Smart Poly(acrylic acid)/Poly(acrylamide) Microgels with Interpenetrating Polymer Network Structure
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. IPN MG Synthesis
2.2.2. Characterization of MG
- Dynamic Light Scattering (DLS)
- Zeta Potential ξ (ZP) measurements
- pH titration
- Transmission Electron Microscopy (TEM)
- Nuclear Magnetic Resonance (NMR)
- Asymmetrical Flow Field-Flow Fractionation (AF4)
3. Results
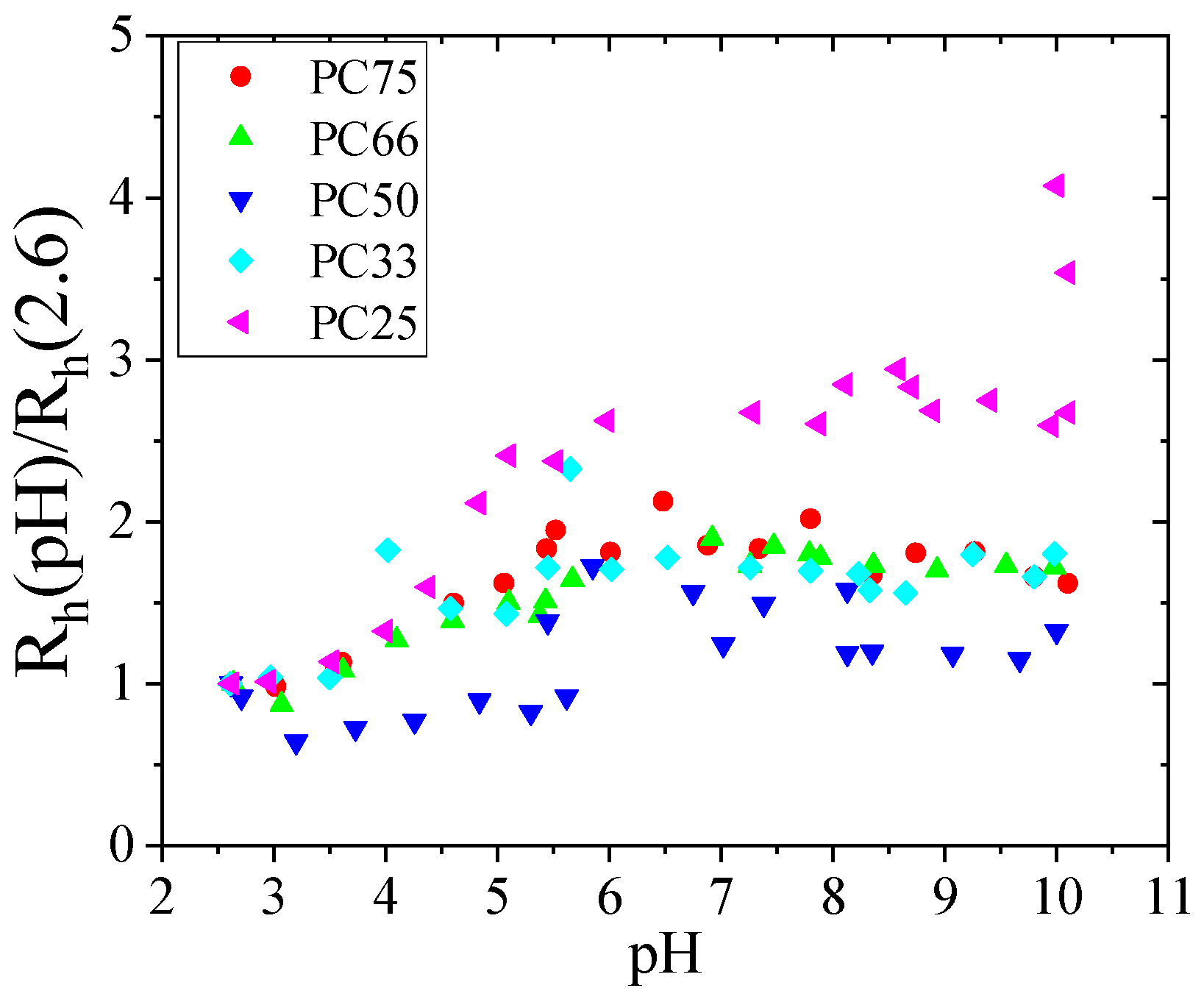
3.1. pH Responsiveness of PAA/PAAM IPN MGs
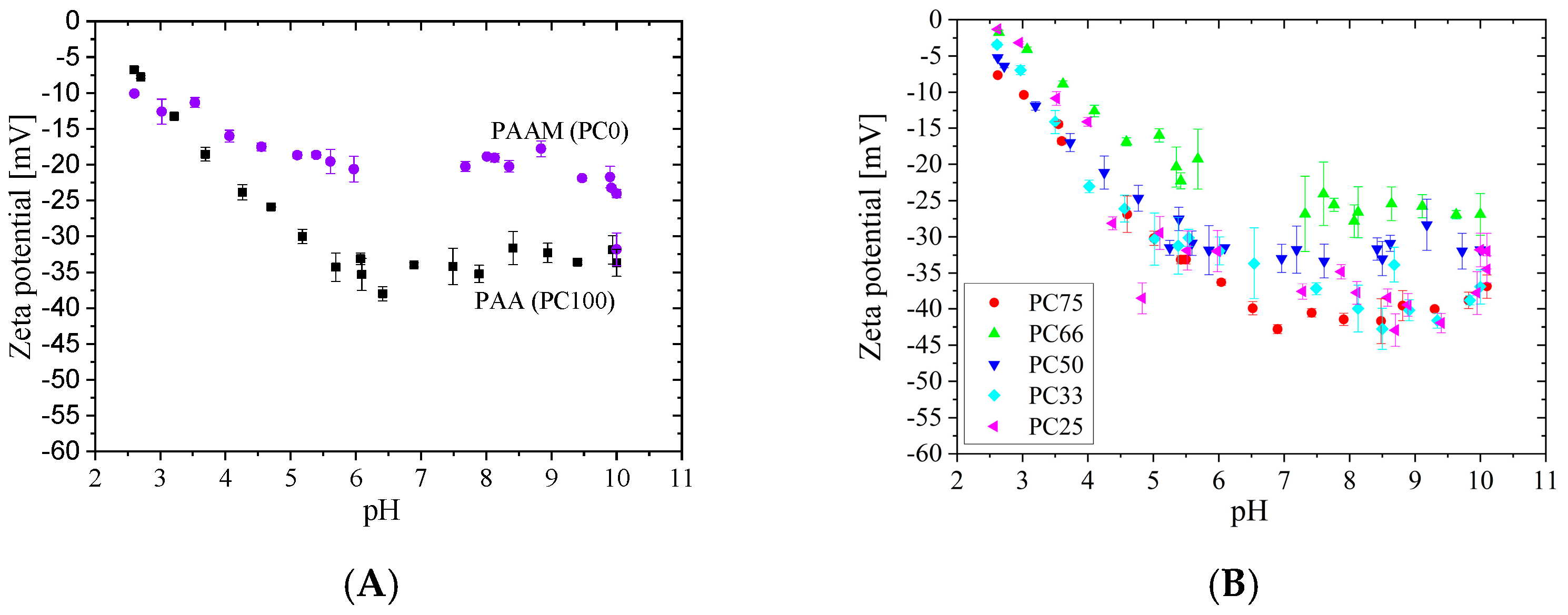
3.2. pH-Dependent Zeta Potential (ZP) of MG
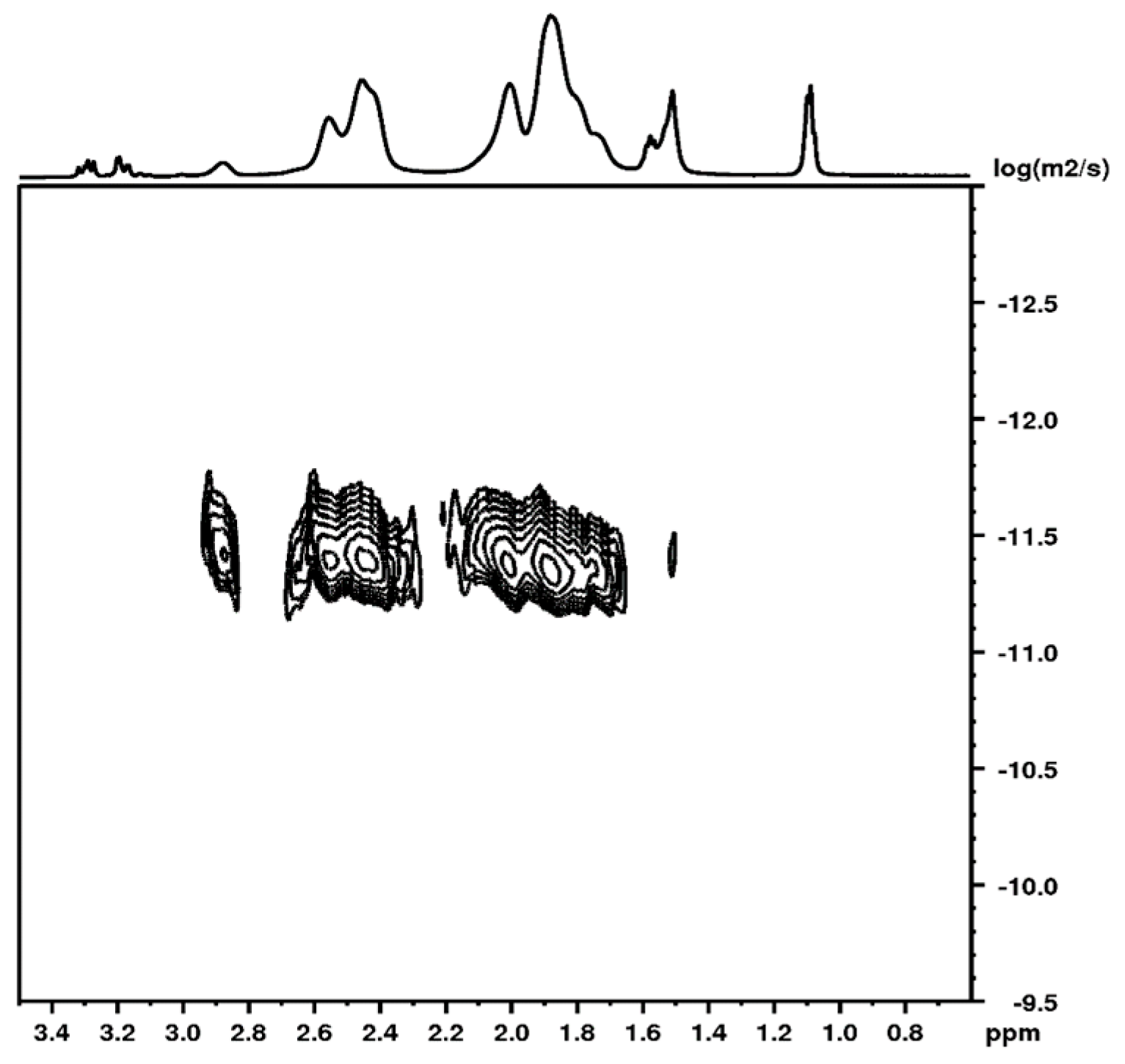
3.3. NMR Study of IPN MGs
3.4. Transmission Electron Microscopy (TEM) Study of IPN MGs
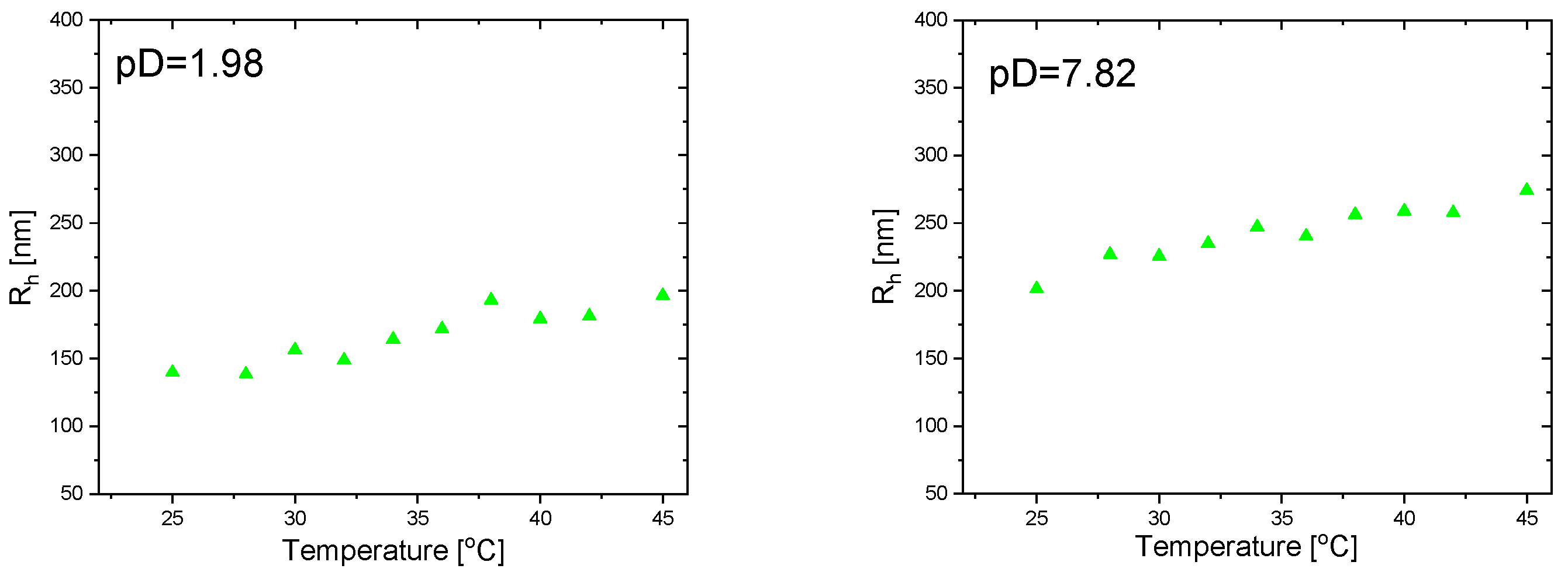
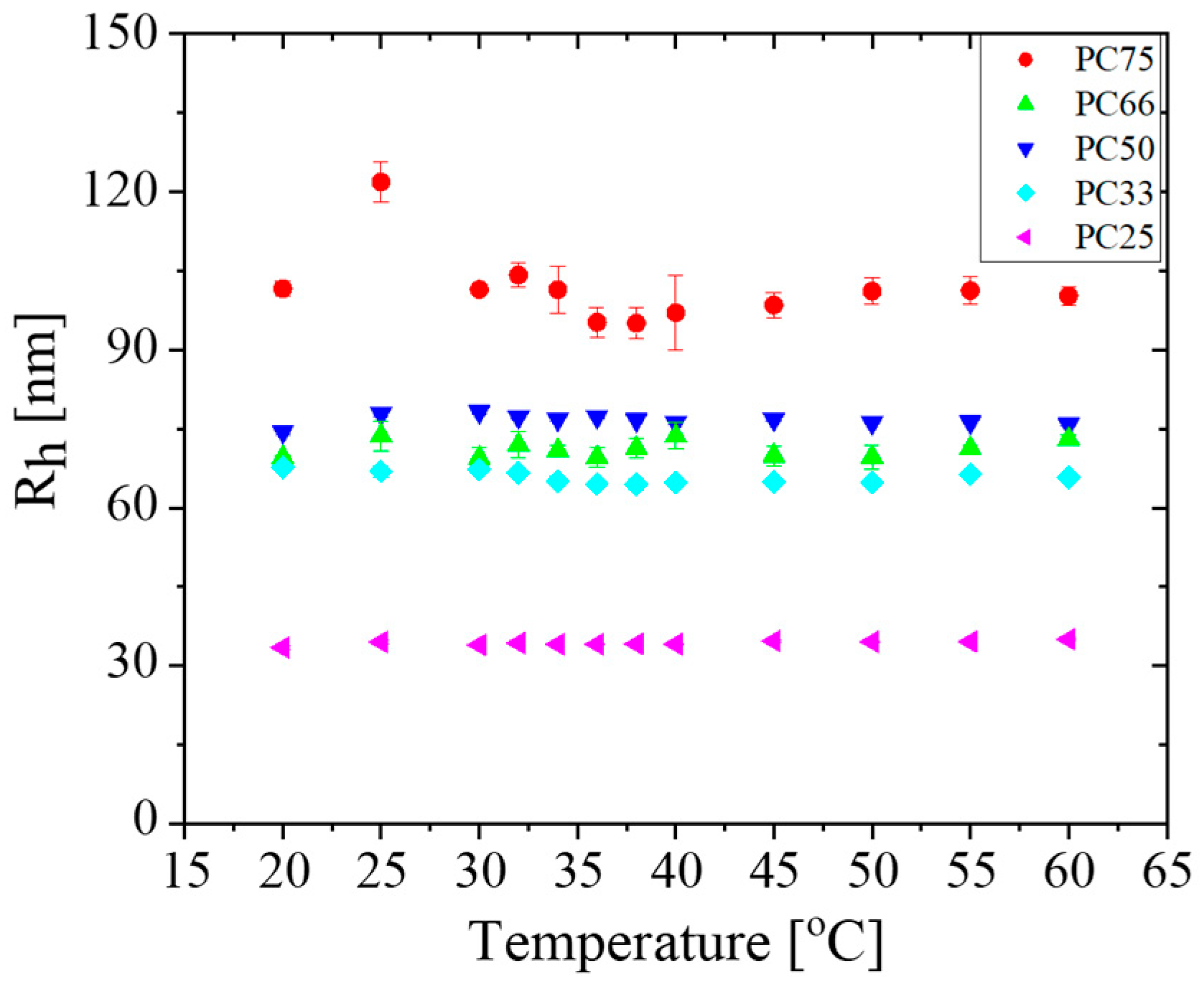
3.5. Temperature Responsiveness of IPN MGs
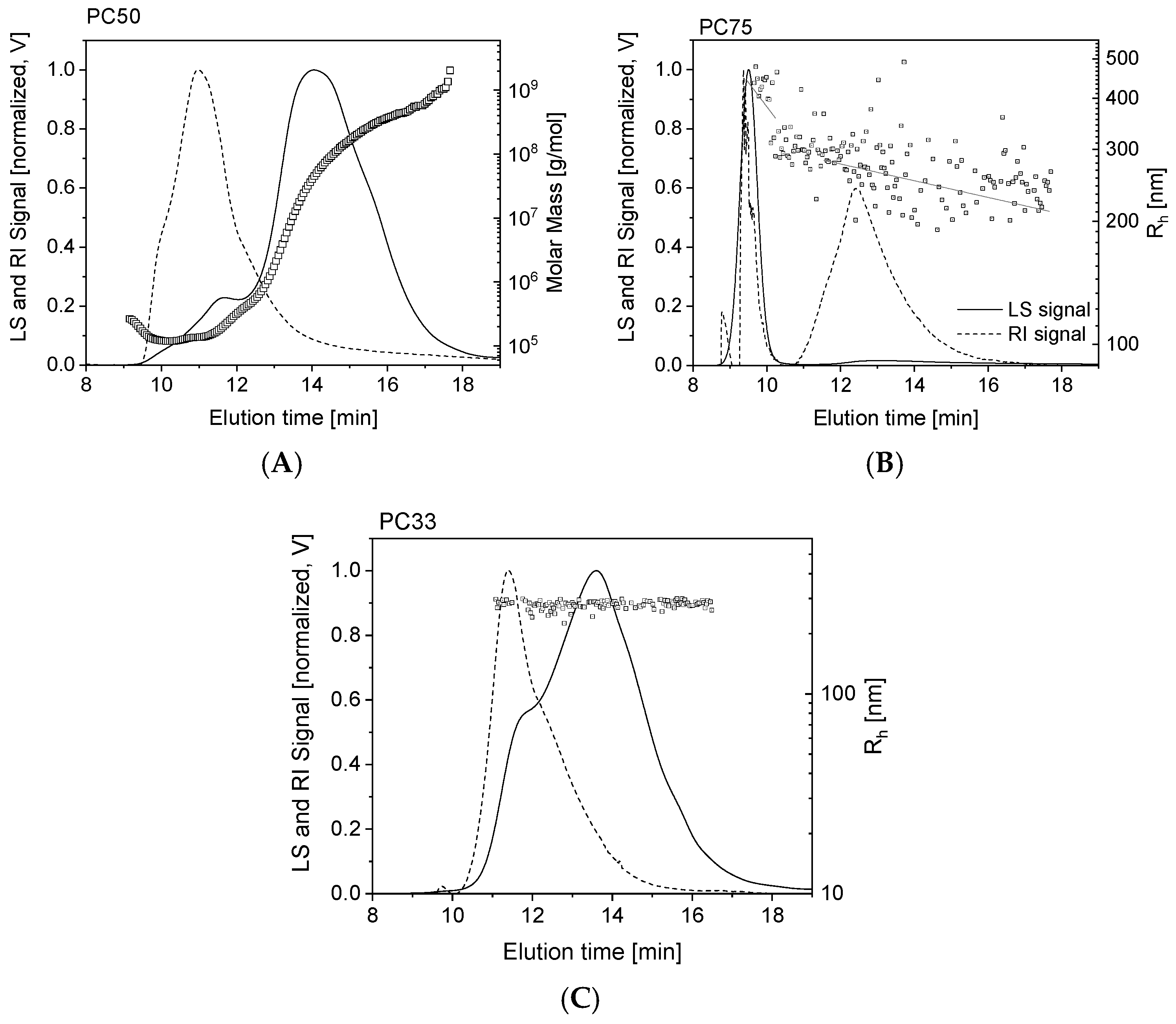
