Self-Etch Adhesive-Loaded ZrO2/Ag3PO4 Nanoparticles on Caries-Affected Dentin: A Tensile Bond Strength, Scanning Electron Microscopy, Energy Dispersive X-ray Spectroscopy, Survival Rate Assessment of S. mutans, and Degree of Conversion Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. ZrO2/Ag3PO4 Nanoparticles Synthesis
2.2. SEM Analysis
2.3. EDX Assessment
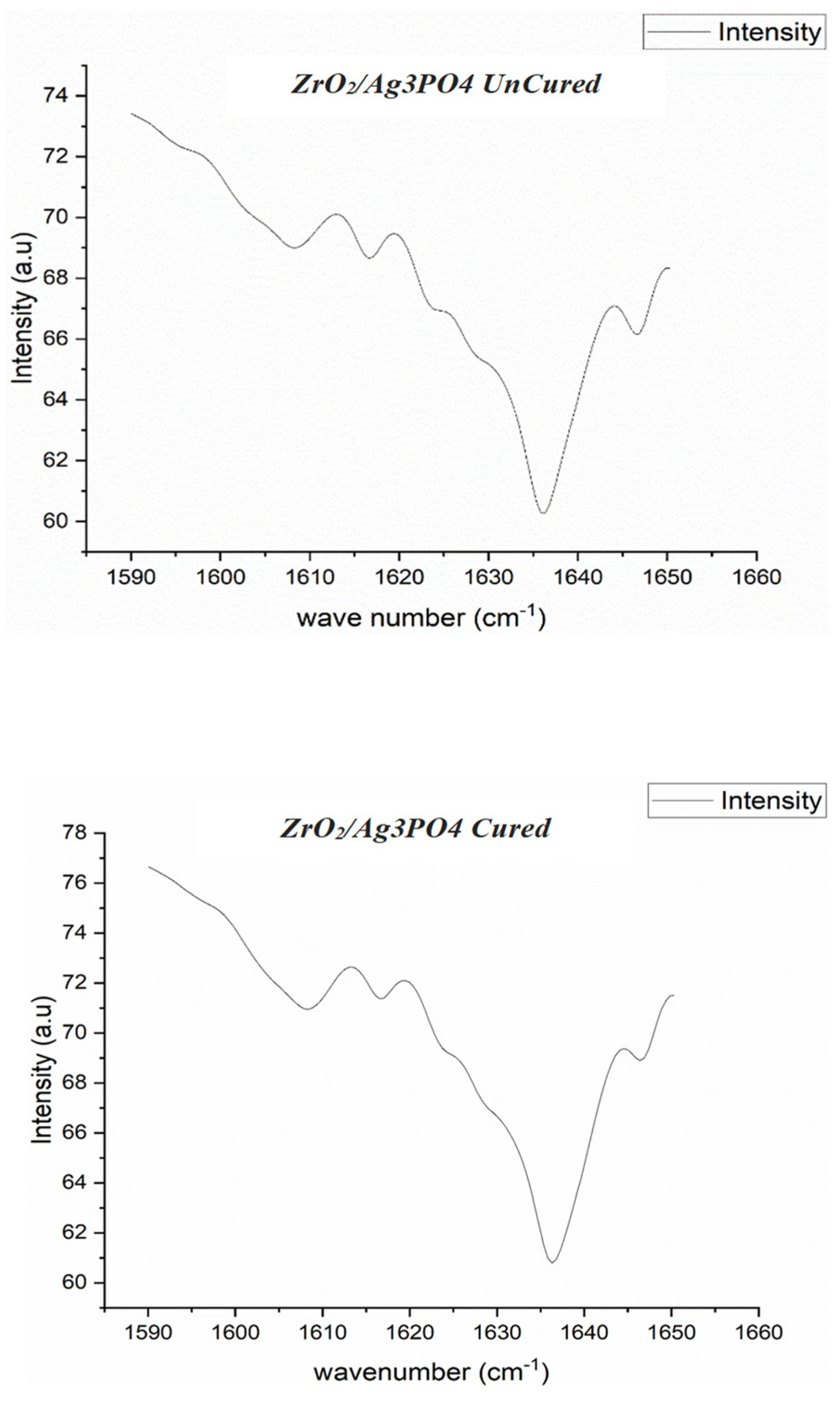
2.4. FTIR Spectroscopy and Degree of Conversion
2.5. Bacterial Culture
group/CFU count of the control.
