Synergism Interactions of Plant-Based Proteins: Their Effect on Emulsifying Properties in Oil/Water-Type Model Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Simplex-Centroid Design
2.3. Emulsion Preparation
2.4. Size and ζ-Potential
2.5. Emulsion Flocculation Index Measurement (FI)
2.6. Flocculation and Coalescence Measurement
2.7. Emulsion Activity Index (EAI) and Emulsion Stability Index (ESI)
2.8. Determination of Centrifugal Stability Constant
2.9. Stability against High Temperatures
2.10. Heat Coagulation Time (HCT)
2.11. Optimization Experimental Design
- OR
2.12. Statistical Analysis
3. Results and Discussion
3.1. Surface Characteristics
3.2. Emulsifying Properties
3.3. Stability to Flocculation and Coalescence
3.4. Accelerated Tests—Stability to Centrifugation and Extreme Heat Treatment
3.5. Surface Response Graphs—Response Optimization Variable
3.6. Optimization of Multiple Parameters
3.7. Verification of Predictive Models
3.8. Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaeger, S.R.; Dupas de Matos, A.; Frempomaa Oduro, A.; Hort, J. Sensory Characteristics of Plant-Based Milk Alternatives: Product Characterisation by Consumers and Drivers of Liking. Food Res. Int. 2024, 180, 114093. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Arendt, E.K. Evaluation of Physicochemical and Glycaemic Properties of Commercial Plant-Based Milk Substitutes. Plant Foods Hum. Nutr. 2017, 72, 26–33. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Grossmann, L. The Science of Plant-Based Foods: Constructing Next-Generation Meat, Fish, Milk, and Egg Analogs. Comprehensive Reviews in Food Science and Food Safety; Blackwell Publishing Inc.: Hoboken, NJ, USA, 2021; pp. 4049–4100. [Google Scholar] [CrossRef]
- Ray, M.; Rousseau, D. Stabilization of Oil-in-Water Emulsions Using Mixtures of Denatured Soy Whey Proteins and Soluble Soybean Polysaccharides. Food Res. Int. 2013, 52, 298–307. [Google Scholar] [CrossRef]
- Huang, L.; Cai, Y.; Liu, T.; Zhao, X.; Chen, B.; Long, Z.; Zhao, M.; Deng, X.; Zhao, Q. Stability of Emulsion Stabilized by Low-Concentration Soybean Protein Isolate: Effects of Insoluble Soybean Fiber. Food Hydrocoll. 2019, 97, 105232. [Google Scholar] [CrossRef]
- Wang, L.; Wu, M.; Liu, H.M. Emulsifying and Physicochemical Properties of Soy Hull Hemicelluloses-Soy Protein Isolate Conjugates. Carbohydr. Polym. 2017, 163, 181–190. [Google Scholar] [CrossRef]
- Tran, T.; Rousseau, D. Stabilization of Acidic Soy Protein-Based Dispersions and Emulsions by Soy Soluble Polysaccharides. Food Hydrocoll. 2013, 30, 382–392. [Google Scholar] [CrossRef]
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, Chickpea and Lentil Protein Isolates: Physicochemical Characterization and Emulsifying Properties. Food Biophys. 2016, 11, 43–51. [Google Scholar] [CrossRef]
- Li, C.; Huang, X.; Peng, Q.; Shan, Y.; Xue, F. Physicochemical Properties of Peanut Protein Isolate-Glucomannan Conjugates Prepared by Ultrasonic Treatment. Ultrason. Sonochem. 2014, 21, 1722–1727. [Google Scholar] [CrossRef]
- Ghumman, A.; Kaur, A.; Singh, N. Impact of Germination on Flour, Protein and Starch Characteristics of Lentil (Lens culinari) and Horsegram (Macrotyloma uniflorum L.) Lines. LWT 2016, 65, 137–144. [Google Scholar] [CrossRef]
- Sharan, S.; Zanghelini, G.; Zotzel, J.; Bonerz, D.; Aschoff, J.; Saint-Eve, A.; Maillard, M.N. Fava Bean (Vicia faba L.) for Food Applications: From Seed to Ingredient Processing and Its Effect on Functional Properties, Antinutritional Factors, Flavor, and Color. Compr. Rev. Food Sci. Food Saf. 2021, 20, 401–428. [Google Scholar] [CrossRef]
