Digital-Focused Approaches in Cancer Patients’ Management in the Post-COVID Era: Challenges and Solutions
Abstract
:1. Introduction
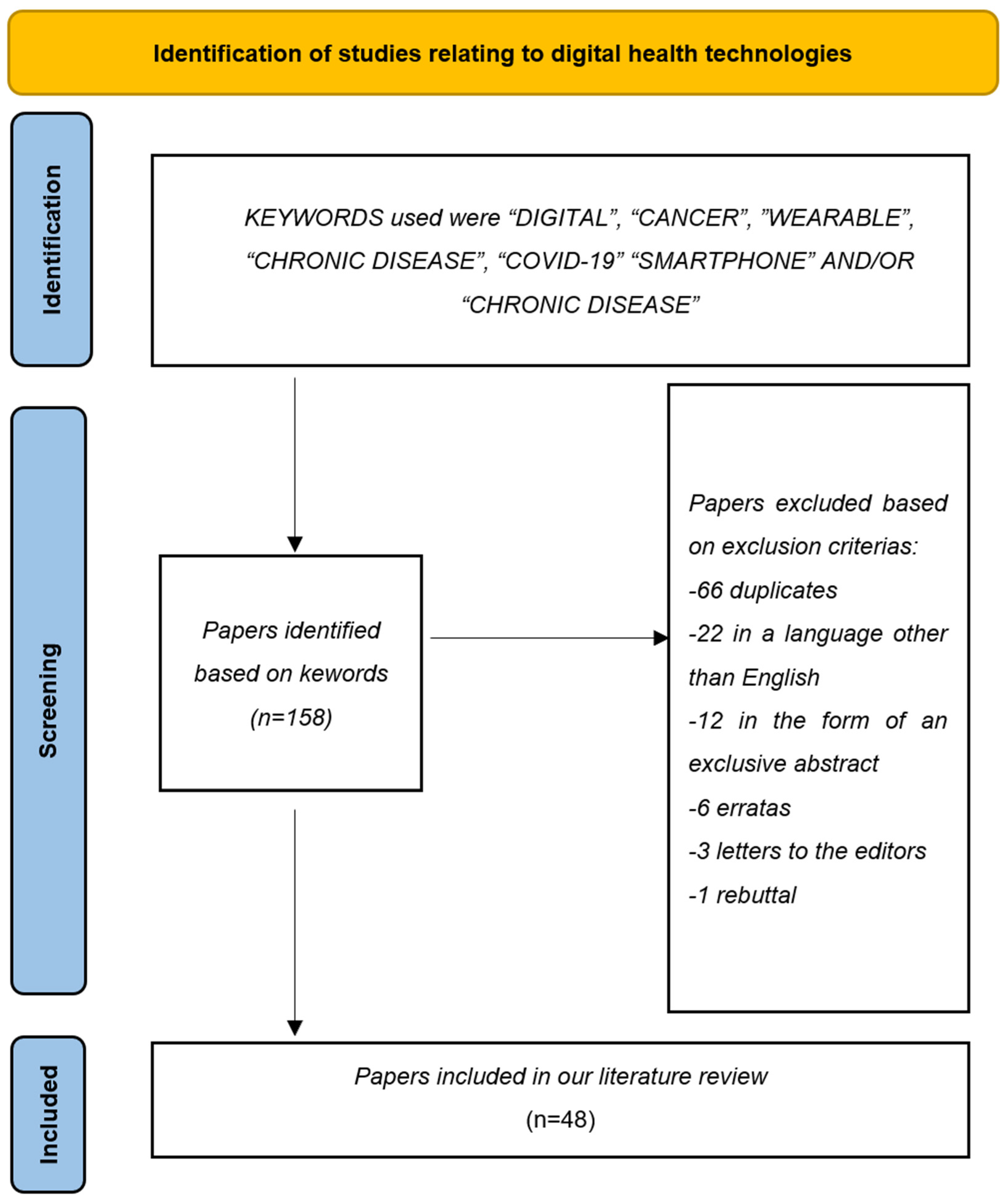
2. Materials and Methods
3. Results
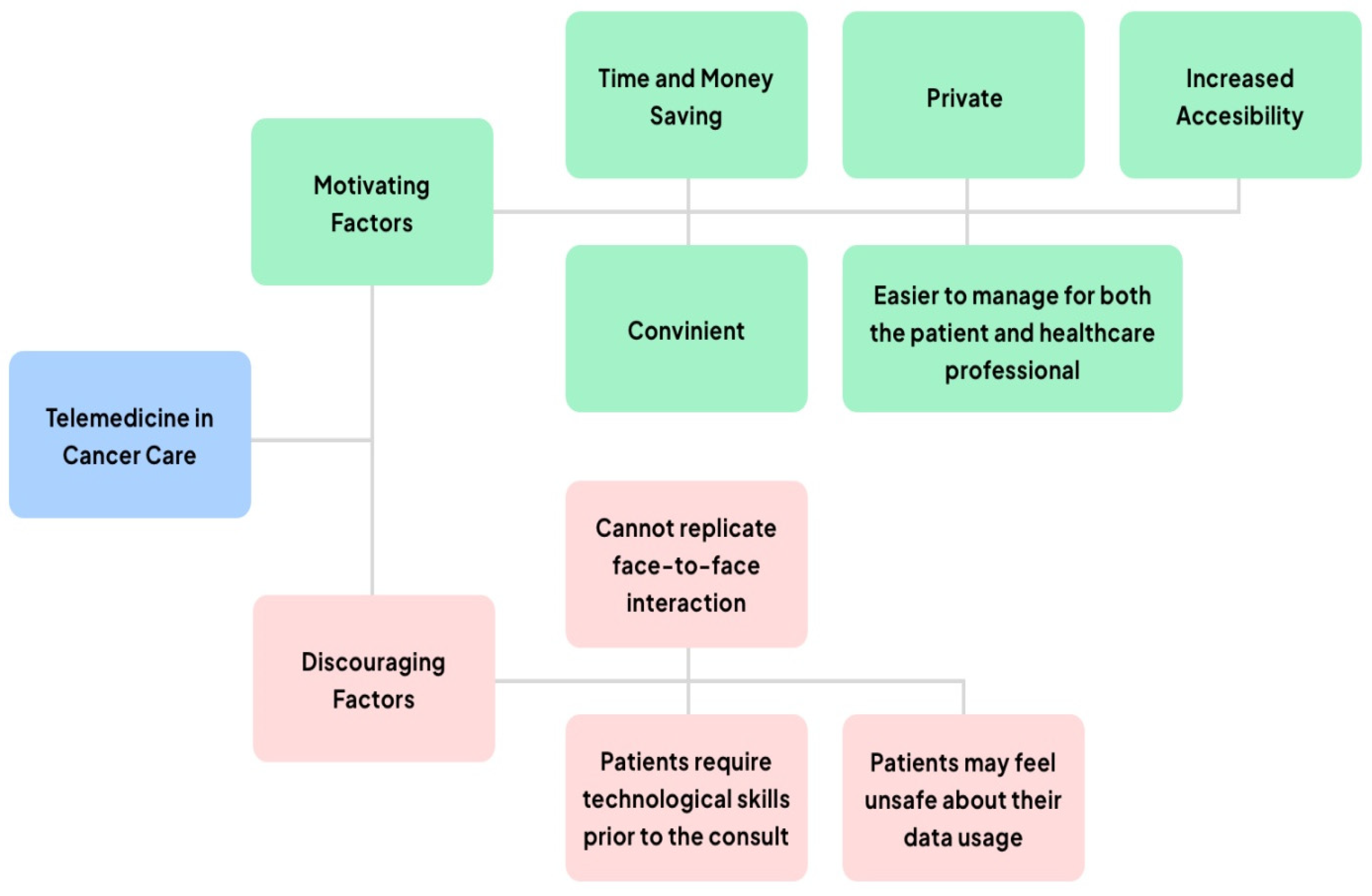
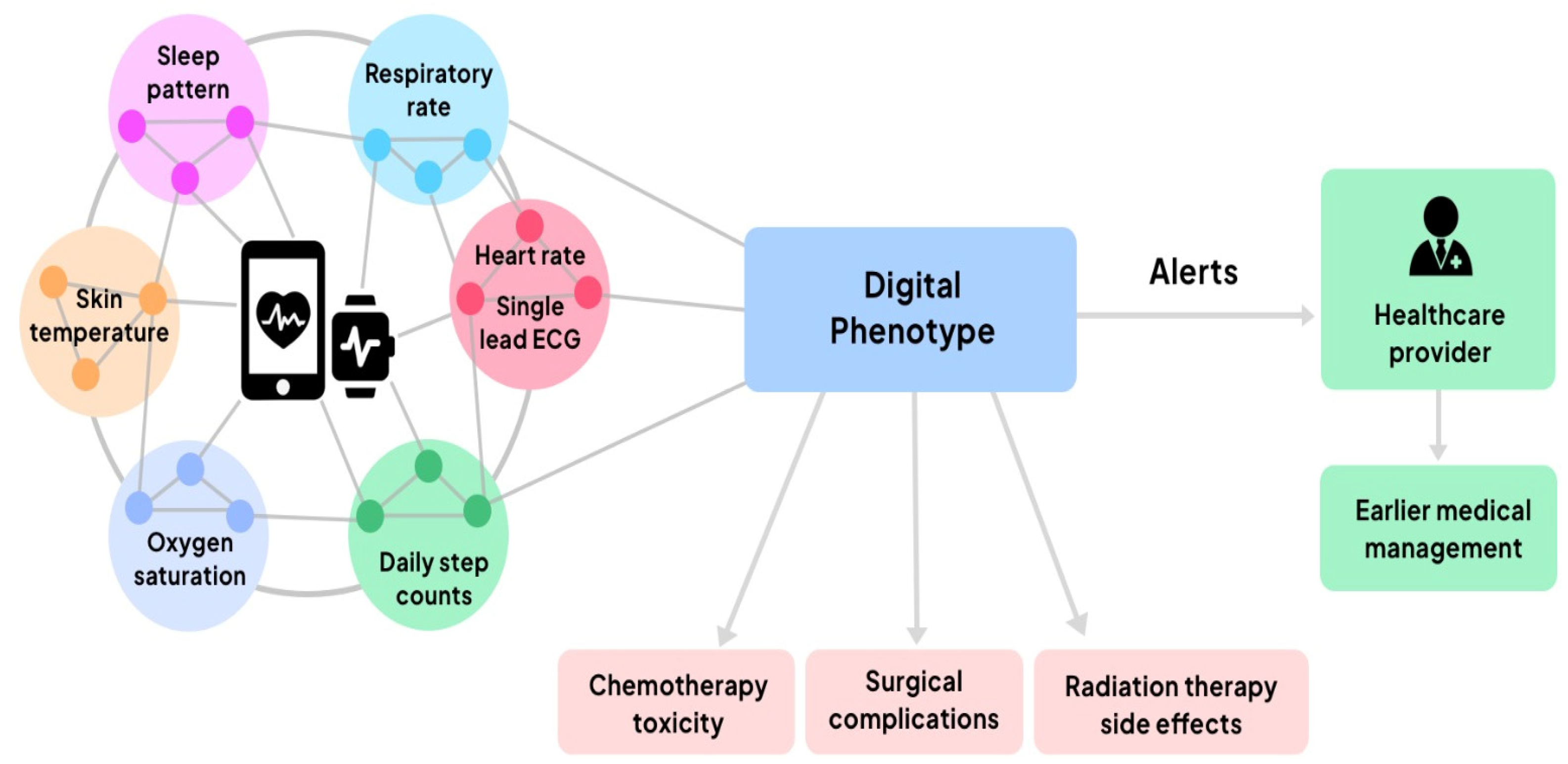
3.1. Digital Health—Telemedicine and Personal Digital Health
3.2. Microneedle Technology
3.3. Artificial Intelligence—Using Data to Improve Cancer Care
3.4. Clinical Trials
3.5. Limitations and Challenges
4. Discussions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the definition of cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Santucci, C.; Carioli, G.; Bertuccio, P.; Malvezzi, M.; Pastorino, U.; Boffetta, P.; Negri, E.; Bosetti, C.; La Vecchia, C. Progress in cancer mortality, incidence, and survival: A global overview. Eur. J. Cancer Prev. 2020, 29, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, A.; Joosten, E.A.J.; van den Beuken-van Everdingen, M.H.J. Personalized Medicine: Recent Progress in Cancer Therapy. Cancers 2021, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Erdem, O.; Es, I.; Akceoglu, G.A.; Saylan, Y.; Inci, F. Recent Advances in Microneedle-Based Sensors for Sampling, Diagnosis and Monitoring of Chronic Diseases. Biosensors 2021, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- West, H.J. Telemedicine in Oncology: Delivering on an Overdue Promise in the COVID-19 Era. Front. Oncol. 2020, 10, 578888. [Google Scholar] [CrossRef] [PubMed]
- Haldane, V.; De Foo, C.; Abdalla, S.M.; Jung, A.S.; Tan, M.; Wu, S.; Chua, A.; Verma, M.; Shrestha, P.; Singh, S.; et al. Health systems resilience in managing the COVID-19 pandemic: Lessons from 28 countries. Nat. Med. 2021, 27, 964–980. [Google Scholar] [CrossRef] [PubMed]
- Legido-Quigley, H.; Mateos-Garcia, J.T.; Campos, V.R.; Gea-Sanchez, M.; Muntaner, C.; McKee, M. The resilience of the Spanish health system against the COVID-19 pandemic. Lancet Public Health 2020, 5, e251–e252. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, W.; Bloem, R.; Ananth, A.; Kanagasabapathi, T.; Amelink, A.; Bouwman, J.; Gelinck, G.; van Veen, S.; Boorsma, A.; Wopereis, S. Digital Resilience Biomarkers for Personalized Health Maintenance and Disease Prevention. Front. Digit. Health 2020, 2, 614670. [Google Scholar] [CrossRef] [PubMed]