2.6. Random Allocation of Samples
2.7. Restoration Bonding
2.8. μTBS and Failure Mode Analysis
2.9. Statistical Analysis
3. Results
3.1. SEM EDX of ZrO2/Ag3PO4 Synthesized Nanoparticles
3.2. Antimicrobial Evaluation
3.3. μTBS, Degree of Conversion, and Failure Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alrahlah, A.; Naseem, M.; Tanveer, S.A.; Abrar, E.; Charania, A.; AlRifaiy, M.Q.; Vohra, F. Influence of Disinfection of Caries Effected Dentin with Different Concentration of Silver Diamine Fluoride, Curcumin and Er, Cr:YSGG on Adhesive Bond Strength to Resin Composite. Photodiagnosis Photodyn. Ther. 2020, 32, 102065. [Google Scholar] [CrossRef]
- Al Ahdal, K.; Maawadh, A.M.; Al Deeb, L.; Alshamrani, A.S.; Almohareb, T.; Alrahlah, A. Effect of Malachite Green, Ocimum Sanctum, and Er, Cr: YSGG Laser on Antimicrobial Activity against S. mutans and CAD Disinfection Bonded to Resin Restoration. Photodiagnosis Photodyn. Ther. 2023, 42, 103571. [Google Scholar] [CrossRef] [PubMed]
- AlSheikh, R.; Abduldaiem, O.Y.; Alkhalifa, M.S.; Jillani, M.S.; Dehailan, L.A.; Barakat, A.; Alazmah, A.; Hameed, M.S.; Niazi, F. Different Cavity Disinfectant Efficacy against S. mutans and Shear Bond Strength of Caries Affected Dentin Bonded to Resin Restoration. Photodiagnosis Photodyn. Ther. 2023, 42, 103560. [Google Scholar] [CrossRef] [PubMed]
- Alfaawaz, Y.F. Disinfection of Caries Affected Dentin Using Rose Bengal, Titanium Sapphire Laser; Ammonium Hexa-Fluorosilicate, and Ozonated Water on Resin Dentin Bond Strength. Photodiagnosis Photodyn. Ther. 2022, 39, 102912. [Google Scholar] [CrossRef] [PubMed]
- Mandava, D.; Ajitha, P.; Narayanan, L.L. Comparative Evaluation of Tensile Bond Strengths of Total-Etch Adhesives and Self-Etch Adhesives with Single and Multiple Consecutive Applications: An in Vitro Study. J. Conserv. Dent. 2009, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Vieira, B.R.; Dantas, E.L.D.A.; Cavalcanti, Y.W.; Santiago, B.M.; Sousa, F.B. De Comparison of Self-Etching Adhesives and Etch-and-Rinse Adhesives on the Failure Rate of Posterior Composite Resin Restorations: A Systematic Review and Meta-Analysis. Eur. J. Dent. 2022, 16, 258–265. [Google Scholar]
- Costa, J.; Bettencourt, A.; Madeira, A.; Nepomuceno, L.S.; Portugal, J.; Neves, C.B. Surface Properties after Chemical Aging of Chlorhexidine Delivery Systems Based on Acrylic Resin. Rev. Port. Estomatol. Med. Dent. E Cir. Maxilofac. 2019, 60, 155–162. [Google Scholar] [CrossRef]
