Analysis of the Existing Air Emissions Detection Methods for Stationary Pollution Sources Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Optical Method for Exhaust Gases Content Determination
2.2. Electrochemical Method for Exhaust Gases’ Content Determination
3. The Results of the Statistical Analysis of the Work of the Optical and Electrochemical Methods
3.1. Analysis of the Optical Method for Measuring the Concentration of Exhaust Gas
3.2. Analysis of an Electrochemical Method for Measuring the Concentration of Exhaust Gas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brauer, M.; Brook, J.R.; Christidis, T.; Chu, Y.; Crouse, D.L.; Erickson, A.; Hystad, P.; Li, C.; Martin, R.V.; Meng, J.; et al. Mortality-Air Pollution Associations in Low-Exposure Environments (MAPLE): Phase 1. Res. Rep. Health Eff. Inst. 2019, 2019, 203. [Google Scholar] [PubMed]
- Surianshah, S.; Hassan, S.; Liwan, A. The Effect of Green Technology Policy on Climate Change Awareness of Youth in Sabah, Malaysia. J. Adv. Res. Appl. Sci. Eng. Technol. 2024, 43, 42–51. [Google Scholar] [CrossRef]
- Cakaj, A.; Qorri, E.; Coulibaly, F.; De Marco, A.; Agathokleous, E.; Leca, Ș.; Sicard, P. Assessing Surface Ozone Risk to Human Health and Forests over Time in Poland. Atmos. Environ. 2023, 309, 119926. [Google Scholar] [CrossRef]
- The Paris Agreement. Available online: https://www.un.org/en/climatechange/paris-agreement (accessed on 20 August 2024).
- The European Green Deal. Available online: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_en (accessed on 20 August 2024).
- The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 20 August 2024).
- U.S. Environmental Protection Agency (EPA). Available online: https://www.epa.gov/ (accessed on 29 September 2024).
- EUR-Lex—Access to European Union Law. Available online: https://eur-lex.europa.eu/homepage.html?locale=en (accessed on 29 September 2024).
- United Nations Economic Commission for Europe (UNECE). Gothenburg Protocol to the UNECE Convention on Long-Range Transboundary Air Pollution. 1999. Available online: https://unece.org/fileadmin/DAM/env/lrtap/full%20text/1999%20Protocol%20to%20Abate.pdf (accessed on 29 September 2024).
- ISO 14001:2015; Environmental Management Systems—Requirements with Guidance for Use. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 12039; Stationary Source Emissions—Determination of Carbon Monoxide, Carbon Dioxide, and Oxygen—Performance Characteristics and Calibration of Automated Measuring Systems. International Organization for Standardization: Geneva, Switzerland, 2001.
- ISO 10473; Ambient Air—Measurement of Sulfur Dioxide—Ultraviolet Fluorescence Method. International Organization for Standardization: Geneva, Switzerland, 2000.
- Decree of the President of the Republic of Kazakhstan Dated 2 February 2023 No. 121 “On Approval of the Strategy for Achieving National Neutrality of the Republic of Kazakhstan Until 2060”. Available online: https://adilet.zan.kz/rus/docs/U2300000121 (accessed on 20 August 2024).
- Order of the Minister of Ecology, Geology and Natural Resources of the Republic of Kazakhstan dated 22 June 2021 No. 208. Registered with the Ministry of Justice of the Republic of Kazakhstan dated 22 July 2021 No. 23659. “On Approval of the Rules for Maintaining an Automated System for Monitoring Emissions into the Environment During Industrial Environmental Control”. Available online: https://adilet.zan.kz/eng/docs/V2100023659 (accessed on 20 August 2024).
- Khan, M.A.H.; Rao, M.V.; Li, Q. Recent Advances in Electrochemical Sensors for Detecting Toxic Gases: NO2, SO2 and H2S. Sensors 2019, 19, 905. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.; Guo, X.; Li, Y.; Li, L.; Pan, L. Recent Advances in Conductive Polymers-Based Electrochemical Sensors for Biomedical and Environmental Applications. Polymers 2024, 16, 1597. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, B.; Liang, J.; Liu, J.; Liang, B.; Li, G.; Long, Y.; Zhang, G.; Liu, H. Research on the Construction of Portable Electrochemical Sensors for Environmental Compounds Quality Monitoring. Mater. Today Adv. 2023, 17, 100340. [Google Scholar] [CrossRef]
- Tang, C.; Tan, J. Quasi-Targeted Analysis of Halogenated Organic Pollutants in Fly Ash, Soil, Ambient Air and Flue Gas Using Gas Chromatography-High Resolution Mass Spectrometry with Isotopologue Distribution Comparison and Predicted Retention Time Alignment. J. Chromatogr. A 2018, 1555, 74–88. [Google Scholar] [CrossRef]
- Lopes, A.F.; Fernandes, T.S.M.; do Nascimento, R.F. Barrier Discharge Ionization Detector in Gas Chromatography: A Review on Applications. Crit. Rev. Anal. Chem. 2021, 53, 614–633. [Google Scholar] [CrossRef]
- Zhao, Y.; Kreisberg, N.M.; Worton, D.R.; Teng, A.P.; Hering, S.V.; Goldstein, A.H. Development of an In Situ Thermal Desorption Gas Chromatography Instrument for Quantifying Atmospheric Semi-Volatile Organic Compounds. Aerosol Sci. Technol. 2012, 47, 258–266. [Google Scholar] [CrossRef]
- Smith, D.; Španěl, P.; Demarais, N.; Langford, V.S.; McEwan, M.J. Recent Developments and Applications of Selected Ion Flow Tube Mass Spectrometry (SIFT-MS). Mass Spectrom. Rev. 2024, 43, 1002–1025. [Google Scholar] [CrossRef]
- Lay, J.O.; Liyanage, R.; Gidden, J.A. The Development of a High-Resolution Mass Spectrometry Method for Ultra-Trace Analysis of Chlorinated Dioxins in Environmental and Biological Samples Including Viet Nam Era Veterans. Mass Spectrom. Rev. 2021, 40, 236–254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-F.; Gao, J.; Cai, Y.-J.; Wang, J.-J.; Pan, J. Real-Time Tracing VOCs, O3, and PM2.5 Emission Sources with Vehicle-Mounted Proton Transfer Reaction Mass Spectrometry Combined with Differential Absorption Lidar. Atmos. Pollut. Res. 2021, 12, 146–153. [Google Scholar] [CrossRef]
- Choi, W.; Yang, J.; Lee, H.; Van Roozendael, M.; Koo, J.-H.; Park, J.; Kim, D. Investigation of Aerosol Peak Height Effect on PBL and Volcanic Air Mass Factors for SO2 Column Retrieval from Space-Borne Hyperspectral UV Sensors. Remote Sens. 2020, 12, 1459. [Google Scholar] [CrossRef]
- Khan, S.; Newport, D.; Le Calvé, S. Gas Detection Using Portable Deep-UV Absorption Spectrophotometry: A Review. Sensors 2019, 19, 5210. [Google Scholar] [CrossRef] [PubMed]
- Barreto, D.; Rodrigues Silva, W.; Mizaikoff, B.; Petruci, J.F. Monitoring Ozone Using Portable Substrate-Integrated Hollow Waveguide-Based Absorbance Sensors in the Ultraviolet Range. ACS Meas. Sci. Au 2021, 2, 39–45. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Yao, Q.; Miao, Y.; Qiu, N. Quantitative Detection of SO2, H2S, and CS2 Based on Ultraviolet Absorption Spectrometry and Least Squares Algorithm. In Proceedings of the 2020 IEEE 4th Conference on Energy Internet and Energy System Integration (EI2), Wuhan, China, 30 October–1 November 2020; pp. 3905–3910. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, J.; Wang, X.; Cui, Z.; Li, Y.; Bian, C.; Chen, X. SO2 and SOF2 Gas Mixture Detection by Mid-Infrared Laser Photoacoustic Spectroscopy Based on IWOA-BP. IEEE Trans. Dielectr. Electr. Insul. 2024. [Google Scholar] [CrossRef]
- Dinh, T.-V.; Choi, I.-Y.; Son, Y.-S.; Kim, J.-C. A Review on Non-Dispersive Infrared Gas Sensors: Improvement of Sensor Detection Limit and Interference Correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.-P.; Li, W.-L. Development of a Multi-Component Infrared Gas Sensor Detection System. J. Phys. Conf. Ser. 2019, 1229, 012068. [Google Scholar] [CrossRef]
- Huang, Q.; Li, W.; Wu, T.; Ma, X.; Jiang, K.; Jin, X. Monoethanolamine-Enabled Electrochemical Detection of H2S in a Hydroxyl-Functionalized Ionic Liquid. Electrochem. Commun. 2018, 88, 93–96. [Google Scholar] [CrossRef]
- Balaish, M.; Rupp, J.L.M. Design of Triple and Quadruple Phase Boundaries and Chemistries for Environmental SO2 Electrochemical Sensing. J. Mater. Chem. A 2021, 9, 14691–14699. [Google Scholar] [CrossRef]
- Melios, C.; Panchal, V.; Edmonds, K.; Lartsev, A.; Yakimova, R.; Kazakova, O. Detection of Ultralow Concentration NO2 in Complex Environment Using Epitaxial Graphene Sensors. ACS Sens. 2018, 3, 1666–1674. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, C.; Xu, L.; Yu, Z.; Wang, D.; Jiang, L.; Jiang, X.; Gao, G. Conductive Polyaniline-Based Microwire Arrays for SO2 Gas Detection. ACS Appl. Mater. Interfaces 2023, 15, 38938–38945. [Google Scholar] [CrossRef]
- Braunger, M.L.; da Silva, E.A.; Fier, I.; Redon, N.; Duc, C. Optimizing Polyaniline-Based Gas Sensors for Hydrogen Sulfide Detection: The Crucial Role of Solvent Choice. Proceedings 2024, 97, 184. [Google Scholar] [CrossRef]
- Varlet, V.; Giuliani, N.; Palmiere, C.; Maujean, G.; Augsburger, M. Hydrogen Sulfide Measurement by Headspace-Gas Chromatography-Mass Spectrometry (HS-GC-MS): Application to Gaseous Samples and Gas Dissolved in Muscle. J. Anal. Toxicol. 2015, 39, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Lushozi, S.; Tshilongo, J.; Chimuka, L. Verification of Nitrous Oxide Primary Standard Gas Mixtures by Gas Chromatography and Cavity Ring-Down Spectroscopy for Ambient Measurements in South Africa. Accred. Qual. Assur. 2019, 24, 203–214. [Google Scholar] [CrossRef]
- Kirk, A.; Last, T.; Zimmermann, S. A Sensitive Gas Chromatography Detector Based on Atmospheric Pressure Chemical Ionization by a Dielectric Barrier Discharge. J. Chromatogr. A 2016, 1483, 120–126. [Google Scholar] [CrossRef]
- Vasquez, K.T.; Allen, H.M.; Crounse, J.D.; Praske, E.; Xu, L.; Noelscher, A.C.; Wennberg, P.O. Low-Pressure Gas Chromatography with Chemical Ionization Mass Spectrometry for Quantification of Multifunctional Organic Compounds in the Atmosphere. Atmos. Meas. Tech. 2018, 11, 6815–6832. [Google Scholar] [CrossRef]
- Risoluti, R.; Materazzi, S. Mass Spectrometry for Evolved Gas Analysis: An Update. Appl. Spectrosc. Rev. 2018, 54, 87–116. [Google Scholar] [CrossRef]
- Roberts, I.J.; Carpenter, L.J.; Shaw, M.D.; Langford, V.S. Selected Ion Flow Tube—Mass Spectrometry (SIFT-MS) Study of the Reactions of H3O+, NO+, and O2+ with a Range of Oxygenated Volatile Organic Carbons (OVOCs). Int. J. Mass Spectrom. 2022, 479, 116892. [Google Scholar] [CrossRef]
- Balsubramaniam, B.G.; Bekal, A.; Rajeshirke, Y.; Mitra, C. Ultraviolet Absorption Spectral Data Analysis for Flue Gas Detection. In Applied Industrial Optics 2021; Miller, G., Smith, A., Capraro, I., Majors, J., Eds.; OSA Technical Digest (Optica Publishing Group, 2021): Washington, DC, USA, 2021; paper F2A.1. [Google Scholar]
- Lu, K. Development of a UV-Based Remote Sensing Technology for Sulphur Dioxide Monitoring from Ship Emissions. Ph.D. Thesis, The Hong Kong Polytechnic University, Hong Kong, China, 2023. Available online: https://theses.lib.polyu.edu.hk/handle/200/12306 (accessed on 18 November 2024).