- Olsmats, E.; Rennie, A.R. Pea Protein [Pisum sativum] as Stabilizer for Oil/Water Emulsions. Adv. Colloid Interface Sci. 2024, 326, 103123. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional Attributes of Pea Protein Isolates Prepared Using Different Extraction Methods and Cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, B.; Liu, Y.; Xiong, Y.L. Interfacial Structural Role of PH-Shifting Processed Pea Protein in the Oxidative Stability of Oil/Water Emulsions. J. Agric. Food Chem. 2014, 62, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Shen, L. Role of Conformational Flexibility in the Emulsifying Properties of Bovine Serum Albumin. J. Agric. Food Chem. 2013, 61, 3097–3110. [Google Scholar] [CrossRef]
- Shewry, P.R.; Croy, R.R.D.; Boulter, D. The Synthesis and Structure of Pea Storage Proteins. Crit. Rev. Plant Sci. 1984, 1, 287–314. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse Proteins: Processing, Characterization, Functional Properties and Applications in Food and Feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Wanasundara, J.P.D. Proteins of Brassicaceae Oilseeds and Their Potential as a Plant Protein Source. Crit. Rev. Food Sci. Nutr. 2011, 51, 635–677. [Google Scholar] [CrossRef]
- Alves, A.C.; Tavares, G.M. Mixing Animal and Plant Proteins: Is This a Way to Improve Protein Techno-Functionalities? Food Hydrocoll. 2019, 97, 105171. [Google Scholar] [CrossRef]
- Grasberger, K.; Sunds, A.V.; Sanggaard, K.W.; Hammershøj, M.; Corredig, M. Behavior of Mixed Pea-Whey Protein at Interfaces and in Bulk Oil-in-Water Emulsions. Innov. Food Sci. Emerg. Technol. 2022, 81, 103136. [Google Scholar] [CrossRef]
- Hinderink, E.B.A.; Münch, K.; Sagis, L.; Schroën, K.; Berton-Carabin, C.C. Synergistic Stabilisation of Emulsions by Blends of Dairy and Soluble Pea Proteins: Contribution of the Interfacial Composition. Food Hydrocoll. 2019, 97, 105206. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Liu, Z.; Zhi, L.; Jiao, B.; Hu, H.; Ma, X.; Agyei, D.; Shi, A. Plant Protein-Based Emulsifiers: Mechanisms, Techniques for Emulsification Enhancement and Applications. Food Hydrocoll. 2023, 144, 109008. [Google Scholar] [CrossRef]
- Hinderink, E.B.A.; Sagis, L.; Schroën, K.; Berton-Carabin, C.C. Behavior of Plant-Dairy Protein Blends at Air-Water and Oil-Water Interfaces. Colloids Surf. B Biointerfaces 2020, 192, 111015. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.K.H.Y.; Schroën, K.; San Martín-González, M.F.; Berton-Carabin, C.C. Synergistic and Antagonistic Effects of Plant and Dairy Protein Blends on the Physicochemical Stability of Lycopene-Loaded Emulsions. Food Hydrocoll. 2018, 81, 180–190. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, A.Y.; Pojchanun, K.; Lee, C.P.; Zhou, A.; An, J.; Hashimoto, M.; Tan, U.X.; Leo, C.H.; Wong, G.; et al. Systematic Engineering Approach for Optimization of Multi-Component Alternative Protein-Fortified 3D Printing Food Ink. Food Hydrocoll. 2022, 131, 107803. [Google Scholar] [CrossRef]
- Hayati, I.N.; Ching, C.W.; Rozaini, M.Z.H. Flow Properties of O/W Emulsions as Affected by Xanthan Gum, Guar Gum and Carboxymethyl Cellulose Interactions Studied by a Mixture Regression Modelling. Food Hydrocoll. 2016, 53, 199–208. [Google Scholar] [CrossRef]
- Batista, R.S.; Costa, H.; Parreira, L.A.; Bernardes, C.O.; Abreu, K.M.P.; Menini, L. Essential Oil of Piper macedoi Yunck. Leaves, Potential Alternative for the Management of Banana Anthracnose Disease. J. Nat. Pestic. Res. 2023, 5, 100039. [Google Scholar] [CrossRef]
- Wiley Analytical Scientific. Gravimetric Analysis: Determining the Density of a Sample for Various Applications; Graf-Utzmann, M., Murtagh, R., Eds.; WILEY-VCH GmbH, Boschstr: Weinheim, Germany; Available online: https://www.sartorius.com/en/products/weighing/lab-weighing-resources/gravimetric-analysis-determining-the-density-of-a-sample-for-various-applications-white-paper#id-1337374 (accessed on 2 January 2024).
