Cationic Lipid Derived from a Basic Amino Acid: Design and Synthesis
Abstract
:1. Introduction
2. Materials and Methods
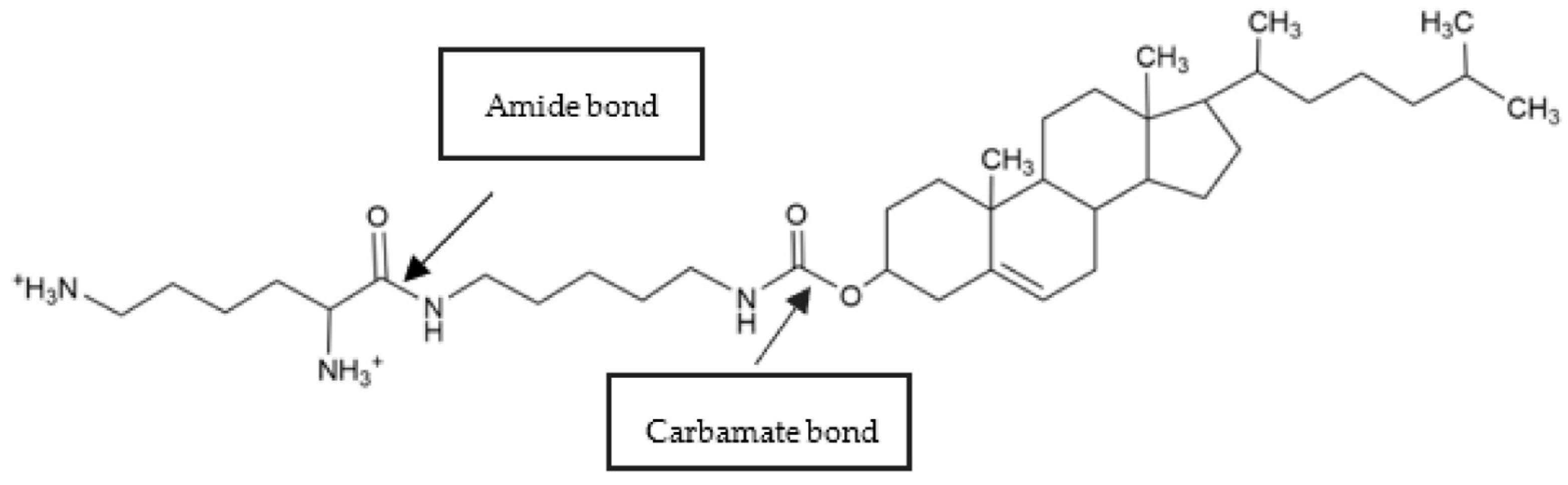
2.1. Design of the Novel CL: Cholesteryl–Cadaverine–Lysine (CholCadLys)
- Structural Conformity: The CholCadLys lipid should conform to the structural scheme consisting of a hydrophobic region, a linker, and a hydrophilic region, the main components of an amphipathic lipid.
- Natural Compatibility: The components used to create the CholCadLys lipid should be molecules that are naturally found as constituents and/or metabolites within eukaryotic cells.
- A steroid for the lipid bilayer: Cholesteryl chloroformate (Sigma-Aldrich, St. Louis, MO, USA) was chosen for its ability to integrate into lipid bilayers.
- A small primary amine as a linker: Cadaverine (Sigma-Aldrich) was selected to function as a connector between the hydrophobic body and the hydrophilic head due to its small size and primary amine group.
- A basic amino acid for the polar head: L-lysine (Sigma-Aldrich) was selected to confer two positive charges to the hydrophilic region, enhancing its ability to associate with DNA.
2.2. Synthesis and Purification of CholCadLys Lipid
2.3. Characterization of CholCadLys Lipid
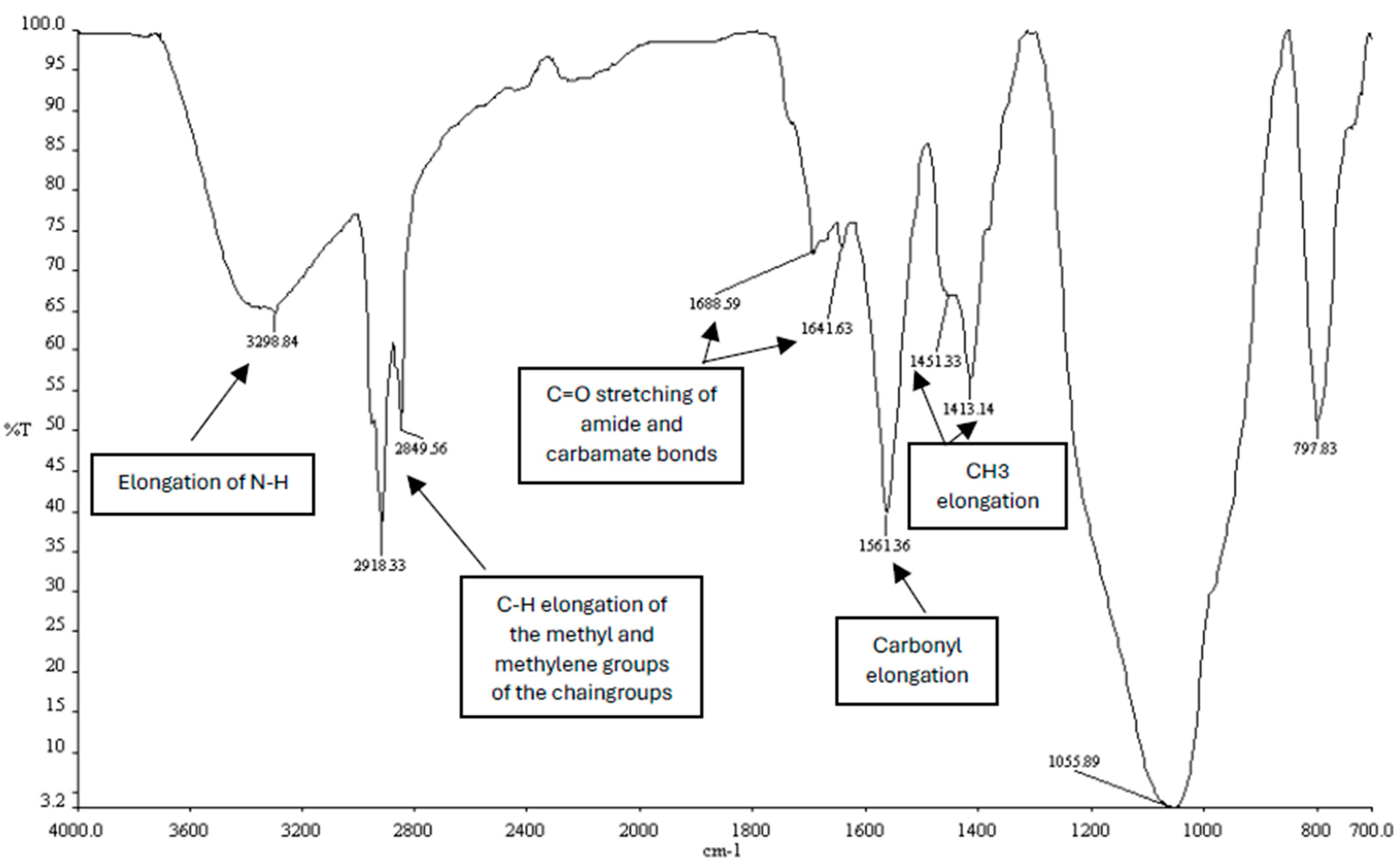
2.3.1. Infrared Spectroscopy
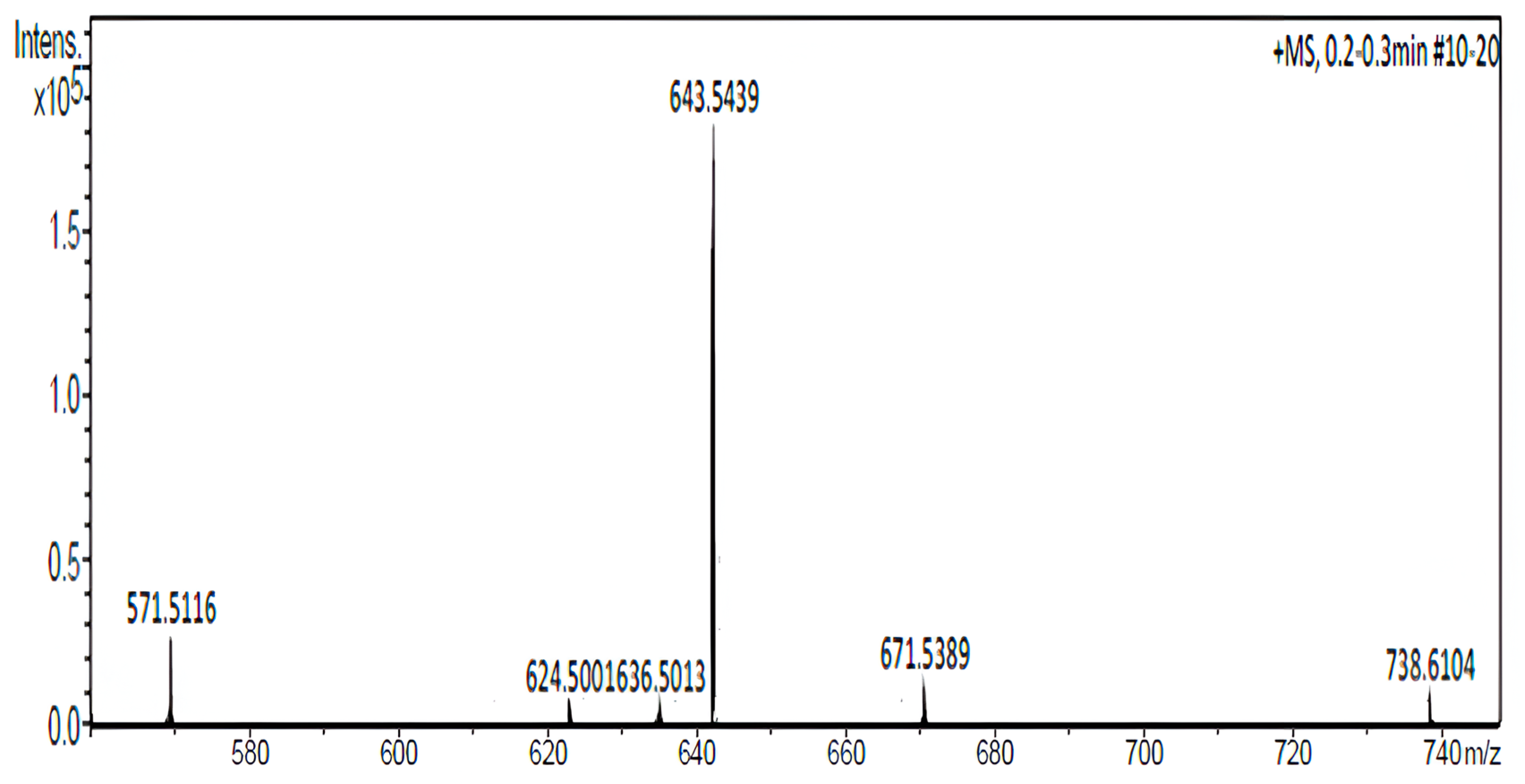
2.3.2. Mass Spectrometry
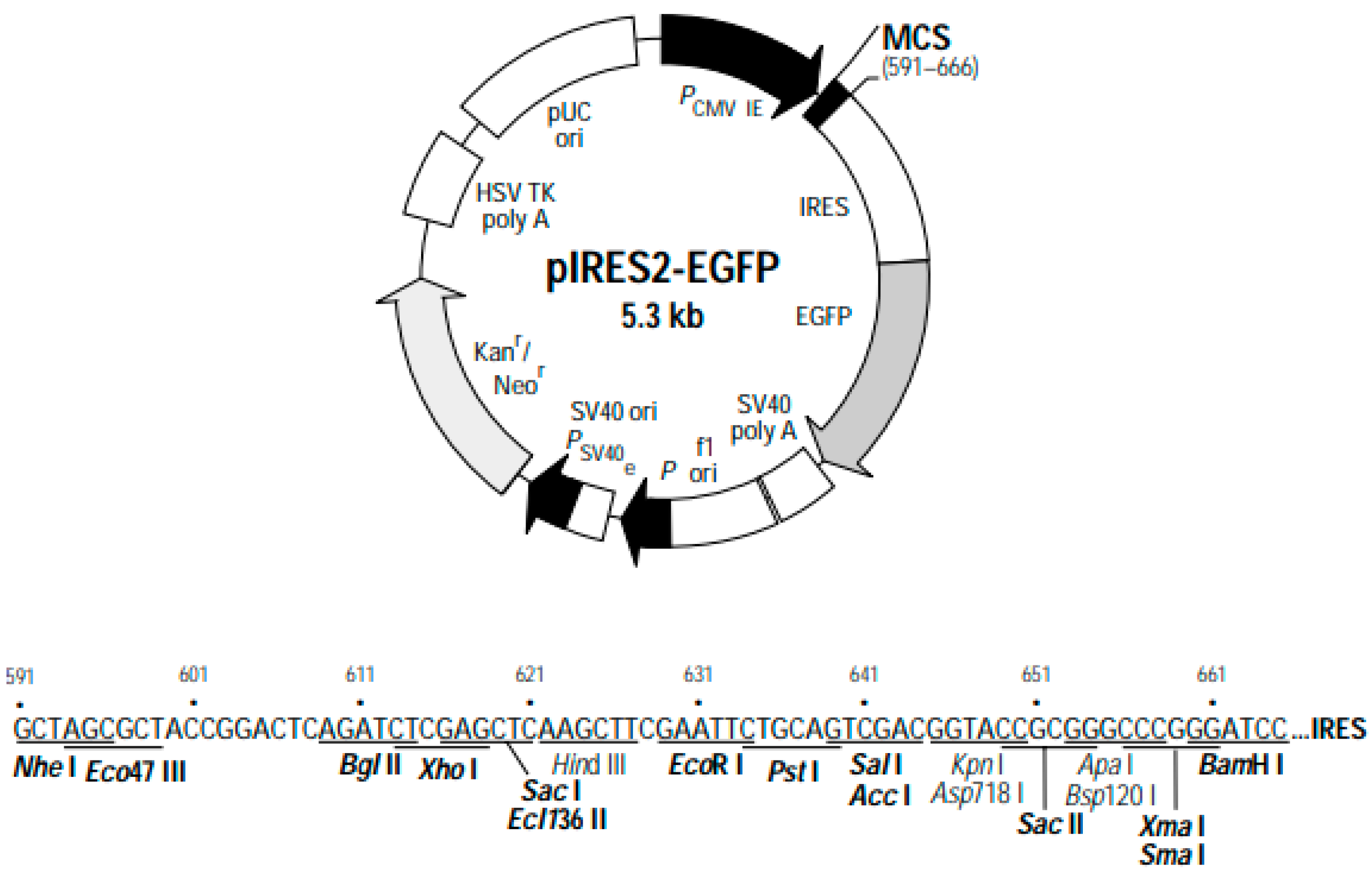
2.4. Isolation and Purification of Plasmid DNA (pDNA)
2.5. Cationic Liposome Formation
2.6. Formation of Lipoplexes
2.7. Characterization of Liposomes and Lipoplexes
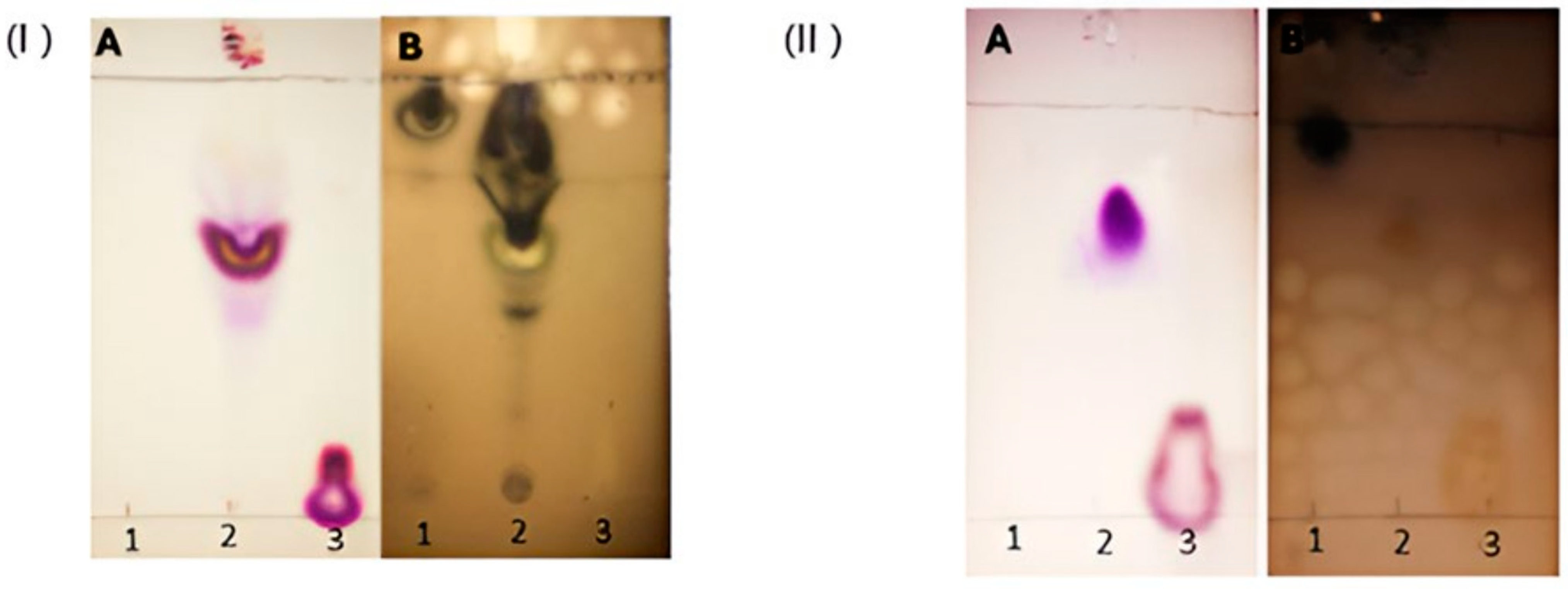
2.7.1. Characterization of Lipoplexes by Agarose Gel Electrophoresis
2.7.2. Transmission Electron Microscopy (TEM)
2.7.3. Nanoparticle Tracking Analysis (NTA)
2.8. Transfection into the Eukaryotic Cell Line
2.9. Determination of Cell Viability Post-Transfection
3. Results
3.1. The Synthesis and Purification of CholCadLys Lipid Enables the High-Yield Formation of Cationic Lipids
3.2. The Elongation of the Carbonyl (C=O) Bond of the Amide and Carbamate Bond Is a Structural Feature of the Cholcadlys Lipid
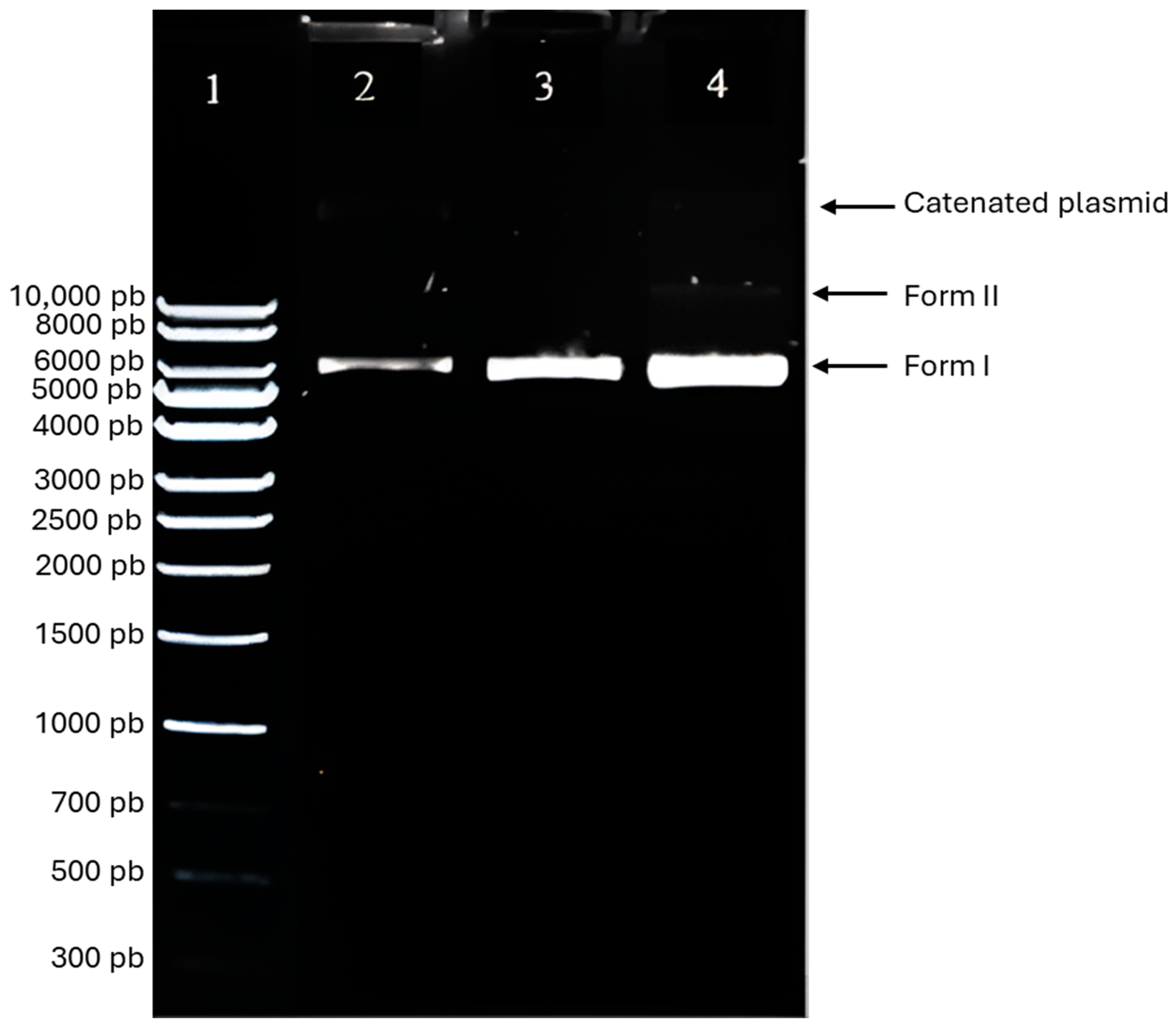
3.3. The Extraction and Characterization of pIRES2-EGFP Plasmid DNA Confirm Its Molecular Integrity
3.4. Formation of Stable Liposomes with Different Molar Ratios of Cholesterol and DOPE as Helper Lipids
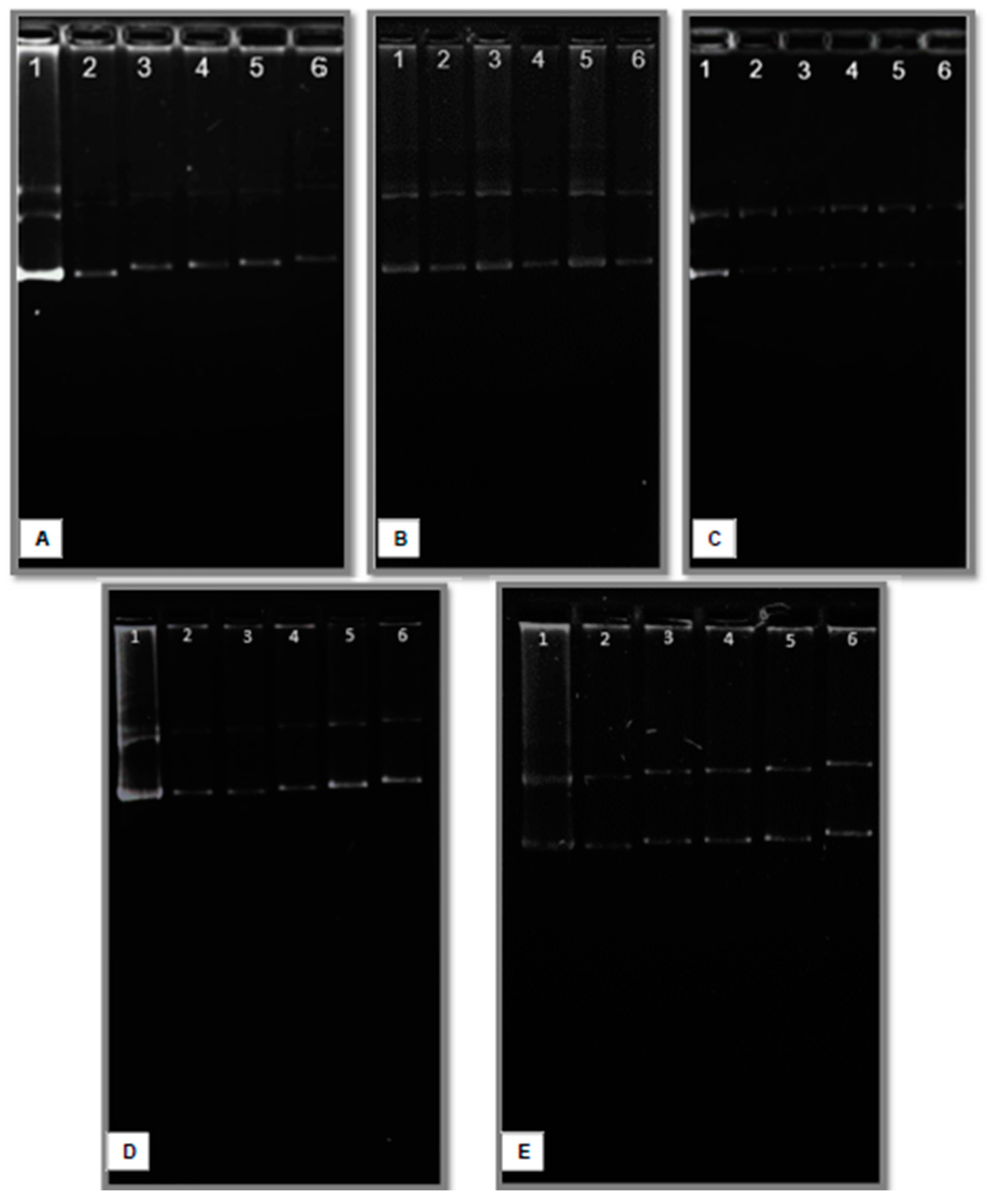
