Fermented Cashew Nut Cheese Alternative Supplemented with Chondrus crispus and Porphyra sp.
Abstract
1. Introduction
2. Materials and Methods
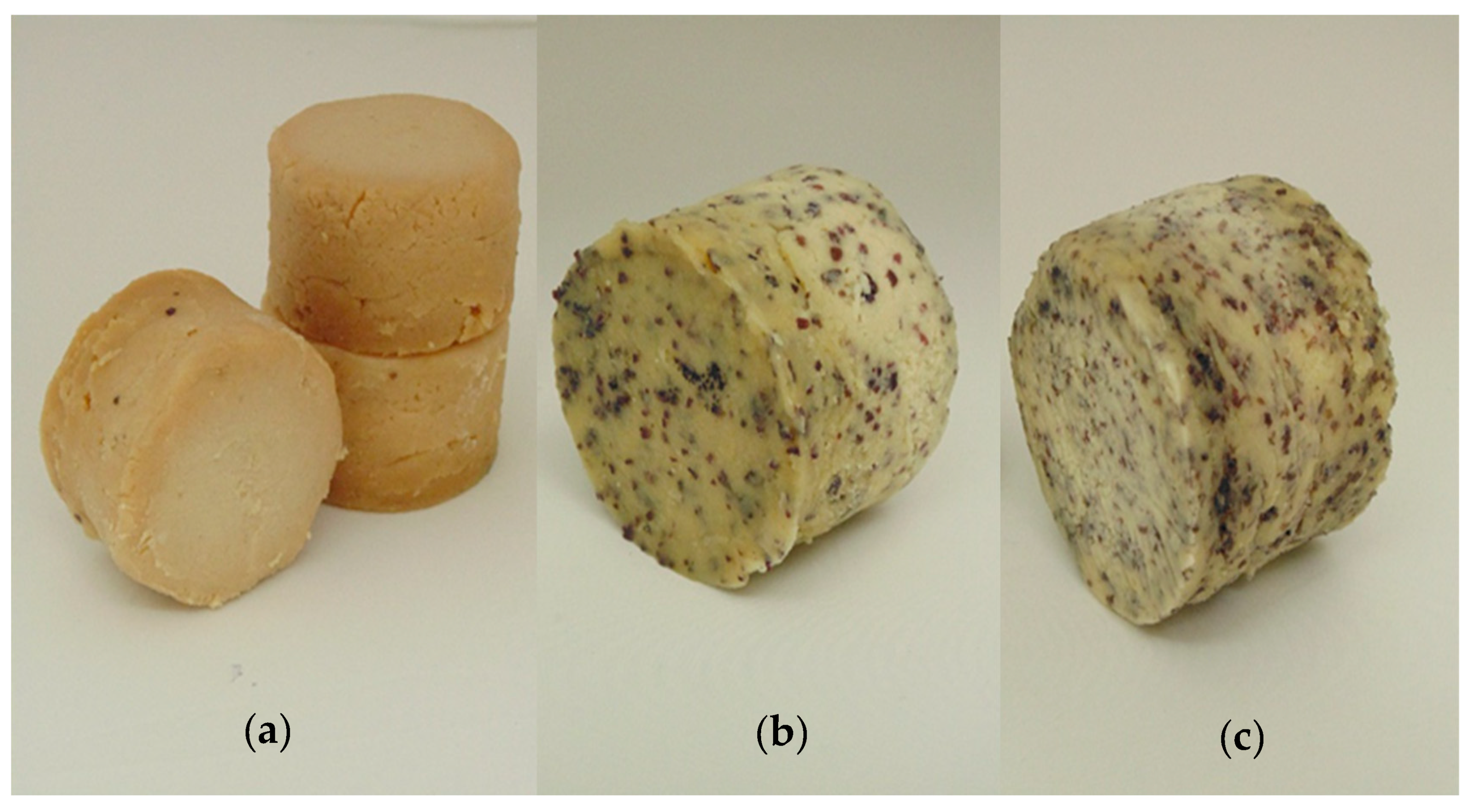
2.1. Preparation of FCNCAs Supplemented with Seaweeds and Sampling Strategies
2.2. Physicochemical Analysis
2.2.1. Total Solid and Moisture Content
2.2.2. Ash Content
2.2.3. NaCl and pH
2.2.4. Total Lipid Content
2.2.5. Crude Protein Content
2.2.6. Elemental Analysis
2.2.7. Color
2.2.8. Texture Profile Analysis (TPA)
2.3. Microbial Load
2.4. Flash Profile (FP)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of FCNCAs
3.2. Total Solid and Moisture Content
3.3. Ash Content
3.4. NaCl and pH
3.5. Total Lipid Content
3.6. Crude Protein Content
3.7. Elemental Analysis
3.8. Color
3.9. Texture Profile Analysis (TPA)
3.10. Microbial Load
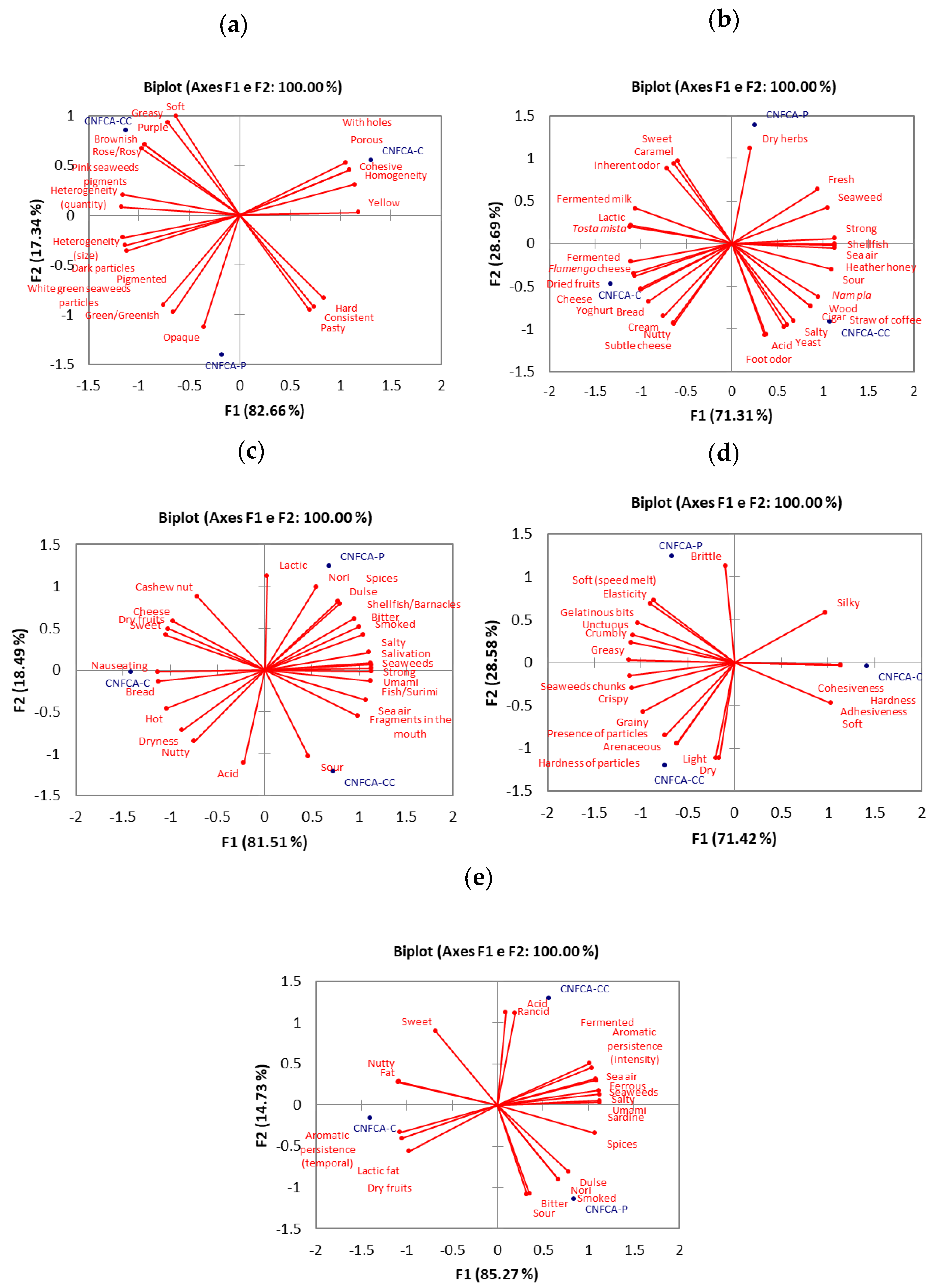
3.11. Flash Profile Methodology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boukid, F.; Lamri, M.; Dar, B.N.; Garron, M.; Castellari, M. Vegan alternatives to processed cheese and yogurt launched in the European market during 2020: A nutritional challenge? Foods 2021, 10, 2782. [Google Scholar] [CrossRef] [PubMed]
- Jeske, S.; Zannini, E.; Elke, K.; Arendt, E.K. Past, present and future: The strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res. Int. 2017, 110, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Mefleh, M.; Pasqualonea, A.; Caponioa, F.; Faccia, M. Legumes as basic ingredient in the production of dairy-free cheese alternatives: A review. J. Sci. Food Agric. 2021, 102, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Short, E.C.; Kinchla, J.; Nolden, A.A. Plant-based cheeses: A systematic review of sensory evaluation studies and strategies to increase consumer acceptance. Foods 2021, 10, 725. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R.; Brothers, C.J. Nutritional profiles of non-dairy plant-based cheese alternatives. Nutrients 2022, 14, 1247. [Google Scholar] [CrossRef]
- Miki, A.J.; Livingston, K.A.; Karlsen, M.C.; Folta, S.C.; McKeown, N.M. Using evidence mapping to examine motivations for following plant-based diets. Curr. Dev. Nutr. 2020, 4, nzaa013. [Google Scholar] [CrossRef]
- Clegg, M.E.; Ribes, A.T.; Reynolds, R.; Kliem, K.; Stergiadis, S. A Comparative assessment of the nutritional composition of dairy and plant-based dairy alternatives available for sale in the UK and the implications for consumers’ dietary intakes. Food Res. Int. 2021, 48, 110586. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Domínguez, R.; Budaraju, S.; Roselló-Soto, E.; Barba, F.J.; Mallikarjunan, K.; Roohinejad, S.; Lorenzo, J.M. Effect of innovative food processing technologies on the physicochemical and nutritional properties and quality of non-dairy plant-based beverages. Foods 2020, 9, 288. [Google Scholar] [CrossRef]