3.6. Asymmetrical Flow Field-Flow Fractionation (AF4)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanson, N.; Rieger, J. Synthesis of Nanogels/Microgels by Conventional and Controlled Radical Crosslinking Copolymerization. Polym. Chem. 2010, 1, 965. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yao, W.; Rao, Y.; Lu, X.; Gao, J. pH-Responsive Carriers for Oral Drug Delivery: Challenges and Opportunities of Current Platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Hiroi, T.; Hashimoto, K.; Fujii, K.; Han, Y.S.; Kim, T.H.; Katsumoto, Y.; Shibayama, M. SANS and DLS Study of Tacticity Effects on Hydrophobicity and Phase Separation of Poly(N-Isopropylacrylamide). Macromolecules 2013, 46, 6225–6232. [Google Scholar] [CrossRef]
- Ruff, K.M.; Roberts, S.; Chilkoti, A.; Pappu, R.V. Advances in Understanding Stimulus-Responsive Phase Behavior of Intrinsically Disordered Protein Polymers. J. Mol. Biol. 2018, 430, 4619–4635. [Google Scholar] [CrossRef]
- Jia, Y.-G.; Chen, K.-F.; Gao, M.; Liu, S.; Wang, J.; Chen, X.; Wang, L.; Chen, Y.; Song, W.; Zhang, H.; et al. Visualizing Phase Transition of Upper Critical Solution Temperature (UCST) Polymers with AIE. Sci. China Chem. 2020, 64, 403–407. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Peppas, N.A. Dynamic and Equilibrium Swelling Behaviour of PH-Sensitive Hydrogels Containing 2-Hydroxyethyl Methacrylate. Biomaterials 1990, 11, 635–644. [Google Scholar] [CrossRef]
- Wang, L.; Ren, J.; Yao, M.; Yang, X.; Yang, W.; Li, Y. Synthesis and Characterization of Self-Oscillating P(AA-Co-AM)/PEG Semi-IPN Hydrogels Based on a PH Oscillator in Closed System. Chin. J. Polym. Sci. 2014, 32, 1581–1589. [Google Scholar] [CrossRef]
- Saruchi; Kaith, B.S.; Jindal, R.; Kumar, V.; Bhatti, M.S. Optimal Response Surface Design of Gum Tragacanth-Based Poly[(Acrylic Acid)-Co-Acrylamide] IPN Hydrogel for the Controlled Release of the Antihypertensive Drug Losartan Potassium. RSC Adv. 2014, 4, 39822–39829. [Google Scholar] [CrossRef]
