Comprehensive Analysis of Teran Red Wine Aroma and Sensory Profiles: Impacts of Maceration Duration, Pre-Fermentation Heating Treatment, and Barrel Aging
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Grapes and Winemaking
2.3. Analysis of Free Volatile Aroma Compounds
2.4. Sensory Analysis
2.5. Statistical Data Analysis
3. Results and Discussion
3.1. Evaluation of Volatile Aroma Compounds
3.1.1. Monoterpenes
3.1.2. C13-Norisoprenoids
3.1.3. Alcohols
3.1.4. Fatty Acids
3.1.5. Ethyl Esters
3.1.6. Acetate Esters
3.1.7. Other Esters
3.1.8. Volatile Phenols
3.1.9. Benzenoids
3.1.10. Furans
3.1.11. Lactones
3.2. Sensory Evaluation of Wine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lambrechts, M.; Pretorius, I. Yeast and Its Importance to Wine Aroma. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Rubio-Bretón, P.; Salinas, M.R.; Nevares, I.; Pérez-Álvarez, E.P.; del Álamo-Sanza, M.; Román, S.M.-S.; Alonso, G.L.; Garde-Cerdán, T. Recent Advances in the Study of Grape and Wine Volatile Composition: Varietal, Fermentative, and Aging Aroma Compounds. In Food Aroma Evolution; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-429-44183-7. [Google Scholar]
- Fischer, U. Wine Aroma. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Berger, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 241–267. ISBN 978-3-540-49339-6. [Google Scholar]
- Styger, G.; Prior, B.; Bauer, F.F. Wine Flavor and Aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Grosch, W. Evaluation of the Key Odorants of Foods by Dilution Experiments, Aroma Models and Omission. Chem. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on Varietal Aromas during Wine Making: A Review of the Impact of Varietal Aromas on the Flavor of Wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science: Principles and Applications; Academic Press: London, UK, 2008; ISBN 978-0-08-056874-4. [Google Scholar]
- Rapp, A.; Mandery, H. Wine Aroma. Experientia 1986, 42, 873–884. [Google Scholar] [CrossRef]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of Wine Fermentation Temperature on the Synthesis of Yeast-Derived Volatile Aroma Compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine—Stabilization and Treatments; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 978-0-470-01038-9. [Google Scholar]
- Slaghenaufi, D.; Ugliano, M. Norisoprenoids, Sesquiterpenes and Terpenoids Content of Valpolicella Wines During Aging: Investigating Aroma Potential in Relationship to Evolution of Tobacco and Balsamic Aroma in Aged Wine. Front. Chem. 2018, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Barros, A.S.; Câmara, J.S.; Rocha, S.M. In-Depth Search Focused on Furans, Lactones, Volatile Phenols, and Acetals As Potential Age Markers of Madeira Wines by Comprehensive Two-Dimensional Gas Chromatography with Time-of-Flight Mass Spectrometry Combined with Solid Phase Microextraction. J. Agric. Food Chem. 2011, 59, 3186–3204. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.; Romero, V.; García, M.; Arroyo, T.; Cabellos, J.M. Influence of Skin-Contact Treatment on Aroma Profile of Malvasia Aromatica Wines in D.O. “Vinos de Madrid”. In Grapes and Wine; Morata, A., Loira, I., González, C., Eds.; IntechOpen: London, UK, 2022; ISBN 978-1-83969-642-8. [Google Scholar]
- Piccardo, D. Influence of the Use of Unripe Grapes to Reduce Ethanol Content and PH on the Color, Polyphenol and Polysaccharide Composition of Conventional and Hot Macerated Pinot Noir and Tannat Wines. Eur. Food Res. Technol. 2019, 245, 1321–1335. [Google Scholar] [CrossRef]
- Rossi, S.; Bestulić, E.; Horvat, I.; Plavša, T.; Lukić, I.; Bubola, M.; Ganić, K.K.; Ćurko, N.; Jagatić Korenika, A.-M.; Radeka, S. Comparison of Different Winemaking Processes for Improvement of Phenolic Composition, Macro- and Microelemental Content, and Taste Sensory Attributes of Teran (Vitis vinifera L.) Red Wines. LWT 2022, 154, 112619. [Google Scholar] [CrossRef]
- Orbanić, F.; Rossi, S.; Bestulić, E.; Budić-Leto, I.; Kovačević Ganić, K.; Horvat, I.; Plavša, T.; Bubola, M.; Lukić, I.; Jeromel, A.; et al. Applying Different Vinification Techniques in Teran Red Wine Production: Impact on Bioactive Compounds and Sensory Attributes. Foods 2023, 12, 3838. [Google Scholar] [CrossRef] [PubMed]
- Bubola, M.; Sivilotti, P.; Janjanin, D.; Poni, S. Early Leaf Removal Has a Larger Effect than Cluster Thinning on Grape Phenolic Composition in Cv. Teran. Am. J. Enol. Vitic. 2017, 68, 234–242. [Google Scholar] [CrossRef]
- Lukić, I.; Horvat, I.; Radeka, S.; Damijanić, K.; Staver, M. Effect of Different Levels of Skin Disruption and Contact with Oxygen during Grape Processing on Phenols, Volatile Aromas, and Sensory Characteristics of White Wine. J. Food Process. Preserv. 2019, 43, e13969. [Google Scholar] [CrossRef]
- Lukić, I.; Budić-Leto, I.; Bubola, M.; Damijanić, K.; Staver, M. Pre-Fermentative Cold Maceration, Saignée, and Various Thermal Treatments as Options for Modulating Volatile Aroma and Phenol Profiles of Red Wine. Food Chem. 2017, 224, 251–261. [Google Scholar] [CrossRef]
- Maletić, E.; Kontić, J.K.; Preiner, D.; Šimon, S.; Staver, M.; PejiĆ, I. Ampelographic and Genetic Studies into “Teran’/’Refošk” Grapes in Istria (Croatia)—One or Two Varieties? Mitteilungen Klosterneubg 2014, 64, 54–62. [Google Scholar]
- Lukić, I.; Lotti, C.; Vrhovsek, U. Evolution of Free and Bound Volatile Aroma Compounds and Phenols during Fermentation of Muscat Blanc Grape Juice with and without Skins. Food Chem. 2017, 232, 25–35. [Google Scholar] [CrossRef]
- Bestulić, E.; Rossi, S.; Plavša, T.; Horvat, I.; Lukić, I.; Bubola, M.; Ilak Peršurić, A.S.; Jeromel, A.; Radeka, S. Comparison of Different Maceration and Non-Maceration Treatments for Enhancement of Phenolic Composition, Colour Intensity, and Taste Attributes of Malvazija Istarska (Vitis vinifera L.) White Wines. J. Food Compos. Anal. 2022, 109, 104472. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Ordinance on Wine and Fruit Wine Sensory Testing. Official Gazette N.N. 106/04, with All Amendments Concluding with N.N. 1/15. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2005_01_2_17.html (accessed on 14 June 2021).
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization (ISO): Geneva, Switzerland, 2007.
- ISO 3591:1977; Sensory Analysis—Apparatus—Wine-Tasting Glass. International Organization for Standardization (ISO): Geneva, Switzerland, 1997.
