Influence of Process Liquids on the Formation of Strengthened Nanocrystalline Structures in Surface Layers of Steel Parts during Thermo-Deformation Treatment
Abstract
1. Introduction
2. White Layers in Transportation Systems: Problems and Technological Advantages
- -
- Plastic deformation: a heterogeneous structure with a fine-grained microstructure appears on the surface as a result of grain grinding;
- -
- Fast heating and hardening, which is due to phase transformations;
- -
- A chemical interaction between the surface of cutting and elements present in the air, such as carbon, nitrogen, oxygen and others.
2.1. Negative Properties of Discontinuous White Layers in Transportation Systems
2.2. Positive Properties of Continuous White Layers in Transportation Systems
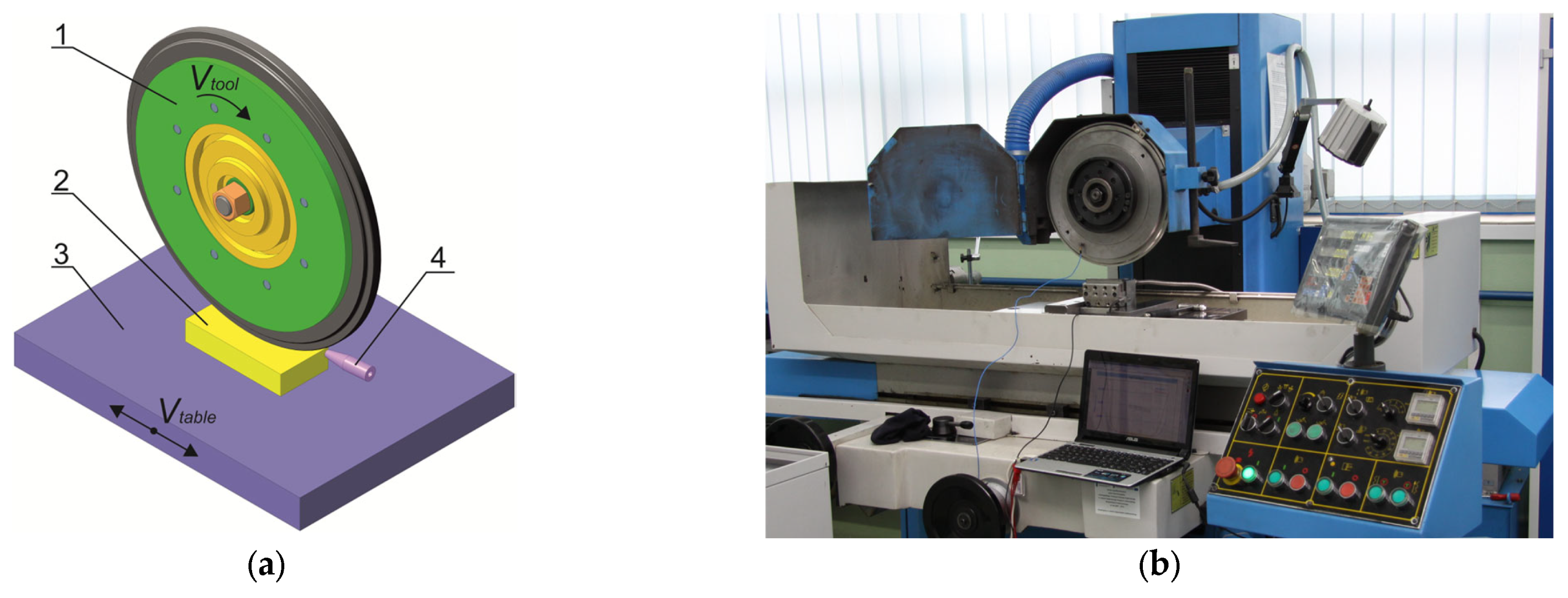
3. Materials and Methods
3.1. The Technology of TDT
3.2. Test Methods
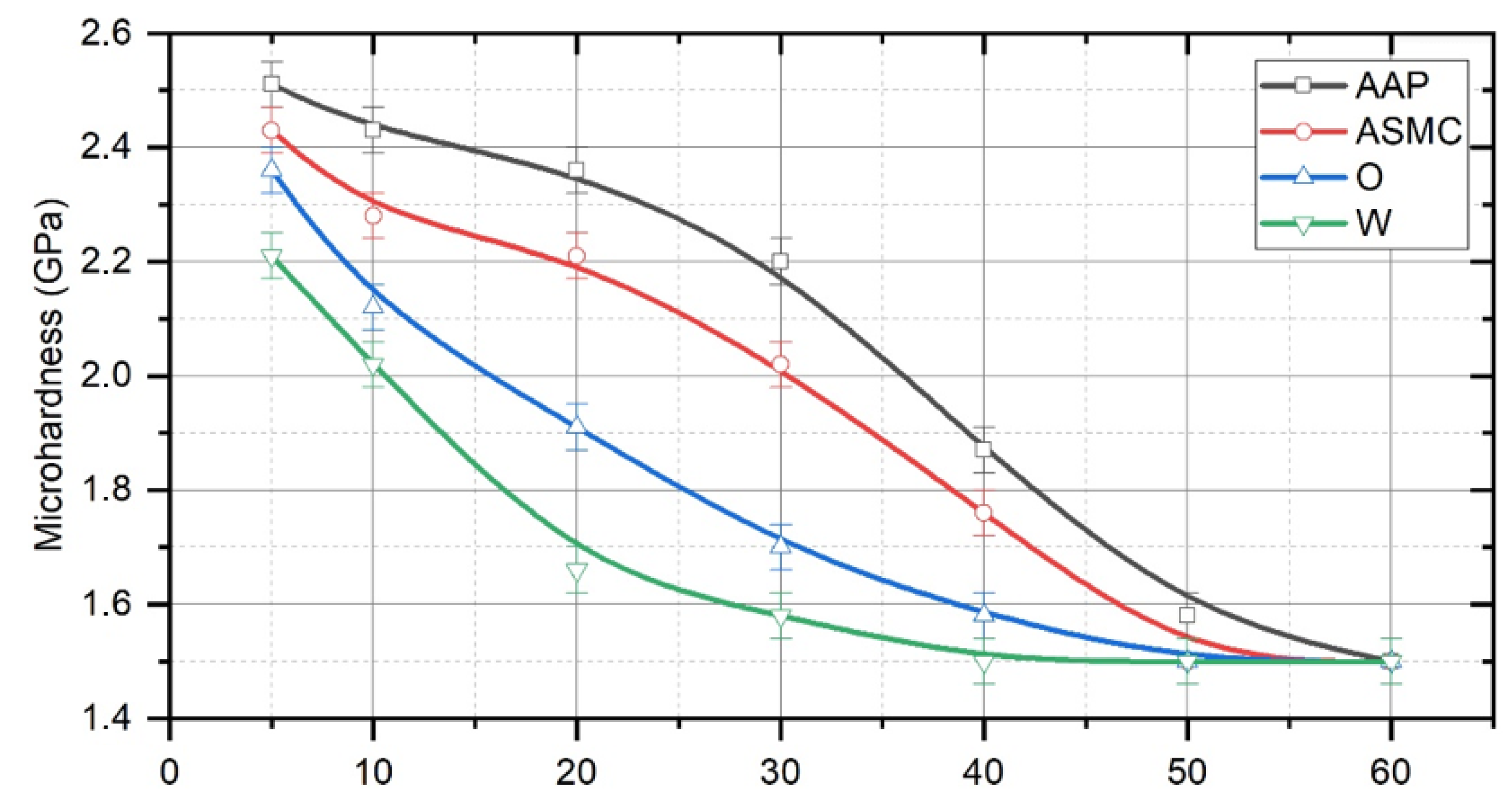
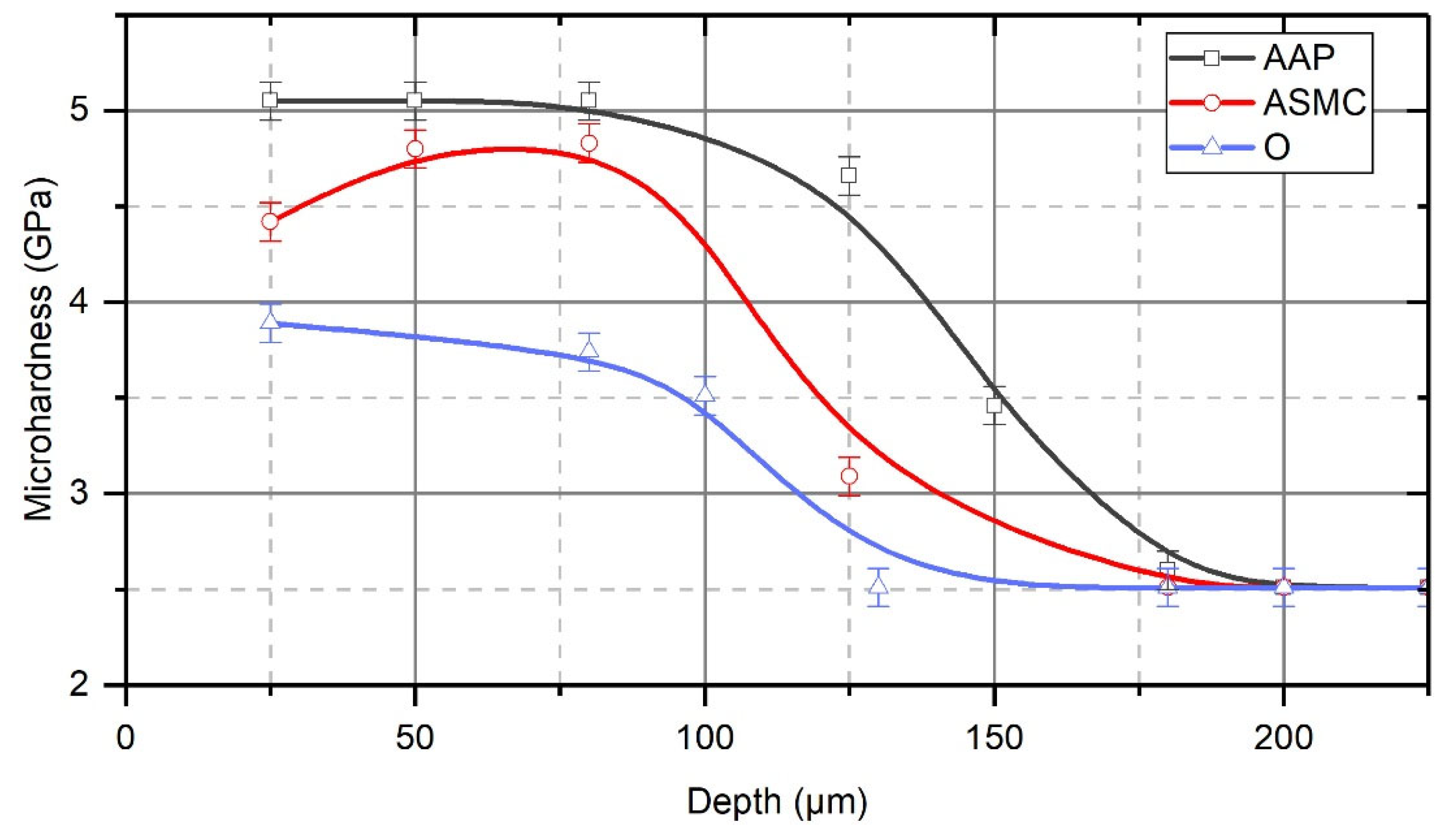
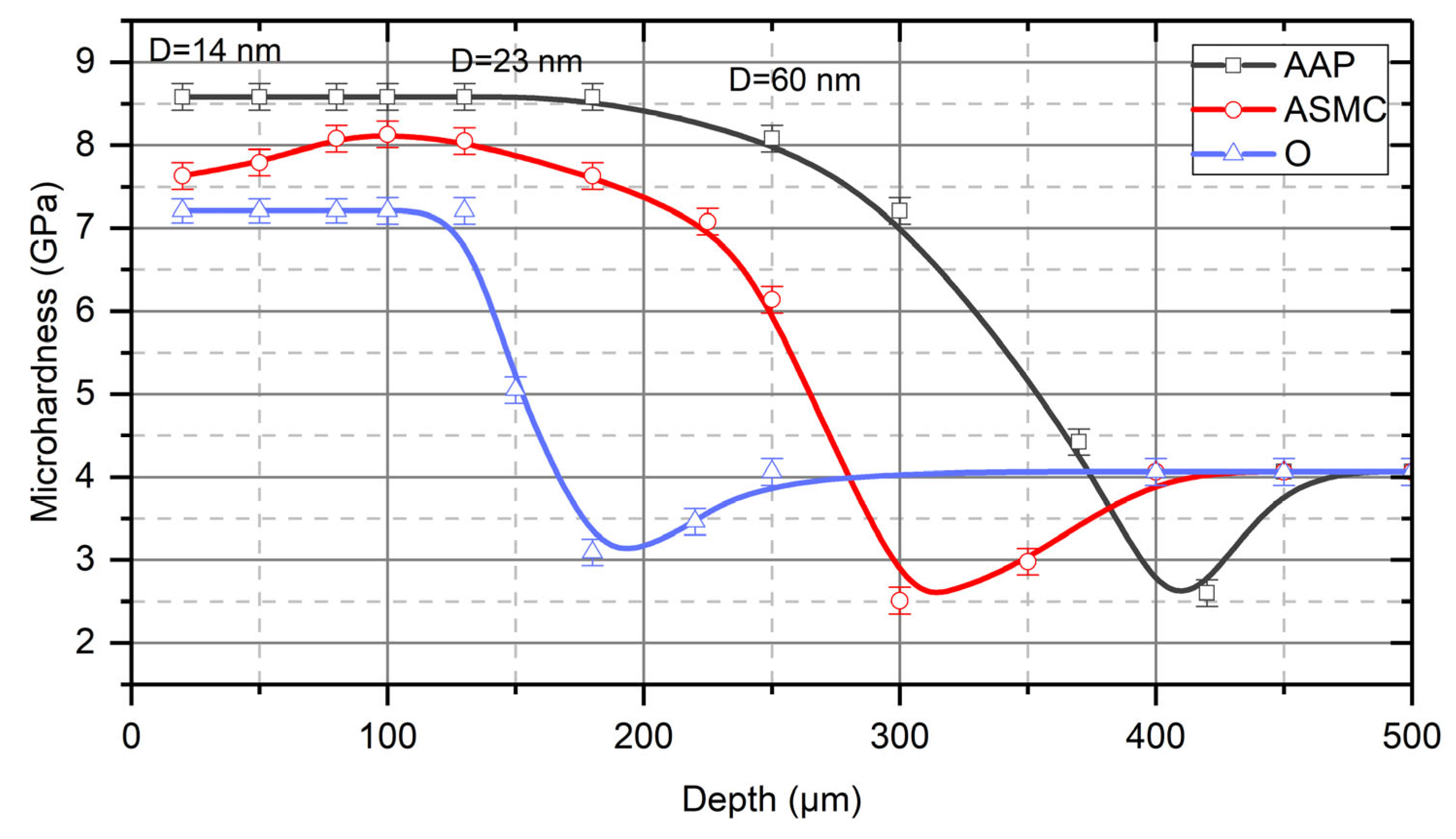
4. Results and Discussion
5. Conclusions
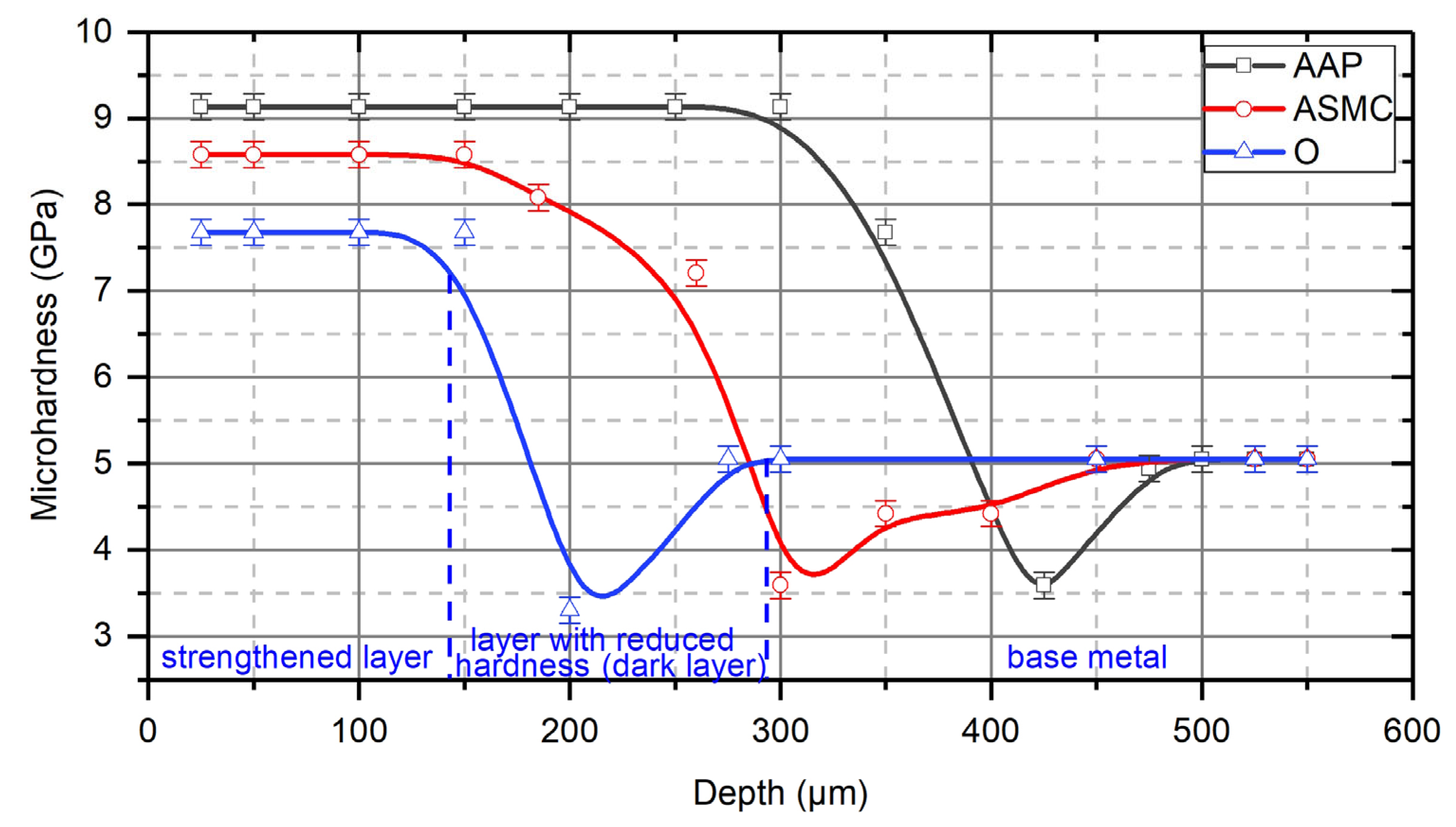
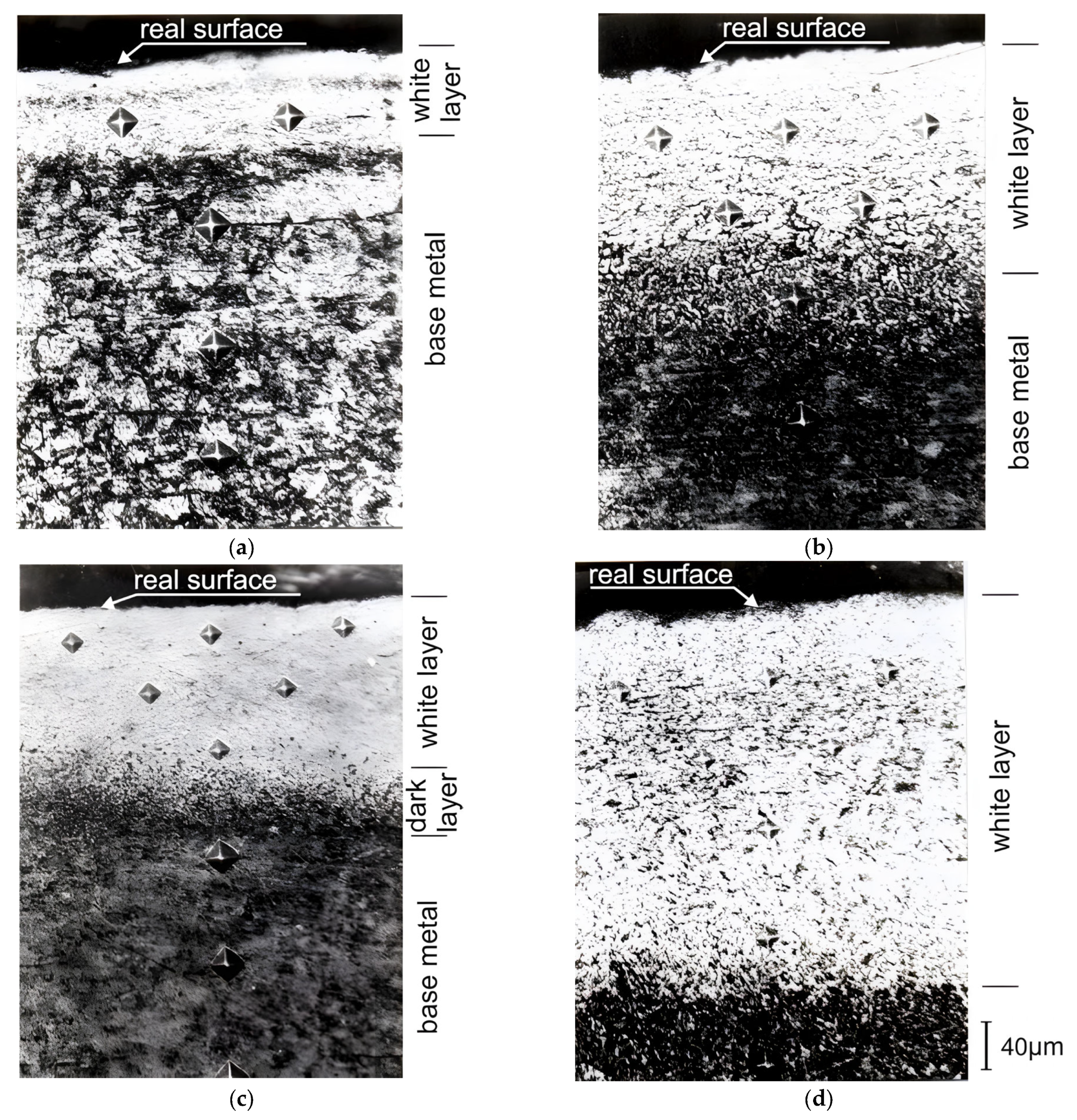
- These studies have shown that in the process of the TDT of steel samples, a strengthened (reinforced) nanocrystalline layer is formed in the surface layer. The grain size of the strengthened layer was 12–20 nm near the treated surface, and the misorientation angle was more than 10°. With an increasing depth in the strengthened layer, the size of nanocrystalline grains increases, and at a depth that is close to the thickness of the strengthened layer, the grain size becomes 54–60 nm.
- The nature of the process liquid used during the TDT significantly affects the thickness of the strengthened layer. The studies have shown that during the TDT using mineral oil with AAP, a uniform strengthened white layer with a nanocrystalline structure of the greatest thickness and hardness is formed on steel samples. Strengthened white layers significantly improve the operational properties of the working surfaces of parts, especially wear resistance.
- It is speculated that molecules of the process liquid, which is fed into the treatment zone, under the action of high temperatures and pressures decompose into component chemical elements which diffuse into the surface layers of the metal. It is considered likely that carbon, which diffuses into the surface layer, significantly affects the formation of a strengthened layer of greater thickness. It may also be that this strengthening is also caused by thermal or mechanical effects, and an avenue of further work would be to determine which of these mechanisms is the primary cause of the strengthening.