- Khan, M.A.H.; Thomson, B.; Debnath, R.; Rani, A.; Motayed, A.; Rao, M.V. Reliable Anatase-Titania Nanoclusters Functionalized GaN Sensor Devices for UV-Assisted NO2 Gas-Sensing in ppb Level. Nanotechnology 2019, 31, 155504. [Google Scholar] [CrossRef]
- da Silveira Petruci, J.F.; Wilk, A.; Cardoso, A.A.; Mizaikoff, B. A Hyphenated Preconcentrator-Infrared-Hollow-Waveguide Sensor System for N2O Sensing. Sci. Rep. 2018, 8, 5909. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, C.; Ci, G.; Zhao, X.; Lv, J.; Liang, J.; Ming, A.; Wei, F.; Mao, C. Development of High-Precision NO2 Gas Sensor Based on Non-Dispersive Infrared Technology. Sensors 2024, 24, 4146. [Google Scholar] [CrossRef]
- Zhalmagambetova, U.; Radelyuk, I.; Omerbayeva, D.; Neftissov, A.; Kazambayev, I. Environmental Monitoring in Kazakhstani Context—State-of-the-Art and Challenges for Industrial Region. Vestn. Toraygyrov Univ. Energy Ser. 2024, 1, 82–93. [Google Scholar] [CrossRef]
- Biloshchytskyi, A.; Kuchanskyi, O.; Andrashko, Y.; Yedilkhan, D.; Neftissov, A.; Biloshchytska, S.; Amirgaliyev, B.; Vatskel, V. Reducing Outdoor Air Pollutants through a Moss-Based Biotechnological Purification Filter in Kazakhstan. Urban Sci. 2023, 7, 104. [Google Scholar] [CrossRef]