- Mateo, J.J.; Jiménez, M. Monoterpenes in Grape Juice and Wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Mihnea, M.; González-SanJosé, M.L.; Ortega-Heras, M.; Pérez-Magariño, S. A Comparative Study of the Volatile Content of Mencía Wines Obtained Using Different Pre-Fermentative Maceration Techniques. LWT–Food Sci. Technol. 2015, 64, 32–41. [Google Scholar] [CrossRef]
- Mahon, H.M.M.; Zoecklein, B.W.; Jasinski, Y.W. The Effects of Prefermentation Maceration Temperature and Percent Alcohol (v/v) at Press on the Concentration of Cabernet Sauvignon Grape Glycosides and Glycoside Fractions. Am. J. Enol. Vitic. 1999, 50, 385–390. [Google Scholar] [CrossRef]
- Lukić, I.; Jedrejčić, N.; Ganić, K.K.; Staver, M.; Peršurić, Đ. Phenolic and Aroma Composition of White Wines Produced by Prolonged Maceration and Maturation in Wooden Barrels. Food Technol. Biotechnol. 2015, 53, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Geffroy, O.; Lopez, R.; Serrano, E.; Dufourcq, T.; Gracia-Moreno, E.; Cacho, J.; Ferreira, V. Changes in Analytical and Volatile Compositions of Red Wines Induced by Pre-Fermentation Heat Treatment of Grapes. Food Chem. 2015, 187, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Escudero, A.; Fernández, P.; Cacho, J.F. Changes in the Profile of Volatile Compounds in Wines Stored under Oxygen and Their Relationship with the Browning Process. Z. Für Leb.-Forsch. A 1997, 205, 392–396. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Oliveira, P.; Baumes, R.L.; Maia, O. Changes in Aromatic Characteristics of Loureiro and Alvarinho Wines during Maturation. J. Food Compos. Anal. 2008, 21, 695–707. [Google Scholar] [CrossRef]
- Slegers, A.; Angers, P.; Ouellet, É.; Truchon, T.; Pedneault, K. Volatile Compounds from Grape Skin, Juice and Wine from Five Interspecific Hybrid Grape Cultivars Grown in Québec (Canada) for Wine Production. Molecules 2015, 20, 10980–11016. [Google Scholar] [CrossRef]
- Daniel, M.A.; Elsey, G.M.; Capone, D.L.; Perkins, M.V.; Sefton, M.A. Fate of Damascenone in Wine: The Role of SO2. J. Agric. Food Chem. 2004, 52, 8127–8131. [Google Scholar] [CrossRef]
- Silva Ferreira, A.C.; Guedes de Pinho, P. Nor-Isoprenoids Profile during Port Wine Ageing—Influence of Some Technological Parameters. Anal. Chim. Acta 2004, 513, 169–176. [Google Scholar] [CrossRef]
- Qian, X.; Jia, F.; Cai, J.; Shi, Y.; Duan, C.; Lan, Y. Characterization and Evolution of Volatile Compounds of Cabernet Sauvignon Wines from Two Different Clones during Oak Barrel Aging. Foods 2022, 11, 74. [Google Scholar] [CrossRef]
- Pereira, V.; Cacho, J.; Marques, J.C. Volatile Profile of Madeira Wines Submitted to Traditional Accelerated Ageing. Food Chem. 2014, 162, 122–134. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of Grape and Wine Aroma. Part 1. Chemical Components and Viticultural Impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- Trdenić, M.; Petek, M.; Marković, Z. Effect of fertilization on the content of volatile compounds in must of variety “Škrlet bijeli” (Vitis vinifera L.). J. Cent. Eur. Agric. 2020, 21, 870–880. [Google Scholar] [CrossRef]
- Yilmaztekin, M.; Kocabey, N.; Hayaloglu, A.A. Effect of Maceration Time on Free and Bound Volatiles of Red Wines from Cv. Karaoğlan (Vitis vinifera L.) Grapes Grown in Arapgir, Turkey. J. Food Sci. 2015, 80, C556–C563. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Bayón, M.Á.; Reineccius, G. Interactions Between Wine Matrix Macro-Components and Aroma Compounds. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 417–435. ISBN 978-0-387-74118-5. [Google Scholar]
- Radeka, S.; Lukić, I. Influence of Different Maceration Treatments on the Aroma Profile of Rosé and Red Wines from Croatian Aromatic Cv. Muškat Ruža Porečki (Vitis vinifera L.). Food Technol. Biotechnol. 2012, 50, 442. [Google Scholar]
- Clodoveo, M.L.; Hbaieb, R.H.; Kotti, F.; Mugnozza, G.S.; Gargouri, M. Mechanical Strategies to Increase Nutritional and Sensory Quality of Virgin Olive Oil by Modulating the Endogenous Enzyme Activities. Compr. Rev. Food Sci. Food Saf. 2014, 13, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Strasser, M.; Gutzler, K. Impact of Fermentation Technology on the Phenolic and Volatile Composition of German Red Wines. Int. J. Food Sci. Technol. 2000, 35, 81–94. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Weightman, C.; Panzeri, V.; Nieuwoudt, H.H.; du Toit, W.J. Effect of Skin Contact before and during Alcoholic Fermentation on the Chemical and Sensory Profile of South African Chenin Blanc White Wines. S. Afr. J. Enol. Vitic. 2015, 36, 366–377. [Google Scholar] [CrossRef]
- Valero, E.; Millán, C.; Ortega, J.M. Influence of Pre-Fermentative Treatment on the Fatty Acid Content of Saccharomyces Cerevisiae (M330-9) during Alcoholic Fermentation of Grape Must. J. Biosci. Bioeng. 2001, 91, 117–122. [Google Scholar] [CrossRef]