- Ohannessian, R.; Duong, T.A.; Odone, A. Global Telemedicine Implementation and Integration Within Health Systems to Fight the COVID-19 Pandemic: A Call to Action. JMIR Public Health Surveill. 2020, 6, e18810. [Google Scholar] [CrossRef] [PubMed]
- Ayyoubzadeh, S.M.; Shirkhoda, M.; Niakan Kalhori, S.R.; Mohammadzadeh, N.; Zakerabasali, S. A Smartphone Remote Monitoring App to Follow Up Colorectal Cancer Survivors: Requirement Analysis. JMIR Cancer 2022, 8, e18083. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, T.; Kawaguchi, T.; Azuma, K.; Suzuki, S.; Sano, Y.; Akatsu, M.; Torii, A.; Kamimura, T.; Ozawa, Y.; Tsuchida, A.; et al. Patient-generated health data collection using a wearable activity tracker in cancer patients—A feasibility study. Support. Care Cancer 2020, 28, 5953–5961. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Handa, M.; Shukla, R.; Rahman, M.; Almalki, W.H.; Afzal, O.; Altamimi, A.S.A. Wearable smart devices in cancer diagnosis and remote clinical trial monitoring: Transforming the healthcare applications. Drug Discov. Today 2022, 27, 103314. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, U.L.; Pappot, H.; Hollander-Mieritz, C. The Use of Wearables in Clinical Trials During Cancer Treatment: Systematic Review. JMIR Mhealth Uhealth 2020, 8, e22006. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, S.; De Bock, M.; Williman, J.; Taylor, B.; Elbalshy, M.; Galland, B.; Hall, R.; Paul, R.; Boucsein, A.; Jones, S.; et al. Study protocol: Safety and efficacy of smart watch integrated do-it-yourself continuous glucose monitoring in adults with Type 1 diabetes, a randomised controlled trial. J. Diabetes Metab. Disord. 2021, 20, 2103–2113. [Google Scholar] [CrossRef] [PubMed]
