 0.05 | 4.3 bc ± 0.01 | 19.37 bc ± 0.06 | 25.74 c ± 0.07 |
| EPC450/15 | 4.77 c ± 0.05 | 62.22 b ± 0.10 | 4.25 bc ± 0.01 | 19.63 bc ± 0.06 | 24.17 b ± 0.12 |
| EPC500/5 | 3.95 bc± 0.03 | 58.63 b ± 0.04 | 3.98 ab ± 0.01 | 18.79 bc ± 0.04 | 23.99 b ± 0.10 |
| EPC500/10 | 4.05 bc ± 0.01 | 64.28 b ± 0.05 | 3.94 ab ± 0.01 | 18.85 bc ± 0.03 | 22.47 a ± 0.02 |
| EPC500/15 | 4.32 c ± 0.02 | 64.47 b ± 0.07 | 3.66 a ± 0.04 | 19.53 c ± 0.03 | 21.45 a ± 0.02 |
| MTC | 3.12 a ± 0.06 | 47.80 a ± 0.07 | 6.90 d ± 0.01 | 5.99 a ± 0.02 | 69.54 g ± 0.12 |
| MTC400/5 | 3.25 ab ± 0.02 | 62.50 b ± 0.04 | 5.41 c ± 0.02 | 15.75 b ± 0.06 | 25.80 f ± 0.12 |
| MTC400/10 | 3.36 ab ± 0.01 | 65.40 bc ± 0.08 | 5.24 c ± 0.01 | 16.14 b ± 0.07 | 25.80 f ± 0.09 |
| MTC400/15 | 3.39 ab ± 0.01 | 66.83 bc ± 0.06 | 5.08 c ± 0.03 | 16.49 bc ± 0.04 | 24.20 e ± 0.13 |
| MTC450/5 | 3.42 ab ± 0.01 | 67.03 bc ± 0.07 | 4.53 bc ± 0.01 | 16.29 bc ± 0.05 | 22.95 de ± 0.09 |
| MTC450/10 | 3.43 ab ± 0.01 | 67.44 bc ± 0.08 | 4.51 bc ± 0.03 | 16.34 bc ± 0.02 | 21.70 d± 0.11 |
| MTC450/15 | 3.47 ab ± 0.02 | 69.56 bc ± 0.06 | 4.34 bc ± 0.01 | 16.87 bc ± 0.04 | 19.33 c ± 0.06 |
| MTC500/5 | 3.54 ab ± 0.02 | 68.44 bc ± 0.06 | 4.16 ab ± 0.01 | 16.81 bc ± 0.04 | 17.47 b ± 0.06 |
| MTC500/10 | 3.65 b ± 0.02 | 70.82 c ± 0.03 | 4.13 ab ± 0.01 | 16.92 bc ± 0.05 | 16.71 ab ± 0.02 |
| MTC500/15 | 3.77 b ± 0.06 | 71.67 c ± 0.07 | 3.87 a ± 0.01 | 17.22 c ± 0.02 | 15.67 a ± 0.12 |
| HC | 4.70 a ± 0.05 | 47.89 a ± 0.03 | 6.34 d ± 0.02 | 6.55 a ± 0.05 | 73.12 f ± 0.04 |
| HC400/5 | 4.88 a ± 0.03 | 59.29 b ± 0.12 | 4.86 c ± 0.04 | 20.39 b ± 0.02 | 23.92 e ± 0.07 |
| HC400/10 | 5.09 a ± 0.01 | 62.06 bc ± 0.05 | 4.74 c ± 0.02 | 20.50 b ± 0.04 | 23.46 de ± 0.08 |
| HC400/15 | 5.19 a ± 0.02 | 62.74 bc ± 0.07 | 4.68 c ± 0.01 | 21.12 b ± 0.03 | 22.26 de ± 0.10 |
| HC450/5 | 5.23 ab ± 0.03 | 61.69 bc ± 0.05 | 4.19 b ± 0.004 | 21.54 b ± 0.04 | 21.35 d± 0.19 |
| HC450/10 | 5.32 ab ± 0.02 | 63.39 bc ± 0.06 | 4.16 b ± 0.02 | 21.93 b ± 0.02 | 19.65 c ± 0.07 |
| HC450/15 | 5.42 ab ± 0.01 | 64.75 bc ± 0.03 | 4.07 ab ± 0.01 | 22.36 b ± 0.03 | 17.71 b ± 0.12 |
| HC500/5 | 5.46 ab ± 0.02 | 62.40 bc ± 0.04 | 3.85 ab ± 0.03 | 21.89 b ± 0.05 | 16.63 b ± 0.10 |
| HC500/10 | 5.63 ab ± 0.02 | 66.42 bc ± 0.02 | 3.81 ab ± 0.01 | 22.00 b ± 0.04 | 15.51 ab ± 0.02 |
| HC500/15 | 5.74 b ± 0.02 | 66.83 c ± 0.04 | 3.67 a ± 0.01 | 22.41 b ± 0.02 | 14.32 a ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saletnik, B.; Czarnota, R.; Maczuga, M.; Saletnik, A.; Bajcar, M.; Zaguła, G.; Puchalski, C. Residues from the Oil Pressing Process as a Substrate for the Production of Alternative Biochar Materials. Appl. Sci. 2024, 14, 8028. https://doi.org/10.3390/app14178028
Saletnik B, Czarnota R, Maczuga M, Saletnik A, Bajcar M, Zaguła G, Puchalski C. Residues from the Oil Pressing Process as a Substrate for the Production of Alternative Biochar Materials. Applied Sciences. 2024; 14(17):8028. https://doi.org/10.3390/app14178028
Chicago/Turabian StyleSaletnik, Bogdan, Radosław Czarnota, Mateusz Maczuga, Aneta Saletnik, Marcin Bajcar, Grzegorz Zaguła, and Czesław Puchalski. 2024. "Residues from the Oil Pressing Process as a Substrate for the Production of Alternative Biochar Materials" Applied Sciences 14, no. 17: 8028. https://doi.org/10.3390/app14178028
APA StyleSaletnik, B., Czarnota, R., Maczuga, M., Saletnik, A., Bajcar, M., Zaguła, G., & Puchalski, C. (2024). Residues from the Oil Pressing Process as a Substrate for the Production of Alternative Biochar Materials. Applied Sciences, 14(17), 8028. https://doi.org/10.3390/app14178028