3.5. Electrophoresis Reveals pDNA Interactions with Liposomes
3.6. TEM Analysis Reveals Size and Morphology for Transfection
3.7. Comparative Analysis of Size and Concentration of Liposomes and Lipoplexes for Transfection Applications
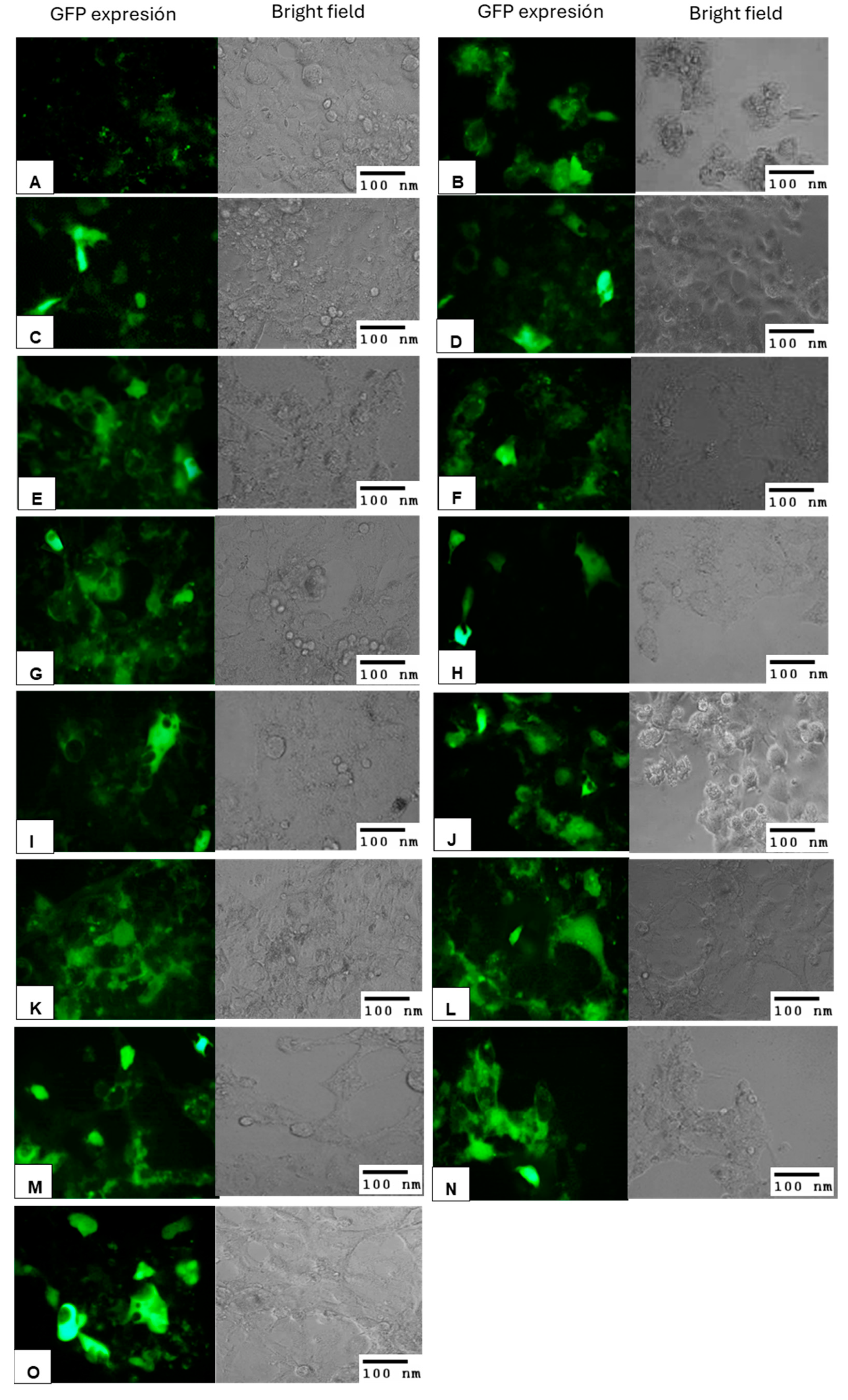
3.8. Lipid Mixtures Containing DOPE as a Helper Lipid Increase Transfection Efficiency
3.9. Lipid Mixtures Containing CholCadLys Do Not Exhibit Cytotoxic Effects in Cell Culture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- High, K.A.; Roncarolo, M.G. Gene Therapy. N. Engl. J. Med. 2019, 381, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.C.; Wang, Z.L.; Xu, T.; He, Z.Y.; Wei, Y.Q. The approved gene therapy drugs worldwide: From 1998 to 2019. Biotechnol. Adv. 2020, 40, 107502. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Rivas-garcía, L.; Baptista, P.V.; Fernandes, A.R. Gene therapy in cancer treatment: Why go nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Afonin, K.A.; Chandler, M. Aptamers as Modular Components of Therapeutic Nucleic Acid Nanotechnology. 2021, pp. 825–882. Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003122005-34/aptamers-modular-components-therapeutic-nucleic-acid-nanotechnology-martin-panigaj-brittany-johnson-weina-ke-jessica-mcmillan-ekaterina-goncharova-morgan-chandler-kirill-afonin (accessed on 28 May 2024).
- Gupta, A.; Andresen, J.L.; Manan, R.S.; Langer, R. Nucleic acid delivery for therapeutic applications. Adv. Drug Deliv. Rev. 2021, 178, 113834. [Google Scholar] [CrossRef]
- Luthra, R.; Kaur, S.; Bhandari, K. Applications of CRISPR as a potential therapeutic. Life Sci. 2021, 284, 119908. [Google Scholar] [CrossRef]
- Kaneda, Y. Gene Therapy: A Battle Against Biological Barriers. Curr. Mol. Med. 2005, 1, 493–499. [Google Scholar] [CrossRef]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef]
- Ibáñez, M.; Oseguera, B. Nanoliposomas catiónicos como vehículos para terapia génica. In Nanobiotecnología: Fundamentos y Perspectivas Tecnología de Frontera para Resolver los Problemas del Mañana; Academica: Ciudad de México, México, 2016; pp. 380–910. ISBN 3841752705. [Google Scholar]
- Zu, H.; Gao, D. Non-viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wang, Y.; Gong, Y.; Lin, X.; Zhao, Y.; Zhi, D.; Zhou, Q.; Zhang, S. Correlation of the cytotoxic effects of cationic lipids with their headgroups. Toxicol. Res. 2018, 7, 473–479. [Google Scholar] [CrossRef]