- Finnane, A.; Dallest, K.; Janda, M.; Soyer, H.P. Teledermatology for the diagnosis and management of skin cancer: A systematic review. JAMA Dermatol. 2017, 153, 319–327. [Google Scholar] [CrossRef]
- Sonagli, M.; Cagnacci Neto, R.; Leite, F.P.M.; Makdissi, F.B.A. The use of telemedicine to maintain breast cancer follow-up and surveillance during the COVID-19 pandemic. J. Surg. Oncol. 2021, 123, 371–374. [Google Scholar] [CrossRef]
- Pang, N.-Q.; Lau, J.; Fong, S.-Y.; Wong, C.Y.-H.; Tan, K.-K. Telemedicine acceptance among older adult patients with cancer: Scoping review. J. Med. Internet Res. 2022, 24, e28724. [Google Scholar] [CrossRef]
- Di Zazzo, A.; Micera, A.; De Piano, M.; Cortes, M.; Bonini, S. Tears and ocular surface disorders: Usefulness of biomarkers. J. Cell Physiol. 2019, 234, 9982–9993. [Google Scholar] [CrossRef]
- Dias, A.; Silva, L.; Moura, J.; Gabriel, D.; Maia, L.F. Fluid biomarkers in stroke: From animal models to clinical care. Acta Neurol. Scand. 2022, 146, 332–347. [Google Scholar] [CrossRef]
- Gaw, G.; Gribben, M. Can we detect biomarkers of oral squamous cell carcinoma from saliva or mouth swabs? Evid. Based Dent. 2022, 23, 32–33. [Google Scholar] [CrossRef]
- Low, C.A. Harnessing consumer smartphone and wearable sensors for clinical cancer research. NPJ Digit. Med. 2020, 3, 140. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, F.; Teymourian, H.; Wuerstle, B.; Kavner, J.; Patel, R.; Furmidge, A.; Aghavali, R.; Hosseini-Toudeshki, H.; Brown, C.; Zhang, F.; et al. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat. Biomed. Eng. 2022, 6, 1214–1224. [Google Scholar] [CrossRef]
- Li, J.; Lu, H.; Wang, Y.; Yang, S.; Zhang, Y.; Wei, W.; Qiao, Y.; Dai, W.; Ge, R.; Dong, H. Interstitial Fluid Biomarkers’ Minimally Invasive Monitoring Using Microneedle Sensor Arrays. Anal. Chem. 2022, 94, 968–974. [Google Scholar] [CrossRef]
- Wang, Z.; Luan, J.; Seth, A.; Liu, L.; You, M.; Gupta, P.; Rathi, P.; Wang, Y.; Cao, S.; Jiang, Q.; et al. Microneedle patch for the ultrasensitive quantification of protein biomarkers in interstitial fluid. Nat. Biomed. Eng. 2021, 5, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yang, H.; Lu, X.; Li, Y.; Liu, Z.; Li, F.; Shang, Z.; Wang, X.; Li, X.; Li, J.; et al. Three-Dimensional-Cultured MSC-Derived Exosome-Hydrogel Hybrid Microneedle Array Patch for Spinal Cord Repair. Nano Lett. 2022, 22, 6391–6401. [Google Scholar] [CrossRef]