- Costa, J.V.; Portugal, J.; Neves, C.B.; Bettencourt, A.F. Should Local Drug Delivery Systems Be Used in Dentistry? Drug Deliv. Transl. Res. 2022, 12, 1395–1407. [Google Scholar] [CrossRef]
- Targino, A.G.R.; Flores, M.A.P.; Dos Santos, V.E.; De Godoy Bené Bezerra, F.; De Luna Freire, H.; Galembeck, A.; Rosenblatt, A. An Innovative Approach to Treating Dental Decay in Children. A New Anti-Caries Agent. J. Mater. Sci. Mater. Med. 2014, 25, 2041–2047. [Google Scholar] [CrossRef]
- Naik, S.V.; Sugandhan, S.; Ramasetty, P.A.; Tripathi, A.P.; Deepak, B.M. Nanotechnology in Dentin Disinfection: Can We Preserve the Bond? Int. J. Clin. Pediatr. Dent. 2018, 11, 468–473. [Google Scholar] [CrossRef]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional Gold Nanoparticles as Potent Antimicrobial Agents against Multi-Drug-Resistant Bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef] [PubMed]
- Giráldez-Pérez, R.M.; Grueso, E.M.; Carbonero, A.; Álvarez Márquez, J.; Gordillo, M.; Kuliszewska, E.; Prado-Gotor, R. Synergistic Antibacterial Effects of Amoxicillin and Gold Nanoparticles: A Therapeutic Option to Combat Antibiotic Resistance. Antibiotics 2023, 12, 1275. [Google Scholar] [CrossRef] [PubMed]
- Giráldez-Pérez, R.M.; Grueso, E.M.; Jiménez-Aguayo, R.; Carbonero, A.; González-Bravo, M.; Kuliszewska, E.; Prado-Gotor, R. Use of Nanoparticles to Prevent Resistance to Antibiotics—Synthesis and Characterization of Gold Nanosystems Based on Tetracycline. Pharmaceutics 2022, 14, 1941. [Google Scholar] [CrossRef] [PubMed]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.S.; Cheng, L.; Zhang, K.; Weir, M.D.; Rodrigues, L.K.A.; Xu, H.H.K. Novel Dental Adhesives Containing Nanoparticles of Silver and Amorphous Calcium Phosphate. Dent. Mater. 2013, 29, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Aljamhan, A.S.; Alrefeai, M.H.; Alhabdan, A.; Alzehiri, M.H.; Naseem, M.; Vohra, F.; Alkhudhairy, F. Interaction of Zirconium Oxide Nanoparticle Infiltrated Resin Adhesive with Dentin Conditioned by Phosphoric Acid and Er, Cr: YSGG Laser. J. Appl. Biomater. Funct. Mater. 2022, 20, 22808000221087349. [Google Scholar] [CrossRef] [PubMed]
- Hatta, M.; Shinya, A.; Yokoyama, D.; Gomi, H.; Vallittu, P.K.; Shinya, A. The Effect of Surface Treatment on Bond Strength of Layering Porcelain and Hybrid Composite Bonded to Zirconium Dioxide Ceramics. J. Prosthodont. Res. 2011, 55, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Bin-Shuwaish, M.S.; Maawadh, A.M.; Al-Hamdan, R.S.; Alresayes, S.; Ali, T.; Almutairi, B.; Vohra, F.; Abduljabbar, T. Influence of Graphene Oxide Filler Content on the Dentin Bond Integrity, Degree of Conversion and Bond Strength of Experimental Adhesive. A SEM, Micro-Raman, FTIR and Microtensile Study. Mater. Res. Express 2020, 7, 115403. [Google Scholar] [CrossRef]