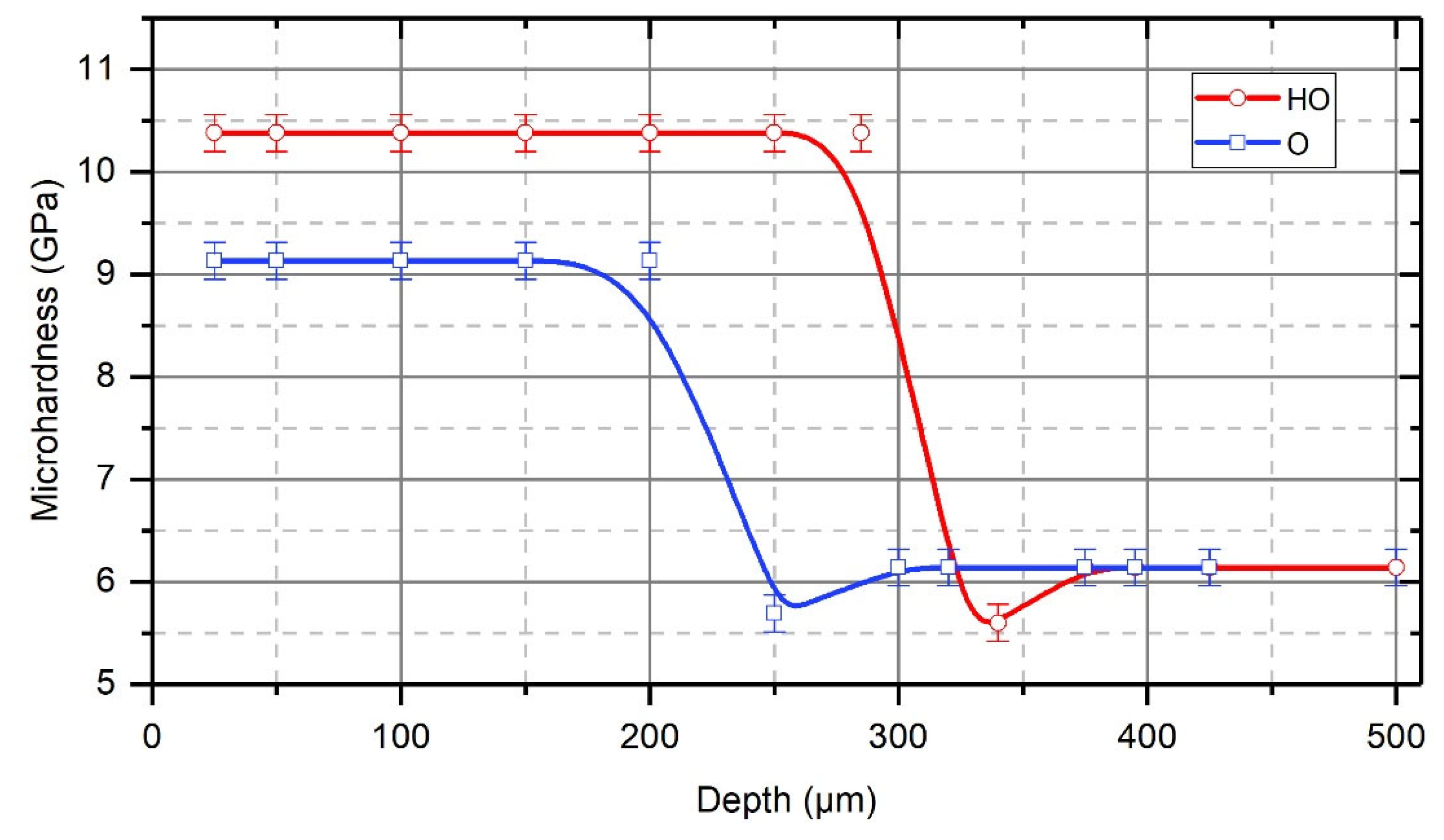
- Hydrogen, which is evolved in the contact zone from the process liquid due to the friction of the tool on the treated surface of the part, likely diffuses into the surface layer of the part, thus significantly affecting the formation of the strengthened layer. Investigations into the strengthening of Steel CT80 samples showed that after treatment of pre-hydrogenated samples, the thickness of the strengthened layer increased 1.5 times, and the microhardness increased from 9.1 GPa to 10.4 GPa.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santecchia, E.; Hamouda, A.M.S.; Musharavati, F.; Zalnezhad, E.; Cabibbo, M.; El Mehtedi, M.; Spigarelli, S. A Review on Fatigue Life Prediction Methods for Metals. Adv. Mater. Sci. Eng. 2016, 2016, 9573524. [Google Scholar] [CrossRef]
- Troshchenko, V.T.; Khamaza, L.A. Stages of Fatigue Failure of Metals and Alloys. Rep. Natl. Acad. Sci. Ukr. 2018, 2, 56–63. [Google Scholar] [CrossRef]
- Davis, J.R. Surface Engineering for Corrosion and Wear Resistance; Davis, J.R., Ed.; ASM International: Novelty, OH, USA, 2001; ISBN 978-1-62708-315-7. [Google Scholar]
- Yushchenko, K.A.; Borysov, Y.S.; Kuznetsov, V.D.; Korzh, V.M. Surface Engineering; Naukova Dumka: Kyiv, Ukraine, 2007. [Google Scholar]
- Pang, J.C.; Li, S.X.; Wang, Z.G.; Zhang, Z.F. General Relation between Tensile Strength and Fatigue Strength of Metallic Materials. Mater. Sci. Eng. A 2013, 564, 331–341. [Google Scholar] [CrossRef]
- Madhukar, S.; Harshith Reddy, B.R.; Kumar, G.A.; Naik, R.P. A Study on Improvement of Fatigue Life of Materials by Surface Coatings. Int. J. Curr. Eng. Technol. 2018, 8, 5–9. [Google Scholar] [CrossRef][Green Version]
- Ropyak, L.; Shihab, T.; Velychkovych, A.; Bilinskyi, V.; Malinin, V.; Romaniv, M. Optimization of Plasma Electrolytic Oxidation Technological Parameters of Deformed Aluminum Alloy D16T in Flowing Electrolyte. Ceramics 2023, 6, 146–167. [Google Scholar] [CrossRef]
- Quintino, L. Overview of Coating Technologies. In Surface Modification by Solid State Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–24. ISBN 9780857094681. [Google Scholar]
- Lukaszkowicz, K. Review of Nanocomposite Thin Films and Coatings Deposited by PVD and CVD Technology. In Nanomaterials; Rahman, M.M., Ed.; InTech: Houston, TX, USA, 2011. [Google Scholar]
- Ropyak, L.; Shihab, T.; Velychkovych, A.; Dubei, O.; Tutko, T.; Bilinskyi, V. Design of a Two-Layer Al–Al2O3 Coating with an Oxide Layer Formed by the Plasma Electrolytic Oxidation of Al for the Corrosion and Wear Protections of Steel. Prog. Phys. Met. 2023, 24, 319–365. [Google Scholar] [CrossRef]
- Prysyazhnyuk, P.; Molenda, M.; Romanyshyn, T.; Ropyak, L.; Romanyshyn, L.; Vytvytskyi, V. Development of a Hardbanding Material for Drill Pipes Based on High-Manganese Steel Reinforced with Complex Carbides. Acta Montan. Slovaca 2022, 27, 685–696. [Google Scholar] [CrossRef]
- Kaplun, P.V.; Honchar, V.A.; Donchenko, T. V Wear Kinetics of Steels with Diffusion Coatings in Rolling Friction. Mater. Sci. 2020, 56, 50–58. [Google Scholar] [CrossRef]
- Klocke, F.; Hensgen, L.; Klink, A.; Ehle, L.; Schwedt, A. Structure and Composition of the White Layer in the Wire-EDM Process. Procedia CIRP 2016, 42, 673–678. [Google Scholar] [CrossRef]
- Javaheri, V.; Sadeghpour, S.; Karjalainen, P.; Lindroos, M.; Haiko, O.; Sarmadi, N.; Pallaspuro, S.; Valtonen, K.; Pahlevani, F.; Laukkanen, A.; et al. Formation of Nanostructured Surface Layer, the White Layer, through Solid Particles Impingement during Slurry Erosion in a Martensitic Medium-Carbon Steel. Wear 2022, 496–497, 204301. [Google Scholar] [CrossRef]
- Zhang, F.; Duan, C.; Wang, M.; Sun, W. White and Dark Layer Formation Mechanism in Hard Cutting of AISI52100 Steel. J. Manuf. Process. 2018, 32, 878–887. [Google Scholar] [CrossRef]
- Olugbade, T.O.; Lu, J. Literature Review on the Mechanical Properties of Materials after Surface Mechanical Attrition Treatment (SMAT). Nano Mater. Sci. 2020, 2, 3–31. [Google Scholar] [CrossRef]