- Taylor, R.M.; Miller, P.R.; Ebrahimi, P.; Polsky, R.; Baca, J.T. Minimally-invasive, microneedle-array extraction of interstitial fluid for comprehensive biomedical applications: Transcriptomics, proteomics, metabolomics, exosome research, and biomarker identification. Lab. Anim. 2018, 52, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Alimardani, V.; Abolmaali, S.S.; Tamaddon, A.M.; Ashfaq, M. Recent advances on microneedle arrays-mediated technology in cancer diagnosis and therapy. Drug Deliv. Transl. Res. 2021, 11, 788–816. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hasan, A.; Attar, F.; Babadaei, M.M.N.; Zeinabad, H.A.; Salehi, M.; Alizadeh, M.; Hassan, M.; Derakhshankhah, H.; Hamblin, M.R.; et al. Diagnostic and drug release systems based on microneedle arrays in breast cancer therapy. J. Control. Release 2021, 338, 341–357. [Google Scholar] [CrossRef]
- Ali, A.A.; McCrudden, C.M.; McCaffrey, J.; McBride, J.W.; Cole, G.; Dunne, N.J.; Robson, T.; Kissenpfennig, A.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomedicine 2017, 13, 921–932. [Google Scholar] [CrossRef]
- Dervisevic, M.; Alba, M.; Adams, T.E.; Prieto-Simon, B.; Voelcker, N.H. Electrochemical immunosensor for breast cancer biomarker detection using high-density silicon microneedle array. Biosens. Bioelectron. 2021, 192, 113496. [Google Scholar] [CrossRef]
- Tawakey, S.H.; Mansour, M.; Soltan, A.; Salim, A.I. Early detection of hypo/hyperglycemia using a microneedle electrode array-based biosensor for glucose ultrasensitive monitoring in interstitial fluid. Lab Chip 2024, 24, 3958–3972. [Google Scholar] [CrossRef]
- García-Guzmán, J.J.; Pérez-Ràfols, C.; Cuartero, M.; Crespo, G.A. Microneedle based electrochemical (Bio) Sensing: Towards decentralised and continuous health status monitoring. TrAC Trends Anal. Chem. 2021, 135, 116148. [Google Scholar] [CrossRef]
