Process Optimization and Techno-Economic Analysis for the Production of Phycocyanobilin from Arthrospira maxima-Derived C-Phycocyanin
Abstract
1. Introduction
2. Materials and Methods
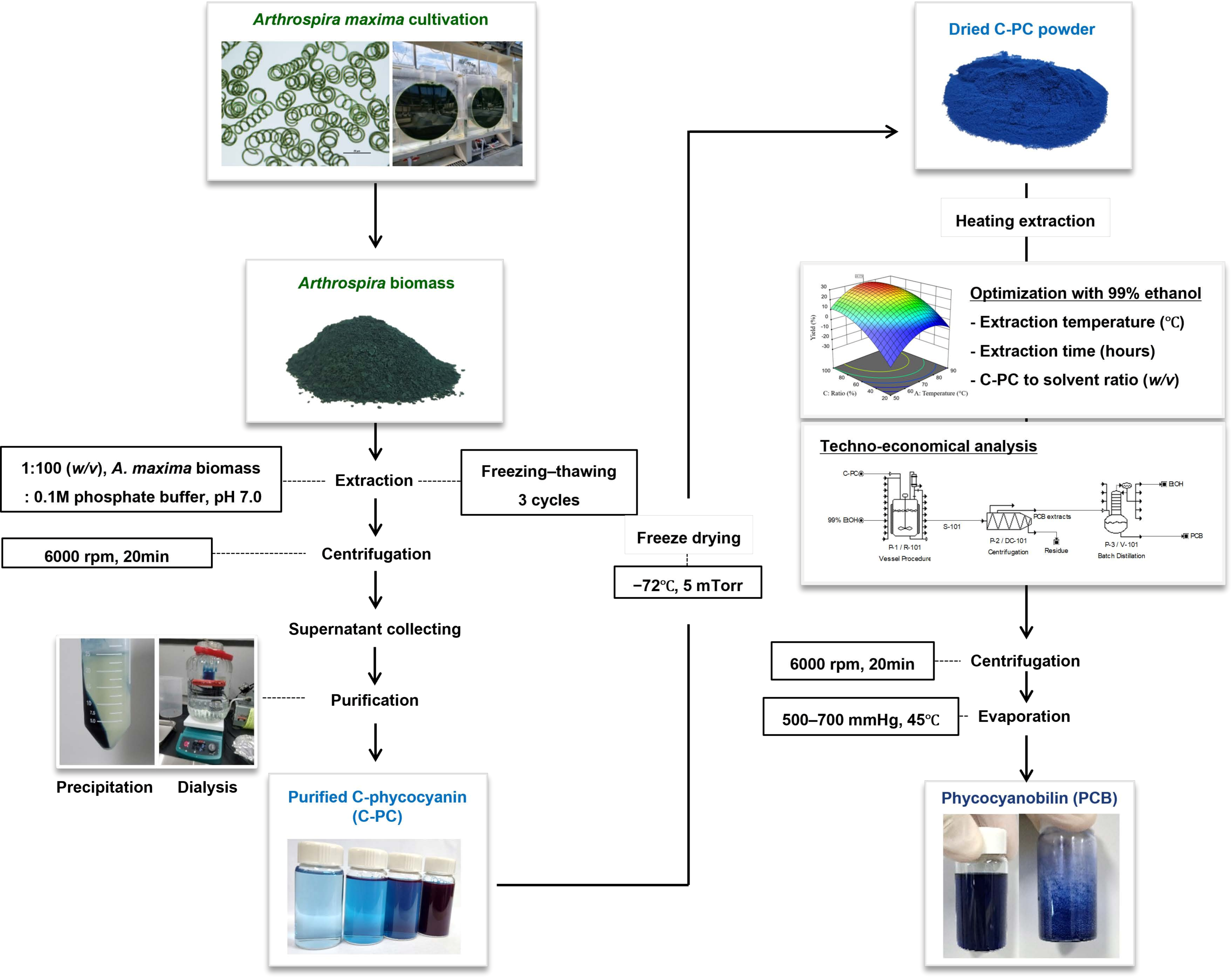
2.1. Microalgae Cultivation and Crude Phycocyanin Production
2.2. Purification of C-PC from Crude Phycocyanin
2.3. Extraction of PCB from C-PC
2.4. Experimental Design
2.5. High-Performance Liquid Chromatography of PCB
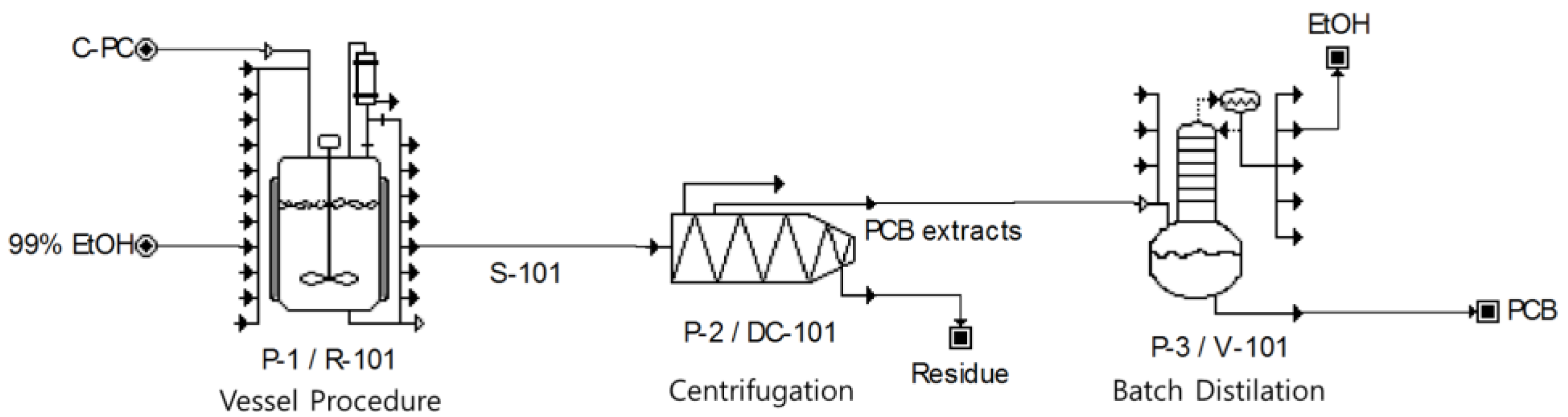
2.6. Techno-Economical Analysis
2.7. Schematic Diagram of Experiments
3. Results and Discussion
3.1. Statistical Analysis of the Quadratic Model
3.2. Process Parameter Interaction and PCB Yield Optimization
3.3. Characterization of PCB Extracted via Optimized Conditions
3.4. Techno-Economical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salazar, M.; Martínez, E.; Madrigal, E.; Ruiz, L.E.; Chamorro, G.A. Subchronic toxicity study in mice fed Spirulina maxima. J. Ethnopharmacol. 1998, 62, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.; Kyewalyanga, M.S.; Lugomela, C.V. Biomass and nutritive value of Spirulina (Arthrospira fusiformis) cultivated in a cost-effective medium. Ann. Microbiol. 2019, 69, 1387–1395. [Google Scholar] [CrossRef]
- Eriksen, N.T. Production of phycocyanin—A pigment with applications in biology, biotechnology, foods and medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.-H.; Zheng, B.-Y.; Ke, M.-R.; Duan, J.-Y.; Zheng, Y.-Q.; Yeh, C.-K.; Huang, J.-D. C-Phycocyanin as a tumour-associated macrophage-targeted photosensitiser and a vehicle of phthalocyanine for enhanced photodynamic therapy. Chem. Commun. 2017, 53, 4112–4115. [Google Scholar] [CrossRef]
- Jespersen, L.; Strømdahl, L.D.; Olsen, K.; Skibsted, L.H. Heat and light stability of three natural blue colorants for use in confectionery and beverages. Eur. Food Res. Technol. 2005, 220, 261–266. [Google Scholar] [CrossRef]
- de O Moreira, I.; Passos, T.S.; Chiapinni, C.; Silveira, G.K.; Souza, J.C.M.; Coca-Vellarde, L.G.; Deliza, R.; de Lima Araújo, K.G. Colour evaluation of a phycobiliprotein-rich extract obtained from Nostoc PCC9205 in acidic solutions and yogurt. J. Sci. Food Agric. 2012, 92, 598–605. [Google Scholar] [CrossRef]