- Saraco, M.N.; Blaxland, J. Dairy-free imitation cheese: Is further development required? Br. Food J. 2020, 122, 3727–3740. [Google Scholar] [CrossRef]
- Frésan, U.; Rippin, H. Nutritional quality of plant-based cheese available in Spanish supermarkets: How do they compare to dairy cheese? Nutrients 2021, 13, 3291. [Google Scholar] [CrossRef]
- Bahrami, M.; Ahmadi, D.; Beigmohammadi, F.; Hosseini, F. Mixing sweet cream buttermilk with whole milk to produce cream cheese. Irish J. Agric. Food Res. 2015, 54, 73–78. [Google Scholar] [CrossRef][Green Version]
- Kumari, P.; Reddy, C.R.K.; Jha, B. Comparative evaluation and selection of a method for lipid and fatty acid extraction from macroalgae. Anal. Biochem. 2011, 415, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, F.; Tomé, D.; Mirand, P. Converting nitrogen into protein—Beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 117–184. [Google Scholar] [CrossRef]
- Uk Health Security Agency. Guidelines for Assessing the Microbiological Safety of Ready-to-Eat Foods Placed on the Market; UKHSA: London, UK, 2024; 73p. [Google Scholar]
- Saraiva, M.; Correia, C.B.; Cunha, I.C.; Maia, C.; Bonito, C.C.; Furtado, R.; Calhau, M.A. Interpretação de Resultados de Ensaios Microbiológicos em Alimentos Prontos para Consumo e em Superfícies do Ambiente de Preparação e Distribuição Alimentar: Valores-Guia; Instituto Nacional de Saúde Doutor Ricardo Jorge: Lisboa, Portugal, 2019; 35p. [Google Scholar]
- European Commission. Comission regulation (EC) No 2073/2005 of 15 november 2005 on microbiological criteria for foodstuffs. OJEU 2005, 338, 1–26. [Google Scholar]
- Wender, L.P.; Liu, J.; Dehlholm, C.; Heymann, H. Flash profile method. In Descriptive Analysis in Sensory Evaluation; Kemp, S.E., Hort, J., Hollowood, T., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 513–533. [Google Scholar]
- Farahat, E.S.A.; Mohamed, A.G.; El-Loly, M.M.; Gafour, W.A.M. Innovative vegetables-processed cheese: I. Physicochemical, rheological and sensory characteristics. Food Biosci. 2021, 42, 01128. [Google Scholar] [CrossRef]
- Chen, J.M.; Al, K.F.; Craven, L.J.; Seney, S.; Coons, M.; McCormick, H.; Reid, G.; O’Connor, C.; Burton, J.P. Nutritional, microbial, and allergenic changes during the fermentation of cashew ‘cheese’ product using a quinoa-based rejuvelac starter culture. Nutrients 2020, 12, 648. [Google Scholar] [CrossRef]
- Campos, B.M.; Ramalho, E.; Marmelo, I.; Noronha, J.P.; Malfeito-Ferreira, M.; Mata, P.; Diniz, M. Proximate composition, physicochemical and microbiological characterization of edible seaweeds available in the Portuguese market. Fronti. Biosci. 2022, 14, 26. [Google Scholar] [CrossRef]
- Salehi, B.; Gültekin-Özgüven, M.; Kırkın, C.; Özçelik, B.; Morais-Braga, M.F.; Carneiro, J.N.; Bezerra, C.F.; Gonçalves da Silva, T.; Coutinho, H.D.; Amina, B.; et al. Anacardium plants: Chemical, nutritional composition and biotechnological applications. Biomolecules 2019, 9, 465. [Google Scholar] [CrossRef]