- Simeonov, M.; Kostova, B.; Vassileva, E. Interpenetrating Polymer Networks of Poly(Acrylic Acid) and Polyacrylamide for Sustained Verapamil Hydrochloride Release. Macromol. Symp. 2015, 358, 225–231. [Google Scholar] [CrossRef]
- Xiao, X.C.; Chu, L.Y.; Chen, W.M.; Zhu, J.H. Monodispersed Thermoresponsive Hydrogel Microspheres with a Volume Phase Transition Driven by Hydrogen Bonding. Polymer 2005, 46, 3199–3209. [Google Scholar] [CrossRef]
- Owens, D.E.; Eby, J.K.; Jian, Y.; Peppas, N.A. Temperature-Responsive Polymer–Gold Nanocomposites as Intelligent Therapeutic Systems. J. Biomed. Mater. Res. A 2007, 83A, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Bouillot, P.; Vincent, B. A Comparison of the Swelling Behaviour of Copolymer and Interpenetrating Network Microgel Particles. Colloid. Polym. Sci. 2000, 278, 74–79. [Google Scholar] [CrossRef]
- Boudraa, K.; Bouchaour, T. Investigating Physical Behavior of Polyacrylamide/Polyacrylic Acid Interpenetrating Polymer Networks through Atomistic Molecular Dynamics Simulations. Mech. Soft Mater. 2021, 3, 8. [Google Scholar] [CrossRef]