- Government of the Republic of Kazakhstan. Approval of the Best Available Techniques Reference Document “Combustion of Fuel in Large Installations for Energy Production.” Resolution No. 23 dated 23 January 2024. Available online: https://adilet.zan.kz/rus/docs/P2400000023 (accessed on 26 October 2024).
- EN 14181; Stationary source emissions—Quality Assurance of Automated Measuring Systems (AMS). European Committee for Standardization: Brussels, Belgium, 2014.
- Scott, C. Introduction to Optics and Optical Imaging; IEEE: Piscataway, NJ, USA, 1998. [Google Scholar] [CrossRef]
- Idemen, M.M. Discontinuities in the Electromagnetic Field; IEEE Press Series on Electromagnetic Wave Theory; Wiley-IEEE Press: Hoboken, NJ, USA, 2011. [Google Scholar]
- Jahnke, J.A. Continuous Emission Monitoring, 3rd ed.; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- SGK-510 SOLER. Available online: https://promanalyt.kz/en/catalog/control-cabinets/portable-and-stationary-gas-analyzers/stationary-oxygen-analyzers/sgk-510-soler (accessed on 20 August 2024).
- Promanalyt. Polar Portable and Stationary Gas Analyzers for Controlling Industrial Emissions. Retrieved 18 November 2024. Available online: https://promanalyt.kz/en/catalog/control-cabinets/portable-and-stationary-gas-analyzers/to-control-industrial-emissions/polar (accessed on 20 August 2024).
- ISO 13752:1998; Environmental Management—Assessment of the Environmental Impact of Activities—Expert Judgement. International Organization for Standardization: Geneva, Switzerland, 1998. Available online: https://www.iso.org/standard/2821.html (accessed on 29 September 2024).
- Breusch, T.S.; Pagan, A.R. A Simple Test for Heteroscedasticity and Random Coefficient Variation. Econometrica 1979, 47, 1287–1294. [Google Scholar] [CrossRef]
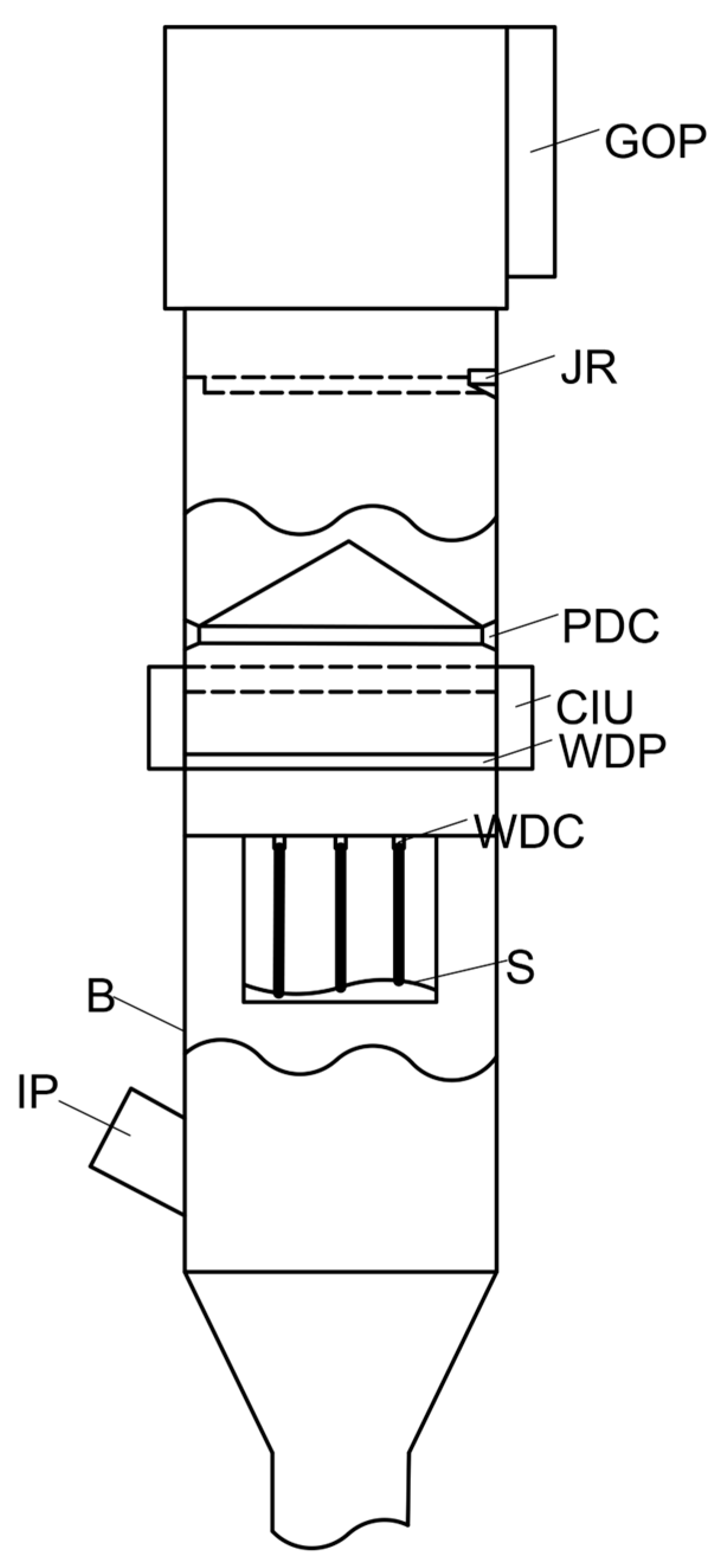
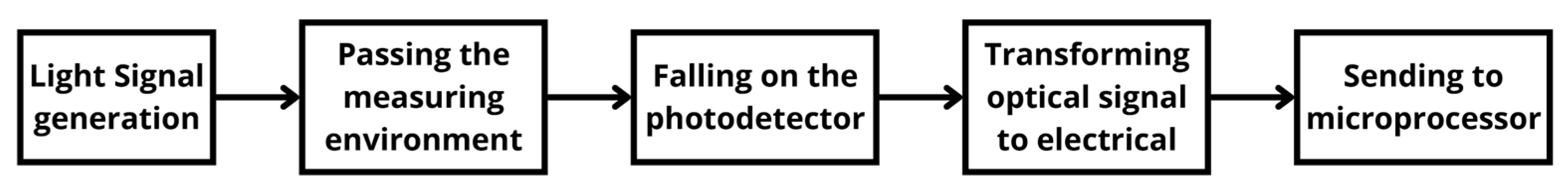

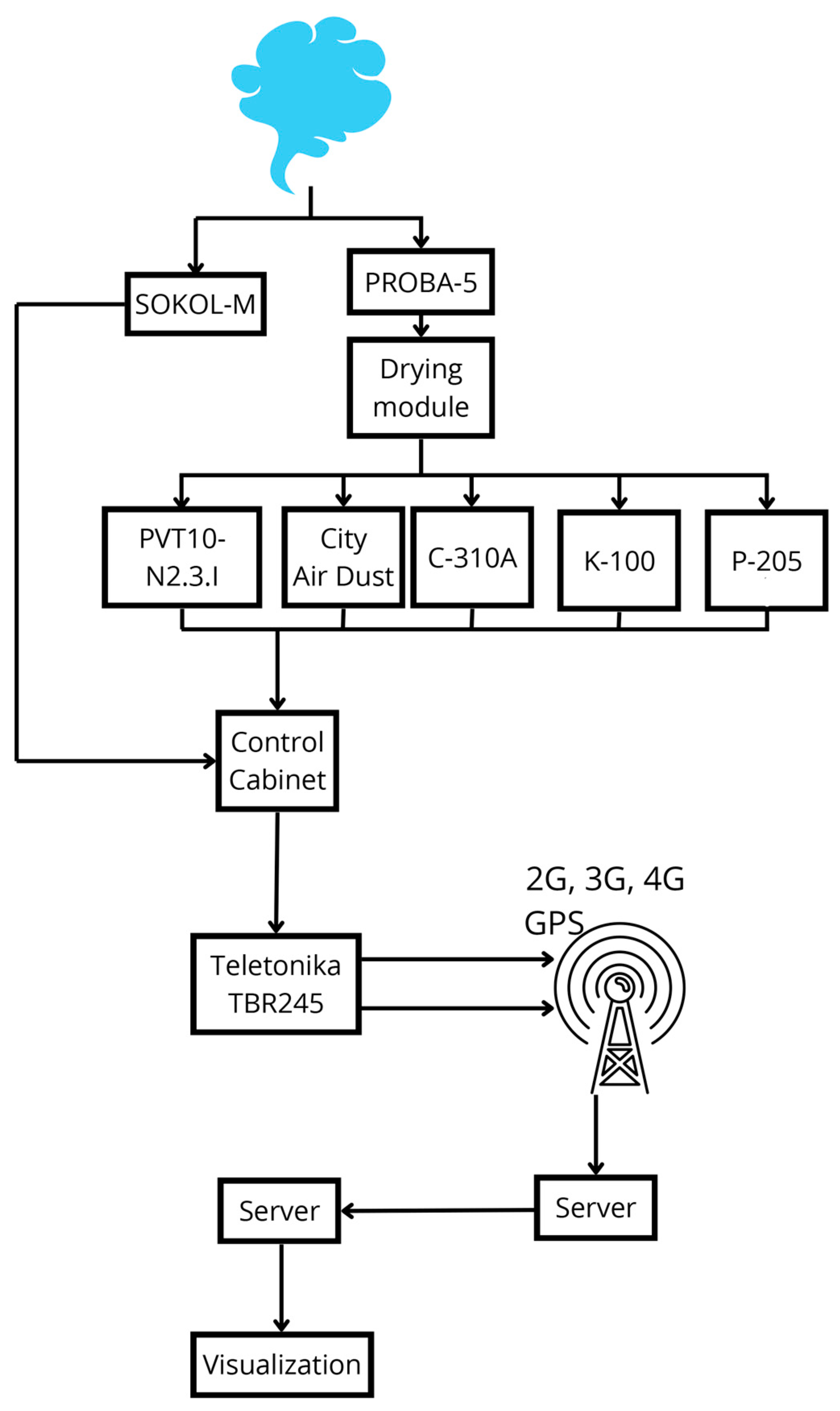

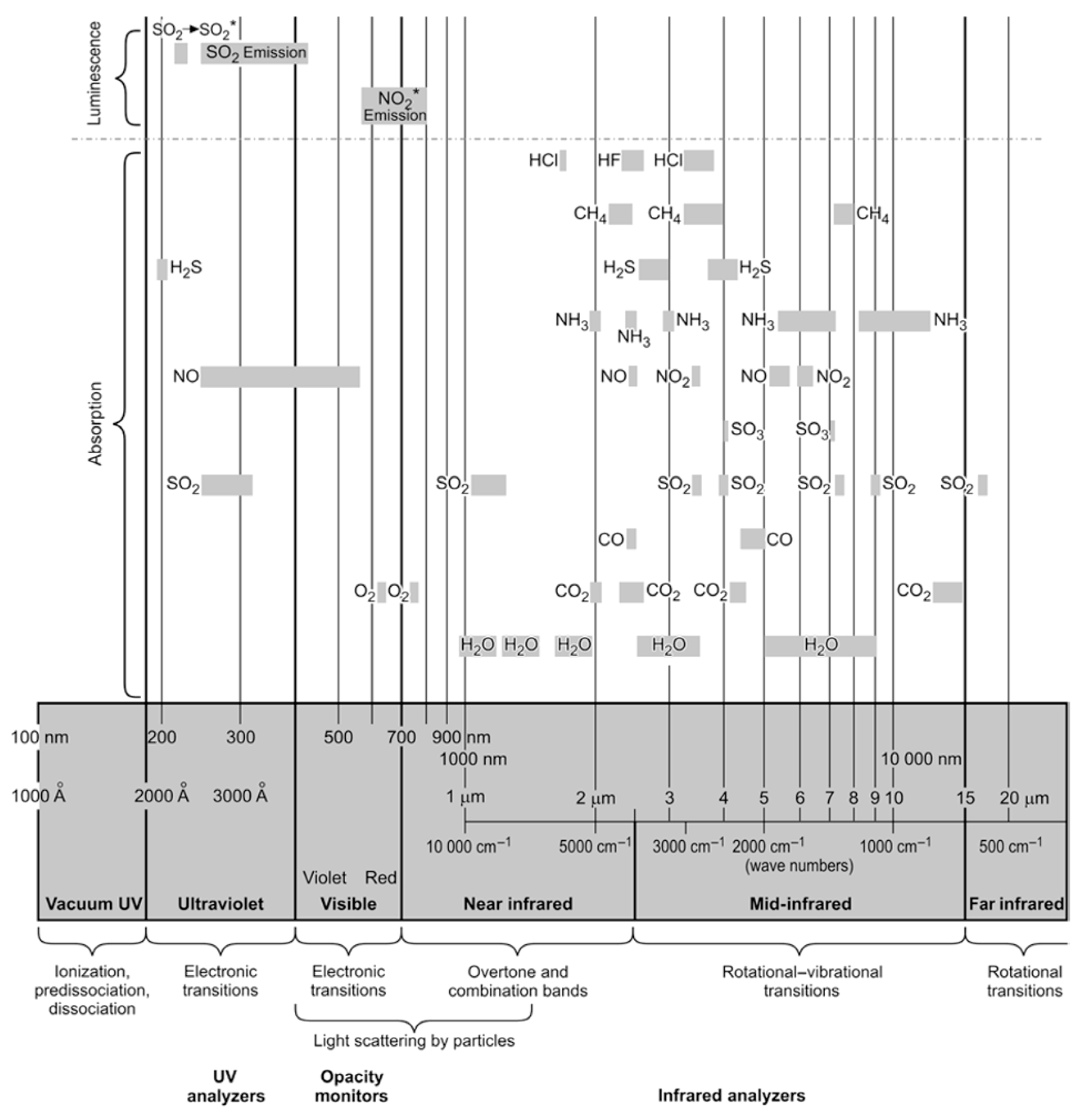


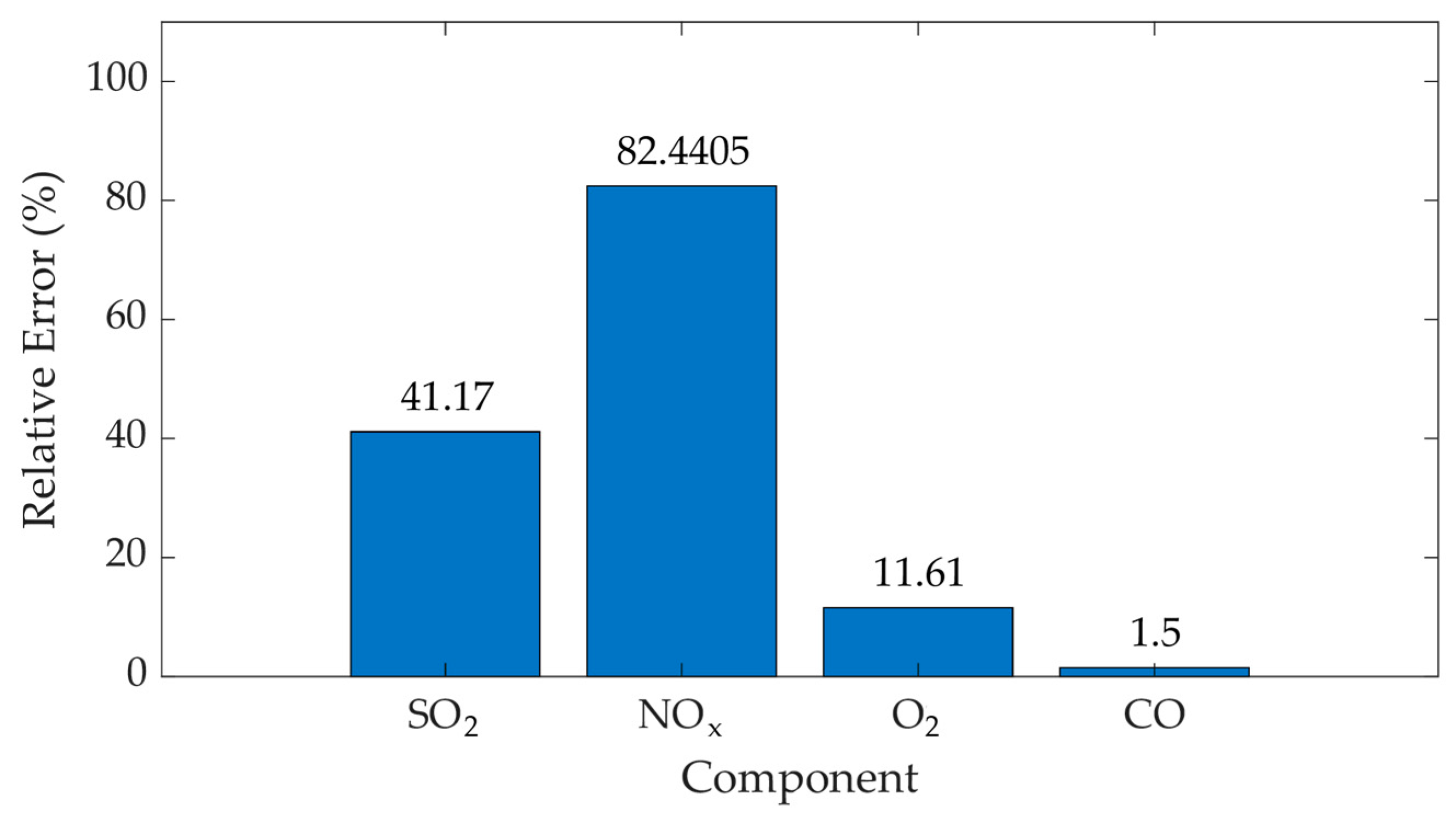
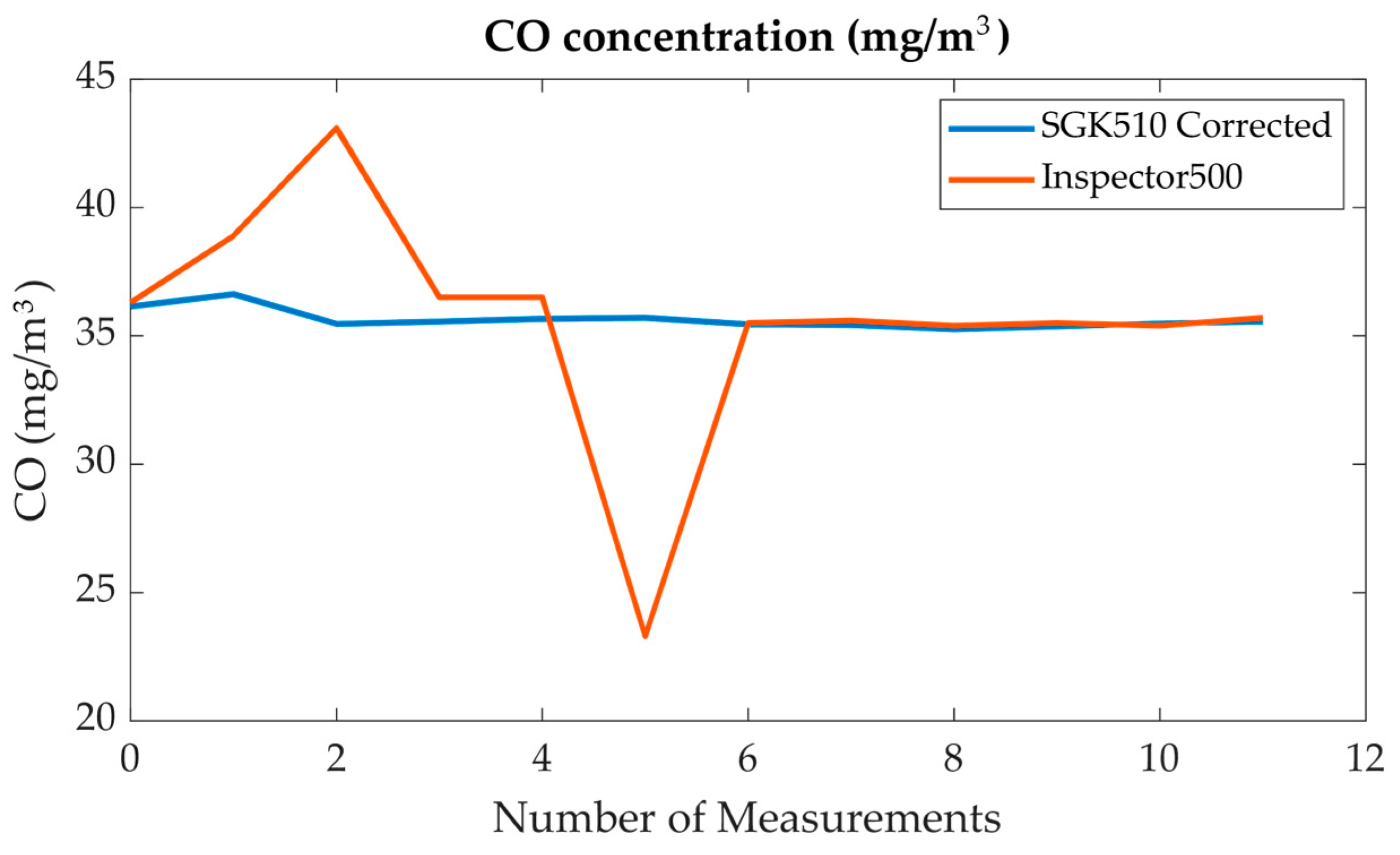
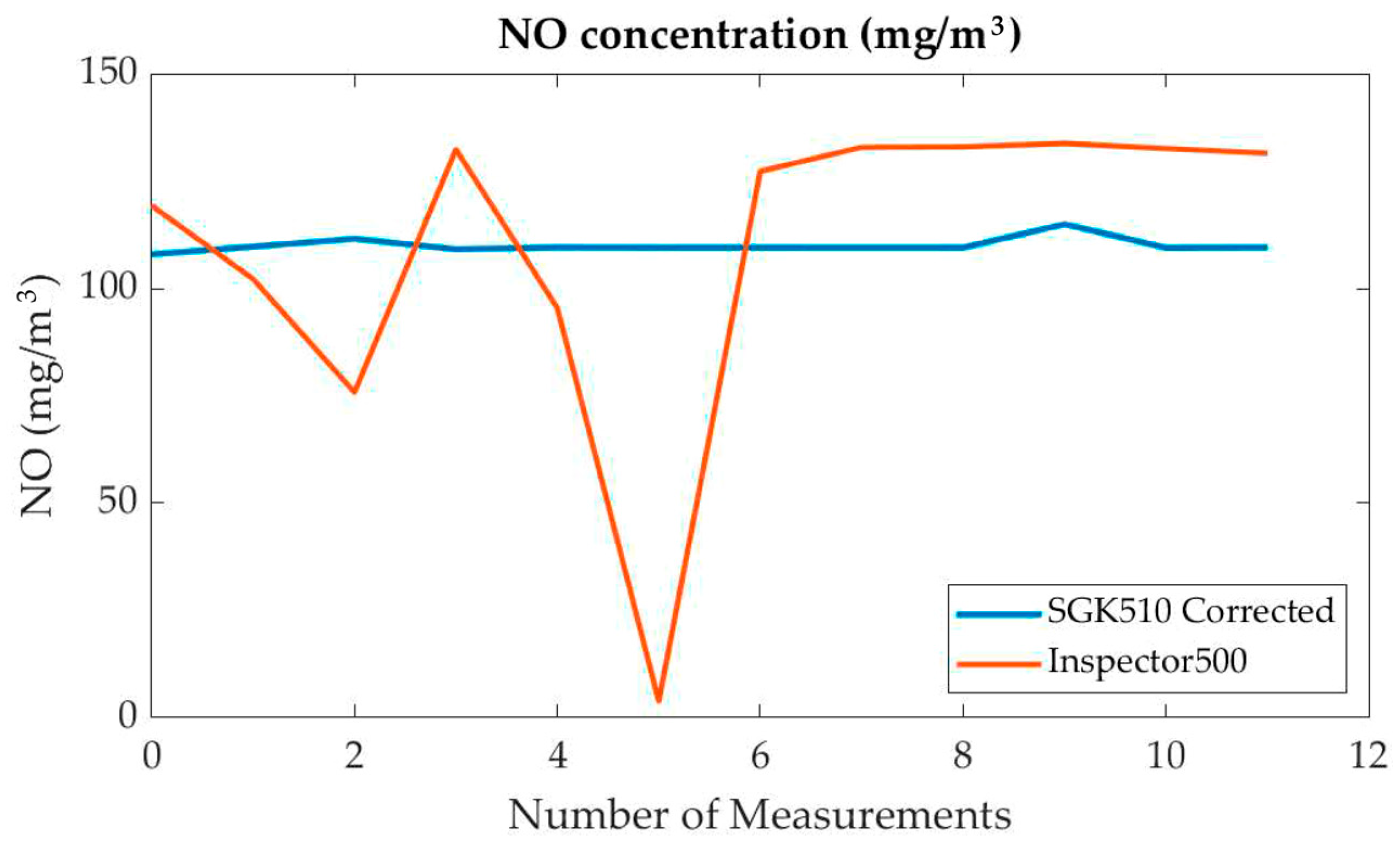
| Time (19 June 2024) | SO2 (mg/m3) (I500) | SO2 (mg/m3) (AMS) | NO (mg/m3) (I500) | NO (mg/m3) (AMS) | NO2 (mg/m3) (I500) | NO2 (mg/m3) (AMS) |
|---|---|---|---|---|---|---|
| 11:00 | 228.2 | 218.3 | 119.3 | 87.0 | 4.3 | 535.3 |
| 11:20 | 246.5 | 285.9 | 102.2 | 64.7 | 8.5 | 398.1 |
| 11:40 | 118.6 | 171.5 | 75.8 | 41.9 | 10.2 | 258 |