- Li, H.; Li, F.; Wu, X.; Wu, W. Effect of Rice Bran Rancidity on the Emulsion Stability of Rice Bran Protein and Structural Characteristics of Interface Protein. Food Hydrocoll. 2021, 121, 107006. [Google Scholar] [CrossRef]
- Du, M.; Xie, J.; Gong, B.; Xu, X.; Tang, W.; Li, X.; Li, C.; Xie, M. Extraction, Physicochemical Characteristics and Functional Properties of Mung Bean Protein. Food Hydrocoll. 2018, 76, 131–140. [Google Scholar] [CrossRef]
- Rahmati, N.F.; Koocheki, A.; Varidi, M.; Kadkhodaee, R. Thermodynamic Compatibility and Interactions between Speckled Sugar Bean Protein and Xanthan Gum for Production of Multilayer O/W Emulsion. J. Food Sci. Technol. 2018, 55, 1143–1153. [Google Scholar] [CrossRef]
- Dumpler, J.; Kulozik, U. Heat Stability of Concentrated Skim Milk as a Function of Heating Time and Temperature on a Laboratory Scale—Improved Methodology and Kinetic Relationship. Int. Dairy J. 2015, 49, 111–117. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Estevão, S.T.; Batista de Almeida e Silva, J.; Lourenço, F.R. Development and Optimization of Beer Containing Malted and Non-Malted Substitutes Using Quality by Design (QbD) Approach. J. Food Eng. 2020, 289, 110182. [Google Scholar] [CrossRef]
- Cui, L.; Bandillo, N.; Wang, Y.; Ohm, J.B.; Chen, B.; Rao, J. Functionality and Structure of Yellow Pea Protein Isolate as Affected by Cultivars and Extraction PH. Food Hydrocoll. 2020, 108, 106008. [Google Scholar] [CrossRef]
- Wei, Y.; Cai, Z.; Wu, M.; Guo, Y.; Tao, R.; Li, R.; Wang, P.; Ma, A.; Zhang, H. Comparative Studies on the Stabilization of Pea Protein Dispersions by Using Various Polysaccharides. Food Hydrocoll. 2020, 98, 105233. [Google Scholar] [CrossRef]
- Lee, H.W.; Lu, Y.; Zhang, Y.; Fu, C.; Huang, D. Physicochemical and Functional Properties of Red Lentil Protein Isolates from Three Origins at Different PH. Food Chem. 2021, 358, 129749. [Google Scholar] [CrossRef] [PubMed]
- Tabilo-Munizaga, G.; Villalobos-Carvajal, R.; Herrera-Lavados, C.; Moreno-Osorio, L.; Jarpa-Parra, M.; Pérez-Won, M. Physicochemical Properties of High-Pressure Treated Lentil Protein-Based Nanoemulsions. LWT 2019, 101, 590–598. [Google Scholar] [CrossRef]
- Galvão, A.M.M.T.; Vélez-Erazo, E.M.; Karatay, G.G.B.; Furtado, G.d.F.; Vidotto, D.C.; Tavares, G.M.; Hubinger, M.D. High Internal Phase Emulsions Stabilized by the Lentil Protein Isolate (Lens culinaris). Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129993. [Google Scholar] [CrossRef]
- Felix, M.; Lopez-Osorio, A.; Romero, A.; Guerrero, A. Faba Bean Protein Flour Obtained by Densification: A Sustainable Method to Develop Protein Concentrates with Food Applications. LWT 2018, 93, 563–569. [Google Scholar] [CrossRef]
- Choe, U.; Chang, L.; Ohm, J.B.; Chen, B.; Rao, J. Structure Modification, Functionality and Interfacial Properties of Kidney Bean (Phaseolus vulgaris L.) Protein Concentrate as Affected by Post-Extraction Treatments. Food Hydrocoll. 2022, 133, 108000. [Google Scholar] [CrossRef]
- Liu, C.; Damodaran, S.; Heinonen, M. Effects of Microbial Transglutaminase Treatment on Physiochemical Properties and Emulsifying Functionality of Faba Bean Protein Isolate. LWT 2019, 99, 396–403. [Google Scholar] [CrossRef]
- Gumus, C.E.; Decker, E.A.; McClements, D.J. Impact of Legume Protein Type and Location on Lipid Oxidation in Fish Oil-in-Water Emulsions: Lentil, Pea, and Faba Bean Proteins. Food Res. Int. 2017, 100, 175–185. [Google Scholar] [CrossRef]
- Presenza, L.; Fabrício, L.F.d.F.; Galvão, J.A.; Vieira, T.M.F.d.S. Simplex-Centroid Mixture Design as a Tool to Evaluate the Effect of Added Flours for Optimizing the Formulation of Native Brazilian Freshwater Fish Burger. LWT 2022, 156, 113008. [Google Scholar] [CrossRef]