- Simeonov, M.S.; Apostolov, A.A.; Vassileva, E.D. In Situ Calcium Phosphate Deposition in Hydrogels of Poly(Acrylic Acid)–Polyacrylamide Interpenetrating Polymer Networks. RSC Adv. 2016, 6, 16274–16284. [Google Scholar] [CrossRef]
- Katono, H.; Maruyama, A.; Sanui, K.; Ogata, N.; Okano, T.; Sakurai, Y. Thermo-Responsive Swelling and Drug Release Switching of Interpenetrating Polymer Networks Composed of Poly(Acrylamide-Co-Butyl Methacrylate) and Poly (Acrylic Acid). J. Control. Release 1991, 16, 215–227. [Google Scholar] [CrossRef]
- Katono, H.; Sanui, K.; Ogata, N.; Okano, T.; Sakurai, Y. Drug Release off Behavior and Deswelling Kinetics of Thermo-Responsive IPNs Composed of Poly(Acrylamide-Co-Butyl Methacrylate) and Poly(Acrylic Acid). Polym. J. 1991, 23, 1179–1189. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Wang, Z.; Liu, H.; Li, C. Preparation and Characterization of a Positive Thermoresponsive Hydrogel for Drug Loading and Release. J. Appl. Polym. Sci. 2009, 111, 1417–1425. [Google Scholar] [CrossRef]