- David, L.; Marx, A.; Adir, N. High-resolution crystal structures of trimeric and rod phycocyanin. J. Mol. Biol. 2011, 405, 201–213. [Google Scholar] [CrossRef]
- Wu, H.-L.; Wang, G.-H.; Xiang, W.-Z.; Li, T.; He, H. Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. Int. J. Food Prop. 2016, 19, 2349–2362. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Abbaspourrad, A. Improved pH stability, heat stability, and functionality of phycocyanin after PEGylation. Int. J. Biol. Macromol. 2022, 222, 1758–1767. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Roda-Serrat, M.C.; Christensen, K.V.; El-Houri, R.B.; Fretté, X.; Christensen, L.P. Fast cleavage of phycocyanobilin from phycocyanin for use in food colouring. Food Chem. 2018, 240, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Yarita, T.; Hasegawa, M.; Asayama, M. Development of a new extraction method and functional analysis of phycocyanobilin from unique filamentous cyanobacteria. J. Biotechnol. 2024, 395, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. The Bioactivities of phycocyanobilin from Spirulina. J. Immunol. Res. 2022, 2022, 4008991. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Clinical potential of Spirulina as a source of phycocyanobilin. J. Med. Food. 2007, 10, 566–570. [Google Scholar] [CrossRef]
- Hirata, T.; Tanaka, M.; Ooike, M.; Tsunomura, T.; Sakaguchi, M. Antioxidant activities of phycocyanobilin prepared from Spirulina platensis. J. Appl. Phycol. 2000, 12, 435–439. [Google Scholar] [CrossRef]
- Chapman, D.J.; Cole, W.J.; Siegelman, H.W. Cleavage of phycocyanobilin from C-phycocyanin. Biochim. Biophys. Acta. 1968, 153, 692–698. [Google Scholar] [CrossRef]
- Malwade, C.R.; Roda-Serrat, M.C.; Christensen, K.V.; Fretté, X.; Christensen, L.P. Kinetics of phycocyanobilin cleavage from C-phycocyanin by methanolysis. In Computer Aided Chemical Engineering; Kravanja, Z., Bogataj, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 61–66. [Google Scholar] [CrossRef]
- Chea, J.D.; Lehr, A.L.; Stengel, J.P.; Savelski, M.J.; Slater, C.S.; Yenkie, K.M. Evaluation of solvent recovery options for economic feasibility through a superstructure-based optimization framework. Ind. Eng. Chem. Res. 2020, 59, 5931–5944. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algal extracts: Technology and advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Box, G.E.P.; Wilson, K.B. On the experimental attainment of optimum conditions. In Breakthroughs in Statistics: Methodology and Distribution; Kotz, S., Johnson, N.L., Eds.; Springer: New York, NY, USA, 1992; pp. 270–310. [Google Scholar] [CrossRef]