- Meskó, B.; Görög, M. A short guide for medical professionals in the era of artificial intelligence. NPJ Digit. Med. 2020, 3, 126. [Google Scholar] [CrossRef]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA A Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef] [PubMed]
- Panayides, A.S.; Amini, A.; Filipovic, N.D.; Sharma, A.; Tsaftaris, S.A.; Young, A.; Foran, D.; Do, N.; Golemati, S.; Kurc, T. AI in medical imaging informatics: Current challenges and future directions. IEEE J. Biomed. Health Inform. 2020, 24, 1837–1857. [Google Scholar] [CrossRef]
- Comes, M.C.; Fanizzi, A.; Bove, S.; Didonna, V.; Diotiaiuti, S.; Fadda, F.; La Forgia, D.; Giotta, F.; Latorre, A.; Nardone, A.; et al. Explainable 3D CNN based on baseline breast DCE-MRI to give an early prediction of pathological complete response to neoadjuvant chemotherapy. Comput. Biol. Med. 2024, 172, 108132. [Google Scholar] [CrossRef] [PubMed]
- Aberle, D.R.; Abtin, F.; Brown, K. Computed tomography screening for lung cancer: Has it finally arrived? Implications of the national lung screening trial. J. Clin. Oncol. 2013, 31, 1002. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.; Aglen, C.F.; Lee, C.I.; Hoff, S.R.; Lund-Hanssen, H.; Lång, K.; Nygård, J.F.; Ursin, G.; Hofvind, S. Artificial intelligence evaluation of 122 969 mammography examinations from a population-based screening program. Radiology 2022, 303, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Twilt, J.J.; van Leeuwen, K.G.; Huisman, H.J.; Fütterer, J.J.; de Rooij, M. Artificial intelligence based algorithms for prostate cancer classification and detection on magnetic resonance imaging: A narrative review. Diagnostics 2021, 11, 959. [Google Scholar] [CrossRef]
- Zeng, J.; Banerjee, I.; Henry, A.S.; Wood, D.J.; Shachter, R.D.; Gensheimer, M.F.; Rubin, D.L. Natural language processing to identify cancer treatments with electronic medical records. JCO Clin. Cancer Inform. 2021, 5, 379–393. [Google Scholar] [CrossRef]