- Primozic, M.; Duchek, A.; Nickerson, M.; Ghosh, S. Effect of Lentil Proteins Isolate Concentration on the Formation, Stability and Rheological Behavior of Oil-in-Water Nanoemulsions. Food Chem. 2017, 237, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying Properties of Chickpea, Faba Bean, Lentil and Pea Proteins Produced by Isoelectric Precipitation and Salt Extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Cui, Z.; Kong, X.; Chen, Y.; Zhang, C.; Hua, Y. Effects of Rutin Incorporation on the Physical and Oxidative Stability of Soy Protein-Stabilized Emulsions. Food Hydrocoll. 2014, 41, 1–9. [Google Scholar] [CrossRef]
- Shao, Y.; Tang, C.H. Characteristics and Oxidative Stability of Soy Protein-Stabilized Oil-in-Water Emulsions: Influence of Ionic Strength and Heat Pretreatment. Food Hydrocoll. 2014, 37, 149–158. [Google Scholar] [CrossRef]
- Chang, C.; Tu, S.; Ghosh, S.; Nickerson, M.T. Effect of PH on the Inter-Relationships between the Physicochemical, Interfacial and Emulsifying Properties for Pea, Soy, Lentil and Canola Protein Isolates. Food Res. Int. 2015, 77, 360–367. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Kennedy, D.; Hogan, S.A.; Kelly, P.M.; Thapa, K.; Murphy, K.M.; Fenelon, M.A. Emulsification Properties of Pea Protein Isolate Using Homogenization, Microfluidization and Ultrasonication. Food Res. Int. 2016, 89, 415–421. [Google Scholar] [CrossRef]
- Yang, J.; Liu, G.; Zeng, H.; Chen, L. Effects of High Pressure Homogenization on Faba Bean Protein Aggregation in Relation to Solubility and Interfacial Properties. Food Hydrocoll. 2018, 83, 275–286. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and Functional Characterization of Kidney Bean and Field Pea Protein Isolates: A Comparative Study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Xu, L.; Ma, H. An Efficient Ultrasound-Assisted Extraction Method of Pea Protein and Its Effect on Protein Functional Properties and Biological Activities. LWT 2020, 127, 109348. [Google Scholar] [CrossRef]
- Jarpa-Parra, M.; Bamdad, F.; Wang, Y.; Tian, Z.; Temelli, F.; Han, J.; Chen, L. Optimization of Lentil Protein Extraction and the Influence of Process PH on Protein Structure and Functionality. LWT 2014, 57, 461–469. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Li, S. Physicochemical and Functional Properties of Chinese Quince Seed Protein Isolate. Food Chem. 2019, 283, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Delahaije, R.J.B.M.; Wierenga, P.A.; Van Nieuwenhuijzen, N.H.; Giuseppin, M.L.F.; Gruppen, H. Protein Concentration and Protein-Exposed Hydrophobicity as Dominant Parameters Determining the Flocculation of Protein-Stabilized Oil-in-Water Emulsions. Langmuir 2013, 29, 11567–11574. [Google Scholar] [CrossRef]
- Tcholakova, S.; Denkov, N.D.; Ivanov, I.B.; Campbell, B. Coalescence Stability of Emulsions Containing Globular Milk Proteins. Adv. Colloid Interface Sci. 2006, 123–126, 259–293. [Google Scholar] [CrossRef]
- Delahaije, R.J.B.M.; Gruppen, H.; Giuseppin, M.L.F.; Wierenga, P.A. Towards Predicting the Stability of Protein-Stabilized Emulsions. Adv. Colloid Interface Sci. 2015, 219, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, E.; Mekhloufi, G.; Huang, N.; Rosilio, V.; Agnely, F. β-Lactoglobulin, Gum Arabic, and Xanthan Gum for Emulsifying Sweet Almond Oil: Formulation and Stabilization Mechanisms of Pharmaceutical Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 77–87. [Google Scholar] [CrossRef]
- Damodaran, S.; Anand, K. Sulfhydryl-Disulfide Interchange-Induced Interparticle Protein Polymerization in Whey Protein-Stabilized Emulsions and Its Relation to Emulsion Stability. 1997. Available online: https://pubs.acs.org/sharingguidelines (accessed on 2 January 2024).