- Wang, J.; Huo, S.; Zhang, Y.; Liu, Y.; Fan, W. Effect of Different Pre-Fermentation Treatments on Polyphenols, Color, and Volatile Compounds of Three Wine Varieties. Food Sci. Biotechnol. 2016, 25, 735–743. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Polo, C. (Eds.) Wine Chemistry and Biochemistry; Springer: New York, NY, USA, 2009; ISBN 978-0-387-74116-1. [Google Scholar]
- Petropulos, V.I.; Bogeva, E.; Stafilov, T.; Stefova, M.; Siegmund, B.; Pabi, N.; Lankmayr, E. Study of the Influence of Maceration Time and Oenological Practices on the Aroma Profile of Vranec Wines. Food Chem. 2014, 165, 506–514. [Google Scholar] [CrossRef]
- Callejon, R.M.; Margulies, B.; Hirson, G.D.; Ebeler, S.E. Dynamic Changes in Volatile Compounds during Fermentation of Cabernet Sauvignon Grapes with and without Skins. Am. J. Enol. Vitic. 2012, 63, 301–312. [Google Scholar] [CrossRef]
- Escalona, H.; Birkmyre, L.; Piggott, J.R.; Paterson, A. Effect of Maturation in Small Oak Casks on the Volatility of Red Wine Aroma Compounds. Anal. Chim. Acta 2002, 458, 45–54. [Google Scholar] [CrossRef]
- Ferreras, D.; Fernández, E.; Falqué, E. Note: Effects of Oak Wood on the Aromatic Composition of Vitis vinifera L. Var. Treixadura Wines. Food Sci. Technol. Int. 2002, 8, 343–349. [Google Scholar] [CrossRef]
- Fukuda, K.; Yamamoto, N.; Kiyokawa, Y.; Yanagiuchi, T.; Wakai, Y.; Kitamoto, K.; Inoue, Y.; Kimura, A. Balance of Activities of Alcohol Acetyltransferase and Esterase in Saccharomyces Cerevisiae Is Important for Production of Isoamyl Acetate. Appl. Environ. Microbiol. 1998, 64, 4076–4078. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Reis, M.; Saraiva, P.; Marques, J.C. Analysis and Assessment of Madeira Wine Ageing over an Extended Time Period through GC-MS and Chemometric Analysis. Anal. Chim. Acta 2010, 660, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative Determination of the Odorants of Young Red Wines from Different Grape Varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Herjavec, S.; Majdak, A. The Influence of Maceration on the Composition of Some Volatile Compounds and Sensory Properties of Traminer Wines. Agric. Conspec. Sci. 2002, 67, 11–17. [Google Scholar]
- Díaz-Maroto, M.C.; Schneider, R.; Baumes, R. Formation Pathways of Ethyl Esters of Branched Short-Chain Fatty Acids during Wine Aging. J. Agric. Food Chem. 2005, 53, 3503–3509. [Google Scholar] [CrossRef]
- Bakker, J.; Clarke, R.J. Wine Flavour Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Michałowicz, J.; Duda, W. Phenols Transformations in the Environment and Living Organisms. Curr. Top. Biophys. 2007, 30, 24–36. [Google Scholar]
- Zhang, B.; Cai, J.; Duan, C.-Q.; Reeves, M.J.; He, F. A Review of Polyphenolics in Oak Woods. Int. J. Mol. Sci. 2015, 16, 6978–7014. [Google Scholar] [CrossRef]
- Palomo, E.S.; González-Viñas, M.A.; Díaz-Maroto, M.C.; Soriano-Pérez, A.; Pérez-Coello, M.S. Aroma Potential of Albillo Wines and Effect of Skin-Contact Treatment. Food Chem. 2007, 103, 631–640. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 978-1-118-62780-8. [Google Scholar]
- Genovese, A.; Gambuti, A.; Piombino, P.; Moio, L. Sensory Properties and Aroma Compounds of Sweet Fiano Wine. Food Chem. 2007, 103, 1228–1236. [Google Scholar] [CrossRef]
- Escudero, A.; Cacho, J.; Ferreira, V. Isolation and Identification of Odorants Generated in Wine during Its Oxidation: A Gas Chromatography–Olfactometric Study. Eur. Food Res. Technol. 2000, 211, 105–110. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Gonçalves, C.; Castillo, M.; Câmara, J.S. An Approach of the Madeira Wine Chemistry. Beverages 2020, 6, 12. [Google Scholar] [CrossRef]
- Kanakaki, E.; Siderakou, D.; Kallithraka, S.; Kotseridis, Y.; Makris, D.P. Effect of the Degree of Toasting on the Extraction Pattern and Profile of Antioxidant Polyphenols Leached from Oak Chips in Model Wine Systems. Eur. Food Res. Technol. 2015, 240, 1065–1074. [Google Scholar] [CrossRef]
- Dumitriu (Gabur), G.-D.; Teodosiu, C.; Gabur, I.; Cotea, V.V.; Peinado, R.A.; López de Lerma, N. Alternative Winemaking Techniques to Improve the Content of Phenolic and Aromatic Compounds in Wines. Agriculture 2021, 11, 233. [Google Scholar] [CrossRef]
- Liberatore, M.T.; Pati, S.; Nobile, M.A.D.; Notte, E.L. Aroma Quality Improvement of Chardonnay White Wine by Fermentation and Ageing in Barrique on Lees. Food Res. Int. 2010, 43, 996–1002. [Google Scholar] [CrossRef]
- Dumitriu (Gabur), G.-D.; Teodosiu, C.; Gabur, I.; Cotea, V.V.; Peinado, R.A.; López de Lerma, N. Evaluation of Aroma Compounds in the Process of Wine Ageing with Oak Chips. Foods 2019, 8, 662. [Google Scholar] [CrossRef]