- Owens, D.E.; Jian, Y.; Fang, J.E.; Slaughter, B.V.; Chen, Y.-H.; Peppas, N.A. Thermally Responsive Swelling Properties of Polyacrylamide/Poly(Acrylic Acid) Interpenetrating Polymer Network Nanoparticles. Macromolecules 2007, 40, 7306–7310. [Google Scholar] [CrossRef]
- Alvarado, A.G.; Cortés, J.A.; Pérez-Carrillo, L.A.; Rabelero, M.; Arellano, J.; Sánchez-Díaz, J.C.; Puig, J.E.; Arellano, M. Temperature and PH-Responsive Polyacrylamide/Poly(Acrylic Acid) Interpenetrating Polymer Network Nanoparticles. J. Macromol. Sci. B 2016, 55, 1086–1098. [Google Scholar] [CrossRef]
- Simeonov, M.; Monova, A.; Kostova, B.; Vassileva, E. Drug Transport in Stimuli Responsive Acrylic and Methacrylic Interpenetrating Polymer Networks. J. Appl. Polym. Sci. 2017, 134, 45380. [Google Scholar] [CrossRef]
- Acrylamide|79-06-1. ChemicalBook. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB4690458.htm (accessed on 15 August 2023).
- Gebhardt, J.E.; Fuerstenau, D.W. Adsorption of Polyacrylic Acid at Oxide/Water Interfaces. Colloids Surf. 1983, 7, 221–231. [Google Scholar] [CrossRef]
- Edwards, M.; Benjamin, M.M.; Ryan, J.N. Role of Organic Acidity in Sorption of Natural Organic Matter (NOM) to Oxide Surfaces. Colloids Surf. A Physicochem. Eng. Asp. 1996, 107, 297–307. [Google Scholar] [CrossRef]
- Jiang, M.; Li, M.; Xiang, M.; Zhou, H. Interpolymer Complexation and Miscibility Enhancement by Hydrogen Bonding. In Polymer Synthesis/Polymer-Polymer Complexation; Springer: Berlin/Heidelberg, Germany, 1999; pp. 121–196. [Google Scholar] [CrossRef]
- Daubreasse, C.; Grandols, C.; Jerome, R.; Teyssie, P. Enzyme immobilization in reactive nanoparticles produced by inverse microemulsion polymerization. Colloid Polym. Sci. 1996, 274, 482–489. [Google Scholar] [CrossRef]
