Machine Learning Model Trained with Finite Element Modeling Can Predict the Risk of Osteoarthritis: Data from the Osteoarthritis Initiative
Abstract
:1. Introduction
2. Materials and Methods
2.1. Workflow
- GPR1—to predict atlas-based FEA method [19] outcomes: contact area, contact pressure, tensile stress, tensile strain, shear stress, shear strain, and pore pressure (peaks and averages over the tibiofemoral contact area), as well as simulated cartilage degeneration using age, weight, and anatomical knee joint dimensions: lateral joint space (JSLAT-MRI), medial joint space (JSMED-MRI), maximum lateral anterior–posterior dimension (APLAT-MRI), maximum medial anterior–posterior dimensions (APMED-MRI), and condyle distance (CDMRI), as a set of predictor variables.
- GPR2—to predict anatomical knee joint dimensions: lateral joint space (JSLAT-XRAY), medial joint space (JSMED-XRAY), and full medial–lateral width of the distal femur (WXRAY) using age, weight, height, and gender as a set of predictor variables.
2.2. Data
2.3. Training Data in GPR1 and GPR2 Models
2.4. Atlas-Based FEA Method
- The tibiofemoral joint spaces (total cartilage thickness) at the medial compartment (JSMED-MRI).
- The tibiofemoral joint spaces (total cartilage thickness) at the lateral compartment (JSLAT-MRI).
- The maximum anterior–posterior dimensions at medial femoral condyles (APMED-MRI).
- The maximum anterior–posterior dimensions at lateral femoral condyles (APLAT-MRI).
- The medial–lateral condyle distance measured as the distance between the medial and lateral contact area (CDMRI).
- The tibiofemoral joint spaces in the medial compartment (JSMED-Xray).
- The tibiofemoral joint spaces in the lateral compartment (JSLAT-Xray).
- The full medial–lateral width of the distal femur (WXray).
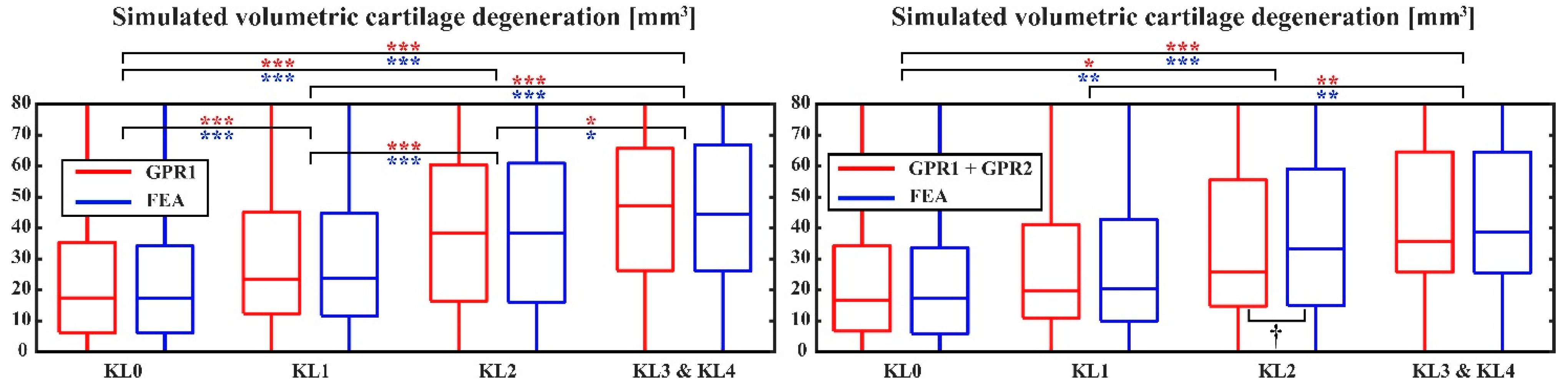
2.5. Simulation of Cartilage Degeneration
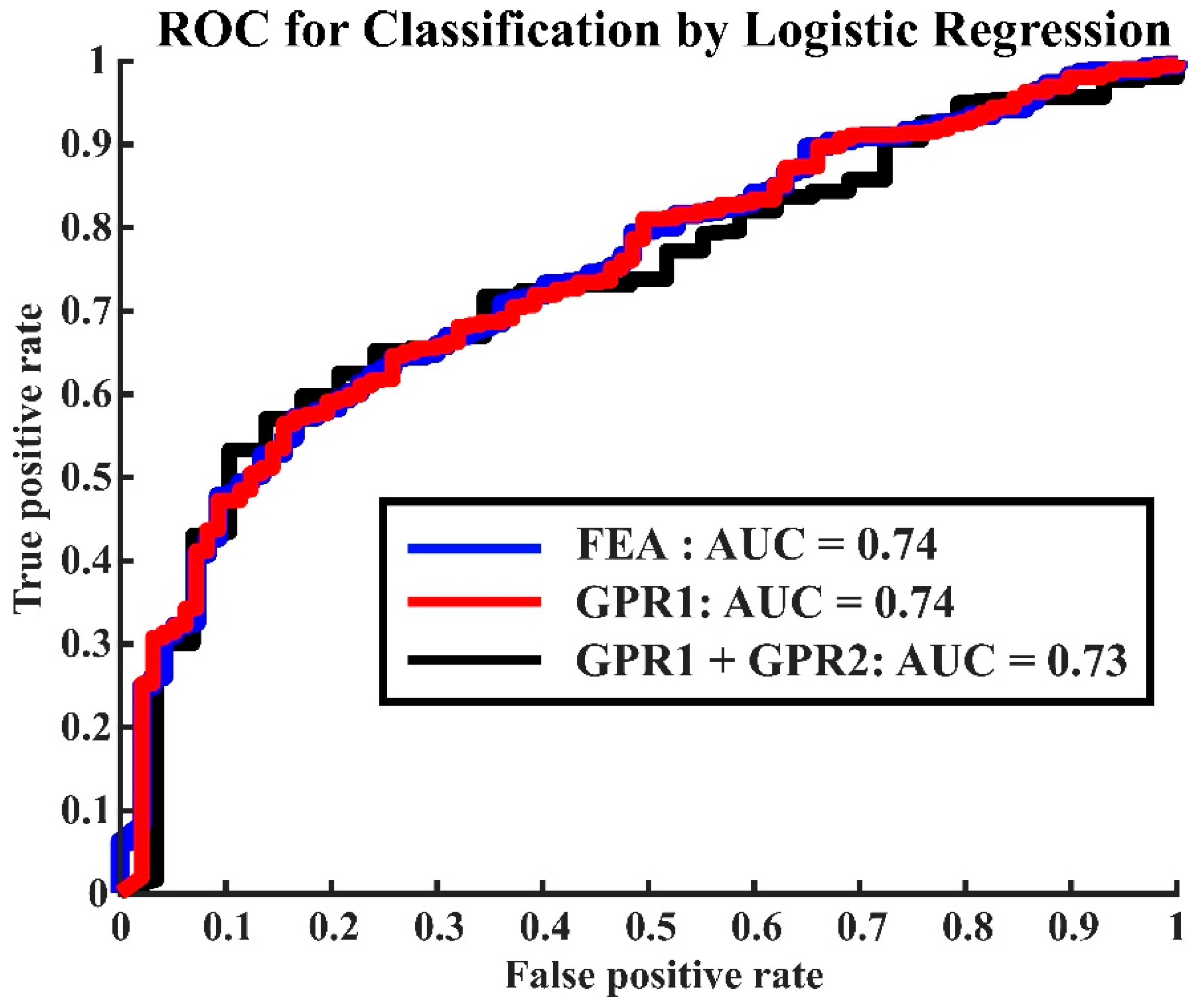
2.6. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; Di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst Century: Risk Factors and Behaviours that Influence Disease Onset and Progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef] [PubMed]