- Yolmeh, M.; Jafari, S.M. Applications of response surface methodology in the food industry processes. Food Bioprocess Technol. 2017, 10, 413–433. [Google Scholar] [CrossRef]
- Choi, W.Y.; Lee, H.Y. Enhancement of chlorophyll a production from marine Spirulina maxima by an optimized ultrasonic extraction process. Appl. Sci. 2018, 8, 26. [Google Scholar] [CrossRef]
- Lee, W.-K.; Ryu, Y.-K.; Kim, T.; Park, A.; Lee, Y.-J.; Sunwoo, I.Y.; Koh, E.-J.; Oh, C.; Choi, W.-Y.; Kang, D.-H. Enhanced photosynthetic pigment production using a scaled-Up continuously circulated bioreactor. Mar. Drugs 2023, 21, 576. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, C. Contribution a I’etude D’une Cyanophycee. Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et la Photosynthese de Spirulina mixima. Ph.D. Thesis, University of Paris, Paris, France, 1966. [Google Scholar]
- Boussiba, S.; Richmond, A.E. Isolation and characterization of phycocyanins from the blue-green alga Spirulina platensis. Arch. Microbiol. 1979, 120, 155–159. [Google Scholar] [CrossRef]
- Kumar, D.; Dhar, D.W.; Pabbi, S.; Kumar, N.; Walia, S. Extraction and purification of C-phycocyanin from Spirulina platensis (CCC540). Indian J. Plant Physiol. 2014, 19, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Patil, G.; Chethana, S.; Sridevi, A.S.; Raghavarao, K.S.M.S. Method to obtain C-phycocyanin of high purity. J. Chromatogr. A 2006, 1127, 76–81. [Google Scholar] [CrossRef]
- Chang, J.; Shi, X.; Kim, M.; Lee, M.-E.; Han, S.O. Enhancing phycocyanobilin production efficiency in engineered Corynebacterium glutamicum: Strategies and potential application. J. Agric. Food Chem. 2024, 72, 12219–12228. [Google Scholar] [CrossRef]
- Sridhar, A.; Vaishampayan, V.; Senthil Kumar, P.; Ponnuchamy, M.; Kapoor, A. Extraction techniques in food industry: Insights into process parameters and their optimization. Food Chem. Toxicol. 2022, 166, 113207. [Google Scholar] [CrossRef]