- Cerdán, T.G.; Ancín-Azpilicueta, C. Effect of Oak Barrel Type on the Volatile Composition of Wine: Storage Time Optimization. LWT–Food Sci. Technol. 2006, 39, 199–205. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, P.; Gómez-Plaza, E. Dependence of Oak-Related Volatile Compounds on the Physicochemical Characteristics of Barrel-Aged Wines. Food Technol. Biotechnol. 2012, 50, 59–65. [Google Scholar]
- Benucci, I.; Luziatelli, F.; Cerreti, M.; Liburdi, K.; Nardi, T.; Vagnoli, P.; Ruzzi, M.; Esti, M. Pre-Fermentative Cold Maceration in the Presence of Non-Saccharomyces Strains: Effect on Fermentation Behaviour and Volatile Composition of a Red Wine. Aust. J. Grape Wine Res. 2018, 24, 267–274. [Google Scholar] [CrossRef]
- Ferreira, V.; San Juan, F.; Escudero, A.; Culleré, L.; Fernández-Zurbano, P.; Saenz-Navajas, M.P.; Cacho, J. Modeling Quality of Premium Spanish Red Wines from Gas Chromatography−Olfactometry Data. J. Agric. Food Chem. 2009, 57, 7490–7498. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, I.; Aleixandre, J.L.; García, M.J.; Lizama, V. Impact of Prefermentative Maceration on the Phenolic and Volatile Compounds in Monastrell Red Wines. Anal. Chim. Acta 2006, 563, 109–115. [Google Scholar] [CrossRef]
- Oliver Simancas, R.; Díaz-Maroto, M.C.; Alañón Pardo, M.E.; Pérez Porras, P.; Bautista-Ortín, A.B.; Gómez-Plaza, E.; Pérez-Coello, M.S. Effect of Power Ultrasound Treatment on Free and Glycosidically-Bound Volatile Compounds and the Sensorial Profile of Red Wines. Molecules 2021, 26, 1193. [Google Scholar] [CrossRef]
- Raposo, R.; Ruiz-Moreno, M.J.; Garde-Cerdán, T.; Puertas, B.; Moreno-Rojas, J.M.; Zafrilla, P.; Gonzalo-Diago, A.; Guerrero, R.F.; Cantos-Villar, E. Replacement of Sulfur Dioxide by Hydroxytyrosol in White Wine: Influence on Both Quality Parameters and Sensory. LWT–Food Sci. Technol. 2016, 65, 214–221. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, A.; Carrer, C.; Palma Lovillo, M.; García Barroso, C. Ultrasonic Treatments during the Alcoholic Fermentation of Red Wines: Effects on “Syrah” Wines. Vitis 2019, 58, 83–88. [Google Scholar]
- Genovese, A.; Lamorte, S.A.; Gambuti, A.; Moio, L. Aroma of Aglianico and Uva Di Troia Grapes by Aromatic Series. Food Res. Int. 2013, 53, 15–23. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- San-Juan, F.; Ferreira, V.; Cacho, J.; Escudero, A. Quality and Aromatic Sensory Descriptors (Mainly Fresh and Dry Fruit Character) of Spanish Red Wines Can Be Predicted from Their Aroma-Active Chemical Composition. J. Agric. Food Chem. 2011, 59, 7916–7924. [Google Scholar] [CrossRef]
- Falcao, L.D.; Lytra, G.; Darriet, P.; Barbe, J.-C. Identification of Ethyl 2-Hydroxy-4-Methylpentanoate in Red Wines, a Compound Involved in Blackberry Aroma. Food Chem. 2012, 132, 230–236. [Google Scholar] [CrossRef]
- Nordestgaard, S. Fermentation: Pre-Fermentation Heating of Red Grapes: A Useful Tool to Manage Compressed Vintages? Aust. New Zealand Grapegrow. Winemak. 2017, 637, 54–61. [Google Scholar]
- Ferreira, V.; Lopez, R. The Actual and Potential Aroma of Winemaking Grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Pons, A.; Lavigne, V.; Eric, F.; Darriet, P.; Dubourdieu, D. Identification of Volatile Compounds Responsible for Prune Aroma in Prematurely Aged Red Wines. J. Agric. Food Chem. 2008, 56, 5285–5290. [Google Scholar] [CrossRef]
- Roldán, A.M.; Sánchez-García, F.; Pérez-Rodríguez, L.; Palacios, V.M. Influence of Different Vinification Techniques on Volatile Compounds and the Aromatic Profile of Palomino Fino Wines. Foods 2021, 10, 453. [Google Scholar] [CrossRef]
- Pozzatti, M.; Guerra, C.C.; Martins, G.; dos Santos, I.D.; Wagner, R.; Ferrão, M.F.; Manfroi, V. Effects of Winemaking on ‘Marselan’ Red Wines: Volatile Compounds and Sensory Aspects. Ciênc. E Téc. Vitivinícola 2020, 35, 63–75. [Google Scholar] [CrossRef]
- De Rosso, M.; Cancian, D.; Panighel, A.; Dalla Vedova, A.; Flamini, R. Chemical Compounds Released from Five Different Woods Used to Make Barrels for Aging Wines and Spirits: Volatile Compounds and Polyphenols. Wood Sci. Technol. 2009, 43, 375–385. [Google Scholar] [CrossRef]
- Sidhu, D.; Lund, J.; Kotseridis, Y.; Saucier, C. Methoxypyrazine Analysis and Influence of Viticultural and Enological Procedures on Their Levels in Grapes, Musts, and Wines. Crit. Rev. Food Sci. Nutr. 2015, 55, 485–502. [Google Scholar] [CrossRef]
- Carpena, M.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Wine Aging Technology: Fundamental Role of Wood Barrels. Foods 2020, 9, 1160. [Google Scholar] [CrossRef]
- Chira, K.; Teissedre, P.-L. Extraction of Oak Volatiles and Ellagitannins Compounds and Sensory Profile of Wine Aged with French Winewoods Subjected to Different Toasting Methods: Behaviour during Storage. Food Chem. 2013, 140, 168–177. [Google Scholar] [CrossRef]
- Reazin, G.H. Chemical Mechanisms of Whiskey Maturation. Am. J. Enol. Vitic. 1981, 32, 283–289. [Google Scholar] [CrossRef]