- Kehl, K.L.; Xu, W.; Lepisto, E.; Elmarakeby, H.; Hassett, M.J.; Van Allen, E.M.; Johnson, B.E.; Schrag, D. Natural language processing to ascertain cancer outcomes from medical oncologist notes. JCO Clin. Cancer Inform. 2020, 4, 680–690. [Google Scholar] [CrossRef] [PubMed]
- de Las Heras, B.; Daehnke, A.; Saini, K.S.; Harris, M.; Morrison, K.; Aguilo, A.; Chico, I.; Vidal, L.; Marcus, R. Role of decentralised clinical trials in cancer drug development: Results from a survey of oncologists and patients. Digit. Health 2022, 8, 20552076221099997. [Google Scholar] [PubMed]
- Sessa, C.; Cortes, J.; Conte, P.; Cardoso, F.; Choueiri, T.; Dummer, R.; Lorusso, P.; Ottmann, O.; Ryll, B.; Mok, T.; et al. The impact of COVID-19 on cancer care and oncology clinical research: An experts’ perspective. ESMO Open 2022, 7, 100339. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. New FDA guidance on general clinical trial conduct in the era of COVID-19. Ther. Innov. Regul. Sci. 2020, 54, 723–724. [Google Scholar] [CrossRef] [PubMed]
- Vyas, D.A.; Eisenstein, L.G.; Jones, D.S. Hidden in Plain Sight—Reconsidering the Use of Race Correction in Clinical Algorithms. N. Engl. J. Med. 2020, 383, 874–882. [Google Scholar] [CrossRef]
- Hossain, M.B.; Kong, Y.; Posada-Quintero, H.F.; Chon, K.H. Comparison of Electrodermal Activity from Multiple Body Locations Based on Standard EDA Indices’ Quality and Robustness against Motion Artifact. Sensors 2022, 22, 3177. [Google Scholar] [CrossRef]
- van der Mee, D.J.; Gevonden, M.J.; Westerink, J.; de Geus, E.J.C. Validity of electrodermal activity-based measures of sympathetic nervous system activity from a wrist-worn device. Int. J. Psychophysiol. 2021, 168, 52–64. [Google Scholar] [CrossRef]
- Fine, J.; Branan, K.L.; Rodriguez, A.J.; Boonya-Ananta, T.; Ajmal Ramella-Roman, J.C.; McShane, M.J.; Cote, G.L. Sources of Inaccuracy in Photoplethysmography for Continuous Cardiovascular Monitoring. Biosensors 2021, 11, 126. [Google Scholar] [CrossRef]