- Vohra, F.; Alamri, R.R.; Almohsen, F.O.; El Mourad, A.M.; Farooq, I.; Alsaif, R. Fiber Post Bonding with Beta-Tricalcium Phosphate Incorporated Root Dentin Adhesive. SEM, EDX, FTIR, Rheometric and Bond Strength Study. Microsc. Res. Tech. 2023, 86, 762–772. [Google Scholar] [CrossRef]
- Khan, A.S.; Alhamdan, Y.; Alibrahim, H.; Almulhim, K.S.; Nawaz, M.; Ahmed, S.Z.; Aljuaid, K.; Ateeq, I.S.; Akhtar, S.; Ansari, M.A.; et al. Analyses of Experimental Dental Adhesives Based on Zirconia/Silver Phosphate Nanoparticles. Polymers 2023, 15, 2614. [Google Scholar] [CrossRef]
- Alsunbul, H.; Alfawaz, Y.F.; Alhamdan, E.M.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Carbon and Graphene Oxide Nanoparticle on the Adhesive Properties of Dentin Bonding Polymer: A SEM, EDX, FTIR Study. J. Appl. Biomater. Funct. Mater. 2023, 21, 22808000231159238. [Google Scholar] [CrossRef]
- Risnes, S.; Saeed, M.; Sehic, A. Scanning Electron Microscopy (SEM) Methods for Dental Enamel. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2019; Volume 1922, pp. 293–308. [Google Scholar]
- Alrefeai, M.H.; Aljamhan, A.S.; Alhabdan, A.; Alzehiri, M.H.; Naseem, M.; Alkhudhairy, F. Influence of Methylene Blue, Riboflavin, and Indocyanine Green on the Bond Strength of Caries Affected Dentin When Bonded to Resin-Modified Glass Ionomer Cement. Photodiagnosis Photodyn. Ther. 2022, 38, 102792. [Google Scholar] [CrossRef] [PubMed]
- Alhenaki, A.M.; Attar, E.A.; Alshahrani, A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Filled and Unfilled Resin Adhesive Enhanced with Silica Nanoparticles—An Sem, Edx, Micro-Raman, Ftir and Micro-Tensile Bond Strength Study. Polymers 2021, 13, 1093. [Google Scholar] [CrossRef] [PubMed]
- Elgamily, H.M.; El-Sayed, H.S.; Abdelnabi, A. The Antibacterial Effect of Two Cavity Disinfectants against One of Cariogenic Pathogen: An in Vitro Comparative Study. Contemp. Clin. Dent. 2018, 9, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Alkhudhairy, F.; Vohra, F.; Naseem, M. Influence of Er,Cr:YSGG Laser Dentin Conditioning on the Bond Strength of Bioactive and Conventional Bulk-Fill Dental Restorative Material. Photobiomodulation Photomed. Laser Surg. 2020, 38, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Al Deeb, L.; Bin-Shuwaish, M.S.; Abrar, E.; Naseem, M.; Al-Hamdan, R.S.; Maawadh, A.M.; Al Deeb, M.; Almohareb, T.; Al Ahdal, K.; Vohra, F.; et al. Efficacy of Chlorhexidine, Er Cr YSGG Laser and Photodynamic Therapy on the Adhesive Bond Integrity of Caries Affected Dentin. An In-Vitro Study. Photodiagnosis Photodyn. Ther. 2020, 31, 101875. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Gu, J.T.; Zare, E.N.; Ashtari, B.; Moeini, A.; Tay, F.R.; Niu, L.N. Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2020, 101, 69–101. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, B.; Kattan, H.F.; BinMahfooz, A.M.; Qutub, O.A.; Basunbul, G.; ArRejaie, A.S.; Farooq, I.; Vohra, F.; Abduljabbar, T. Synergistic Effect of Graphene Oxide/Calcium Phosphate Nanofiller in a Dentin Adhesive on Its Dentin Bond Integrity and Degree of Conversion. A Scanning Electron Microscopy, Energy Dispersive X-Ray Spectroscopy, Fourier Transform Infrared, Micro-Raman, An. Microsc. Res. Tech. 2021, 84, 2082–2094. [Google Scholar] [CrossRef]
- Al-Saleh, S.; Alateeq, A.; Alshaya, A.H.; Al-Qahtani, A.S.; Tulbah, H.I.; Binhasan, M.; Shabib, S.; Farooq, I.; Vohra, F.; Abduljabbar, T. Article Influence of TiO2 and ZrO2 Nanoparticles on Adhesive Bond Strength and Viscosity of Dentin Polymer: A Physical and Chemical Evaluation. Polymers 2021, 13, 3794. [Google Scholar] [CrossRef]
- Lee, M.J.; Seo, Y.B.; Seo, J.Y.; Ryu, J.H.; Ahn, H.J.; Kim, K.M.; Kwon, J.S.; Choi, S.H. Development of a Bioactive Flowable Resin Composite Containing a Zinc-Doped Phosphate-Based Glass. Nanomaterials 2020, 10, 2311. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic Extract of Propolis (EEP) Enhances the Apoptosis- Inducing Potential of TRAIL in Cancer Cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Chatzistavrou, X.; Faulk, D.; Badylak, S.; Zheng, L.; Papagerakis, S.; Ge, L.; Liu, H.; Papagerakis, P. Biological and Bactericidal Properties of Ag-Doped Bioactive Glass in a Natural Extracellular Matrix Hydrogel with Potential Application in Dentistry. Eur. Cells Mater. 2015, 29, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.G.; Nirmala, M.; Rekha, K.; Anukaliani, A. Structural, Optical, Photo Catalytic and Antibacterial Activity of ZnO and Co Doped ZnO Nanoparticles. Mater. Lett. 2011, 65, 1797–1800. [Google Scholar] [CrossRef]
- Salar, R.; Kumar, N.; Sharma, P. Enhanced Antibacterial Activity of Streptomycin against Some Human Pathogens Using Green Synthesized Silver Nanoparticles. Resour. Technol. 2015, 1, 106–115. [Google Scholar] [CrossRef]
- Ayanwale, A.P.; Ruíz-Baltazar, A.d.J.; Espinoza-Cristóbal, L.; Reyes-López, S.Y. Bactericidal Activity Study of ZrO2-Ag2O Nanoparticles. Dose-Response 2020, 18, 3. [Google Scholar] [CrossRef]