- Nan, L.; Liu, L.; Li, Y.; Huang, J.; Wang, Y.; Wang, C.; Wang, Z.; Xu, C. Comparison of Aroma Compounds in Cabernet Sauvignon Red Wines from Five Growing Regions in Xinjiang in China. J. Food Qual. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
| Volatile Compounds | Young/Aged Wine | Treatments | |||
|---|---|---|---|---|---|
| TM7 | TM10 | TM21 | TPHT | ||
| Monoterpenes | |||||
| Limonene | Y | 1.03 ± 0.12 a* | 0.98 ± 0.04 a* | 0.23 ± 0.02 c | 0.37 ± 0.06 b* |
| A | 0.45 ± 0.05 a | 0.18 ± 0.02 b | 0.62 ± 0.14 a* | 0.17 ± 0.10 b | |
| β-pinene | Y | 2.17 ± 0.24 a* | 2.17 ± 0.16 a* | 0.42 ± 0.03 b | 0.34 ± 0.04 b* |
| A | 0.62 ± 0.03 b | 0.26 ± 0.03 c | 1.37 ± 0.20 a* | 0.11 ± 0.07 c | |
| Linalool | Y | 25.28 ± 1.18 a* | 24.01 ± 0.95 a* | 5.22 ± 0.76 b | 4.94 ± 0.73 b |
| A | 18.24 ± 0.45 a | 3.56 ± 0.25 c | 14.08 ± 1.04 b* | 14.09 ± 0.62 b* | |
| 4-Terpineol | Y | 5.07 ± 0.18 a* | 4.36 ± 0.8 a | 0.97 ± 0.11 b | 1.07 ± 0.17 b |
| A | 4.16 ± 0.10 a | 4.40 ± 0.17 a | 2.84 ± 0.38 b* | 1.34 ± 0.15 c | |
| α-Terpineol | Y | 28.32 ± 2.66 a* | 28.15 ± 2.46 a* | 4.64 ± 0.42 b | 6.10 ± 0.39 b |
| A | 8.88 ± 0.98 b | 9.12 ± 0.52 b | 20.41 ± 1.41 a* | 9.86 ± 1.08 b* | |
| Citronellol | Y | 16.02 ± 0.76 a* | 13.96 ± 1.45 b* | 7.07 ± 0.46 c | 4.99 ± 0.60 d* |
| A | 7.70 ± 0.10 a | 3.24 ± 0.08 b | 7.64 ± 0.76 a | 3.02 ± 0.13 b | |
| Geraniol | Y | 3.47 ± 0.33 a | 1.62 ± 0.26 c | 2.59 ± 0.28 b | 1.58 ± 0.33 c |
| A | 3.49 ± 0.23 b | 2.12 ± 0.18 c | 7.46 ± 0.77 a* | 1.64 ± 0.43 c | |
| Geranyl acetone | Y | 6.80 ± 0.51 a* | 4.58 ± 0.49 b* | 0.99 ± 0.19 c | 1.07 ± 0.15 c* |
| A | 0.98 ± 0.14 b | 0.80 ± 0.18 b | 2.44 ± 0.48 a* | 0.56 ± 0.17 b | |
| trans-Nerolidol | Y | 2.23 ± 0.17 a* | 1.42 ± 0.09 b* | 0.41 ± 0.06 c | 0.48 ± 0.10 c* |
| A | 0.54 ± 0.05 b | 0.45 ± 0.03 b | 1.37 ± 0.27 a* | 0.12 ± 0.09 c | |
| Eucalyptol | Y | 1.65 ± 0.16 a* | 1.12 ± 0.05 b* | 0.19 ± 0.03 d | 0.52 ± 0.07 c* |
| A | 0.90 ± 0.02 a | 0.40 ± 0.04 c | 0.74 ± 0.01 b* | 0.13 ± 0.10 d | |
| Menthol | Y | 62.96 ± 3.86 a* | 41.79 ± 2.11 b* | 8.37 ± 0.73 c | 10.68 ± 1.73 c* |
| A | 11.55 ± 1.28 b | 8.65 ± 1.06 b | 23.89 ± 3.05 a* | 2.20 ± 1.63 c | |
| trans-Rose oxide | Y | 4.05 ± 0.70 a* | 3.98 ± 0.76 a* | 0.57 ± 0.07 b | 0.87 ± 0.14 b* |
| A | 2.28 ± 0.11 a | 0.81 ± 0.07 c | 1.04 ± 0.07 b* | 0.19 ± 0.12 d | |
| Total monoterpenes | Y | 159.1 ± 9.3 a* | 128.2 ± 6.4 b* | 31.68 ± 2.70 c | 33.01 ± 4.24 c |
| A | 59.80 ± 1.60 b | 34.00 ± 2.29 c | 83.89 ± 7.96 a* | 33.43 ± 3.08 c | |
| C13-norisoprenoids | |||||
| Vitispirane I | Y | 8.43 ± 0.42 a* | 6.12 ± 0.45 b* | 1.96 ± 0.15 d | 2.59 ± 0.16 c* |
| A | 2.30 ± 0.23 b | 0.96 ± 0.07 c | 3.74 ± 1.12 a | 0.39 ± 0.25 c | |
| Vitispirane II | Y | 2.98 ± 0.58 b* | 4.32 ± 0.18 a* | 1.25 ± 0.19 c | 1.49 ± 0.12 c* |
| A | 0.05 ± 0.01 b | 0.09 ± 0.07 b | 1.86 ± 1.46 a | 0.24 ± 0.17 b | |
| Actinidol ethyl ether I | Y | n.d. | n.d. | n.d. | n.d. |
| A | 80.49 ± 0.96 a | 77.05 ± 4.35 ab | 68.80 ± 5.68 b | 55.32 ± 7.51 c | |
| Actinidol ethyl ether II | Y | n.d. | n.d. | n.d. | n.d. |
| A | 47.56 ± 0.33 a | 45.68 ± 2.36 a | 41.78 ± 4.05 a | 31.90 ± 5.16 b | |
| TDN | Y | n.d. | n.d. | n.d. | n.d. |
| A | 1.42 ± 0.03 b | 1.41 ± 0.08 b | 1.85 ± 0.29 a | 1.15 ± 0.21 b | |
| β- Damascenone | Y | 8.33 ± 0.86 b* | 8.31 ± 0.81 b* | 10.27 ± 0.12 a* | 1.65 ± 0.35 c* |
| A | 1.66 ± 0.13 b | 1.41 ± 0.15 b | 3.73 ± 0.48 a | 0.45 ± 0.33 c | |
| β- Ionone | Y | 4.69 ± 1.08 a* | 3.00 ± 0.60 b* | 0.47 ± 0.13 c | 0.63 ± 0.07 c |
| A | 0.84 ± 0.07 b | 0.58 ± 0.11 bc | 1.72 ± 0.24 a* | 0.31 ± 0.22 c | |
| TPB | Y | n.d. | n.d. | n.d. | n.d. |
| A | 1.27 ± 0.08 b | 2.39 ± 0.13 a | 2.83 ± 0.41 a | 1.69 ± 0.22 b | |
| Actinidol I | Y | n.d. | n.d. | n.d. | n.d. |
| A | 14.21 ± 0.25 | 16.7 ± 1.42 | 18.65 ± 2.68 | 16.72 ± 3.71 | |
| Actinidol II | Y | n.d. | n.d. | n.d. | n.d. |
| A | 27.00 ± 0.46 | 25.1 ± 1.78 | 27.99 ± 4.06 | 24.29 ± 4.85 | |
| Total C13-norisoprenoides | Y | 24.43 ± 1.93 a | 21.75 ± 0.91 b | 13.95 ± 0.47 c | 6.35 ± 0.61 d |
| A | 176.8 ± 1.4 a* | 171.4 ± 10.0 a* | 172.9 ± 18.3 a* | 132.5 ± 22.4 b* | |
| Alcohols | |||||
| 1-Hexanol | Y | 4245 ± 149 b* | 4593 ± 292 a* | 1372 ± 87 c | 1473 ± 79 c |
| A | 1578± 38 b | 1617 ± 18 b | 2211 ± 100 a* | 1236 ± 43 c | |
| trans-3-Hexen-1-ol | Y | 43.67 ± 2.89 b* | 52.16 ± 2.05 a* | 17.55 ± 1.83 c | 16.29 ± 1.13 c |
| A | 11.29 ± 4.71 c | 19.18 ± 0.61 b | 24.69 ± 2.43 a | 16.9 ± 0.33 b | |
| cis-3-Hexen-1-ol | Y | 97.88 ± 3.45 b | 102.7 ± 1.80 a* | 25.09 ± 2.38 c | 26.26 ± 1.18 c |
| A | 14.98 ± 0.37 b | 23.59 ± 2.21 b | 45.23 ± 8.06 a | 24.99 ± 5.83 b | |
| Benzyl alcohol | Y | 2.03 ± 0.49 a | 1.53 ± 0.15 b | 1.34 ± 0.03 bc | 1.02 ± 0.04 c |
| A | 2.05 ± 0.07 a | 2.01 ± 0.09 ab* | 1.80 ± 0.12 bc* | 1.69 ± 0.19 c* | |
| 2-Phenylethyl Alcohol | Y | 152,349 ± 5115 a* | 131,289 ± 6406 b* | 70,524 ± 5296 c | 67,290 ± 2106 c |