- Patel, V.; Orchanian-Cheff, A.; Wu, R. Evaluating the Validity and Utility of Wearable Technology for Continuously Monitoring Patients in a Hospital Setting: Systematic Review. JMIR Mhealth Uhealth 2021, 9, e17411. [Google Scholar] [CrossRef]
- Knight, S.R.; Ng, N.; Tsanas, A.; McLean, K.; Pagliari, C.; Harrison, E.M. Mobile devices and wearable technology for measuring patient outcomes after surgery: A systematic review. NPJ Digit. Med. 2021, 4, 157. [Google Scholar] [CrossRef]
- Rong, G.; Zheng, Y.; Sawan, M. Energy Solutions for Wearable Sensors: A Review. Sensors 2021, 21, 3806. [Google Scholar] [CrossRef] [PubMed]
- Cilliers, L. Wearable devices in healthcare: Privacy and information security issues. Health Inf. Manag. 2020, 49, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Avcil, M.; Celik, A. Microneedles in Drug Delivery: Progress and Challenges. Micromachines 2021, 12, 1321. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Gupta, R.; Vanshita. Microneedle Technology: An Insight into Recent Advancements and Future Trends in Drug and Vaccine Delivery. Assay Drug Dev. Technol. 2021, 19, 97–114. [Google Scholar] [CrossRef]
- Dong, Y.; Mao, S.; Chen, S.; Ma, J.; Jaffrezic-Renault, N.; Guo, Z. Opportunities and Challenges of Microneedle Electrochemical Sensors for Interstitial Fluid Detection. TrAC Trends Anal. Chem. 2024, 180, 117891. [Google Scholar] [CrossRef]
- Ji, W.; Zhu, J.; Wu, W.; Wang, N.; Wang, J.; Wu, J.; Wu, Q.; Wang, X.; Yu, C.; Wei, G.; et al. Wearable Sweat Biosensors Refresh Personalized Health/Medical Diagnostics. Research 2021, 2021, 9757126. [Google Scholar] [CrossRef]
- Taha, B.A.; Al-Jubouri, Q.; Chahal, S.; Al Mashhadany, Y.; Rustagi, S.; Chaudhary, V.; Arsad, N. State-of-the-art telemodule-enabled intelligent optical nano-biosensors for proficient SARS-CoV-2 monitoring. Microchem. J. 2024, 197, 109774. [Google Scholar] [CrossRef]
- Muller, A.; Haneke, H.; Kirchberger, V.; Mastella, G.; Dommasch, M.; Merle, U.; Heinze, O.; Siegmann, A.; Spinner, C.; Buiatti, A.; et al. Integration of mobile sensors in a telemedicine hospital system: Remote-monitoring in COVID-19 patients. Z. Gesundh. Wiss. 2022, 30, 93–97. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, M.; Cao, X.; Zhao, Y. Functional microneedles for wearable electronics. Smart Med. 2023, 2, e20220023. [Google Scholar] [CrossRef]