- Perren, R.; Escher, F.E. Impact of roasting on nut quality. In Improving the Safety and Quality of Nuts, 1st ed.; Harris, L.J., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 173–197. [Google Scholar]
- El-Bakry, M. Salt in cheese: A review. Curr. Res. Dairy Sci. 2012, 4, 1–5. [Google Scholar] [CrossRef]
- Medina, E.; de Castro, A.; Romero, C.; Ramirez, E.M.; Brenes, M. Safety of fermented fruits and vegetables. In Regulating Safety of Traditional and Ethnic Foods, 1st ed.; Vishweshwaraiah, P., Martín-Belloso, O., Keener, L., Astley, S., Braun, S., McMahon, H., Lelieveld, H., Eds.; Academic Press: Waltham, WA, USA, 2016; pp. 355–367. [Google Scholar]
- Tabanelli, G.; Pasini, F.; Riciputi, Y.; Vannini, L.; Gozzi, G.; Balestra, F.; Caboni, M.F.; Gardini, F.; Montanari, C. Fermented nut-based vegan food: Characterization of a home made product and scale-up to an industrial pilot-scale production. J. Food Sci. 2018, 83, 711–722. [Google Scholar] [CrossRef]
- Carvalho, J.M.; Figueiredo, R.W.; Machado de Sousa, P.H.; Tavares de Luna, F.M.; Maia, G.A. Cashew nut oil: Effect of kernel grade and a microwave preheating extraction step on chemical composition, oxidative stability and bioactivity. Int. J. Food Sci. Technol. 2018, 53, 930–937. [Google Scholar] [CrossRef]
- Toschi, T.G.; Caboni, M.F.; Penazzi, G.; Lercker, G.; Capella, P. A study on cashew nut oil composition. J. Am. Oil Chem. Soc. 1993, 70, 1017–1020. [Google Scholar] [CrossRef]
- Trox, J.; Vadivel, V.; Vetters, W.; Stuetz, W.; Scherbaum, V.; Gola, U.; Nohr, D.; Biesalski, H.K. Bioactive compounds in cashew nut (Anacardium occidentale L.) kernels: Effect of different shelling methods. J. Agric. Food Chem. 2010, 58, 5341–5346. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, R.; Devi, P. Evaluation of physiochemical properties, proximate and nutritional composition of Gracilaria edulis collected from Palk Bay. Food Chem. 2015, 174, 68–74. [Google Scholar] [CrossRef]
- Cian, R.E.; Drago, S.R.; de Medina, S.F.; Martínez-Augustin, O. Proteins and carbohydrates from red seaweeds: Evidence for beneficial effects on gut function and microbiota. Mar. Drugs. 2015, 13, 5358–5383. [Google Scholar] [CrossRef]
- Leandro, A.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweed’s bioactive candidate compounds to food industry and global food security. Life 2020, 10, 140. [Google Scholar] [CrossRef]
- Rico, R.; Bulló, M.; Salas-Salvadó, J. Nutritional composition of raw fresh cashew (Anacardium occidentale L.) kernels from different origin. Food Sci. Nutr. 2015, 4, 329–338. [Google Scholar] [CrossRef]
- Loveday, S.M. Plant protein ingredients with food functionality potential. Nutr. Bull. 2020, 45, 321–327. [Google Scholar] [CrossRef]