- Yin, H.; Kauffman, K.J.; Anderson, D.G. Delivery technologies for genome editing. Nat. Rev. Drug Discov. 2017, 16, 387–399. [Google Scholar] [CrossRef]
- Sayed, N.; Allawadhi, P.; Khurana, A.; Singh, V.; Navik, U.; Pasumarthi, S.K.; Khurana, I.; Banothu, A.K.; Weiskirchen, R.; Bharani, K.K. Gene therapy: Comprehensive overview and therapeutic applications. Life Sci. 2022, 294, 120375. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.H.; Zaman, M.; Ahmad, A.; Khan, R.; Mallhi, T.H.; Hasan, M.M.; Khan, Y.H.; Hafeez, S.; Massoud, E.E.S.; Rahman, M.H.; et al. Appraisal for the Potential of Viral and Nonviral Vectors in Gene Therapy: A Review. Genes 2022, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Sainz-ramos, M.; Gallego, I.; Villate-beitia, I.; Zarate, J.; Maldonado, I.; Puras, G.; Pedraz, J.L. How Far Are Non-Viral Vectors to Come of Age and Reach Clinical Translation in Gene Therapy? Int. J. Mol. Sci. 2021, 22, 7545. [Google Scholar] [CrossRef]
- Kanvinde, S.; Kulkarni, T.; Deodhar, S.; Bhattacharya, D.; Dasgupta, A. Non-Viral Vectors for Delivery of Nucleic Acid Therapies for Cancer. BioTech 2022, 11, 6. [Google Scholar] [CrossRef]
- Ewert, K.K.; Scodeller, P.; Simón-Gracia, L.; Steffes, V.M.; Wonder, E.A.; Teesalu, T.; Safinya, C.R. Cationic Liposomes as Vectors for Nucleic Acid and Hydrophobic Drug Therapeutics. Pharmaceutics 2021, 13, 1365. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Agrawal, M.K. A historical perspective of liposomes-a bio nanomaterial. Mater. Today Proc. 2021, 45, 2963–2966. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.C.P.; Radaic, A.; Garcia-Fossa, F.; da Cruz-Höfling, M.A.; Vinolo, M.A.R.; de Jesus, M.B. The in vivo toxicological profile of cationic solid lipid nanoparticles. Drug Deliv. Transl. Res. 2020, 10, 34–42. [Google Scholar] [CrossRef]
- Thongbamrer, C.; Roobsoong, W.; Sattabongkot, J.; Opanasopit, P.; Yingyongnarongkul, B. ek Serum Compatible Spermine-Based Cationic Lipids with Nonidentical Hydrocarbon Tails Mediate High Transfection Efficiency. ChemBioChem 2022, 23, e202100672. [Google Scholar] [CrossRef]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef]