| 12:00 | 321.0 | 277.5 | 132.4 | 72.2 | 4.8 | 444.5 |
| 12:20 | 316.2 | 336.8 | 95.4 | 66.9 | 6.0 | 411.9 |
| 12:40 | 133.2 | 302.8 | 3.8 | 67.6 | 4.0 | 415.8 |
| 13:00 | 375.1 | 387.1 | 127.3 | 67.4 | 3.2 | 415 |
| 13:20 | 405.4 | 416.7 | 132.9 | 67.8 | 3.5 | 417 |
| 13:40 | 416.2 | 419.4 | 133.0 | 67.5 | 4.2 | 415.6 |
| 14:00 | 419.7 | 414.6 | 133.8 | -0.4 | 4.0 | −2.3 |
| 14:20 | 430.8 | 442.2 | 132,6 | 68.2 | 4.2 | 419.7 |
| 14:40 | 432 | 446.4 | 131.5 | 67.1 | 4.2 | 412.7 |
| Time (19 June 2024) | NOx (mg/m3) (I500) | NOx (mg/m3) (AMS) | O2 (%) (I500) | O2 (%) (AMS) | CO (mg/m3) (I500) | CO (mg/m3) (AMS) |
|---|---|---|---|---|---|---|
| 11:00 | 165.5 | 139.3 | 16.7 | 16.3 | 36.3 | 16.7 |
| 11:20 | 148.2 | 166.6 | 15.1 | 16.0 | 38.9 | 19.3 |
| 11:40 | 115.3 | 115.9 | 15.1 | 17.7 | 43.1 | 13.0 |
| 12:00 | 183.9 | 151.2 | 15.3 | 16.7 | 36.5 | 13.5 |
| 12:20 | 135.7 | 176.2 | 15.9 | 15.9 | 36.5 | 14.1 |
| 12:40 | 10.4 | 176.1 | 19.7 | 15.9 | 23.3 | 14.3 |
| 13:00 | 174.8 | 177.8 | 15.4 | 15.9 | 35.5 | 12.9 |
| 13:20 | 182.5 | 178.1 | 15.1 | 15.9 | 35.6 | 12.8 |
| 13:40 | 183.6 | 174.1 | 15.1 | 16 | 35.4 | 11.9 |
| 14:00 | 184.5 | 174.8 | 15.2 | 16.1 | 35.5 | 12.5 |
| 14:20 | 183,2 | 179 | 15.3 | 15.9 | 35.4 | 13.1 |