- Zhang, F.; Duan, C.; Sun, W.; Ju, K. Effects of Cutting Conditions on the Microstructure and Residual Stress of White and Dark Layers in Cutting Hardened Steel. J. Mater. Process. Technol. 2019, 266, 599–611. [Google Scholar] [CrossRef]
- Hosseini, S.B.; Klement, U. A Descriptive Phenomenological Model for White Layer Formation in Hard Turning of AISI 52100 Bearing Steel. CIRP J. Manuf. Sci. Technol. 2021, 32, 299–310. [Google Scholar] [CrossRef]
- Xu, Y.; Fang, L.; Cen, Q.; Zhu, J. Nano Structure and Transformation Mechanism of White Layer for AISI1045 Steel during Impact Wear. Wear 2005, 258, 537–544. [Google Scholar] [CrossRef]
- Inoue, A.; Hashimoto, K. (Eds.) Amorphous and Nanocrystalline Materials; Advances in Materials Research; Springer: Berlin/Heidelberg, Germany, 2001; Volume 3, ISBN 978-3-642-08664-9. [Google Scholar]
- Lei, S.; Liu, Q.; Ma, Y.; Ren, J. Wear Performance of Laser Surface Hardened GCr15 Steel. J. Wuhan Univ. Technol. Sci. Ed. 2010, 25, 84–88. [Google Scholar] [CrossRef]
- Bartkowska, A. Production and Properties of FeB-Fe2B-Fe3(B,C) Surface Layers Formed on Tool Steel Using Combination of Diffusion and Laser Processing. Coatings 2020, 10, 1130. [Google Scholar] [CrossRef]
- Korzhyk, V.; Tyurin, Y.; Kolisnichenko, O. Theory and Practice of Plasma-Detonation Technology of Surface Hardening Metal Products; Privat Company Technology Center: Kharkiv, Ukraine, 2021; ISBN 9786177319466. [Google Scholar]
- Bosheh, S.S.; Mativenga, P.T. White Layer Formation in Hard Turning of H13 Tool Steel at High Cutting Speeds Using CBN Tooling. Int. J. Mach. Tools Manuf. 2006, 46, 225–233. [Google Scholar] [CrossRef]
- Vilaça, P. Friction Surfacing. In Surface Modification by Solid State Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 25–72. [Google Scholar]
- Garcia-Giron, A.; Romano, J.M.; Liang, Y.; Dashtbozorg, B.; Dong, H.; Penchev, P.; Dimov, S.S. Combined Surface Hardening and Laser Patterning Approach for Functionalising Stainless Steel Surfaces. Appl. Surf. Sci. 2018, 439, 516–524. [Google Scholar] [CrossRef]
- Huang, X.; Ren, Y.; Zhou, Z.; Xiao, H. Experimental Study on White Layers in High-Speed Grinding of AISI52100 Hardened Steel. J. Mech. Sci. Technol. 2015, 29, 1257–1263. [Google Scholar] [CrossRef]
- Wu, S.; Liu, G.; Zhang, W.; Chen, W.; Wang, C. Formation Mechanism of White Layer in the High-Speed Cutting of Hardened Steel under Cryogenic Liquid Nitrogen Cooling. J. Mater. Process. Technol. 2022, 302, 117469. [Google Scholar] [CrossRef]
- Guo, Y.B.; Warren, A.W.; Hashimoto, F. The Basic Relationships between Residual Stress, White Layer, and Fatigue Life of Hard Turned and Ground Surfaces in Rolling Contact. CIRP J. Manuf. Sci. Technol. 2010, 2, 129–134. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, A.; Herbig, M.; Brinckmann, S.; Dehm, G.; Kirchlechner, C. Micro Fracture Investigations of White Etching Layers. Mater. Des. 2019, 180, 107892. [Google Scholar] [CrossRef]
- Dmytrakh, I.M.; Leshchak, R.L.; Syrotyuk, A.M.; Lutyts’kyi, O.L. Influence of the bulk concentration of hydrogen in the metal on the specific features of deformation of low-alloy pipe steel. Mater. Sci. 2014, 50, 170–178. [Google Scholar] [CrossRef]
- Ostash, O.P.; Vytvyts’kyi, V.I. Duality of the action of hydrogen on the mechanical behavior of steels and structural optimization of their hydrogen resistance. Mater Sci. 2012, 47, 421–437. [Google Scholar] [CrossRef]
- Lynch, S. Hydrogen Embrittlement Phenomena and Mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Martin, M.L.; Dadfarnia, M.; Nagao, A.; Wang, S.; Sofronis, P. Enumeration of the Hydrogen-Enhanced Localized Plasticity Mechanism for Hydrogen Embrittlement in Structural Materials. Acta Mater. 2019, 165, 734–750. [Google Scholar] [CrossRef]
- Tkachov, V.I. Mechanism of Reversible Effect of Hydrogen on Mechanical Properties of Steel. Mater. Sci. 1999, 35, 477–484. [Google Scholar] [CrossRef]
- Robertson, I.M. The effect of hydrogen on dislocation dynamics. Eng. Fract. Mech. 2001, 68, 671–692. [Google Scholar] [CrossRef]
- Somerday, B., Sofronis, P., Jones, R., Eds.; Effects of hydrogen on materials. In Proceedings of the 2008 International Hydrogen Conference, Jackson Lake Lodge, WY, USA, 7–10 September 2008; ASM International: Novelty, OH, USA, 2009. 766p. [Google Scholar]