- Hu, Y.Q.; Yin, S.W.; Zhu, J.H.; Qi, J.R.; Guo, J.; Wu, L.Y.; Tang, C.H.; Yang, X.Q. Fabrication and Characterization of Novel Pickering Emulsions and Pickering High Internal Emulsions Stabilized by Gliadin Colloidal Particles. Food Hydrocoll. 2016, 61, 300–310. [Google Scholar] [CrossRef]
- Petursson, S.; Decker, E.A.; McClements, D.J. Stabilization of Oil-in-Water Emulsions by Cod Protein Extracts. J. Agric. Food Chem. 2004, 52, 3996–4001. [Google Scholar] [CrossRef]
- McClements, D.J. Protein-Stabilized Emulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 305–313. [Google Scholar] [CrossRef]
- Joshi, M.; Adhikari, B.; Aldred, P.; Panozzo, J.F.; Kasapis, S. Physicochemical and Functional Properties of Lentil Protein Isolates Prepared by Different Drying Methods. Food Chem. 2011, 129, 1513–1522. [Google Scholar] [CrossRef]
- Jeske, S.; Bez, J.; Arendt, E.K.; Zannini, E. Formation, Stability, and Sensory Characteristics of a Lentil-Based Milk Substitute as Affected by Homogenisation and Pasteurisation. Eur. Food Res. Technol. 2019, 245, 1519–1531. [Google Scholar] [CrossRef]
- Burger, T.G.; Singh, I.; Mayfield, C.; Baumert, J.L.; Zhang, Y. The Impact of Spray Drying Conditions on the Physicochemical and Emulsification Properties of Pea Protein Isolate. LWT 2022, 153, 112495. [Google Scholar] [CrossRef]
- Alonso-Miravalles, L.; Zannini, E.; Bez, J.; Arendt, E.K.; O’Mahony, J.A. Thermal and Mineral Sensitivity of Oil-in-Water Emulsions Stabilised Using Lentil Proteins. Foods 2020, 9, 453. [Google Scholar] [CrossRef] [PubMed]

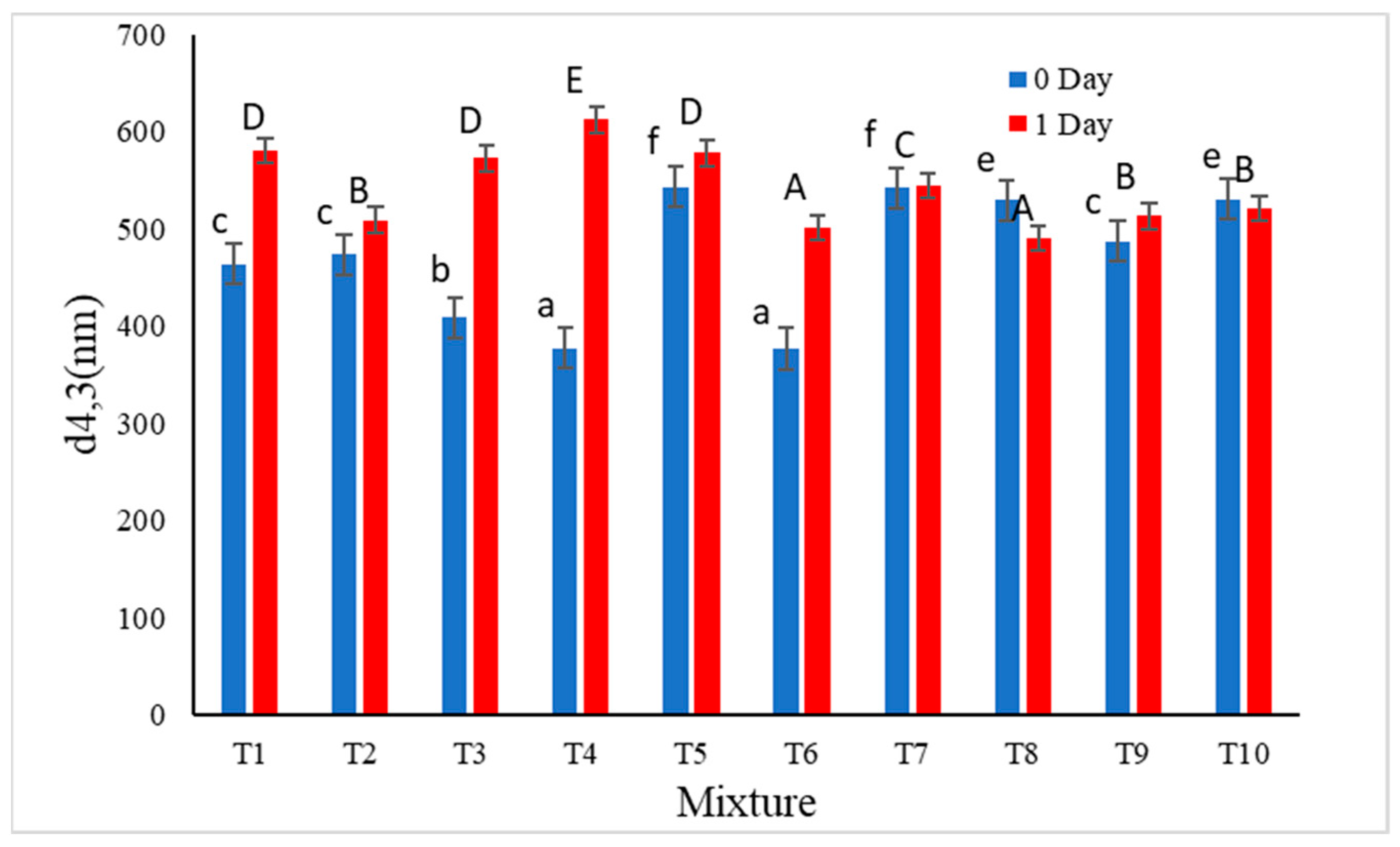