- Belluzzi, E.; Stocco, E.; Pozzuoli, A.; Granzotto, M.; Porzionato, A.; Vettor, R.; De Caro, R.; Ruggieri, P.; Ramonda, R.; Rossato, M.; et al. Contribution of Infrapatellar Fat Pad and Synovial Membrane to Knee Osteoarthritis Pain. BioMed Res. Int. 2019, 2019, 6390182. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.S.; Loeser, R.F. Why is osteoarthritis an age-related disease? Best Pr. Res. Clin. Rheumatol. 2010, 24, 15–26. [Google Scholar] [CrossRef]
- Theis, K.A.; Murphy, L.B.; Guglielmo, D.; Boring, M.A.; Okoro, C.A.; Duca, L.M.; Helmick, C.G. Prevalence of Arthritis and Arthritis-Attributable Activity Limitation—United States, 2016–2018. Mmwr. Morb. Mortal. Wkly. Rep. 2021, 70, 1401–1407. [Google Scholar] [CrossRef]
- Törmälehto, S.; Mononen, M.E.; Aarnio, E.; Arokoski, J.P.A.; Korhonen, R.K.; Martikainen, J. Health-related quality of life in relation to symptomatic and radiographic definitions of knee osteoarthritis: Data from Osteoarthritis Initiative (OAI) 4-year follow-up study. Heal. Qual. Life Outcomes 2018, 16, 154. [Google Scholar] [CrossRef]
- Duffell, L.D.; Southgate, D.F.; Gulati, V.; McGregor, A.H. Balance and gait adaptations in patients with early knee osteoarthritis. Gait Posture 2014, 39, 1057–1061. [Google Scholar] [CrossRef]
- Felson, D.T.; Hodgson, R. Identifying and Treating Preclinical and Early Osteoarthritis. Rheum. Dis. Clin. N. Am. 2014, 40, 699–710. [Google Scholar] [CrossRef]
- Murphy, L.B.; Cisternas, M.G.; Pasta, D.J.; Helmick, C.G.; Yelin, E.H. Medical Expenditures and Earnings Losses Among US Adults With Arthritis in 2013. Arthritis Care Res. 2018, 70, 869–876. [Google Scholar] [CrossRef]
- Bliddal, H.; Leeds, A.R.; Christensen, R. Osteoarthritis, obesity and weight loss: Evidence, hypotheses and horizons—A scoping review. Obes. Rev. 2014, 15, 578–586. [Google Scholar] [CrossRef]