- Beuhler, R.J.; Pierce, R.C.; Friedman, L.; Siegelman, H.W. Cleavage of phycocyanobilin from C-phycocyanin. Separation and mass spectral identification of the products. J. Biol. Chem. 1976, 251, 2405–2411. [Google Scholar] [CrossRef]
- Toxics Relase Inventory (TRI) Program. Available online: https://www.epa.gov/toxics-release-inventory-tri-program/tri-listed-chemicals (accessed on 12 January 2022).


| Factor | Level | ||||
|---|---|---|---|---|---|
| –2 | –1 | 0 | 1 | 2 | |
| A: Extraction temperature (°C) | 50 | 60 | 70 | 80 | 90 |
| B: Extraction time (h) | 8 | 12 | 16 | 20 | 24 |
| C: C-PC to solvent ratio (w/v) | 20 (1:20) | 40 (1:40) | 60 (1:60) | 80 (1:80) | 100 (1:100) |
| Run | A: Temperature (°C) | B: Time (h) | C: C-PC to Solvent Ratio (w/v) | Yield (%) |
|---|---|---|---|---|
| 1 | 60 | 12 | 40 (1:40) | 3.8 |
| 2 | 80 | 12 | 40 (1:40) | 9.21 |
| 3 | 70 | 16 | 60 (1:60) | 26.28 |
| 4 | 70 | 16 | 60 (1:60) | 24.91 |
| 5 | 70 | 16 | 60 (1:60) | 24.01 |
| 6 | 70 | 24 | 60 (1:60) | 22.45 |
| 7 | 60 | 12 | 80 (1:80) | 22.33 |
| 8 | 70 | 16 | 20 (1:20) | 1.42 |
| 9 | 70 | 16 | 60 (1:60) | 24.61 |
| 10 | 70 | 16 | 60 (1:60) | 23.25 |
| 11 | 50 | 16 | 60 (1:60) | 4.68 |
| 12 | 60 | 20 | 80 (1:80) | 18.29 |
| 13 | 70 | 16 | 60 (1:60) | 24.36 |
| 14 | 60 | 20 | 40 (1:40) | 5.14 |
| 15 | 80 | 12 | 80 (1:80) | 23.34 |
| 16 | 80 | 20 | 80 (1:80) | 27.92 |
| 17 | 70 | 8 | 60 (1:60) | 17.58 |
| 18 | 80 | 20 | 40 (1:40) | 15.54 |
| 19 | 90 | 16 | 60 (1:60) | 16.77 |
| 20 | 70 | 16 | 100 (1:100) | 29.89 |
| Source | Sum of Squares | Df | Mean Squares | F Value | p Value |
|---|---|---|---|---|---|
| Model | 1448.13 | 9 | 160.90 | 62.84 | <0.0001 a |
| A: Extraction temperature | 160.21 | 1 | 160.21 | 62.57 | <0.0001 a |
| B: Extraction time | 20.14 | 1 | 20.14 | 7.87 | 0.0186 a |
| C: C-PC to solvent ratio | 828.43 | 1 | 828.43 | 323.56 | <0.0001 a |
| AB | 23.15 | 1 | 23.15 | 9.04 | 0.0132 a |
| AC | 3.34 | 1 | 3.34 | 1.30 | 0.2799 b |
| BC | 6.35 | 1 | 6.35 | 2.48 | 0.1462 b |
| A2 | 334.44 | 1 | 334.44 | 130.63 | <0.0001 a |
| B2 | 44.12 | 1 | 44.12 | 17.23 | 0.0020 a |
| C2 | 146.60 | 1 | 146.60 | 57.26 | <0.0001 a |
| Residual | 25.60 | 10 | 2.56 | ||
| Lack-of-Fit | 20.46 | 5 | 4.09 | 3.98 | 0.0779 b |
| Pure Error | 5.14 | 5 | 1.03 | ||
| Cor Total | 1473.73 | 19 | |||