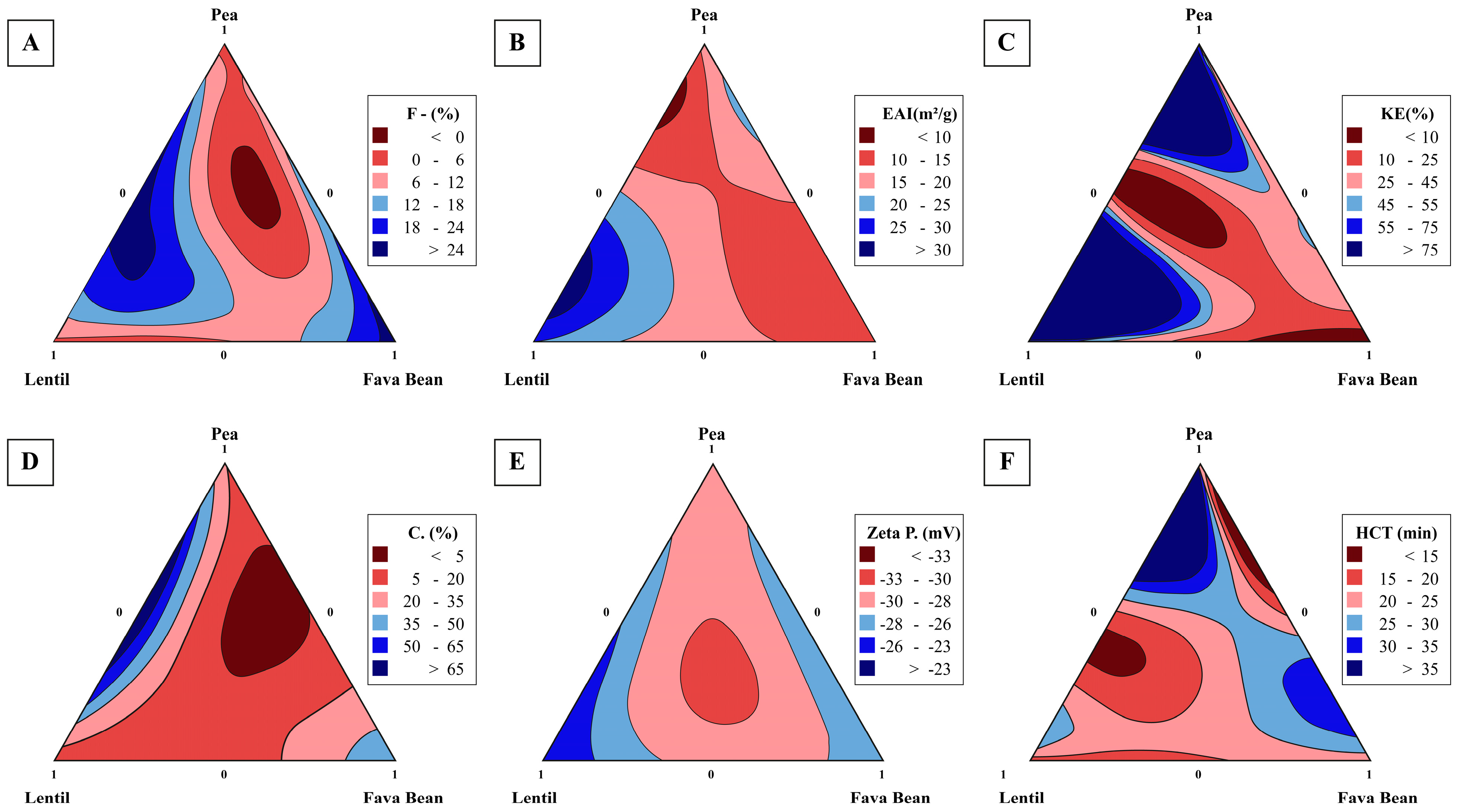
| Emulsion Code | Component Proportion | ||
|---|---|---|---|
| Pea (%) | Lentil (%) | Fava Bean (%) | |
| T1 | 100 | 0 | 0 |
| T2 | 0 | 100 | 0 |
| T3 | 0 | 0 | 100 |
| T4 | 50 | 50 | 0 |
| T5 | 50 | 0 | 50 |
| T6 | 0 | 50 | 50 |
| T7 | 50 | 25 | 25 |
| T8 | 25 | 50 | 25 |
| T9 | 25 | 25 | 50 |
| T10 | 33.33 | 33.33 | 33.33 |
| Treatment | Coalescence Stability (%) | Flocculation Stability (%) | Flocculation Index (%) | HCT (Minutes) | KE (%) | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| T1 | 22.18 ± 0.03 c | 4.13 ± 0.014 j | 5.7 2 ± 0.01 e | 18.34 ± 3.99 ab | 24.46 ± 0.03 d | −30.28 ± 2.62 b |
| T2 | 6.92 ± 0.016 e | 4.77 ± 0.014 h | 3.93 ± 0.09 f | 13.53 ± 2.11 c | 65.58 ± 3.84 b | −23.77 ± 2.33 a |
| T3 | 49.41 ± 0.23 b | 27.18 ± 0.13 a | 25.80 ± 0.12 a | 20.3 ± 1.80 ab | 3.45 ± 2.05 h | −26.25 ± 2.33 a |
| T4 | 78.39 ± 0.38 a | 26.69 ± 0.13 b | 26.02 ± 0.12 a | 19.7 ± 0.83 ab | 40.53 ± 0.05 c | −26.75 ± 2.34 a |
| T5 | 5. 25 ± 0.02 f | 15.15 ± 0.06 d | 13.13 ± 0.05 b | 18.4 ± 4.14 ab | 10.91 ± 2.65 f | −26.15 ± 2.19 ab |
| T6 | 11.58 ± 0.04 d | 4.49 ± 0.07 h | 12.79 ± 0.04 c | 18.96 ± 0.56 ab | 16.11 ± 0.11 e | −28.05 ± 1.20 b |
| T7 | 4.93 ± 0.07 f | 1.31 ± 0.07 k | 3.21 ± 0.07 g | 25.78 ± 0.67 a | 16.39 ± 0.09 e | −28.10 ± 2.12 b |
| T8 | 11.67 ± 0.02 d | 18.56 ± 0.03 c | 0.53 ± 0.07 i | 16.85 ± 0.50 b | 74.01 ± 0.07 a | −29.90 ± 2.69 b |
| T9 | 7.07 ± 0.01 e | 4.24 ± 0.01 j | 5.76 ± 0.06 e | 25.00 ± 0.79 a | 17.41 ± 0.49 e | −32.15 ± 1.27 c |