| A | 122,741 ± 2219 a | 60,919 ± 2600 c | 122,662 ± 8255 a* | 73,310 ± 1367 b* | |
| Total alcohols | Y | 156,739 ± 5051 a* | 136,039 ± 6350 b* | 71,940 ± 5385 c | 68,807 ± 2119.28 c |
| A | 124,347 ± 2222 a | 62,581 ± 2607 c | 124,945 ± 8161 a* | 74,590 ± 1326 b* | |
| Fatty acids | |||||
| Butanoic acid | Y | 1544 ± 77 a | 1642 ± 97 a | 702.7 ± 86.4 b | 741.9 ± 46.6 b |
| A | 1663 ± 25 a | 1655 ± 57 a | 1072 ± 54 b* | 655.3 ± 51 c | |
| Hexanoic acid | Y | 1022 ± 96 ab | 1100 ± 147 a | 862.2 ± 46.9 bc | 746.2 ± 62.1 c |
| A | 1200 ± 11 b* | 1092 ± 17 b | 1399 ± 72 a* | 941.0 ± 95.6 c* | |
| Octanoic Acid | Y | 385.6 ± 53.2 a | 272.5 ± 60.0 b | 386.7± 35.1 a | 402.8 ± 29.2 a* |
| A | 329.4 ± 18.7 b | 260.2 ± 22.4 b | 471.4 ± 71.7 a* | 257.3 ± 19.4 b | |
| Nonanoic acid | Y | 281.3 ± 70.6 ab | 311.4 ± 57.3 a* | 198.0 ± 16.3 b | 61.93 ± 13.46 c |
| A | 264.5 ± 19.5 a | 175.9 ± 18.7 b | 199.5 ± 2.8 b | 71.76 ± 21.78 c | |
| Decanoic acid | Y | 177.3 ± 20.3 a* | 149.9 ± 18.3 a* | 146.7 ± 17.2 ab | 111.9 ± 22.6 b* |
| A | 84.45 ± 4.5 b | 84.49 ± 9.22 b | 185.3 ± 13.3 a* | 36.4 ± 0.6 c | |
| Total fatty acids | Y | 3411 ± 147 a | 3476 ± 242 a | 2296 ± 150 b | 2064 ± 78 b |
| A | 3542 ± 24 a | 3268 ± 105 b | 3328 ± 82 b* | 1961 ± 88 c | |
| Ethyl esters | |||||
| Ethyl butanoate | Y | 147.4 ± 1.9 b* | 192.1 ± 3.8 a* | 83.20 ± 4.60 d | 94.78 ± 3.38 c* |
| A | 73.60 ± 1.49 b | 72.71 ± 2.10 b | 117.4 ± 6.4 a* | 73.81 ± 6.25 b | |
| Ethyl 2-methylbutanoate | Y | 26.11 ± 2.30 b | 36.06 ± 0.79 a* | 17.62 ± 1.27 c | 23.62 ± 1.26 b |
| A | 25.13 ± 0.81 c | 25.85 ± 1.28 bc | 35.30 ± 3.27 a* | 30.38 ± 3.69 b* | |
| Ethyl 3-methylbutanoate | Y | 59.73 ± 4.62 b | 77.26 ± 4.54 a* | 29.53 ± 1.92 d | 43.31 ± 1.01 c |
| A | 48.43 ± 1.09 b | 50.97 ± 2.24 ab | 56.74 ± 1.97 a* | 52.65 ± 7.96 ab | |
| Ethyl pentanoate | Y | 13.38 ± 1.22 a* | 14.86 ± 1.58 a* | 6.46 ± 1.14 c | 3.65 ± 0.20 b |
| A | 7.72 ± 0.42 a | 3.83 ± 1.45 b | 7.02 ± 0.58 a | 6.26 ± 0.44 a* | |
| Ethyl hexanoate | Y | 156.9 ± 6.2 b* | 177.1 ± 1.9 a* | 120.1 ± 4.5 c | 181.0 ± 8.4 a* |
| A | 114.5 ± 1.7 b | 105.2 ± 1.2 c | 137.2 ± 1.3 a* | 70.5 ± 3.5 d | |
| Ethyl octanoate | Y | 492.4 ± 26.6 a* | 201.7 ± 44.6 b* | 199.8 ± 21.7 b* | 151.6 ± 24.9 b* |
| A | 11.92 ± 2.09 b | 13.69 ± 1.85 b | 32.01 ± 3.04 a | 12.09 ± 0.71 b | |
| Total ethyl esters | Y | 896.0 ± 4.6 a* | 699.1 ± 5.0 b* | 456.7 ± 3.4 d* | 498.0 ± 4.9 c* |
| A | 281.3 ± 1.3 b | 272.3 ± 2.2 c | 385.7 ± 1.7 a | 245.7 ± 5.0 d | |
| Acetate esters | |||||
| Butyl acetate | Y | 0.59 ± 0.05 a | 0.38 ± 0.22 a | 0.09 ± 0.02 b | 0.11 ± 0.01 b |
| A | 0.53 ± 0.07 a | 0.10 ± 0.00 c | 0.13 ± 0.02 c | 0.21 ± 0.02 b* | |
| Isoamyl acetate | Y | 1217 ± 264 a | 1178 ± 98 a* | 613.9 ± 69.9 b | 262.3 ± 17.8 b |
| A | 1317 ± 83 a | 849.9 ± 31.1 c | 1031 ± 117 b* | 751.5 ± 51.7 c* | |
| Hexyl acetate | Y | 6.36 ± 0.46 a | 2.44 ± 0.31 b | 0.88 ± 0.08 c | 2.36 ± 0.09 b |
| A | 6.34 ± 0.21 a | 5.70 ± 0.52 b* | 3.50 ± 0.12 c* | 2.54 ± 0.11 d | |
| 2-Phenethyl acetate | Y | 123.2 ± 8.1 a | 42.81 ± 1.79 b | 30.31 ± 3.27 c | 36.59 ± 5.62 bc |
| A | 126.1 ± 4.9 a | 46.57 ± 3.12 c | 72.56 ± 3.43 b* | 50.80 ± 0.21 c* | |
| Total acetate esters | Y | 1347 ± 259 a | 1224 ± 99 a* | 645.2 ± 73.2 b | 301.3 ± 19.5 c |
| A | 1450 ± 88 a | 902.3 ± 28.6 c | 1107 ± 116 b* | 805.0 ± 52.0 c* | |
| Other esters | |||||
| Ethyl lactate | Y | 142,057 ± 2847 b | 137,925 ± 6529 b | 162,549 ± 10,898 a | 55,354 ± 6695 c |
| A | 153,191 ± 2942 bc* | 171,138 ± 5786 b* | 235,716 ± 26,846 a* | 131,284 ± 6124 c* | |
| Diethyl succinate | Y | 8079 ± 397 a | 7363 ± 238 b | 2265 ± 186 d | 2773 ± 151 c |
| A | 11,982 ± 536 b* | 9060 ± 593 c* | 17,668 ± 1157 a* | 6882 ± 461 d* | |
| Total other esters | Y | 150,136 ± 2574 b | 145,288 ± 6434 b | 164,815 ± 11,083 a | 58,127 ± 6646 c |
| A | 165,173 ± 3473 b* | 180,198 ± 5298 b* | 253,384 ± 26,412 a* | 138,166 ± 5687 c* | |
| Volatile phenols | |||||
| Guaiacol | Y | n.d. | n.d. | n.d. | n.d. |
| A | 2.28 ± 0.34 bc | 2.20 ± 0.25 c | 3.82 ± 0.58 a | 3.03 ± 0.36 b | |
| Eugenol | Y | n.d. | n.d. | n.d. | n.d. |
| A | 3.66 ± 0.21 b | 6.47 ± 2.42 b | 19.73 ± 8.04 a | 2.03 ± 1.09 b | |
| 4-Ethylguaiacol | Y | n.d. | n.d. | n.d. | n.d. |
| A | 531.4 ± 34.2 a | 345.8 ± 29.3 b | 233.3 ± 29.5 c | 326.7 ± 56.3 b | |
| 4-Ethylphenol | Y | 17.77 ± 1.54 a | 7.79 ± 0.23 b | 1.78 ± 0.32 c | 2.93 ± 0.64 c |
| A | 352.7 ± 6 a* | 376.6 ± 27.8 a* | 75.55 ± 8.16 b* | 356.2 ± 49.6 a* | |
| 4-Vinylguaiacol | Y | 24.82 ± 0.96 b | 19.91 ± 1.13 c | 28.25 ± 1.95 a | 23.59 ± 1.67 b |
| A | 25.44 ± 0.98 b | 23.32 ± 1.24 b* | 41.29 ± 4.88 a* | 21.30 ± 3.70 b | |
| Total volatile phenols | Y | 42.59 ± 2.40 a | 27.70 ± 1.25 b | 30.02 ± 2.12 b | 26.52 ± 2.07 b |
| A | 915.5 ± 29.1 a* | 754.4 ± 59.9 b* | 373.7 ± 49.3 c* | 709.2 ± 110.1 b* | |
| Benzenoids | |||||