- Zhang, L.; An, B.; Fukuyama, S.; Yokogawa, K. Hydrogen effects on localized plasticity in SUS310S stainless steel investigated by nanoindentation and atomic force microscopy. Jan. J. Appl. Phys. 2009, 48, 08JB08. [Google Scholar] [CrossRef]
- Ren, Y.; Nie, H.; Dong, Y.; Xu, X.; He, J.; Li, Z.; Cai, Z.; Shen, H.; Zhu, M. Fretting wear behavior of different surface modified layers of a tight fit spline used for a gauge-changeable railway vehicle. Tribol. Int. 2024, 193, 109359. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Z.; Wang, P.; Chen, C.; Jin, X.; Zhao, X.; Ren, R.; Li, Z.; Zhang, H. An investigation on the formation and hardening mechanisms of white etching layer in hypoeutectoid rail surface. Wear 2023, 530–531, 205010. [Google Scholar] [CrossRef]
- Nguyen, B.H.; Camacho, E.T.; Al-Juboori, A.; Ma, Y.; Li, H.; Zhu, H. Tribological behaviors of two distinct classes of white etching layers on rail surface. Wear 2023, 532–533, 205097. [Google Scholar] [CrossRef]
- Ribamar, G.G.; Pereira, J.I.; Escobar, J.D.; Avila, J.A.; Lopes, J.G.; Maawad, E.; Schell, N.; Oliveira, J.P.; Goldenstein, H.; Souza, R.M. Microstructure gradients across the white etching and transition layers of a heavy haul pearlitic steel. Mater. Charact. 2024, 210, 113811. [Google Scholar] [CrossRef]
- Papazoglou, E.L.; Markopoulos, A.P.; Manolakos, D.E. Experimental research on EDM of AISI O1 tool steel and study of the surface white layer formation. Procedia Struct. Integr. 2018, 10, 235–242. [Google Scholar] [CrossRef]
- Zhang, J.; Du, J.; Li, B.; Su, G. Investigation on White Layer Formation in Dry High-Speed Milling of Nickel-Based Superalloy GH4169. Machines 2023, 11, 406. [Google Scholar] [CrossRef]
- Hieu Nguyen, B.; Al-Juboori, A.; Zhu, H.; Zhu, Q.; Li, H.; Tieu, K. Formation mechanism and evolution of white etching layers on different rail grades. Int. J. Fatigue 2022, 163, 107100. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Zhang, Y.; Xu, L.; Wang, J.; Han, E.-H. Formation Mechanisms and Crack Propagation Behaviors of White Etching Layers and Brown Etching Layers on Raceways of Failure Bearings. Lubricants 2024, 12, 59. [Google Scholar] [CrossRef]
- Schöfer, J.; Rehbein, P.; Stolz, U.; Löhe, D.; Zum Gahr, K.-H. Formation of tribochemical films and white layers on self-mated bearing steel surfaces in boundary lubricated slidingcontact. Wear 2001, 248, 7–15. [Google Scholar] [CrossRef]
- Akulovich, L.M.; Galgo, S.I.; Sergeev, L.E.; Babich, V.E. Processing of Materials by Cutting: Educational and Methodological Complex; BSATU: Minsk, Belarus, 2011; 272p. [Google Scholar]
- Shaw, L.L. The Surface Deformation and Mechanical Behavior of Nanostructured Alloys. In Nanostructured Metals and Alloys; Woodhead Publishing: Sawston, UK, 2011; pp. 481–506. ISBN 9781845696702. [Google Scholar]
- Gurey, V.; Shynkarenko, H.; Kuzio, I. Mathematical Model of the Thermoelasticity of the Surface Layer of Parts During Discontinuous Friction Treatment. Lect. Notes Mech. Eng. 2021, 1, 12–22. [Google Scholar] [CrossRef]
- Hurey, I.; Hurey, T.; Lanets, O.; Dmyterko, P. The Durability of the Nanocrystalline Hardened Layer During the Fretting Wear. In Design, Simulation, Manufacturing: The Innovation Exchange; Lecture Notes in Mechanical Engineering; Springer: Berlin/Heidelberg, Germany, 2021; pp. 23–32. [Google Scholar] [CrossRef]
- Gurey, V.; Hurey, I.; Hurey, T.; Wojtowicz, W. Fatigue Strength of Steel Samples After Friction Treatment. Lect. Notes Mech. Eng. 2023, 1, 274–283. [Google Scholar] [CrossRef]
- Kyryliv, V.; Maksymiv, O.; Gurey, V.; Hurey, I.; Kyryliv, Y.; Zvirko, O. The Mode Deformation Effect on Surface Nanocrystalline Structure Formation and Wear Resistance of Steel 41Cr4. Coatings 2023, 13, 249. [Google Scholar] [CrossRef]
- Gurey, V.; Hurey, I. Influence of Surface Hardened Nanocrystalline Layers on the Resistance of Contact Fatigue Destruction. In Proceedings of the Lecture Notes in Mechanical Engineering, Teton, WY, USA, 7-10 September 2008; Ivanov, V., Trojanowska, J., Pavlenko, I., Zajac, J., Peraković, D., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 483–491. [Google Scholar]