| T10 | 5.19 ± 0.01 f | 6.82 ± 0.02 g | 3.38 ± 0.04 g | 21.63 ± 0.60 ab | 6.87 ± 0.02 g | −30.40 ± 1.27 b |
| Response Variable | Model | F-Test | R2 | p-Value |
|---|---|---|---|---|
| Zeta potential (mV) | Linear | 5.04 | 76.81 | 0.022 |
| Coalescence (%) | Linear | 1008.87 | 99.88 | <0.001 |
| Flocculation Index (%) | Quadratic | 139.34 | 98.00 | <0.001 |
| Centrifugal stability (%) | Special cubic | 140.81 | 99.96 | <0.001 |
| HCT (min) | Quadratic | 8.62 | 74.36 | 0.010 |
| EAI (m2/g) | Quadratic | 7.75 | 73.76 | 0.002 |
| Centrifugal stability constant—KE (%) | Special Quadratic | 79.63 | 99.58 | <0.001 |
| Evaluated Parameters | Pea (%) | Lentil (%) | Fava Bean (%) | Experimental Value | Adjusted R2 |
|---|---|---|---|---|---|
| Zeta potential (mV) | 33.33 | 33.33 | 33.33 | −30.43 | 0.99 |
| Coalescence | 34.87 | 27.76 | 37.37 | 2.99 | 0.98 |
| Flocculation Index | 36.73 | 21.85 | 41.41 | 1.0 | 0.99 |
| Centrifugal stability (%) | 41.41 | 30.60 | 27.99 | 0.5 | 0.98 |
| HCT (min) | 56.73 | 43.26 | 0 | 27.00 | 0.99 |
| EAI (m2/g) | 19.19 | 80.81 | 0 | 32.85 | 0.95 |
| Sample 1 | Sample 2 | Correlation | p-Value |
|---|---|---|---|
| EAI (m2/g) | Centrifugal stability (%) | 0.759 | 0.024 |
| EAI (m2/g) | IPD | 0.695 | 0.002 |
| HCT (min) | Centrifugal stability (%) | 0.725 | 0.008 |
| Coalescence stability (%) | Flocculation stabilit (%) | 0.765 | 0.000 |
| Coalescence stability (%) | Flocculation index (%) | 0.845 | 0.000 |
| Flocculation stability (%) | Flocculation index (%) | 0.725 | 0.000 |
| Flocculation stability (%) | D4,3 (nm) | 0.758 | 0.008 |
| Zeta Potential (mV) | ESI (min) | 0.657 | 0.005 |