| 14:40 | 181,6 | 176.8 | 15.1 | 15.9 | 35.7 | 13.5 |
| Component | RMD1 | RMD2 | S1 | S2 | f | ||
|---|---|---|---|---|---|---|---|
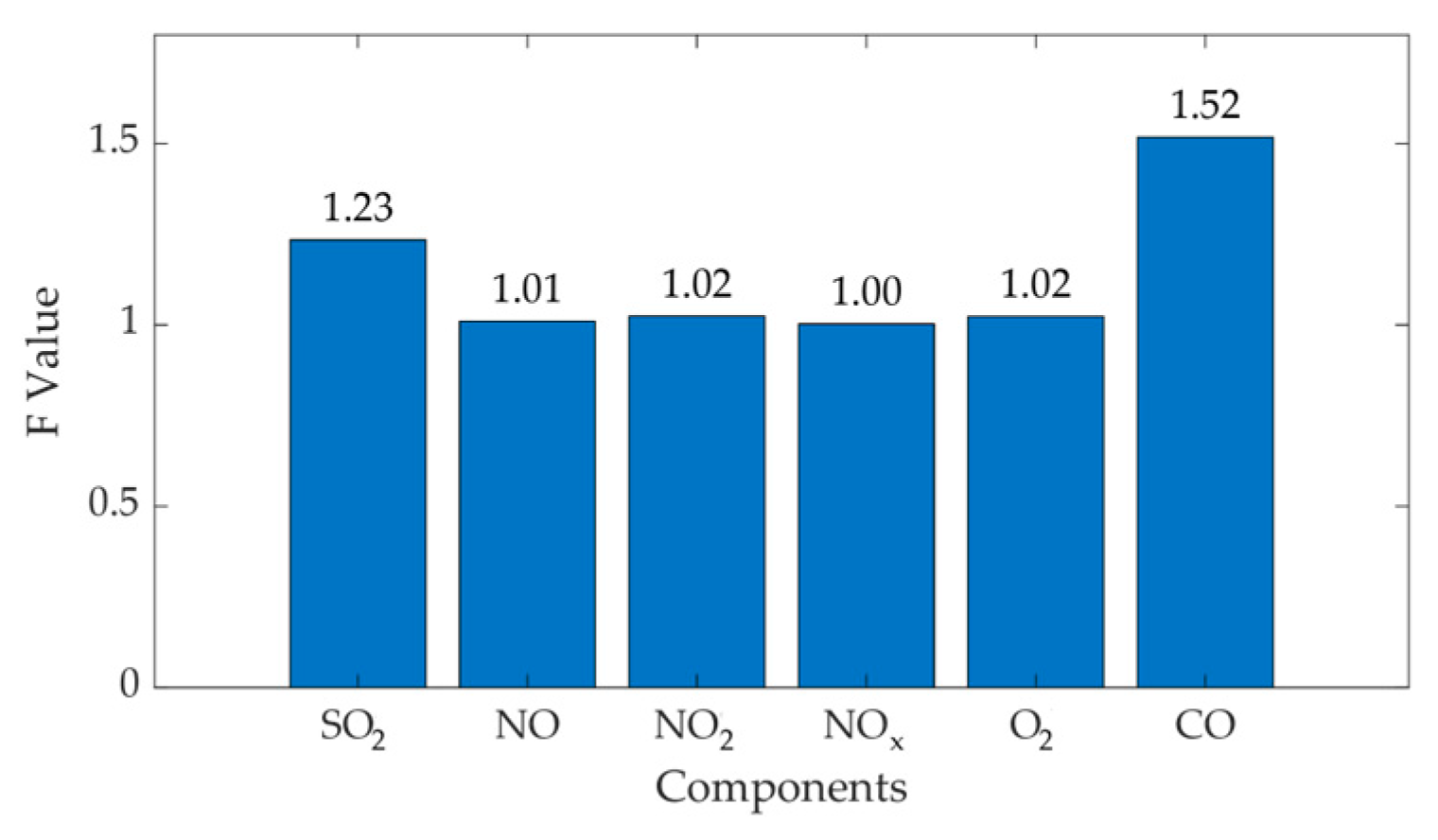
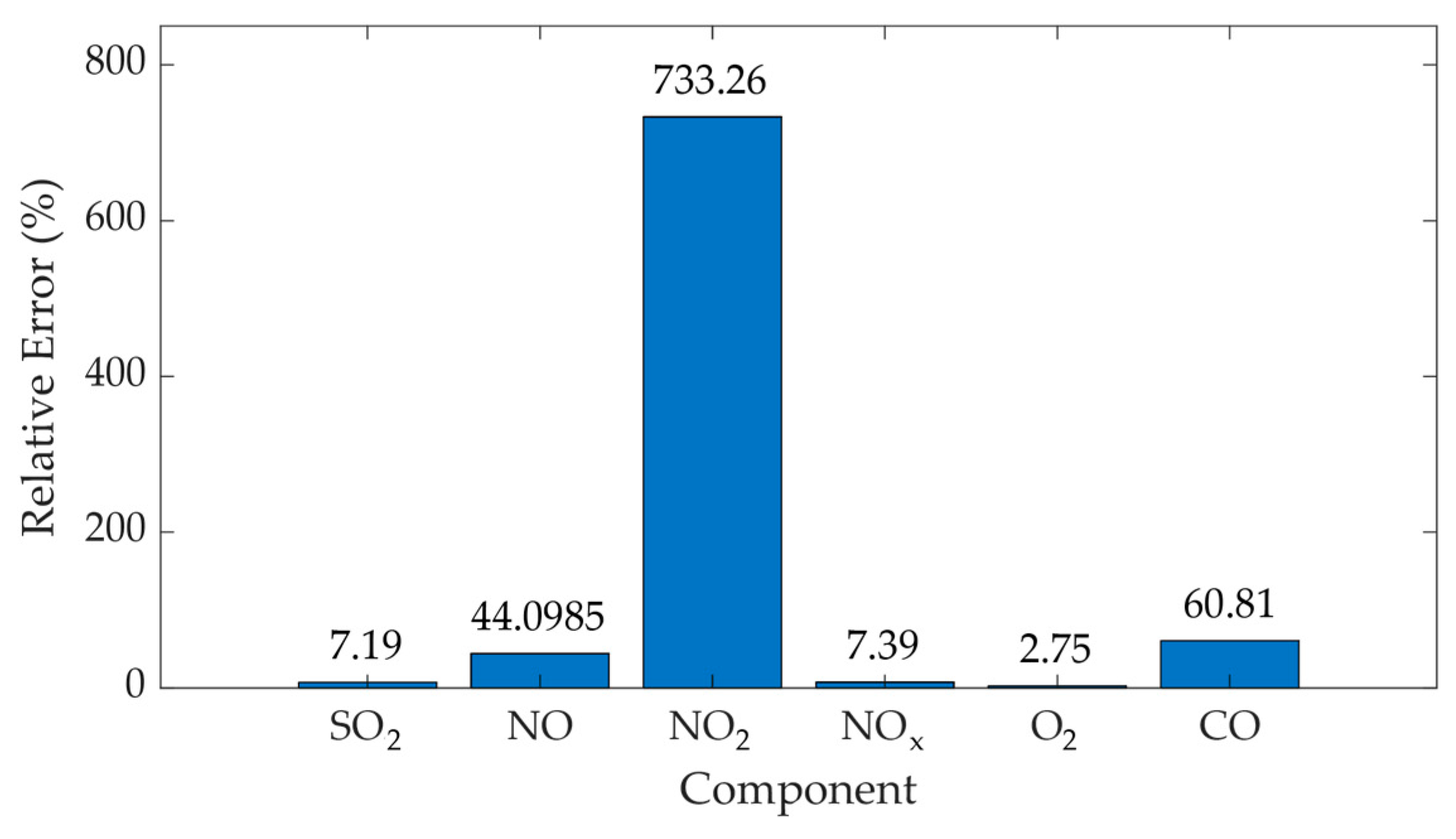
| SO2 | 343.27 | 320.24 | 88.05 | 109.41 | 8458.08 | 13,058.62 | 1.54 |
| NO | 61.49 | 110 | 20.9 | 36.75 | 476.72 | 1472.95 | 3.09 |
| NO2 | 378.44 | 5.09 | 128.59 | 1.96 | 4.18 | 18,038.19 | 3958.2 |
| NOx | 165.49 | 154.1 | 19.06 | 48.46 | 396.33 | 2561.76 | 6.46 |
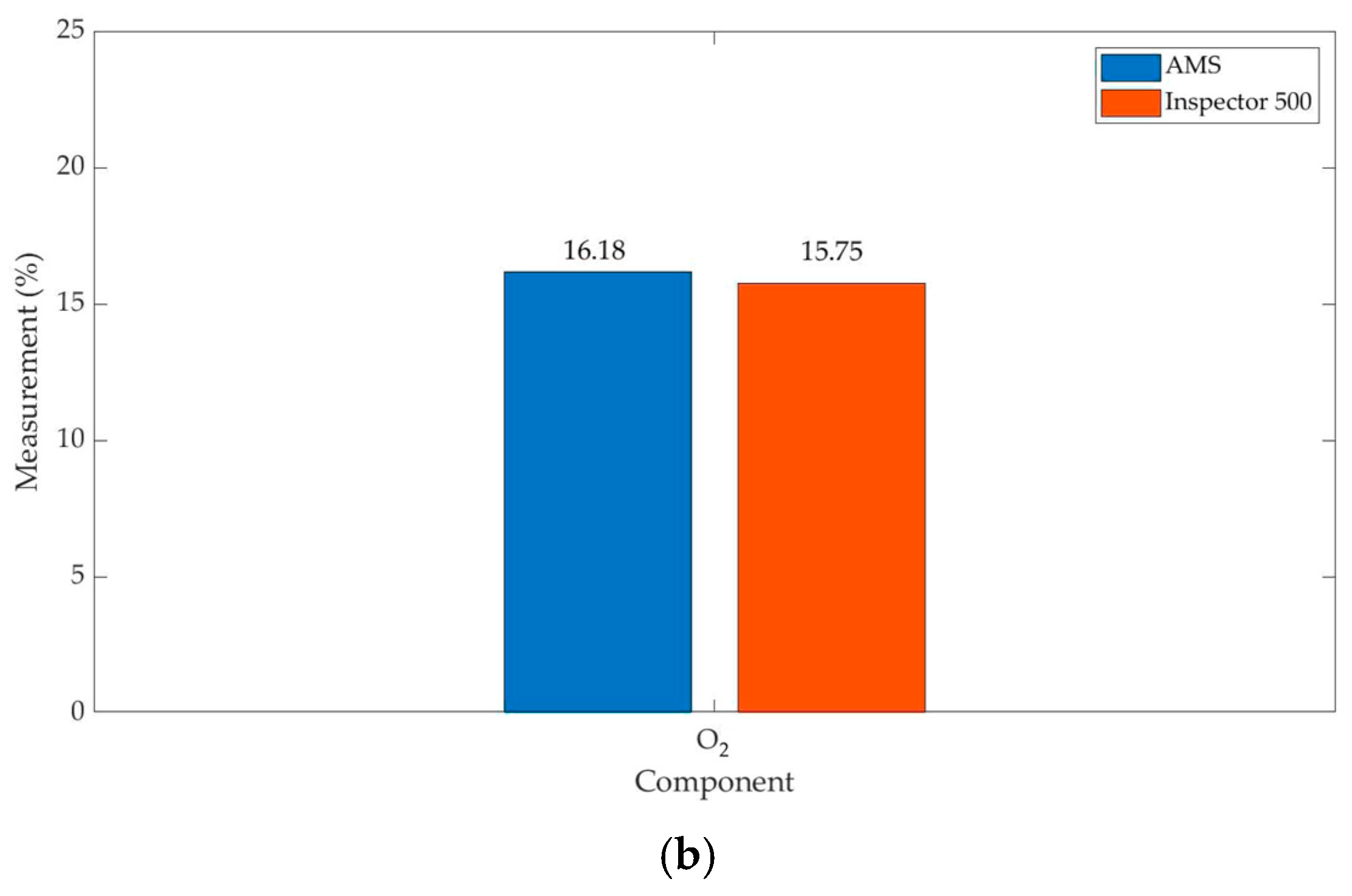
| O2 | 16.18 | 15.75 | 0.51 | 1.2738 | 0.2852 | 1.77 | 6.21 |
| CO | 13.97 | 35.64 | 1.98 | 4.29 | 4.28 | 2.01 | 4.69 |
| Component | f (Fisher’s Test) | t-Value (t-Test) | F-Value (ANOVA) | p-Value (t-Test) | p-Value (ANOVA) |
|---|---|---|---|---|---|
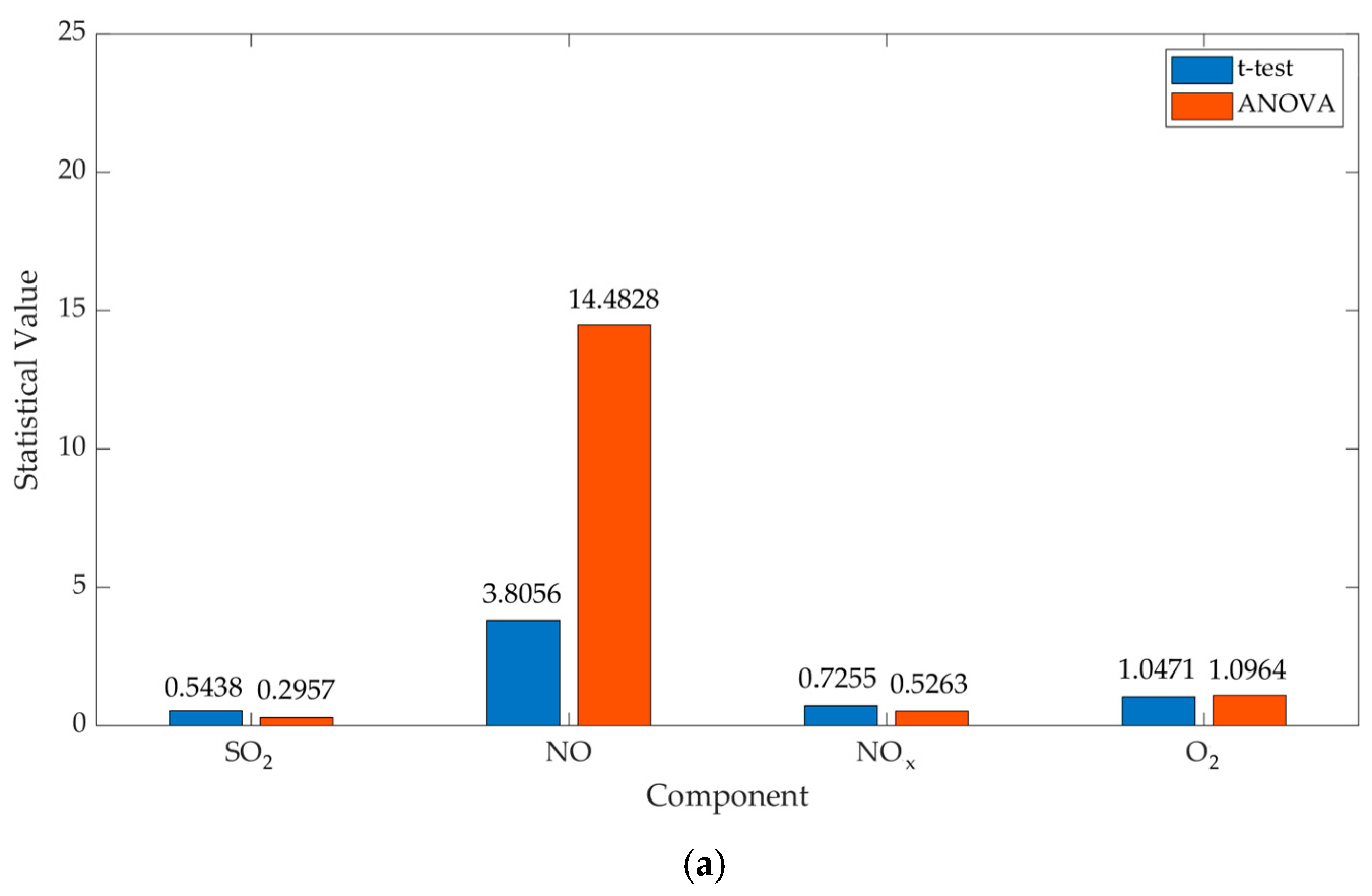
| SO2 | 1.54 | 0.5438 | 0.2957 | 0.5923 | 0.5921 |
| NO | 3.09 | 3.8056 | 14.4828 | 0.0014 | 0.001 |
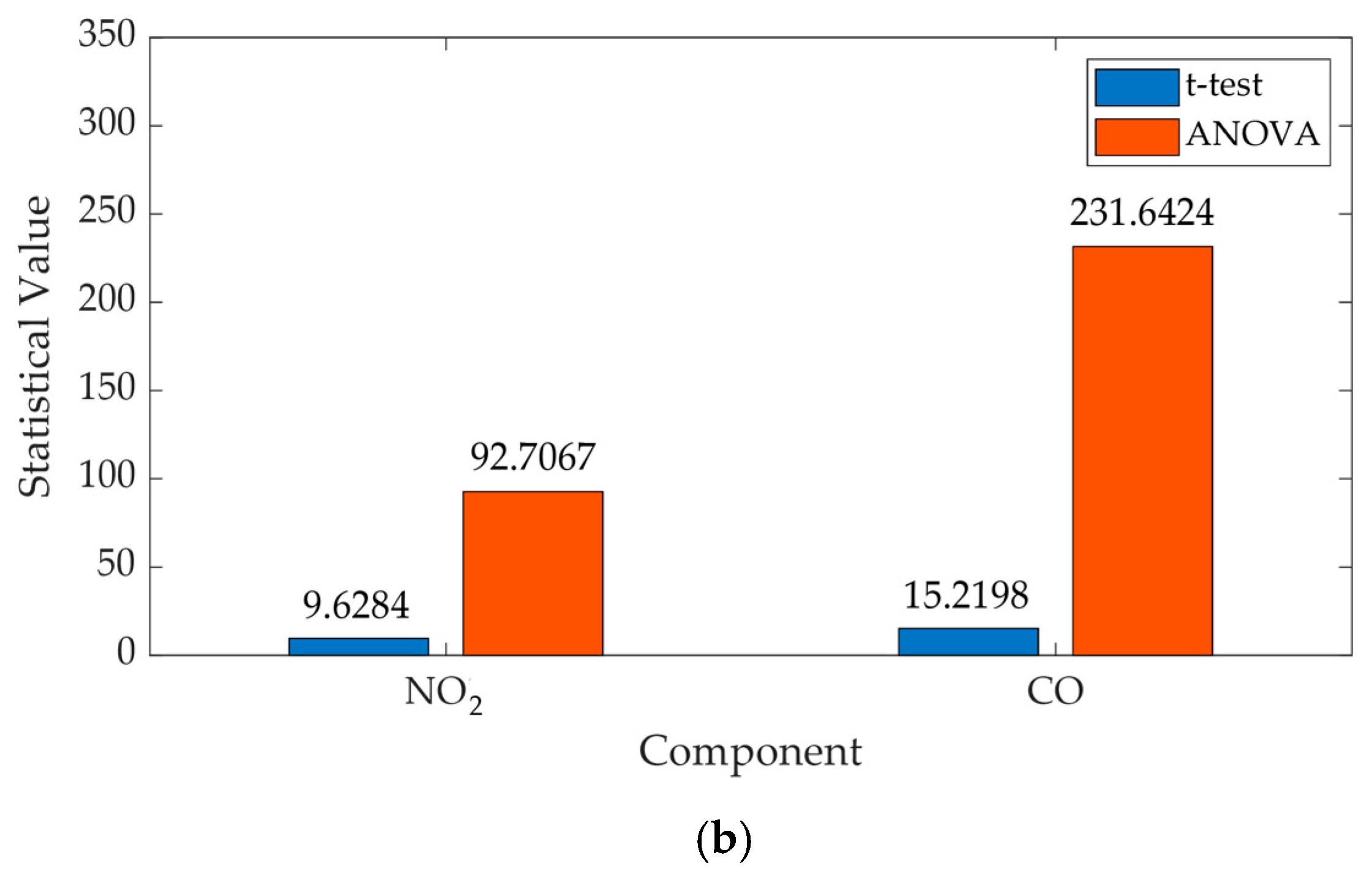
| NO2 | 3958.2 | 9.6284 | 92.7067 | 0 | 0 |
| NOx | 6.46 | 0.7255 | 0.5263 | 0.4799 | 0.4758 |
| O2 | 6.21 | 1.0471 | 1.0964 | 0.3122 | 0.3064 |
| CO | 4.69 | 15.2198 | 231.6424 | 0 | 0 |
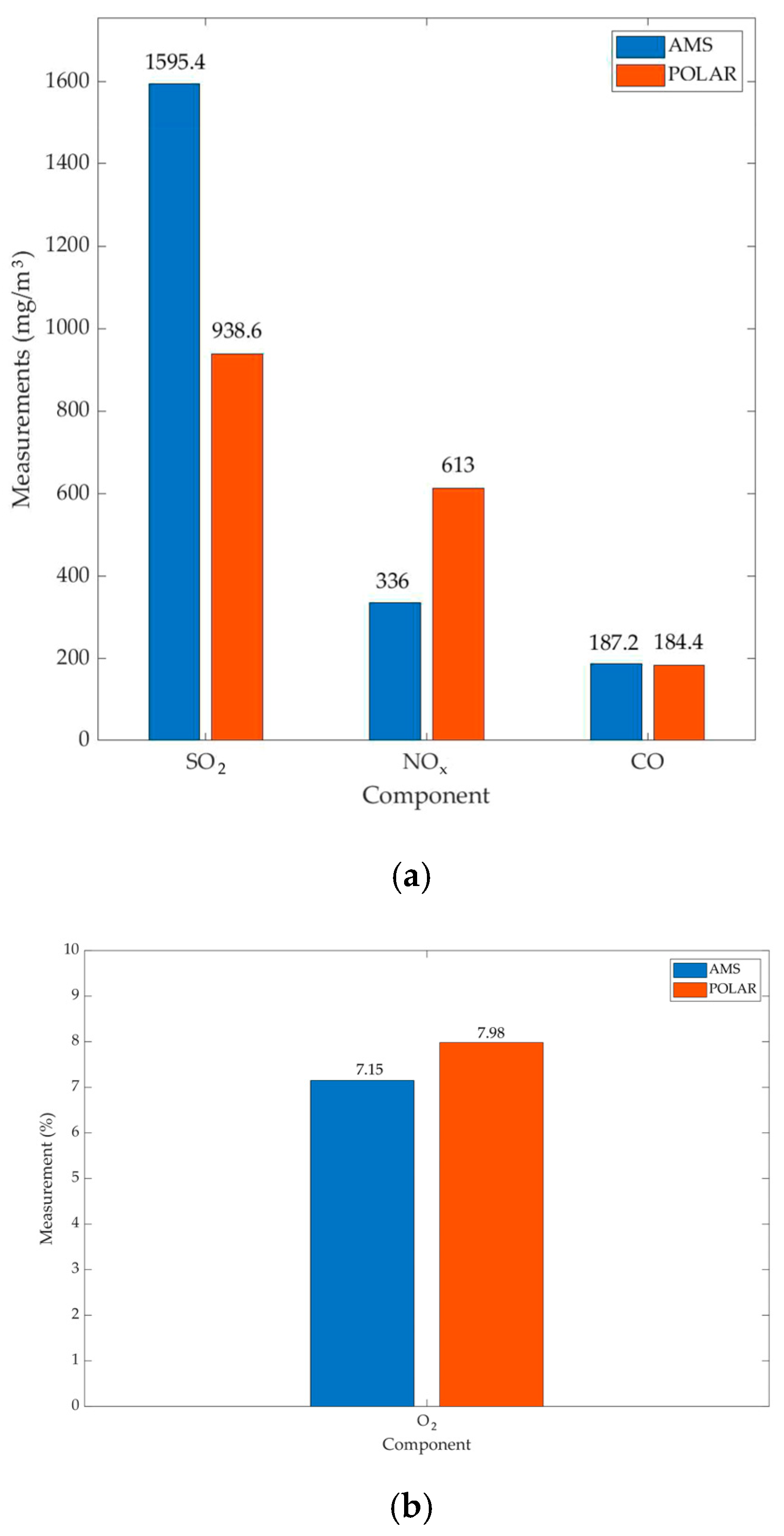
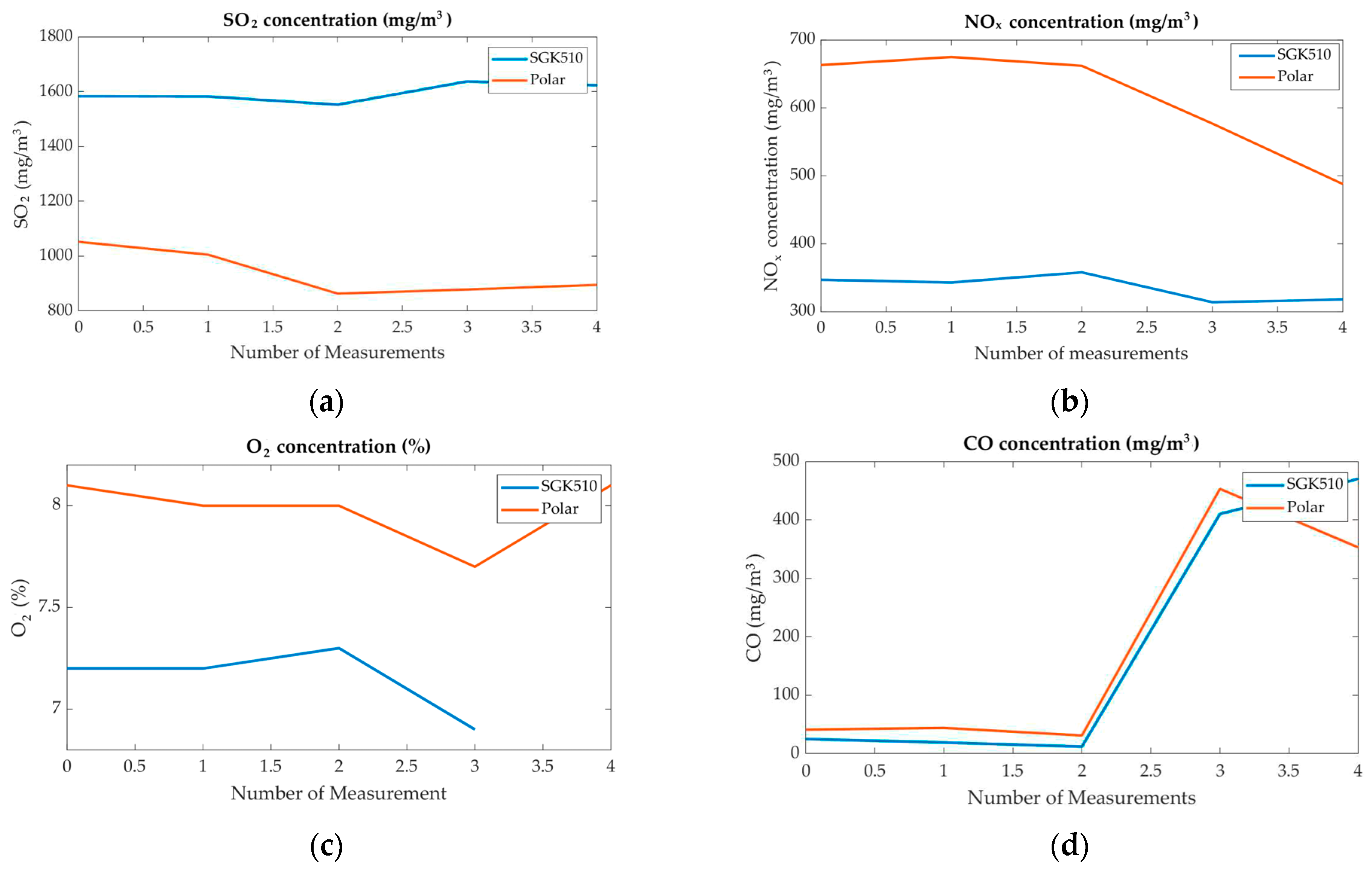