- Hirvasniemi, J.; Runhaar, J.; van der Heijden, R.; Zokaeinikoo, M.; Yang, M.; Li, X.; Tan, J.; Rajamohan, H.; Zhou, Y.; Deniz, C.; et al. The KNee OsteoArthritis Prediction (KNOAP2020) challenge: An image analysis challenge to predict incident symptomatic radiographic knee osteoarthritis from MRI and X-Ray images. Osteoarthr. Cartil. 2023, 31, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Ramazanian, T.; Fu, S.; Sohn, S.; Taunton, M.J.; Kremers, H.M. Prediction Models for Knee Osteoarthritis: Review of Current Models and Future Directions. Arch Bone Jt Surg. 2023, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, T.; Thomas, M.J.; Antcliff, D.; Peat, G. Prediction Models to Estimate the Future Risk of Osteoarthritis in the General Population: A Systematic Review. Arthritis Care Res. 2023, 75, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Mononen, M.E.; Turunen, M.J.; Stenroth, L.; Saarakkala, S.; Boesen, M. Biomechanical modeling and imaging for knee osteoarthritis—is there a role for AI? Osteoarthr. Imaging 2024, 4, 100182. [Google Scholar] [CrossRef]
- Cooper, R.J.; Wilcox, R.K.; Jones, A.C. Finite element models of the tibiofemoral joint: A review of validation approaches and modelling challenges. Med. Eng. Phys. 2019, 74, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.; Orozco, G.A.; Korhonen, R.K.; García, J.J.; Mononen, M.E. Expediting Finite Element Analyses for Subject-Specific Studies of Knee Osteoarthritis: A Literature Review. Appl. Sci. 2021, 11, 11440. [Google Scholar] [CrossRef]
- Halonen, K.S.; Dzialo, C.M.; Mannisi, M.; Venäläinen, M.S.; de Zee, M.; Andersen, M.S. Workflow assessing the effect of gait alterations on stresses in the medial tibial cartilage-combined musculoskeletal modelling and finite element analysis. Sci. Rep. 2017, 7, 17396. [Google Scholar] [CrossRef]
- Liukkonen, M.K.; Mononen, M.E.; Vartiainen, P.; Kaukinen, P.; Bragge, T.; Suomalainen, J.-S.; Malo, M.K.H.; Venesmaa, S.; Käkelä, P.; Pihlajamäki, J.; et al. Evaluation of the Effect of Bariatric Surgery-Induced Weight Loss on Knee Gait and Cartilage Degeneration. J. Biomech. Eng. 2018, 140, 041008. [Google Scholar] [CrossRef]
- Mononen, M.E.; Liukkonen, M.K.; Turunen, M.J. X-Ray with finite element analysis is a viable alternative for MRI to predict knee osteoarthritis: Data from the Osteoarthritis Initiative. J. Orthop. Res. 2024, 42, 1964–1973. [Google Scholar] [CrossRef]