- Koynova, R.; Tenchov, B. Cationic Lipids: Molecular Structure/Transfection Activity Relationships and Interactions with Biomembranes. Top. Curr. Chem. 2010, 296, 51–93. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.; Nietupski, J.B.; Lee, E.R.; Siegel, C.S.; Rafter, P.W.; Rudginsky, S.A.; Chang, C.D.; Eastman, S.J.; Harris, D.J.; Scheule, R.K.; et al. Cationic Lipid Structure and Formulation Considerations for Optimal Gene Transfection of the Lung. J. Drug Target. 2000, 7, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Bai, Y.; Yang, J.; Cui, S.; Zhao, Y.; Chen, H.; Zhang, S. A review on cationic lipids with different linkers for gene delivery. Adv. Colloid Interface Sci. 2018, 253, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhi, D.; Zhang, S. Cationic Liposomes in Different Structural Levels for Gene Delivery. In Non-Viral Gene Therapy; In Tech Open: London, UK, 2011. [Google Scholar] [CrossRef]
- Paecharoenchai, O.; Niyomtham, N.; Apirakaramwong, A.; Ngawhirunpat, T.; Rojanarata, T.; Yingyongnarongkul, B.E.; Opanasopit, P. Structure relationship of cationic lipids on gene transfection mediated by cationic liposomes. AAPS PharmSciTech 2012, 13, 1302–1308. [Google Scholar] [CrossRef]
- Kim, B.K.; Hwang, G.B.; Seu, Y.B.; Choi, J.S.; Jin, K.S.; Doh, K.O. DOTAP/DOPE ratio and cell type determine transfection efficiency with DOTAP-liposomes. Biochim. Biophys. Acta—Biomembr. 2015, 1848, 1996–2001. [Google Scholar] [CrossRef]
- Ciccarone, V.; Anderson, D.; Lan, J.; Schifferli, K.; Jessee, J. DMRIE-C reagent for transfection of suspension cells and for RNA transfection. Focus 1995, 17, 84–86. [Google Scholar]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. DNA Transfection Mediated by Cationic Lipid Reagents. Cold Spring Harb. Protoc. 2019, 2019, pdb.prot095414. [Google Scholar] [CrossRef]
- Zhen, S.; Li, X. Liposomal delivery of CRISPR/Cas9. Cancer Gene Ther. 2019, 27, 515–527. [Google Scholar] [CrossRef]
- Yang, S.Y.; Zheng, Y.; Chen, J.Y.; Zhang, Q.; Zhao, D.; Han, D.; Chen, X. Comprehensive study of cationic liposomes composed of DC-Chol and cholesterol with different mole ratios for gene transfection. Colloids Surf. B Biointerfaces 2013, 101, 6–13. [Google Scholar] [CrossRef]
- Soenen, S.J.H.; Brisson, A.R.; De Cuyper, M. Addressing the problem of cationic lipid-mediated toxicity: The magnetoliposome model. Biomaterials 2009, 30, 3691–3701. [Google Scholar] [CrossRef]
- Cui, K.; Liang, D.; Sweedler, D. Compositions Comprising Cationic Amphiphiles and Colipids for Delivering Therapeutic Molecules. U.S. Patent 9,080,186, 15 December 2012. pp. 1–48. [Google Scholar]