| Time | SO2 (mg/m3) (P) | SO2 (mg/m3) (AMS) | NOx (mg/m3) (P) | NOx (mg/m3) (AMS) | O2 (%) (P) | O2 (%) (AMS) | CO (mg/m3) (I500) | CO (mg/m3) (AMS) | |
|---|---|---|---|---|---|---|---|---|---|
| 9:25 | 1052 | 1583 | 663 | 347 | 8.1 | 7.2 | 41 | 25 | |
| 9:30 | 1005 | 1582 | 675 | 343 | 8 | 7.2 | 44 | 19 | |
| 10:23 | 863 | 1552 | 662 | 358 | 8 | 7.3 | 31 | 12 | |
| 11:21 | 878 | 1637 | 577 | 314 | 7.7 | 6.9 | 453 | 410 | |
| 14:10 | 895 | 1623 | 488 | 318 | 8.1 | - | 353 | 470 | |
| Component | RMD1 | RMD2 | S1 | S2 | f | ||
|---|---|---|---|---|---|---|---|
| SO2 | 1595.4 | 938.6 | 30.69 | 75.57 | 1177.3 | 7139.3 | 6.06 |
| NOx | 336 | 613 | 17.1 | 80.1 | 365.5 | 6416.5 | 17.56 |
| O2 | 7.15 | 7.98 | 0.15 | 0.147 | 0.03 | 0.0027 | 11.11 |
| CO | 187.2 | 184.4 | 207.32 | 181.32 | 53,727.7 | 41,094.8 | 1.31 |
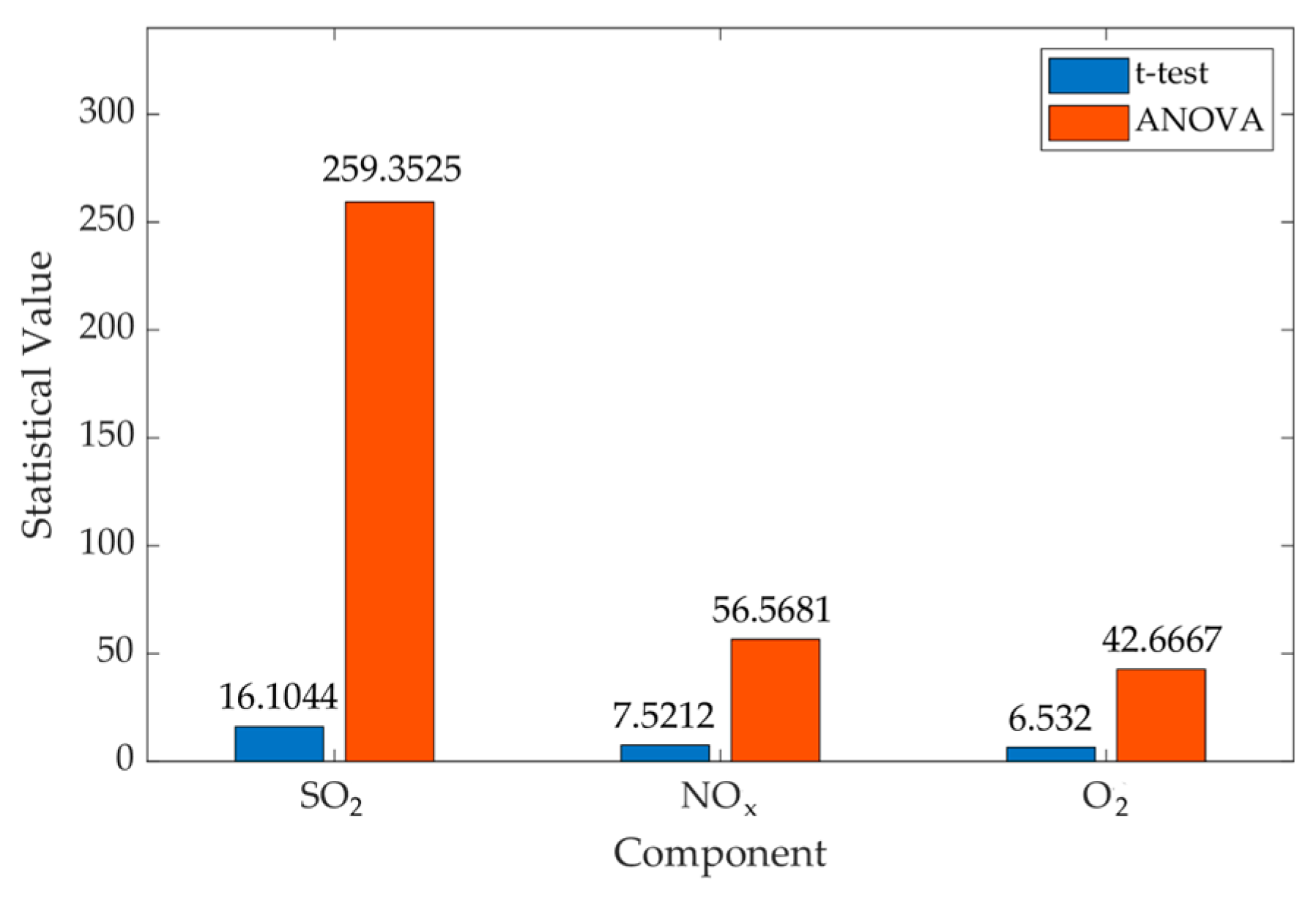
| Component | f (Fisher’s Test) | t-Value (t-Test) | F-Value (ANOVA) | p-Value (t-Test) | p-Value (ANOVA) |
|---|---|---|---|---|---|
| SO2 | 6.06 | 16.1044 | 259.3525 | 0 | 0 |
| NOx | 17.56 | 7.5212 | 56.5681 | 0.0011 | 0.0001 |
| O2 | 11.11 | 6.5320 | 42.6667 | 0.0006 | 0.0006 |