- Lonnie, M.; Hooker, E.; Brunstrom, J.M.; Corfe, B.M.; Green, M.A.; Watson, A.W.; Williams, E.A.; Stevenson, E.J.; Penson, S.; Johnstone, A.M. Protein for life: Review of optimal protein intake, sustainable dietary sources and the effect on appetite in ageing adults. Nutrients 2018, 10, 360. [Google Scholar] [CrossRef]
- Lima, J.R.; Bruno, L.M.; Wurlitzer, N.J.; Machado de Sousa, P.H.; Holanda, S.A. Cashew nut-based beverage: Development, characteristics and stability during refrigerated storage. Food Sci. Technol. 2020, 41, 60–64. [Google Scholar] [CrossRef]
- Mohammed, H.O.; O’Grady, M.N.; O’Sullivan, M.G.; Hamill, R.M.; Kilcawley, K.N.; Kerry, J.P. An assessment of selected nutritional, bioactive, thermal and technological properties of brown and red Irish seaweed species. Foods 2021, 10, 2784. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, E.; Yokota, H.; Matsui, T. The in vitro digestibility and absorption of magnesium in some edible seaweeds. J. Sci. Food Agric. 2012, 92, 2305–2309. [Google Scholar] [CrossRef] [PubMed]
- Pelczyńska, M.; Moszak, M.; Bogdański, P. The role of magnesium in the pathogenesis of metabolic disorders. Nutrients 2022, 14, 1714. [Google Scholar] [CrossRef]
- Bougma, K.; Aboud, F.E.; Harding, K.B.; Marquis, G.S. Iodine and mental development of children 5 years old and under: A systematic review and meta-analysis. Nutrients 2013, 5, 1384–1416. [Google Scholar] [CrossRef]
- Batool, M.; Naddem, M.; Imran, M.; Gulzar, N.; Shahid, M.Q.; Shahbaz, M.; Ajmal, M.; Khan, I.T. Impact of vitamin E and selenium on antioxidant capacity and lipid oxidation of cheddar cheese in accelerated ripening. Lipids Health Dis. 2018, 17, 79. [Google Scholar] [CrossRef]
- Bonanno, G.; Orlando-Bonaca, M. Chemical elements in Mediterranean macroalgae. A review. Ecotoxicol. Environ. Saf. 2018, 148, 44–71. [Google Scholar] [CrossRef]
- Freitas, M.V.; Pacheco, D.; Cotas, J.; Mouga, T.; Afonso, C. Red seaweed pigments from a biotechnological perspective. Phycology 2022, 2, 1–29. [Google Scholar] [CrossRef]
- Carpena, M.; Caleja, C.; Pereira, E.; Pereira, C.; Ćirić, A.; Soković, M.; Soria-Lopez, A.; Fraga-Corral, M.; Simal-Gandara, J.; Ferreira, I.C.F.; et al. Red seaweeds as a source of nutrients and bioactive compounds: Optimization of the extraction. Chemosensors 2021, 9, 132. [Google Scholar] [CrossRef]
- Pina, A.L.; Costa, A.R.; Lage-Yusty, M.A.; López-Hernández, J. An evaluation of edible red seaweed (Chondrus crispus) components and their modification during the cooking process. LWT-Food Sci. Technol. 2014, 56, 175–180. [Google Scholar] [CrossRef]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B. Pigments content (chlorophylls, fucoxanthin and phycobiliproteins) of different commercial dried algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Queiroga, R.; Santos, B.M.; Gomes, A.M.; Monteiro, M.J.; Teixeira, S.M.; Leite de Souza, E.; Pereira, C.J.; Pintado, M.M. Nutritional, textural and sensory properties of coalho cheese made of goats’, cows’ milk and their mixture. LWT-Food Sci. Technol. 2013, 50, 538–544. [Google Scholar] [CrossRef]