- Paz, A.; García, J.J.; Korhonen, R.K.; Mononen, M.E. Towards a Transferable Modeling Method of the Knee to Distinguish Between Future Healthy Joints from Osteoarthritic Joints: Data from the Osteoarthritis Initiative. Ann. Biomed. Eng. 2023, 51, 2192–2203. [Google Scholar] [CrossRef]
- Mononen, M.E.; Liukkonen, M.K.; Korhonen, R.K. Utilizing Atlas-Based Modeling to Predict Knee Joint Cartilage Degeneration: Data from the Osteoarthritis Initiative. Ann. Biomed. Eng. 2019, 47, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Widén, E.; Junna, N.; Ruotsalainen, S.; Surakka, I.; Mars, N.; Ripatti, P.; Partanen, J.J.; Aro, J.; Mustonen, P.; Tuomi, T.; et al. How Communicating Polygenic and Clinical Risk for Atherosclerotic Cardiovascular Disease Impacts Health Behavior: An Observational Follow-up Study. Circ. Genom. Precis. Med. 2022, 15, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, J.F.; Mongelard, K.B.G.; Radev, D.I.; Kappel, A.; Rasmussen, L.E.; Østgaard, S.E.; Odgaard, A. MRi of the knee compared to specialized radiography for measurements of articular cartilage height in knees with osteoarthritis. J. Orthop. 2021, 25, 191–198. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Mononen, M.E.; Paz, A.; Liukkonen, M.K.; Turunen, M.J. Atlas-based finite element analyses with simpler constitutive models predict personalized progression of knee osteoarthritis: Data from the osteoarthritis initiative. Sci. Rep. 2023, 13, 8888. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Camblor, P.; Pérez-Fernández, S.; Díaz-Coto, S. The area under the generalized receiver-operating characteristic curve. Int. J. Biostat. 2021, 18, 293–306. [Google Scholar] [CrossRef]
- Chan, L.; Li, H.; Chan, P.; Wen, C. A machine learning-based approach to decipher multi-etiology of knee osteoarthritis onset and deterioration. Osteoarthr. Cartil. Open 2021, 3, 100135. [Google Scholar] [CrossRef]
- Cai, G.; Jiang, M.; Cicuttini, F.; Jones, G. Association of age, sex and BMI with the rate of change in tibial cartilage volume: A 10.7-year longitudinal cohort study. Arthritis Res. Ther. 2019, 21, 273. [Google Scholar] [CrossRef]