- Kraus, W.; Nolze, G. POWDER CELL—A Program for the Representation and Manipulation of Crystal Structures and Calculation of the Resulting X-ray Powder Patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley Publishing Company: Boston, MA, USA, 1978; 555p. [Google Scholar]
- Cappellini, C.; Attanasio, A.; Rotella, G.; Umbrello, D. Formation of White and Dark Layers in Hard Cutting: Influence of Tool Wear. Int. J. Mater. Form. 2010, 3, 455–458. [Google Scholar] [CrossRef]
- Hurey, I.; Hurey, T.; Gurey, V. Wear Resistance of Hardened Nanocrystalline Structures in the Course of Friction of Steel-Grey Cast Iron Pair in Oil-Abrasive Medium. In Design, Simulation, Manufacturing: The Innovation Exchange; Ivanov, V., Trojanowska, J., Machado, J., Liaposhchenko, O., Zajac, J., Pavlenko, I., Edl, M., Perakovic, D., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 572–580. [Google Scholar]
- Katzarov, I.H.; Pashov, D.L.; Paxton, A.T. Hydrogen Embrittlement I. Analysis of Hydrogen-Enhanced Localized Plasticity: Effect of Hydrogen on the Velocity of Screw Dislocations in α-Fe. Phys. Rev. Mater. 2017, 1, 033602. [Google Scholar] [CrossRef]
- Yasniy, P.V.; Okipnyi, I.B.; Maruschak, P.O.; Bishchak, R.T.; Sorochak, A.P. Toughness and failure of heat resistant steel before and after hydrogenation. Theor. Appl. Fract. Mech. 2011, 56, 63–67. [Google Scholar] [CrossRef]
- Yasniy, P.V.; Okipnyi, I.B.; Maruschak, P.O.; Panin, S.V.; Konovalenko, I.V. Crack tip strain localisation on mechanics of fracture of heat resistant steel after hydrogenation. Theor. Appl. Fract. Mech. 2013, 63–64, 63–68. [Google Scholar] [CrossRef]
- Fukai, Y.; Sugimoto, H. Diffusion of Hydrogen in Metals. Adv. Phys. 1985, 34, 263–326. [Google Scholar] [CrossRef]
- Guzanová, A.; Brezinová, J.; Bronček, J.; Maruschak, P.; Landová, M. Study of selected properties of coatings devoted to extreme tribo-corrosive conditions. Mater. Sci. Forum 2015, 818, 32–36. [Google Scholar] [CrossRef]
- Ali Al-Juboori; Huijun Li; Hongato Zhu Formation of white etching layer on rails due to coupled thermal and mechanical actions. Wear 2023, 530–531, 205063. [CrossRef]
- Dzyura, V.O.; Maruschak, P.O.; Zakiev, I.M.; Sorochak, A.P. Analysis of inner surface roughness parameters of load-carrying and support elements of mechanical systems. Int. J. Eng. Trans. B Appl. 2017, 30, 1170–1175. [Google Scholar]

| White Layers | Properties of the Surface with the Formed White Layers | ||||
|---|---|---|---|---|---|
| Technology | Wear Stability | Resistance to Cracking | Crack Resistance | Fatigue Strength | |
| Intermittent | − | − | − | − | − |
| Solid | + | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurey, I.; Augousti, A.; Maruschak, P.; Flowers, A.; Gurey, V.; Dzyura, V.; Prentkovskis, O. Influence of Process Liquids on the Formation of Strengthened Nanocrystalline Structures in Surface Layers of Steel Parts during Thermo-Deformation Treatment. Appl. Sci. 2024, 14, 8053. https://doi.org/10.3390/app14178053
Hurey I, Augousti A, Maruschak P, Flowers A, Gurey V, Dzyura V, Prentkovskis O. Influence of Process Liquids on the Formation of Strengthened Nanocrystalline Structures in Surface Layers of Steel Parts during Thermo-Deformation Treatment. Applied Sciences. 2024; 14(17):8053. https://doi.org/10.3390/app14178053
Chicago/Turabian StyleHurey, Ihor, Andy Augousti, Pavlo Maruschak, Alan Flowers, Volodymyr Gurey, Volodymyr Dzyura, and Olegas Prentkovskis. 2024. "Influence of Process Liquids on the Formation of Strengthened Nanocrystalline Structures in Surface Layers of Steel Parts during Thermo-Deformation Treatment" Applied Sciences 14, no. 17: 8053. https://doi.org/10.3390/app14178053
APA StyleHurey, I., Augousti, A., Maruschak, P., Flowers, A., Gurey, V., Dzyura, V., & Prentkovskis, O. (2024). Influence of Process Liquids on the Formation of Strengthened Nanocrystalline Structures in Surface Layers of Steel Parts during Thermo-Deformation Treatment. Applied Sciences, 14(17), 8053. https://doi.org/10.3390/app14178053