- Ganeson, K.; Alias, A.H.; Murugaiyah, V.; Amirul, A.A.; Ramakrishna, S.; Vigneswari, S. Microneedles for Efficient and Precise Drug Delivery in Cancer Therapy. Pharmaceutics 2023, 15, 744. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Z.; Huang, H.; Liang, X. Pioneering healthcare with soft robotic devices: A review. Smart Med. 2024, 3, e20230045. [Google Scholar] [CrossRef] [PubMed]
- Butt, S.R.; Soulat, A.; Lal, P.M.; Fakhor, H.; Patel, S.K.; Ali, M.B.; Arwani, S.; Mohan, A.; Majumder, K.; Kumar, V.; et al. Impact of artificial intelligence on the diagnosis, treatment and prognosis of endometrial cancer. Ann. Med. Surg. 2024, 86, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hu, Z.; Wang, S.; Zhang, Y. Deep Learning for Medical Image-Based Cancer Diagnosis. Cancers 2023, 15, 3608. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgescu, I.; Dricu, A.; Artene, S.-A.; Vrăjitoru, N.-R.; Barcan, E.; Tache, D.E.; Giubelan, L.-I.; Staicu, G.-A.; Manea, E.-V.; Pană, C.; et al. Digital-Focused Approaches in Cancer Patients’ Management in the Post-COVID Era: Challenges and Solutions. Appl. Sci. 2024, 14, 8097. https://doi.org/10.3390/app14188097
Georgescu I, Dricu A, Artene S-A, Vrăjitoru N-R, Barcan E, Tache DE, Giubelan L-I, Staicu G-A, Manea E-V, Pană C, et al. Digital-Focused Approaches in Cancer Patients’ Management in the Post-COVID Era: Challenges and Solutions. Applied Sciences. 2024; 14(18):8097. https://doi.org/10.3390/app14188097
Chicago/Turabian StyleGeorgescu, Ilona, Anica Dricu, Stefan-Alexandru Artene, Nicolae-Răzvan Vrăjitoru, Edmond Barcan, Daniela Elise Tache, Lucian-Ion Giubelan, Georgiana-Adeline Staicu, Elena-Victoria Manea (Carneluti), Cristina Pană, and et al. 2024. "Digital-Focused Approaches in Cancer Patients’ Management in the Post-COVID Era: Challenges and Solutions" Applied Sciences 14, no. 18: 8097. https://doi.org/10.3390/app14188097