- Si, L.; Xuan, K.; Zhong, J.; Huo, J.; Xing, Y.; Geng, J.; Hu, Y.; Zhang, H.; Wang, Q.; Yao, W. Knee Cartilage Thickness Differs Alongside Ages: A 3-T Magnetic Resonance Research Upon 2481 Subjects via Deep Learning. Front. Med. 2021, 7, 600049. [Google Scholar] [CrossRef]
- Karvonen, R.L.; Negendank, W.G.; A Teitge, R.; Reed, A.H.; Miller, P.R.; Fernandez-Madrid, F. Factors affecting articular cartilage thickness in osteoarthritis and aging. J. Rheumatol. 1994, 21, 1310–1318. [Google Scholar]
- Gwynne-Jones, J.H.; Wilson, R.A.; Wong, J.M.; Abbott, J.H.; Gwynne-Jones, D.P. The Outcomes of Nonoperative Management of Patients With Hip and Knee Osteoarthritis Triaged to a Physiotherapy-Led Clinic at Minimum 5-Year Follow-Up and Factors Associated With Progression to Surgery. J. Arthroplast. 2020, 35, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Pagnon, D.; Domalain, M.; Reveret, L. Pose2Sim: An End-to-End Workflow for 3D Markerless Sports Kinematics—Part 1: Robustness. Sensors 2021, 21, 6530. [Google Scholar] [CrossRef] [PubMed]
- Di Raimondo, G.; Willems, M.; Killen, B.A.; Havashinezhadian, S.; Turcot, K.; Vanwanseele, B.; Jonkers, I. Peak Tibiofemoral Contact Forces Estimated Using IMU-Based Approaches Are Not Significantly Different from Motion Capture-Based Estimations in Patients with Knee Osteoarthritis. Sensors 2023, 23, 4484. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Myller, K.A.; Tanska, P.; Hirvasniemi, J.; Saarakkala, S.; Töyräs, J.; Korhonen, R.K.; Mononen, M.E. Rapid CT-based Estimation of Articular Cartilage Biomechanics in the Knee Joint Without Cartilage Segmentation. Ann. Biomed. Eng. 2020, 48, 2965–2975. [Google Scholar] [CrossRef] [PubMed]
- Kempson, G.E. Relationship between the tensile properties of articular cartilage from the human knee and age. Ann. Rheum. Dis. 1982, 41, 508–511. [Google Scholar] [CrossRef]
- Tiulpin, A.; Saarakkala, S. Automatic Grading of Individual Knee Osteoarthritis Features in Plain Radiographs Using Deep Convolutional Neural Networks. Diagnostics 2020, 10, 932. [Google Scholar] [CrossRef]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef]
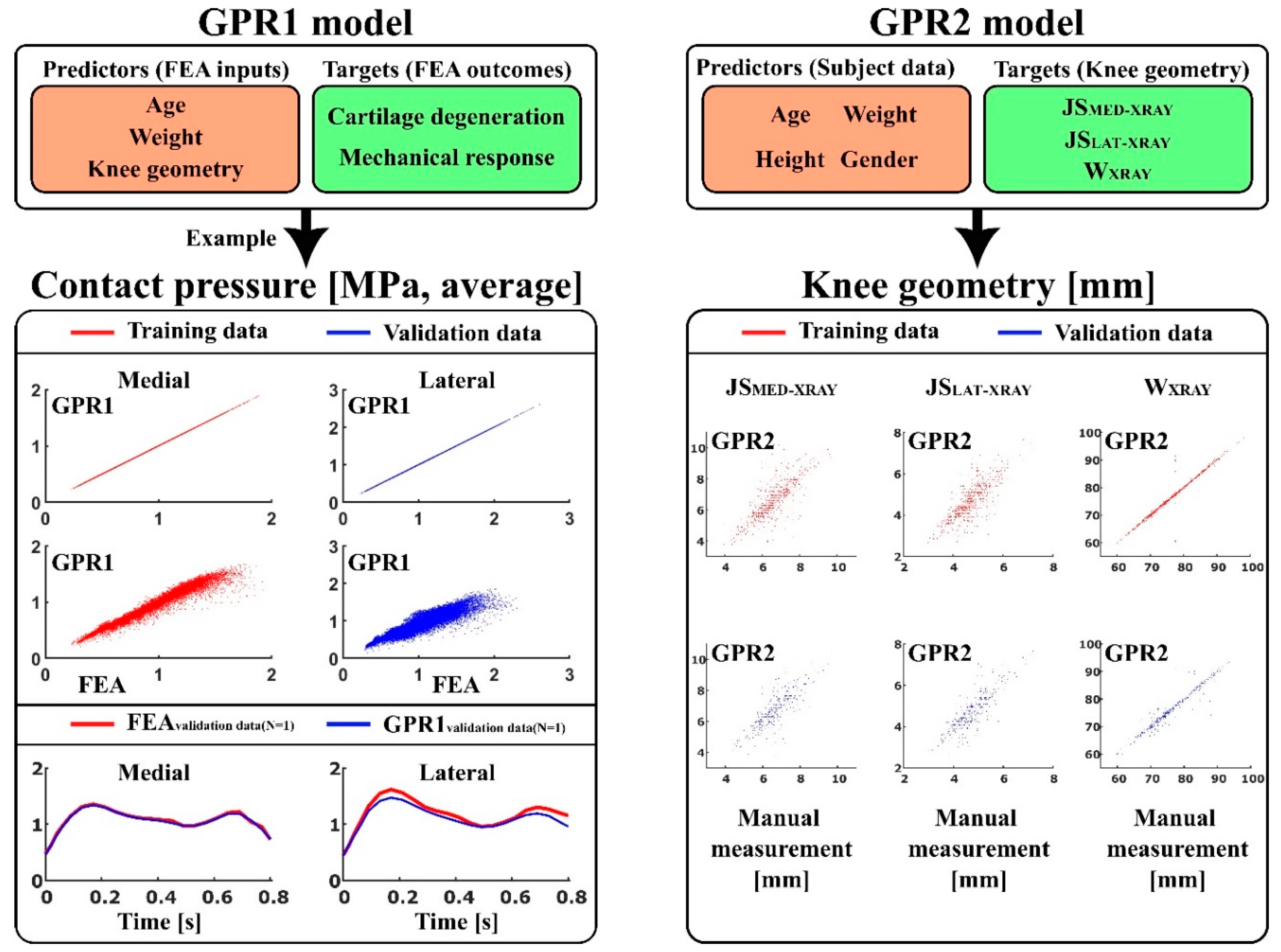