| Benzaldehyde | Y | 2.58 ± 0.30 | 2.49 ± 0.19 | 2.18 ± 0.05 | 2.97 ± 0.1 |
| A | 2.32 ± 0.24 b | 2.23 ± 0.14 b | 2.89 ± 0.36 b* | 7.02 ± 0.65 a* | |
| Furans | |||||
| Furfuryl ether | Y | n.d. | n.d. | n.d. | n.d. |
| A | 104.2 ± 2.7 c | 104.8 ± 3.0 c | 126.4 ± 5.7 b | 166.6 ± 11.1 a | |
| Furfural | Y | n.d. | n.d. | n.d. | n.d. |
| A | 16.88 ± 0.33 b | 17.81 ± 0.77 b | 17.91 ± 0.37 b | 20.08 ± 2.17 a | |
| 5-Methylfurfural | Y | n.d. | n.d. | n.d. | n.d. |
| A | 5.70 ± 0.23 b | 9.88 ± 0.48 a | 5.99 ± 0.78 b | 4.71 ± 0.23 c | |
| Ethyl-3-furoate | Y | 153.0 ± 4.7 a* | 139.3 ± 2.5 b* | 34.62 ± 1.57 d | 44.94 ± 4.42 c |
| A | 83.84 ± 4.21 a | 81.83 ± 7.07 a | 91.05 ± 8.03 a* | 59.03 ± 6.58 b* | |
| Total furans | Y | 153.0 ± 4.7 a | 139.3 ± 2.5 b | 34.62 ± 1.57 d | 44.94 ± 4.42 c |
| A | 210.6 ± 6.1 c* | 214.3 ± 4.5 c* | 241.3 ± 3.6 b* | 250.4 ± 2.3 a* | |
| Lactones | |||||
| trans-oak lactone | Y | n.d. | n.d. | n.d. | n.d. |
| A | 88.74 ± 1.08 b | 88.00 ± 6.78 b | 146.4 ± 14.7 a | 109.3 ± 18.8 b | |
| cis- oak lactone | Y | n.d. | n.d. | n.d. | n.d. |
| A | 108.9 ± 1.3 c | 152.3 ± 5.0 b | 197.4 ± 16.5 a | 173.4 ± 22.7 b | |
| γ- Nonalactone | Y | 31.08 ± 1.68 | 31.75 ± 2.61 | 28.58 ± 0.92 | 28.21 ± 2.08 |
| A | 33.23 ± 0.31 | 33.04 ± 1.45 | 32.84 ± 2.99 | 29.82 ± 3.50 | |
| γ- Decalactone | Y | n.d. | n.d. | n.d. | n.d. |
| A | 14.49 ± 0.24 b | 16.41 ± 1.15 b | 38.56 ± 3.18 a | 16.33 ± 2.93 b | |
| Total lactones | Y | 31.08 ± 1.68 | 31.75 ± 2.61 | 28.58 ± 0.92 | 28.21 ± 2.08 |
| A | 245.3 ± 0.6 c* | 289.7 ± 14.0 bc* | 415.2 ± 36.0 a* | 328.8 ± 47.8 b* | |
| Aroma Descriptors | Young/Aged Wine | Treatments | |||
|---|---|---|---|---|---|
| TM7 | TM10 | TM21 | TPHT | ||
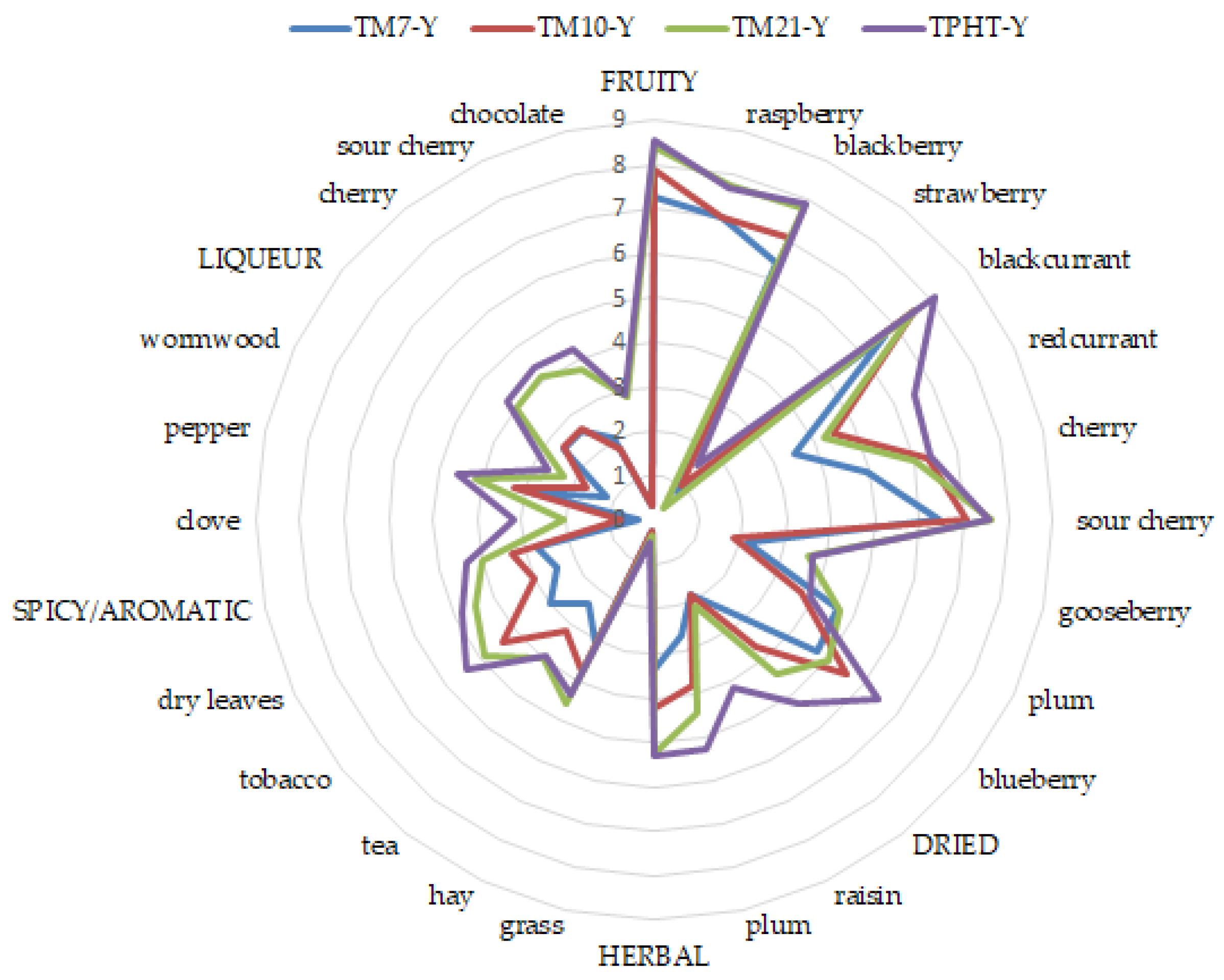
| Fruity | Y | 7.25 ± 0.22 c* | 7.88 ± 0.13 b* | 8.42 ± 0.19 a* | 8.54 ± 0.19 a* |
| A | 4.17 ± 0.21 d | 5.17 ± 0.21 c | 6.07 ± 0.21 b | 6.77 ± 0.12 a | |
| raspberry | Y | 6.96 ± 0.26 b* | 7.00 ± 0.38 b* | 7.71 ± 0.26 a* | 7.67 ± 0.14 a* |
| A | 2.67 ± 0.23 b | 3.33 ± 0.50 ab | 4.20 ± 1.25 ab | 4.27 ± 0.90 a | |
| blackberry | Y | 6.42 ± 0.47 b* | 7.04 ± 0.31 ab* | 7.79 ± 0.38 ab* | 7.88 ± 0.94 a |
| A | 3.87 ± 0.23 b | 4.40 ± 0.35 b | 5.80 ± 0.60 a | 6.33 ± 0.31 a | |
| strawberry | Y | 0.83 ± 0.72 | 1.00 ± 0.87 | 0.33 ± 0.58 | 1.58 ± 1.77 |
| A | n.d. | n.d. | n.d. | n.d. | |
| blackcurrant | Y | 6.67 ± 0.29 * | 7.54 ± 0.31 * | 7.54 ± 1.68 * | 8.08 ± 0.29 * |
| A | 2.13 ± 1.01 c | 2.80 ± 1.51 bc | 4.33 ± 0.50 ab | 5.47 ± 0.31 a | |
| redcurrant | Y | 3.50 ± 0.90 b | 4.50 ± 1.32 b | 4.25 ± 1.25 b* | 6.50 ± 1.21 a* |
| A | 1.80 ± 0.60 | 2.13 ± 1.14 | 1.60 ± 0.69 | 2.73 ± 0.12 | |
| cherry | Y | 4.92 ± 0.38 * | 6.33 ± 0.69 * | 6.04 ± 1.77 * | 6.42 ± 0.72 * |
| A | 1.27 ± 0.31 b | 1.60± 0.60 ab | 2.93 ± 1.62 ab | 3.20 ± 0.53 a | |
| cherry sour | Y | 6.42 ± 0.52 b* | 7.04 ± 0.38 ab* | 7.63 ± 0.50 a* | 7.58 ± 0.63 a* |
| A | 4.83 ± 0.32 c | 5.07 ± 0.31 bc | 5.53 ± 0.42 b | 6.47 ± 0.12 a | |
| gooseberry | Y | 2.17 ± 1.89 | 1.83 ± 1.71 | 3.58 ± 0.14 | 3.67 ± 0.38 * |
| A | n.d. | n.d. | 2.80 ± 0.72 | 2.07 ± 0.81 | |
| plum | Y | 4.58 ± 0.38 * | 3.71 ± 1.70 | 4.67 ± 1.01 | 3.92 ± 1.18 |
| A | 1.20 ± 0.20 c | 2.20 ± 0.20 b | 2.80 ± 0.72 ab | 3.40 ± 0.35 a | |
| blueberry | Y | 4.71 ± 0.85 b* | 5.58 ± 0.29 ab* | 5.04 ± 0.26 b | 6.46 ± 0.64 a* |
| A | 1.53 ± 0.83 c | 2.60 ± 0.92 bc | 4.13 ± 0.64 a | 4.00 ± 0.53 ab | |
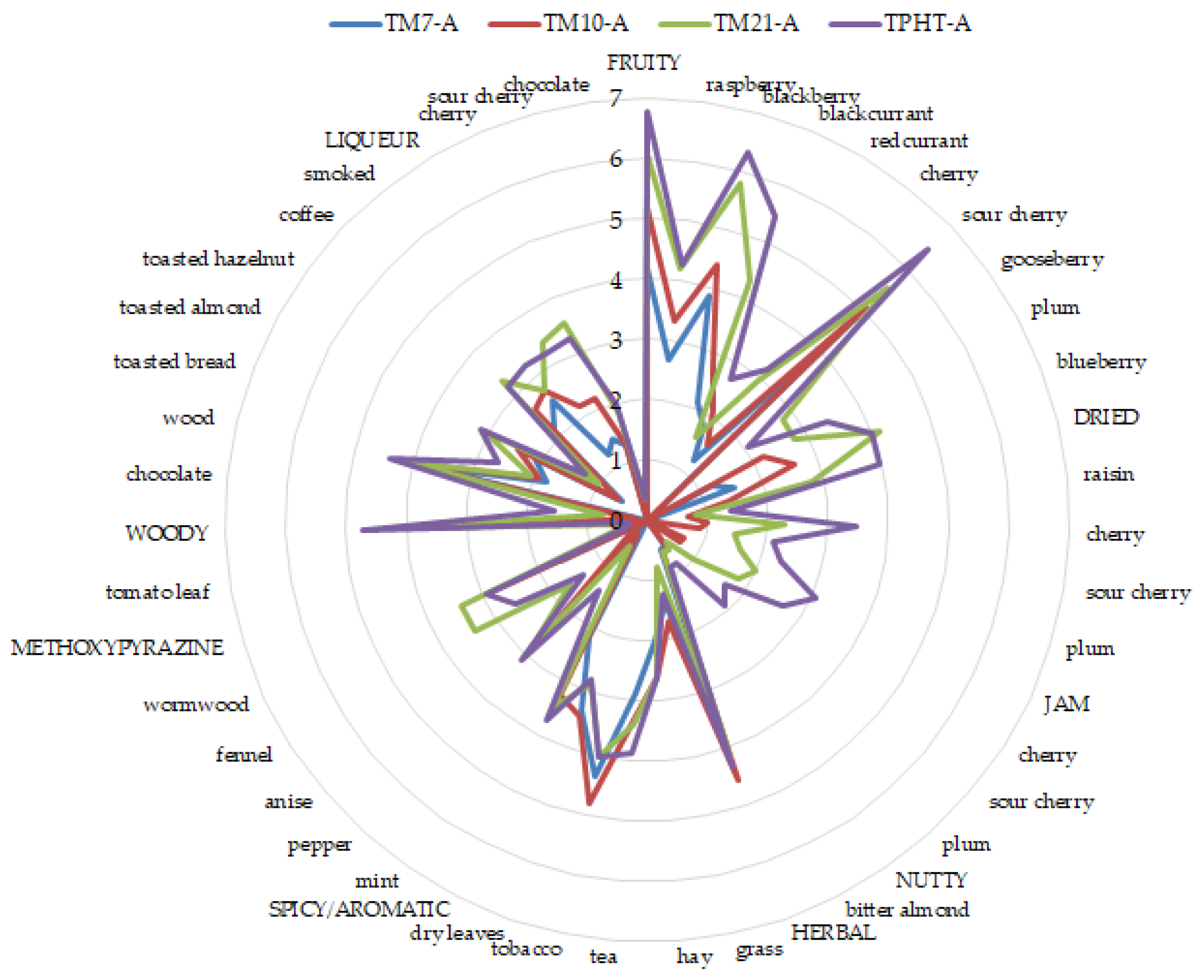
| Dried Fruit | Y | 2.58 ± 0.31 d | 3.67 ± 0.07 c* | 4.42 ± 0.19 b* | 5.25 ± 0.45 a* |
| A | n.d. | 1.40 ± 0.35 c | 2.80 ± 0.20 b | 3.97 ± 0.25 a | |
| raisin | Y | 1.88 ± 0.82 | 1.88 ± 1.72 | 2.17 ± 1.61 | 4.17 ± 0.63 * |
| A | n.d. | 0.67 ± 0.46 b | 0.80 ± 0.20 ab | 1.40 ± 0.53 a | |
| sour cherry | Y | n.d. | n.d. | n.d. | n.d. |
| A | n.d. | 1.00 ± 0.20 c | 2.27 ± 0.42 b | 3.47 ± 0.64 a | |
| cherry | Y | n.d. | n.d. | n.d. | n.d. |
| A | n.d. | 0.87 ± 0.12 c | 1.47 ± 0.23 b | 2.13 ± 0.50 a | |
| plum | Y | 2.67 ± 0.19 d | 3.83 ± 0.26 c | 4.42 ± 0.31 b* | 5.29 ± 0.36 a* |
| A | n.d. | n.d. | 1.60 ± 0.40 b | 2.33 ± 0.31 a | |
| Jam | Y | n.d. | n.d. | n.d. | n.d. |
| A | n.d. | 0.87 ± 0.12 c | 2.00 ± 0.53 b | 3.07 ± 0.15 a | |
| sour cherry | Y | n.d. | n.d. | n.d. | n.d. |
| A | n.d. | 0.67 ± 0.23 c | 1.80 ± 0.40 b | 2.67 ± 0.50 a | |
| cherry | Y | n.d. | n.d. | n.d. | n.d. |
| A | n.d. | n.d. | 1.00 ± 0.35 b | 1.67 ± 0.46 a | |
| plum | Y | n.d. | n.d. | n.d. | n.d. |
| A | n.d. | n.d. | 0.47 ± 0.42 b | 1.93 ± 0.58 a | |
| Nutty | Y | n.d. | n.d. | n.d. | n.d. |
| A | 0.53 ± 0.50 | 0.60 ± 0.60 | 0.60 ± 0.00 | 0.87 ± 0.46 | |
| bitter almond (marzipan) | Y | n.d. | n.d. | n.d. | n.d. |
| A | 0.53 ± 0.50 | 0.60 ± 0.60 | 0.60 ± 0.00 | 0.87 ± 0.46 | |
| Herbal | Y | 3.33 ± 0.59 c | 4.25 ± 0.13 b | 5.25 ± 0.22 a* | 5.29 ± 0.36 a* |
| A | 3.53 ± 0.49 b | 4.56 ± 0.48 a | 4.56 ± 0.48 a | 4.56 ± 0.48 a | |