- Paz, N.F.; Gonçalvez de Oliveira, E.; Villalva, F.J.; Armada, M.; Ramón, A.N. Effect of pH at drainage on the physicochemical, textural and microstructural characteristics of Mozzarella cheese from goat milk. Food Sci. Technol. 2017, 37, 193–201. [Google Scholar] [CrossRef]
- Milovanovic, B.; Djekic, I.; Miocinovic, J.; Djordjevic, V.; Lorenzo, J.M.; Barba, F.J.; Mörlein, D.; Tomasevic, I. What Is the Color of Milk and Dairy Products and How Is It Measured? Foods 2020, 9, 1629. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Ak, M.M. Cheese Rheology and Texture, 1st ed.; CRC Press: New York, NY, USA, 2003. [Google Scholar]
- Lepesioti, S.; Zoidou, E.; Lioliou, D.; Moschopoulou, E.; Moatsou, G. Quark-type cheese: Effect of fat content, homogenization, and heat treatment of cheese milk. Foods 2021, 10, 184. [Google Scholar] [CrossRef]
- Devi, A.; Khatkar, B.S. Effects of fatty acids composition and microstructure properties of fats and oils on textural properties of dough and cookie quality. J. Food Sci. Technol. 2017, 55, 321–330. [Google Scholar] [CrossRef]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. An overview to the health benefits of seaweeds consumption. Mar. Drugs. 2021, 19, 341. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and potential properties of seaweeds and their recent applications: A review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science, 2nd ed.; Springer: New York, NY, USA, 2017; pp. 494–503. [Google Scholar]
- Sołowiej, B.; Cheung, I.W.Y.; Li-Chan, E.C.Y. Texture, rheology and meltability of processed cheese analogues prepared using rennet or acid casein with or without added whey proteins. Int. Dairy J. 2014, 37, 87–94. [Google Scholar] [CrossRef]
- Rupérez, P.; Saura-Calixto, F. Dietary fibre and physicochemical properties of edible Spanish seaweeds. Eur. Food Res. Technol. 2001, 212, 349–354. [Google Scholar] [CrossRef]
- Bourne, M. Food Texture and Viscosity: Concept and Measurement, 2nd ed.; Academic Press: New York, NY, USA, 2002; pp. 259–276. [Google Scholar]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Rani, R.P. Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnol. Res. Int. 2014, 2014, 250424. [Google Scholar] [CrossRef]
- Pisano, M.B.; Deplano, M.; Fadda, M.E.; Cosentino, S. Microbiota of Sardinian goat’s milk and preliminary characterization of prevalent LAB species for a starter or adjunct cultures development. Biomed. Res. Int. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Muniz, C.R.; Freire, F.-C.; Lemos, É.H.; Pinto, G.A.S.; Teixeira de Figueiredo, E.A.; Figueiredo, R.W. Effect of processing conditions on the microbiological quality of cashew nuts. Braz. J. Food Technol. 2006, 9, 33–38. [Google Scholar]