- Jiang, T.; Gonzalez, K.M.; Cordova, L.E.; Lu, J. Nanotechnology-enabled gene delivery for cancer and other genetic diseases. Expert Opin. Drug Deliv. 2023, 20, 523. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Pan, C.; Yong, H.; Wang, F.; Bo, T.; Zhao, Y.; Ma, B.; He, W.; Li, M. Emerging non-viral vectors for gene delivery. J. Nanobiotechnol. 2023, 21, 272. [Google Scholar] [CrossRef] [PubMed]
- Simões, S.; Filipe, A.; Faneca, H.; Mano, M.; Penacho, N.; Düzgünes, N.; de Lima, M.P. Cationic liposomes for gene delivery. Expert Opin. Drug Deliv. 2005, 2, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Jang, W.Y.; Ko, Y.T. The Effect of Surface Charges on the Cellular Uptake of Liposomes Investigated by Live Cell Imaging. Pharm. Res. 2017, 34, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Tseu, G.Y.W.; Kamaruzaman, K.A. A Review of Different Types of Liposomes and Their Advancements as a Form of Gene Therapy Treatment for Breast Cancer. Molecules 2023, 28, 1498. [Google Scholar] [CrossRef]
- Ju, J.; Huan, M.L.; Wan, N.; Qiu, H.; Zhou, S.Y.; Zhang, B. Le Novel Cholesterol-Based Cationic Lipids as Transfecting Agents of DNA for Efficient Gene Delivery. Int. J. Mol. Sci. 2015, 16, 5666. [Google Scholar] [CrossRef]
- Shi, J.; Yu, S.; Zhu, J.; Zhi, D.; Zhao, Y.; Cui, S.; Zhang, S. Carbamate-linked cationic lipids with different hydrocarbon chains for gene delivery. Colloids Surf. B. Biointerfaces 2016, 141, 417–422. [Google Scholar] [CrossRef]
- Zhi, D.; Zhang, S.; Zhao, Y.; Cui, S.; Wang, B.; Chen, H.; Zhi, D.; Zhao, D.; Zhi, D.; Zhang, S.; et al. In Vitro Study of Carbamate-Linked Cationic Lipid for Gene Delivery Against Cervical Cancer Cells. Adv. Mater. Phys. Chem. 2013, 2, 229–232. [Google Scholar] [CrossRef]
- Liu, D.; Qiao, W.; Li, Z.; Cui, X.; Li, K.; Yu, L.; Yan, K.; Zhu, L.; Cheng, L. Carbamate-linked cationic lipids for gene delivery. Bioorg. Med. Chem. 2008, 16, 995–1005. [Google Scholar] [CrossRef]
- Vogel, A.; Tatchell, A.; Furnis, B.; Hannaford, A.; Smith, P. Vogel’s Textbook of Practical Organic Chemistry; Longman Scientific & Technical: London, UK, 1996. [Google Scholar]
- Coskun, O. Separation techniques: Chromatography. North. Clin. Istanbul 2016, 3, 156. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Murase, O.; Sugishita, K.; Yoneyama, S.; Akada, K.; Ueha, M.; Nakamura, A.; Kobayashi, S. Optical characterization of liposomes by right angle light scattering and turbidity measurement. Biochim. Biophys. Acta—Biomembr. 