- Lohbauer, U.; Wagner, A.; Belli, R.; Stoetzel, C.; Hilpert, A.; Kurland, H.D.; Grabow, J.; Müller, F.A. Zirconia Nanoparticles Prepared by Laser Vaporization as Fillers for Dental Adhesives. Acta Biomater. 2010, 6, 4539–4546. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, B.; Binhasan, M.; Shabib, S.; Al-Qahtani, A.S.; Tulbah, H.I.; Al-Aali, K.A.; Vohra, F.; Abduljabbar, T. Adhesive Bond Integrity of Silanized Zirconia Nanoparticles in Polymeric Resin Dentin Bonding Agent. An FTIR, SEM, and Micro-Tensile Experiment. Int. J. Adhes. Adhes. 2022, 114, 103069. [Google Scholar] [CrossRef]
- Khurana, C.; Sharma, P.; Pandey, O.P.; Chudasama, B. Synergistic Effect of Metal Nanoparticles on the Antimicrobial Activities of Antibiotics against Biorecycling Microbes. J. Mater. Sci. Technol. 2016, 32, 524–532. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial Activity of Silver Nanoparticles: A Surface Science Insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Heinlaan, M.; et al. Size-Dependent Toxicity of Silver Nanoparticles to Bacteria, Yeast, Algae, Crustaceans and Mammalian Cells in Vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Aljamhan, A.S.; Alrefeai, M.H.; Alhabdan, A.; Alhusseini, S.A.; Farooq, I.; Vohra, F.; Naseem, M.; Alkhudhairy, F. Influence of ER-CR-YSGG Laser and Photodynamic Therapy on the Dentin Bond Integrity of Nano-Hydroxyapatite Containing Resin Dentin Adhesive: SEM-EDX. Polymers 2021, 13, 1903. [Google Scholar] [CrossRef] [PubMed]
- Fathima, J.B.; Pugazhendhi, A.; Venis, R. Synthesis and Characterization of ZrO2 Nanoparticles-Antimicrobial Activity and Their Prospective Role in Dental Care. Microb. Pathog. 2017, 110, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Vohra, F.; Bukhari, I.A.; Sheikh, S.A.; Naseem, M.; Hussain, M. Photodynamic Activation of Irrigation (Using Different Laser Prototypes) on Push out Bond Strength of Fiber Posts. Photodiagnosis Photodyn. Ther. 2020, 30, 101716. [Google Scholar] [CrossRef] [PubMed]
- Zampetti, P.; Scribante, A. Historical and Bibliometric Notes on the Use of Fluoride in Caries Prevention. Eur. J. Paediatr. Dent. 2020, 21, 148–152. [Google Scholar] [CrossRef]
- Desai, S.; Rao, D.; Panwar, S.; Kothari, N.; Gupta, S. An in Vitro Comparative Evaluation of Casein Phosphopeptide-Amorphous Calcium Phosphate Fluoride, Tricalcium Phosphate and Grape Seed Extract on Remineralization of Artificial Caries Lesion in Primary Enamel. J. Clin. Pediatr. Dent. 2022, 46, 72–80. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Trapani, B.; Gallo, S.; Radu, M.; Scribante, A. Biomimetic Hydroxyapatite Paste for Molar–Incisor Hypomineralization: A Randomized Clinical Trial. Oral Dis. 2023, 29, 2789–2798. [Google Scholar] [CrossRef]