| R2 | 0.9826 |
| Variable | Yield | |||
|---|---|---|---|---|
| Extraction Temperature (°C) | Extraction Time (h) | C-PC to Solvent Ratio (w/v) | (%) | |
| CCD (theoretical) | 68.81 | 14.91 | 1:95 | 29.18 |
| Validation (actual) | 69 | 14.91 | 1:95 | 29.67 ± 1.33 |
| Parameter | Methanol Extraction | Ethanol Extraction |
|---|---|---|
| Investment charged to this project | USD 21,633,680 | USD 27,867,186 |
| Annual operating cost | USD 131,959,686/year | USD 207,987,258/year |
| Annual revenues | USD 134,902,668/year | USD 235,414,870/year |
| Unit production ref. rate | 84.31 kg MP 1/year | 147.13 kg MP 1/year |
| Unit production cost | USD 1,565,095/kg MP 1 | USD 1,413,588/kg MP 1 |
| Unit production revenue | USD 1,600,000/kg MP 1 | USD 1,600,000/kg MP 1 |
| Gross margin | 2.18% | 11.65% |
| Return on investment (ROI) | 12.75% | 62.61% |
| Payback time | 7.84 years | 1.60 years |
| Internal rate of return (IRR) | 8.20% | 53.36% |
| Net present value (NPV, at 7.00%) | USD 1,749,872 | USD 103,815,276 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-K.; Sunwoo, I.-Y.; Kim, J.; Ryu, Y.-K.; Koh, E.-J.; Kim, T.; Choi, W.-Y. Process Optimization and Techno-Economic Analysis for the Production of Phycocyanobilin from Arthrospira maxima-Derived C-Phycocyanin. Appl. Sci. 2024, 14, 11440. https://doi.org/10.3390/app142311440
Lee W-K, Sunwoo I-Y, Kim J, Ryu Y-K, Koh E-J, Kim T, Choi W-Y. Process Optimization and Techno-Economic Analysis for the Production of Phycocyanobilin from Arthrospira maxima-Derived C-Phycocyanin. Applied Sciences. 2024; 14(23):11440. https://doi.org/10.3390/app142311440
Chicago/Turabian StyleLee, Won-Kyu, In-Yung Sunwoo, Junseong Kim, Yong-Kyun Ryu, Eun-Jeong Koh, Taeho Kim, and Woon-Yong Choi. 2024. "Process Optimization and Techno-Economic Analysis for the Production of Phycocyanobilin from Arthrospira maxima-Derived C-Phycocyanin" Applied Sciences 14, no. 23: 11440. https://doi.org/10.3390/app142311440
APA StyleLee, W.-K., Sunwoo, I.-Y., Kim, J., Ryu, Y.-K., Koh, E.-J., Kim, T., & Choi, W.-Y. (2024). Process Optimization and Techno-Economic Analysis for the Production of Phycocyanobilin from Arthrospira maxima-Derived C-Phycocyanin. Applied Sciences, 14(23), 11440. https://doi.org/10.3390/app142311440