2000, 1467, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; de Kruijff, B.; Hope, M.J.; Nayar, R.; Schmid, S.L. Phospholipids and membrane transport. Can. J. Biochem. 1980, 58, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Karanth, H.; Murthy, R.S.R. pH-Sensitive liposomes-principle and application in cancer therapy. J. Pharm. Pharmacol. 2010, 59, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Hirsch-Lerner, D.; Zhang, M.; Eliyahu, H.; Ferrari, M.E.; Wheeler, C.J.; Barenholz, Y. Effect of “helper lipid” on lipoplex electrostatics. Biochim. Biophys. Acta—Biomembr. 2005, 1714, 71–84. [Google Scholar] [CrossRef]
- Ermilova, I.; Swenson, J. DOPC versus DOPE as a helper lipid for gene-therapies: Molecular dynamics simulations with DLin-MC3-DMA. Phys. Chem. Chem. Phys. 2020, 22, 28256–28268. [Google Scholar] [CrossRef]
- Nsairat, H.; Alshaer, W.; Odeh, F.; Esawi, E.; Khater, D.; Bawab, A.A.; El-Tanani, M.; Awidi, A.; Mubarak, M.S. Recent advances in using liposomes for delivery of nucleic acid-based therapeutics. OpenNano 2023, 11, 100132. [Google Scholar] [CrossRef]
- Dan, N.; Danino, D. Structure and kinetics of lipid–nucleic acid complexes. Adv. Colloid Interface Sci. 2014, 205, 230–239. [Google Scholar] [CrossRef]
- Lebrón, J.A.; Ostos, F.J.; López-lópez, M.; Moyá, M.L.; Sales, C.; García, E.; García-calderón, C.B.; García-calderón, M.; Peña-gómez, M.J.; Rosado, I.V.; et al. Metallo-Liposomes of Ruthenium Used as Promising Vectors of Genetic Material. Pharmaceutics 2020, 12, 482. [Google Scholar] [CrossRef]
- Crook, K.; McLachlan, G.; Stevenson, B.J.; Porteous, D.J. Plasmid DNA molecules complexed with cationic liposomes are protected from degradation by nucleases and shearing by aerosolisation. Gene Ther. 1996, 3, 834–839. [Google Scholar]
- dos Santos Rodrigues, B.; Banerjee, A.; Kanekiyo, T.; Singh, J. Functionalized Liposomal Nanoparticles for Efficient Gene Delivery System to Neuronal Cell Transfection. Int. J. Pharm. 2019, 566, 717. [Google Scholar] [CrossRef]
- Durymanov, M.; Reineke, J. Non-viral Delivery of Nucleic Acids: Insight Into Mechanisms of Overcoming Intracellular Barriers. Front. Pharmacol. 2018, 9, 971. [Google Scholar] [CrossRef]
- Du, Z.; Munye, M.M.; Tagalakis, A.D.; Manunta, M.D.I.; Hart, S.L. The Role of the Helper Lipid on the DNA Transfection Efficiency of Lipopolyplex Formulations. Sci. Rep. 2014, 4, 7107. [Google Scholar] [CrossRef] [PubMed]