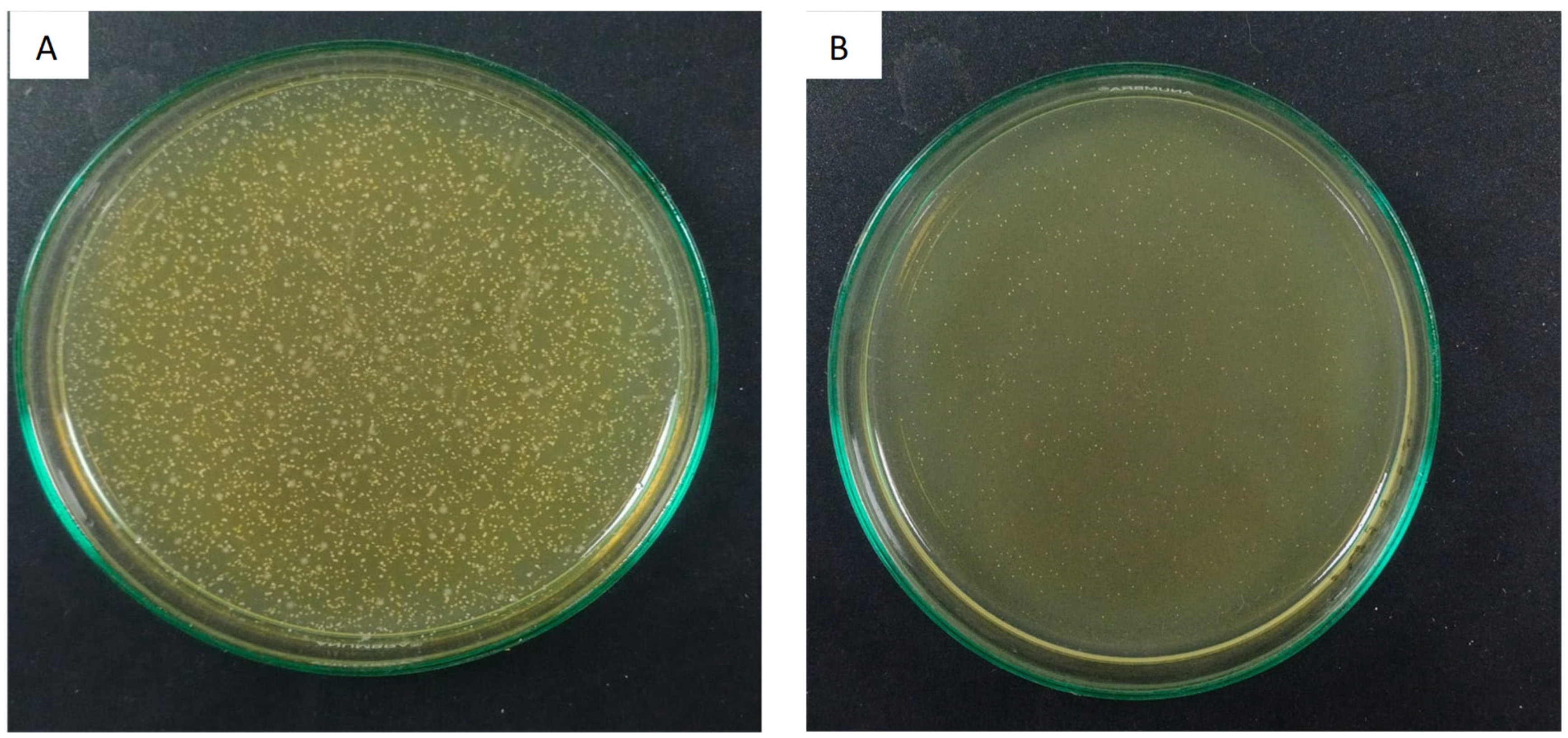
| Experimental Groups | Survival Rate CFU/mL | Standard Deviation (SD) |
|---|---|---|
| Group 1: Unmodified SE (Control) | 0.40 A | 0.09 |
| Group 2: 0.15 wt% ZrO2/Ag3PO4 + SE adhesive | 0.19 B | 0.05 |
| Group 3: 0.25 wt% ZrO2/Ag3PO4 + SE adhesive | 0.17 B | 0.04 |
| Group 4: 0.5 wt% ZrO2/Ag3PO4 + SE adhesive | 0.12 B | 0.01 |
| Investigated Groups | Mean ± SD (MPa) | p-Value |
|---|---|---|
| Group 1: Unmodified SE (Control) | 15.28 ± 0.22 a | <0.05 |
| Group 2: 0.15 wt% ZrO2/Ag3PO4 + SE adhesive | 19.16 ± 0.45 b | |
| Group 3: 0.25 wt% ZrO2/Ag3PO4 + SE adhesive | 19.85 ± 0.57 b | |
| Group 4: 0.5 wt% ZrO2/Ag3PO4 + SE adhesive | 20.12 ± 0.79 b |
| Failure Type | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| Adhesive | 20% | 10% | 10% | 10% |
| Cohesive | 40% | 60% | 70% | 80% |
| Admixed | 40% | 30% | 20% | 10% |
| Experimental Groups | Degree of Conversion % * |
|---|---|
| Group 1: Unmodified SE (Control) | 69.85 ± 8.37 B |
| Group 2: 0.15 wt% ZrO2/Ag3PO4 + SE adhesive | 41.89 ± 8.11 A |
| Group 3: 0.25 wt% ZrO2/Ag3PO4 + SE adhesive | 43.74 ± 6.91 A |
| Group 4: 0.5 wt% ZrO2/Ag3PO4 + SE adhesive | 49.68 ± 6.59 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhudhairy, F.; AlRefeai, M.H. Self-Etch Adhesive-Loaded ZrO2/Ag3PO4 Nanoparticles on Caries-Affected Dentin: A Tensile Bond Strength, Scanning Electron Microscopy, Energy Dispersive X-ray Spectroscopy, Survival Rate Assessment of S. mutans, and Degree of Conversion Analysis. Appl. Sci. 2024, 14, 563. https://doi.org/10.3390/app14020563
Alkhudhairy F, AlRefeai MH. Self-Etch Adhesive-Loaded ZrO2/Ag3PO4 Nanoparticles on Caries-Affected Dentin: A Tensile Bond Strength, Scanning Electron Microscopy, Energy Dispersive X-ray Spectroscopy, Survival Rate Assessment of S. mutans, and Degree of Conversion Analysis. Applied Sciences. 2024; 14(2):563. https://doi.org/10.3390/app14020563
Chicago/Turabian StyleAlkhudhairy, Fahad, and Mohammad H. AlRefeai. 2024. "Self-Etch Adhesive-Loaded ZrO2/Ag3PO4 Nanoparticles on Caries-Affected Dentin: A Tensile Bond Strength, Scanning Electron Microscopy, Energy Dispersive X-ray Spectroscopy, Survival Rate Assessment of S. mutans, and Degree of Conversion Analysis" Applied Sciences 14, no. 2: 563. https://doi.org/10.3390/app14020563