| Dependent Variable Response | Goal | Lower Limit | Upper Limit | Predicted Value | Experimental Value | Relative Error (%) |
|---|---|---|---|---|---|---|
| EAI (m2/g) | Maximize | 16.00 | 32.00 | 16.09 | 23.75 ± 1.18 | 41.0 |
| Zeta potential (mV) | Minimize | −28.00 | −31.00 | −30.9 | −29.16 ± 1.46 | 0.90 |
| Coalescence stability (%) | Minimize | 4.0 | 7.0 | 3.56 | 2.53 ± 0.69 | 0.91 |
| D4.3 (nm) | Minimize | 400.00 | 420.00 | 421.28 | 436.16 ± 16.06 | 3.56 |
| HCT (min) | Maximize | 18.00 | 26.00 | 24.69 | 22.40 ± 2.54 | 6.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, R.R.; Vieira, M.E.M.; Campos, N.d.S.; Perrone, Í.T.; Stephani, R.; Casanova, F.; de Carvalho, A.F. Synergism Interactions of Plant-Based Proteins: Their Effect on Emulsifying Properties in Oil/Water-Type Model Emulsions. Appl. Sci. 2024, 14, 8086. https://doi.org/10.3390/app14178086
Lima RR, Vieira MEM, Campos NdS, Perrone ÍT, Stephani R, Casanova F, de Carvalho AF. Synergism Interactions of Plant-Based Proteins: Their Effect on Emulsifying Properties in Oil/Water-Type Model Emulsions. Applied Sciences. 2024; 14(17):8086. https://doi.org/10.3390/app14178086
Chicago/Turabian StyleLima, Raquel Reis, Maria Eduarda Martins Vieira, Nathalia da Silva Campos, Ítalo Tuler Perrone, Rodrigo Stephani, Federico Casanova, and Antônio Fernandes de Carvalho. 2024. "Synergism Interactions of Plant-Based Proteins: Their Effect on Emulsifying Properties in Oil/Water-Type Model Emulsions" Applied Sciences 14, no. 17: 8086. https://doi.org/10.3390/app14178086