- Colosimo, A.; Serafino, A.; Sangiuolo, F.; Di Sario, S.; Bruscia, E.; Amicucci, P.; Novelli, G.; Dallapiccola, B.; Mossa, G. Gene transfection efficiency of tracheal epithelial cells by DC-Chol–DOPE/DNA complexes. Biochim. Biophys. Acta—Biomembr. 1999, 1419, 186–194. [Google Scholar] [CrossRef]
- Mochizuki, S.; Kanegae, N.; Nishina, K.; Kamikawa, Y.; Koiwai, K.; Masunaga, H.; Sakurai, K. The role of the helper lipid dioleoylphosphatidylethanolamine (DOPE) for DNA transfection cooperating with a cationic lipid bearing ethylenediamine. Biochim. Biophys. Acta—Biomembr. 2013, 1828, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Betker, J.L.; Kullberg, M.; Gomez, J.; Anchordoquy, T.J. Cholesterol Domains Enhance Transfection. Ther. Deliv. 2013, 4, 453. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, E.S.; Nikkhah, M.; Hosseinkhani, S. Cholesterol-rich lipid-mediated nanoparticles boost of transfection efficiency, utilized for gene editing by CRISPR-Cas9. Int. J. Nanomed. 2019, 14, 4353–4366. [Google Scholar] [CrossRef] [PubMed]
- de Santis, A.; Scoppola, E.; Ottaviani, M.F.; Koutsioubas, A.; Barnsley, L.C.; Paduano, L.; D’Errico, G.; Krauss, I.R. Order vs. Disorder: Cholesterol and Omega-3 Phospholipids Determine Biomembrane Organization. Int. J. Mol. Sci. 2022, 23, 5322. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Stegmann, T.; Legendre, J.Y. Gene transfer mediated by cationic lipids: Lack of a correlation between lipid mixing and transfection. Biochim. Biophys. Acta—Biomembr. 1997, 1325, 71–79. [Google Scholar] [CrossRef]
- Zhi, D.; Zhang, S.; Wang, B.; Zhao, Y.; Yang, B.; Yu, S. Transfection efficiency of cationic lipids with different hydrophobic domains in gene delivery. Bioconjug. Chem. 2010, 21, 563–577. [Google Scholar] [CrossRef]
- Zhu, L.; Mahato, R.I. Lipid and polymeric carrier-mediated nucleic acid delivery. Expert Opin. Drug Deliv. 2010, 7, 1209. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, S.; Tang, K.C.; Raj, M. Amide Bond Activation of Biological Molecules. Molecules 2018, 23, 2615. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.J.M.; Topp, E.M. Release from polymeric prodrugs: Linkages and their degradation. J. Pharm. Sci. 2004, 93, 1962–1979. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.; Yarrarapu, S.N.S.; Dimri, M. Biochemistry, Cholesterol. StatPearls 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513326/ (accessed on 22 October 2024).