- Göçer, E.M.; Koptagel, E. Microbiological and rheological properties of nut-based beverages fermented with kefir. SSRN J. 2022. [Google Scholar] [CrossRef]
- Schmitt, N.; Yu, G.; Greve, R.; McIntyre, L. Outbreak of S. Weltevreden linked to fermented cashew nut cheese in Victoria, BC. Environ. Health Rev. 2018, 61, 74–81. [Google Scholar] [CrossRef]
- Renard, C.M.; Maingonnat, J.F. Thermal processing of fruits and fruit juices. In Thermal Food Processing: New Technologies and Qualities Issues, 2nd ed.; Sun, D.-W., Ed.; CRC Press: New York, NY, USA, 2012; pp. 413–438. [Google Scholar]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Mendes, M.; Pereira, R.; Sousa Pinto, I.; Carvalho, A.P.; Gomes, A.M. Antimicrobial activity and lipid profile of seaweed extracts from the North Portuguese Coast. Int. Food Res. J. 2013, 20, 3337–3345. [Google Scholar]
- Zhang, G.; Hu, L.; Melka, D.; Wang, H.; Laasri, A.; Brown, E.W.; Strain, E.; Allard, M.; Bunning, V.K.; Musser, S.M.; et al. Prevalence of salmonella in cashews, hazelnuts, macadamia nuts, pecans, pine nuts, and walnuts in the United States. J. Food Prot. 2017, 80, 45–466. [Google Scholar] [CrossRef]
- Eglezos, S. The bacteriological quality of retail-level peanut, almond, cashew, hazelnut, Brazil, and mixed nut kernels produced in two Australian nut-processing facilities over a period of 3 years. Foodborne Pathog. Dis. 2010, 7, 863–866. [Google Scholar] [CrossRef]
- Lima, J.R.; Garruti, D.S.; Bruno, L.M. Physicochemical, microbiological and sensory characteristics of cashew nut butter made from different kernel grades-quality. LWT-Food Sci. Technol. 2012, 45, 180–185. [Google Scholar] [CrossRef]
- Baldermann, S.; Yamamoto, M.; Yang, Z.; Kawahashi, T.; Kuwano, K.; Watanabe, N. C 13 -Apocarotenoids: More than flavor compounds? In Carotenoid Cleavage Products; Winterhalter, P., Ebeler, S.E., Eds.; American Chemical Society: Washington, DC, USA, 2013; pp. 73–80. [Google Scholar]
- Du, X.; Xu, Y.; Jiang, Z.; Zhu, Y.; Li, Z.; Ni, H.; Chen, F. Removal of the fishy malodor from Bangia fusco-purpurea via fermentation of Saccharomyces cerevisiae, Acetobacter pasteurianus, and Lactobacillus plantarum. J. Food Biochem. 2021, 45, e13728. [Google Scholar] [CrossRef]
- Vilar, E.G.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawleya, K.N. Volatile compounds of six species of edible seaweed: A review. Algal Res. 2020, 45, 101740. [Google Scholar] [CrossRef]
- Figueroa, V.; Bunger, A.; Ortiz, J.; Aguilera, J.M. Sensory descriptors for three edible Chilean seaweeds and their relations to umami components and instrumental texture. J. Appl. Phycol. 2022, 34, 3141–3156. [Google Scholar] [CrossRef] [PubMed]