| CO | 1.31 | 0.0203 | 0.0004 | 0.9843 | 0.9843 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neftissov, A.; Biloshchytskyi, A.; Kazambayev, I.; Kirichenko, L.; Zhalmagambetova, U.; Biloshchytska, S. Analysis of the Existing Air Emissions Detection Methods for Stationary Pollution Sources Monitoring. Appl. Sci. 2024, 14, 10934. https://doi.org/10.3390/app142310934
Neftissov A, Biloshchytskyi A, Kazambayev I, Kirichenko L, Zhalmagambetova U, Biloshchytska S. Analysis of the Existing Air Emissions Detection Methods for Stationary Pollution Sources Monitoring. Applied Sciences. 2024; 14(23):10934. https://doi.org/10.3390/app142310934
Chicago/Turabian StyleNeftissov, Alexandr, Andrii Biloshchytskyi, Ilyas Kazambayev, Lalita Kirichenko, Ultuar Zhalmagambetova, and Svitlana Biloshchytska. 2024. "Analysis of the Existing Air Emissions Detection Methods for Stationary Pollution Sources Monitoring" Applied Sciences 14, no. 23: 10934. https://doi.org/10.3390/app142310934
APA StyleNeftissov, A., Biloshchytskyi, A., Kazambayev, I., Kirichenko, L., Zhalmagambetova, U., & Biloshchytska, S. (2024). Analysis of the Existing Air Emissions Detection Methods for Stationary Pollution Sources Monitoring. Applied Sciences, 14(23), 10934. https://doi.org/10.3390/app142310934