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and Strategies of Cationic Liposomes for Cancer Gene Therapy. Mol. Ther. Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef]
- Elsana, H.; Olusanya, T.O.B.; Carr-wilkinson, J.; Darby, S.; Faheem, A.; Elkordy, A.A. Evaluation of novel cationic gene based liposomes with cyclodextrin prepared by thin film hydration and microfluidic systems. Sci. Rep. 2019, 9, 15120. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, S.; Jiang, H.; Zhao, B.; Lv, H. Lipoplex morphologies and their influences on transfection efficiency in gene delivery. J. Control. Release 2007, 123, 184–194. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Gao, Y.; Zhu, J.; Wientjes, M.G.; Au, J.L.S. Relationships between Liposome Properties, Cell Membrane Binding, Intracellular Processing, and Intracellular Bioavailability. AAPS J. 2011, 13, 585. [Google Scholar] [CrossRef]
- Gaspar, R.; Coelho, F.; Silva, B.F.B. Lipid-Nucleic Acid Complexes: Physicochemical Aspects and Prospects for Cancer Treatment. Molecules 2020, 25, 5006. [Google Scholar] [CrossRef]
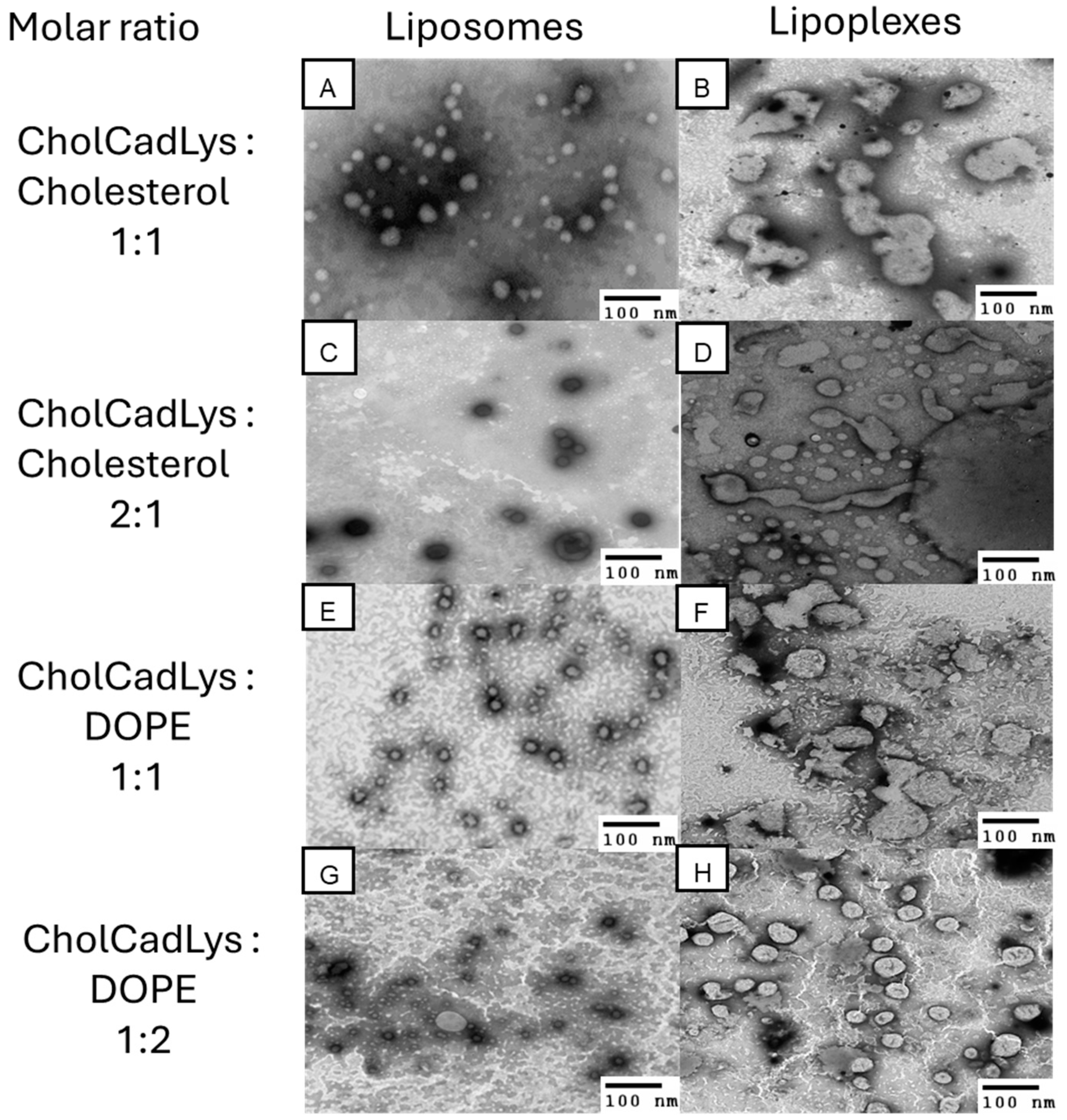
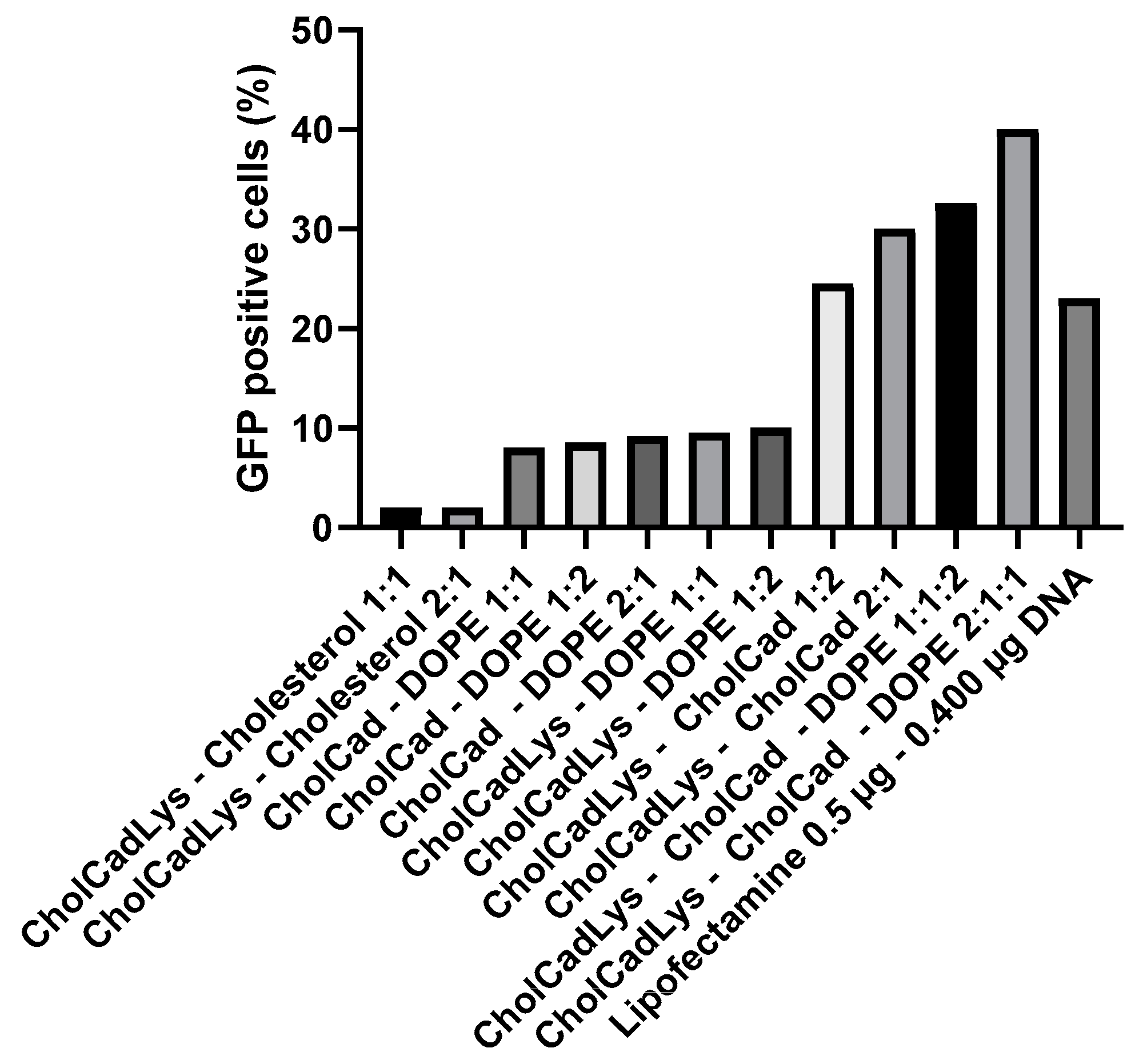

| Molar Ratios (CholCadLys–Helper Lipid) | Average Size (nm) | Concentration (Particles/mL) | |||
|---|---|---|---|---|---|
| Liposomes | Lipoplexes | Liposomes | Lipoplexes | ||
| Cholesterol | 1:1 | 136.6 | 132 | ||
| 2:1 | 114.8 | 92.3 | |||
| DOPE | 1:1 | 102.4 | 142 | ||
| 1:2 | 116.7 | 107.2 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo-Estupiñan, D.M.; Montaño-Samaniego, M.; Mora-Rodríguez, R.A.; Ibáñez-Hernández, M. Cationic Lipid Derived from a Basic Amino Acid: Design and Synthesis. Appl. Sci. 2024, 14, 10892. https://doi.org/10.3390/app142310892
Bravo-Estupiñan DM, Montaño-Samaniego M, Mora-Rodríguez RA, Ibáñez-Hernández M. Cationic Lipid Derived from a Basic Amino Acid: Design and Synthesis. Applied Sciences. 2024; 14(23):10892. https://doi.org/10.3390/app142310892
Chicago/Turabian StyleBravo-Estupiñan, Diana M., Mariela Montaño-Samaniego, Rodrigo A. Mora-Rodríguez, and Miguel Ibáñez-Hernández. 2024. "Cationic Lipid Derived from a Basic Amino Acid: Design and Synthesis" Applied Sciences 14, no. 23: 10892. https://doi.org/10.3390/app142310892
APA StyleBravo-Estupiñan, D. M., Montaño-Samaniego, M., Mora-Rodríguez, R. A., & Ibáñez-Hernández, M. (2024). Cationic Lipid Derived from a Basic Amino Acid: Design and Synthesis. Applied Sciences, 14(23), 10892. https://doi.org/10.3390/app142310892