| grass | Y | 0.25 ± 0.43 | 0.25 ± 0.43 | 0.33 ± 0.58 | 0.50 ± 0.43 |
| A | 1.38 ± 0.56 * | 1.72 ± 0.93 | 1.20 ± 1.13 | 1.27 ± 0.61 | |
| hay | Y | 3.08 ± 0.75 c | 3.79 ± 0.26 bc | 4.58 ± 0.14 a* | 4.33 ± 0.14 ab* |
| A | 2.00 ± 0.53 | 2.63 ± 1.10 | 2.60 ± 0.35 | 2.60 ± 0.72 | |
| tea | Y | 2.42 ± 0.58 b | 3.17 ± 0.29 ab | 4.00 ± 0.87 ab | 3.92 ± 0.72 a |
| A | 2.87 ± 0.42 b | 3.33 ± 0.31 ab | 3.40 ± 0.60 ab | 3.87 ± 0.61 a | |
| tobacco | Y | 3.00 ± 0.66 c | 4.38 ± 0.22 b | 4.92 ± 0.38 ab | 5.42 ± 0.36 a* |
| A | 4.33 ± 0.12 * | 4.81 ± 0.72 | 4.00 ± 0.53 | 4.00 ± 0.53 | |
| dry leaves | Y | 2.42 ± 0.72 c | 3.00 ± 1.39 bc | 4.50 ± 0.66 ab* | 4.83 ± 0.29 a* |
| A | 3.33 ± 0.81 | 3.43 ± 0.97 | 2.87 ± 0.64 | 2.80 ± 0.69 | |
| Spicy/Aromatic | Y | 2.75 ± 0.38 b | 3.29 ± 0.47 ab | 3.96 ± 0.19 a | 4.33 ± 0.52 a |
| A | 2.17 ± 0.29 b | 3.23 ± 0.25 a | 3.50 ± 0.26 a | 3.70 ± 0.26 a | |
| clove | Y | 0.33 ± 0.29 b | 0.79 ± 0.75 b | 2.04 ± 1.82 ab | 3.17 ± 0.63 a |
| A | n.d. | n.d. | n.d. | n.d. | |
| mint | Y | n.d. | n.d. | n.d. | n.d. |
| A | n.d. | 0.27 ± 0.46 b | 0.53 ± 0.12 b | 1.40 ± 0.35 a | |
| pepper | Y | 2.83 ± 0.29 b* | 3.25 ± 0.38 b* | 4.13 ± 0.45 a* | 4.54 ± 0.47 a* |
| A | 1.20 ± 0.20 c | 2.20 ± 0.20 b | 2.87 ± 0.46 a | 3.13 ± 0.42 a | |
| anis | Y | n.d. | n.d. | n.d. | n.d. |
| A | n.d. | 0.40 ± 0.35 b | 1.67 ± 0.31 a | 1.40 ± 0.00 a | |
| fennel | Y | n.d. | n.d. | n.d. | n.d. |
| A | n.d. | n.d. | 3.40 ± 0.00 a | 2.60 ± 0.35 b | |
| wormwood | Y | 1.21 ± 0.19 c | 1.71 ± 0.40 bc | 2.25 ± 0.25 ab | 2.67 ± 0.38 a |
| A | 1.60 ± 0.35 b | 2.93 ± 0.58 a* | 3.40 ± 0.35 a* | 2.93 ± 0.31 a | |
| Methoxypyrazine | Y | n.d. | n.d. | n.d. | n.d. |
| A | n.d. | n.d. | 0.47 ± 0.23 | 0.33 ± 0.42 | |
| tomato leaf | Y | n.d. | n.d. | n.d. | n.d. |
| A | n.d. | n.d. | 0.47 ± 0.23 | 0.33 ± 0.42 | |
| Woody | Y | n.d. | n.d. | n.d. | n.d. |
| A | 3.63 ± 0.25 c | 4.07 ± 0.31 bc | 4.30 ± 0.40 ab | 4.73 ± 0.15 a | |
| chocolate | Y | n.d. | n.d. | n.d. | n.d. |
| A | n.d. | 0.20 ± 0.35 b | 0.73 ± 0.42 ab | 1.53 ± 0.70 a | |
| wood | Y | n.d. | n.d. | n.d. | n.d. |
| A | 3.80 ± 0.35 | 4.13 ± 0.23 | 4.07 ± 0.31 | 4.40 ± 0.69 | |
| toasted bread | Y | n.d. | n.d. | n.d. | n.d. |
| A | 1.80 ± 0.20 b | 1.93 ± 0.42 b | 2.07 ± 0.46 ab | 2.67 ± 0.12 a | |
| toasted almond | Y | n.d. | n.d. | n.d. | n.d. |
| A | 2.13 ± 0.70 | 2.47 ± 0.95 | 3.07 ± 0.42 | 3.13 ± 0.12 | |
| toasted hazelnut | Y | n.d. | n.d. | n.d. | n.d. |
| A | 0.53 ± 0.23 b | 0.60 ± 0.20 b | 1.00 ± 0.20 a | 1.27 ± 0.12 a | |
| coffee | Y | n.d. | n.d. | n.d. | n.d. |
| A | 2.13 ± 0.23 b | 2.60 ± 0.72 ab | 3.33 ± 0.50 ab | 3.20 ± 0.40 a | |
| smoked | Y | n.d. | n.d. | n.d. | n.d. |
| A | 2.53 ± 0.46 | 2.73 ± 0.12 | 2.73 ± 0.61 | 3.27 ± 0.31 | |
| Liqueur | Y | 2.58 ± 0.26 b* | 2.63 ± 0.13 b | 4.00 ± 0.38 a | 4.25 ± 0.50 a |
| A | 1.27 ± 0.31 b | 2.20 ± 0.87 b | 3.43 ± 0.35 a | 3.23 ± 0.40 a | |
| sour cherry | Y | 2.58 ± 0.38 b* | 2.63 ± 0.13 b | 4.13 ± 0.54 a | 4.38 ± 0.38 a* |
| A | 1.47 ± 0.42 b | 2.20 ± 0.87 b | 3.53 ± 0.23 a | 3.27 ± 0.46 a | |
| cherry | Y | 2.08 ± 0.76 b | 1.79 ± 0.64 b | 3.75 ± 0.5 a* | 4.29 ± 0.51 a* |
| A | 1.30 ± 0.17 b | 1.33 ± 0.23 b | 1.87 ± 0.12 a | 2.00 ± 0.20 a | |
| chocolate | Y | 0.33 ± 0.29 b | 0.33 ± 0.38 b | 2.83 ± 1.01 a* | 2.88 ± 0.70 a* |
| A | n.d. | n.d. | 0.33 ± 0.31 | 0.33 ± 0.31 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, S.; Bestulić, E.; Orbanić, F.; Horvat, I.; Lukić, I.; Ilak Peršurić, A.S.; Bubola, M.; Plavša, T.; Radeka, S. Comprehensive Analysis of Teran Red Wine Aroma and Sensory Profiles: Impacts of Maceration Duration, Pre-Fermentation Heating Treatment, and Barrel Aging. Appl. Sci. 2024, 14, 8729. https://doi.org/10.3390/app14198729
Rossi S, Bestulić E, Orbanić F, Horvat I, Lukić I, Ilak Peršurić AS, Bubola M, Plavša T, Radeka S. Comprehensive Analysis of Teran Red Wine Aroma and Sensory Profiles: Impacts of Maceration Duration, Pre-Fermentation Heating Treatment, and Barrel Aging. Applied Sciences. 2024; 14(19):8729. https://doi.org/10.3390/app14198729
Chicago/Turabian StyleRossi, Sara, Ena Bestulić, Fumica Orbanić, Ivana Horvat, Igor Lukić, Anita Silvana Ilak Peršurić, Marijan Bubola, Tomislav Plavša, and Sanja Radeka. 2024. "Comprehensive Analysis of Teran Red Wine Aroma and Sensory Profiles: Impacts of Maceration Duration, Pre-Fermentation Heating Treatment, and Barrel Aging" Applied Sciences 14, no. 19: 8729. https://doi.org/10.3390/app14198729
APA StyleRossi, S., Bestulić, E., Orbanić, F., Horvat, I., Lukić, I., Ilak Peršurić, A. S., Bubola, M., Plavša, T., & Radeka, S. (2024). Comprehensive Analysis of Teran Red Wine Aroma and Sensory Profiles: Impacts of Maceration Duration, Pre-Fermentation Heating Treatment, and Barrel Aging. Applied Sciences, 14(19), 8729. https://doi.org/10.3390/app14198729