- Afonso, N.C.; Catarino, M.D.; Silva, A.M.; Cardoso, S.M. Brown macroalgae as valuable food ingredients. Antioxidants 2019, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Osako, K.; Ohshima, T. Identification and characterisation of headspace volatiles of fish miso, a Japanese fish meat based fermented paste, with special emphasis on effect of fish species and meat washing. Food Chem. 2010, 120, 621–631. [Google Scholar] [CrossRef]
- Peinado, I.; Girón, J.; Koutsidis, G.; Ames, J.M. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res. Int. 2014, 66, 36–44. [Google Scholar] [CrossRef]

| Physicochemical Parameters | Control | Supplemented Products | |
|---|---|---|---|
| FCNCA-C | FCNCA-CC | FCNCA-P | |
| Total solids (% WW) | 64.63 ± 0.88 a | 67.96 ± 0.53 b | 69.79 ± 0.59 c |
| Moisture (% WW) | 35.37 ± 0.88 a | 32.04 ± 0.53 b | 30.21 ± 0.59 c |
| Ash (% DW) | 2.81 ± 0.08 a | 3.63 ± 0.15 b | 3.12 ± 0.09 c |
| NaCl (g/100 g) | 2.67 ± 0.21 a | 2.50 ± 0.10 a | 2.43 ± 0.15 a |
| pH | 5.27 ± 0.12 a | 5.07 ± 0.15 a,b | 5.07 ± 0.06 b |
| Lipids (% DW) | 37.45 ± 0.44 a | 34.51 ± 3.85 b | 32.91 ± 0.16 b |
| Crude protein (% DW) | 18.92 ± 0.00 a | 18.26 ± 0.45 b | 18.73 ± 0.20 c |
| Minerals and Trace Elements | C. crispus | Porphyra sp. | FCNCA-C | FCNCA-CC | FCNCA-P | Certified Values *** |
|---|---|---|---|---|---|---|
| Ca (g·kg−1 DW) | 4.29 ± 0.17 A | 1.77 ± 0.05 B | 0.81 ± 0.09 a | 1.07 ± 0.06 b | 1.77 ± 0.19 ab | 12.35 ± 0.22 |
| K (g·kg−1 DW) | 36.24 ± 1.09 A | 16.55 ± 0.48 B | 2.59 ± 0.24 a | 3.79 ± 0.31 b | 2.79 ± 1.05 ab | 75.18 ± 1.66 |
| Mg (g·kg−1 DW) | 6.82 ± 0.07 A | 4.26 ± 0.12 B | 3.13 ± 0.19 a | 3.51 ± 0.15 b | 3.19 ± 0.50 ab | 6.08 ± 0.21 |
| Na (g·kg−1 DW) | 31.81 ± 0.58 A | 22.40 ± 0.95 B | 6.19 ± 0.24 a | 9.53 ± 0.18 b | 7.38 ± 1.10 c | 16.56 ± 0.49 |
| P (g·kg−1 DW) | 1.70 ± 0.05 A | 2.31 ± 0.09 B | 7.59 ± 0.41 a | 6.64 ± 0.65 b | 7.98 ± 0.66 ab | 4.58 ± 0.48 |
| Fe (mg·kg−1 DW) | 123.95 ± 5.8 A | 79.96 ± 1.73 B | 49.48 ± 2.14 a | 60.14 ± 0.21 b | 48.21 ± 2.97 a | 661.03 ± 33.11 |
| I (mg·kg−1 DW) | 29.33 ± 0.11 A | 15.80 ± 0.82 B | <LOQ ** | 1.26 ± 0.30 | <LOQ ** | 918.05 ± 49.52 |
| Mn (mg·kg−1 DW) | 26.18 ± 0.88 A | 20.77 ± 0.79 B | 15.03 ± 0.18 a | 15.84 ± 1.29 a | 14.88 ± 1.31 a | 23.90 ± 1.81 |
| Se (mg·kg−1 DW) | 0.84 ± 0.10 A | 2.49 ± 0.10 B | 2.05 ± 0.02 a | 2.54 ± 0.33 b | 2.15 ± 0.19 ab | n.a. |
| Zn (mg·kg−1 DW) | 66.24 ± 3.97 A | 48.05 ± 1.89 B | 40.13 ± 2.52 a | 65.76 ± 2.51 b | 46.78 ± 6.55 a | 26.52 ± 0.63 |
| Parameters | Control | Supplemented Products | |
|---|---|---|---|
| FCNCA-C | FCNCA-CC | FCNCA-P | |
| L* | 53.33 ± 1.91 a | 43.77 ± 2.25 b | 38.30 ± 2.21 c |
| a* | 10.56 ± 0.47 a | 3.19 ± 0.50 b | 6.68 ± 0.94 c |
| b* | 25.74 ± 0.46 a | 15.62 ± 2.28 b | 13.71 ± 1.43 c |
| C* | 27.83 ± 0.52 a | 15.95 ± 2.29 b | 15.26 ± 1.50 b |
| WI | 45.67 ± 1.88 a | 41.55 ± 2.16 b | 36.44 ± 2.46 c |
| Supplemented Products | Hardness (N) | Adhesiveness (J) | Cohesiveness (―) | Springiness (mm) | Gumminess (N) |
|---|---|---|---|---|---|