| Training Data for GPR1 | AGE [years] | BMI [kg/m2] | JSMED-MRI [mm] | JSLAT-MRI [mm] |
|---|---|---|---|---|
| N = 2092 | 56.8 ± 6.1 | 27.6 ± 4.7 | 5.0 ± 1.0 | 6.0 ± 1.0 |
| Validation Data for GPR1 | AGE [years] | BMI [kg/m2] | JSMED-XRAY [mm] | JSLAT-XRAY [mm] |
| KL01 N = 845 | 56.3 ± 6.3 | 26.4 ± 4.4 | 4.7 ± 0.8 | 6.7 ± 1.1 |
| KL2 N = 114 | 57.8 ± 5.1 | 29.3 ± 4.8 | 4.6 ± 0.8 | 6.5 ± 1.2 |
| KL34 N = 97 | 59.3 ± 5.6 | 28.3 ± 3.9 | 4.6 ± 1.0 | 6.5 ± 1.4 |
| Training Data for GPR2 | AGE [years] | BMI [kg/m2] | JSMED-XRAY [mm] | JSLAT-XRAY [mm] |
| N = 1669 | 56.8 ± 6.1 | 27.8 ± 4.8 | 4.7 ± 0.9 | 6.7 ± 1.2 |
| Validation Data for GPR2 | AGE [years] | BMI [kg/m2] | JSMED-XRAY [mm] | JSLAT-XRAY [mm] |
| KL01 N = 303 | 56.1 ± 6.3 | 26.1 ± 4.2 | 4.7 ± 0.8 | 6.7 ± 1.1 |
| KL2 N = 39 | 56.9 ± 5.5 | 29.4 ± 4.6 | 4.7 ± 0.8 | 6.5 ± 1.1 |
| KL34 N = 33 | 58.5 ± 5.9 | 28.6 ± 4.2 | 4.6 ± 1.2 | 6.6 ± 1.4 |
| GPR1 Model (Training Data) | ||||
|---|---|---|---|---|
| Target Variable | Medial | Lateral | ||
| R2 | RMSE | R2 | RMSE | |
| Contact area [mm2] | 1.00 | 0.045 | 1.00 | 0.046 |
| Contact pressure [MPa, peak] | 1.00 | 0.0020 | 1.00 | 0.018 |
| Contact pressure [MPa, average] | 1.00 | 0.00077 | 1.00 | 0.00072 |
| Tensile stress [MPa, peak] | 1.00 | 0.0030 | 0.99 | 0.12 |
| Tensile stress [MPa, average] | 1.00 | 0.0015 | 1.00 | 0.00083 |
| Tensile strain [-, peak] | 0.97 | 0.0022 | 0.92 | 0.0035 |
| Tensile strain [-, average] | 0.98 | 0.00066 | 0.98 | 0.00076 |
| Shear stress [MPa, peak] | 1.00 | 0.0015 | 1.00 | 0.040 |
| Shear stress [MPa, average] | 1.00 | 0.00082 | 1.00 | 0.00067 |
| Shear strain [-, peak] | 0.98 | 0.0029 | 0.89 | 0.0049 |
| Shear strain [-, average] | 0.99 | 0.00064 | 0.99 | 0.00073 |
| Pore pressure [MPa, peak] | 1.00 | 0.0043 | 1.00 | 0.055 |
| Pore pressure [MPa, average] | 1.00 | 0.0021 | 1.00 | 0.0013 |
| Degeneration [mm3] | 1.00 | 0.031 | 1.00 | 0.070 |
| GPR1 Model (Validation Data) | ||||
| Target Variable | Medial | Lateral | ||
| R2 | RMSE | R2 | RMSE | |
| Contact area [mm2] | 0.98 | 2.67 | 0.80 | 16.46 |
| Contact pressure [MPa, peak] | 0.95 | 0.19 | 0.88 | 0.30 |
| Contact pressure [MPa, average] | 0.97 | 0.040 | 0.85 | 0.14 |
| Tensile stress [MPa, peak] | 0.92 | 0.44 | 0.82 | 0.67 |
| Tensile stress [MPa, average] | 0.94 | 0.14 | 0.83 | 0.33 |
| Tensile strain [-, peak] | 0.86 | 0.0046 | 0.66 | 0.0068 |
| Tensile strain [-, average] | 0.94 | 0.0012 | 0.88 | 0.0025 |
| Shear stress [MPa, peak] | 0.92 | 0.17 | 0.83 | 0.27 |
| Shear stress [MPa, average] | 0.95 | 0.058 | 0.85 | 0.13 |
| Shear strain [-, peak] | 0.88 | 0.0053 | 0.68 | 0.0084 |
| Shear strain [-, average] | 0.96 | 0.0015 | 0.89 | 0.0033 |
| Pore pressure [MPa, peak] | 0.94 | 0.49 | 0.79 | 0.86 |
| Pore pressure [MPa, average] | 0.96 | 0.19 | 0.86 | 0.36 |
| Degeneration [mm3] | 1.00 | 1.74 | 1.00 | 2.61 |
| GPR2 Model (Training Data) | ||
|---|---|---|
| Target Variable | R2 | RMSE |
| JSMED-Xray [mm] | 0.62 | 0.73 |
| JSLAT-Xray [mm] | 0.65 | 0.50 |
| WXray [mm] | 0.96 | 1.35 |
| GPR2 Model (Validation Data) | ||
| Target Variable | R2 | RMSE |
| JSMED-Xray [mm] | 0.67 | 0.67 |
| JSLAT-Xray [mm] | 0.72 | 0.47 |
| WXray [mm] | 0.90 | 2.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mononen, M.E.; Liukkonen, M.K.; Turunen, M.J. Machine Learning Model Trained with Finite Element Modeling Can Predict the Risk of Osteoarthritis: Data from the Osteoarthritis Initiative. Appl. Sci. 2024, 14, 9538. https://doi.org/10.3390/app14209538
Mononen ME, Liukkonen MK, Turunen MJ. Machine Learning Model Trained with Finite Element Modeling Can Predict the Risk of Osteoarthritis: Data from the Osteoarthritis Initiative. Applied Sciences. 2024; 14(20):9538. https://doi.org/10.3390/app14209538
Chicago/Turabian StyleMononen, Mika E., Mimmi K. Liukkonen, and Mikael J. Turunen. 2024. "Machine Learning Model Trained with Finite Element Modeling Can Predict the Risk of Osteoarthritis: Data from the Osteoarthritis Initiative" Applied Sciences 14, no. 20: 9538. https://doi.org/10.3390/app14209538
APA StyleMononen, M. E., Liukkonen, M. K., & Turunen, M. J. (2024). Machine Learning Model Trained with Finite Element Modeling Can Predict the Risk of Osteoarthritis: Data from the Osteoarthritis Initiative. Applied Sciences, 14(20), 9538. https://doi.org/10.3390/app14209538