| FCNCA-C | 6.01± 0.56 a | 0.07 ± 0.06 a | 0.33 ± 0.04 a | 1.70 ± 0.25 a | 2.00 ± 0.23 a |
| FCNCA-CC | 7.90 ± 0.68 b | 0.17 ± 0.09 a | 0.37 ± 0.03 a | 1.91 ± 0.30 a | 2.93 ± 0.18 b |
| FCNCA-P | 9.69 ± 0.66 c | 0.03 ± 0.04 a | 0.42 ± 0.02 b | 2.29 ± 0.19 b | 4.08 ± 0.45 c |
| Microbiological Parameters (Log CFU·g−1) | Control | Supplemented Products | |
|---|---|---|---|
| FCNCA-C | FCNCA-CC | FCNCA-P | |
| Enterococcus | 4.48 ± 000 a | 4.48 ± 0.00 a | 4.48 ± 0.00 a |
| LAB | 6.56 ± 0.00 a | 5.48 ± 0.00 b | 5.48 ± 0.00 b |
| AMB | 7.83 ± 0.86 a | 7.36 ± 0.80 b | 7.48 ± 0.00 c |
| MAC | <2 a | 6.18 ± 1.63 b | 6.08 ± 0.18 b |
| GYP (Molds) | <2 a | <2 a | <2 a |
| GYP (Yeasts) | 3.76 ± 1.64 a | <2 b | <2 b |
| E. coli | 2.11 ± 0.33 a | <1 b | 1.9 ± 1.89 a |
| TC | 0.7 ± 0.15 a | 0.7 ± 0.15 a | 2.57 ± 0.00 b |
| S. aureus | 2.18 ± 1.63 a | 2.54 ± 0.37 a,b | 2.72 ± 0.42 b |
| Salmonella spp. | Absent in 25 g | Absent in 25 g | Absent in 25 g |
| L. monocytogenes | Absent in 25 g | Absent in 25 g | Absent in 25 g |
| Attributes | Object | Residual (%) |
|---|---|---|
| FCNCA-C | 12.412 | |
| Appearance | FCNCA-CC | 29.920 |
| FCNCA-P | 13.578 | |
| FCNCA-C | 9.729 | |
| Aroma | FCNCA-CC | 11.096 |
| FCNCA-P | 17.230 | |
| FCNCA-C | 6.051 | |
| Flavor | FCNCA-CC | 18.133 |
| FCNCA-P | 13.764 | |
| FCNCA-C | 20.604 | |
| Texture | FCNCA-CC | 16.473 |
| FCNCA-P | 15.811 | |
| FCNCA-C | 7.781 | |
| After-taste | FCNCA-CC | 22.348 |
| FCNCA | 12.737 |
| Attributes | Rc (%) |
|---|---|
| Appearance | 33.8% |
| Aroma | 31.2% |
| Flavor | 29.1% |
| Texture | 11.6% |
| After-taste | 23.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, B.M.; Moreira-Leite, B.; Salgado, A.; Ramalho, E.; Marmelo, I.; Malfeito-Ferreira, M.; Sousa, P.; Diniz, M.S.; Mata, P. Fermented Cashew Nut Cheese Alternative Supplemented with Chondrus crispus and Porphyra sp. Appl. Sci. 2024, 14, 11082. https://doi.org/10.3390/app142311082
Campos BM, Moreira-Leite B, Salgado A, Ramalho E, Marmelo I, Malfeito-Ferreira M, Sousa P, Diniz MS, Mata P. Fermented Cashew Nut Cheese Alternative Supplemented with Chondrus crispus and Porphyra sp. Applied Sciences. 2024; 14(23):11082. https://doi.org/10.3390/app142311082
Chicago/Turabian StyleCampos, Bruno M., Bruno Moreira-Leite, Abigail Salgado, Edgar Ramalho, Isa Marmelo, Manuel Malfeito-Ferreira, Paulo Sousa, Mário S. Diniz, and Paulina Mata. 2024. "Fermented Cashew Nut Cheese Alternative Supplemented with Chondrus crispus and Porphyra sp." Applied Sciences 14, no. 23: 11082. https://doi.org/10.3390/app142311082
APA StyleCampos, B. M., Moreira-Leite, B., Salgado, A., Ramalho, E., Marmelo, I., Malfeito-Ferreira, M., Sousa, P., Diniz, M. S., & Mata, P. (2024). Fermented Cashew Nut Cheese Alternative Supplemented with Chondrus crispus and Porphyra sp. Applied Sciences, 14(23), 11082. https://doi.org/10.3390/app142311082