- Boye, S.; Ennen, F.; Scharfenberg, L.; Appelhans, D.; Nilsson, L.; Lederer, A. From 1D Rods to 3D Networks: A Biohybrid Topological Diversity Investigated by Asymmetrical Flow Field-Flow Fractionation. Macromolecules 2015, 48, 4607–4619. [Google Scholar] [CrossRef]
- Karnoosh-Yamchi, J.; Mobasseri, M.; Akbarzadeh, A.; Davaran, S.; Ostad-Rahimi, A.R.; Hamishehkar, H.; Salehi, R.; Bahmani, Z.; Nejati-Koshki, K.; Darbin, A.; et al. Preparation of PH Sensitive Insulin-Loaded Nano Hydrogels and Evaluation of Insulin Releasing in Different PH Conditions. Mol. Biol. Rep. 2014, 41, 6705–6712. [Google Scholar] [CrossRef]
- Wahlgren, M.; Christensen, K.L.; Jørgensen, E.V.; Svensson, A.; Ulvenlund, S. Oral-Based Controlled Release Formulations Using Poly(Acrylic Acid) Microgels. Drug Dev. Ind. Pharm. 2009, 35, 922–929. [Google Scholar] [CrossRef]
- Knipe, J.M.; Strong, L.E.; Peppas, N.A. Enzyme- and PH-Responsive Microencapsulated Nanogels for Oral Delivery of SiRNA to Induce TNF-α Knockdown in the Intestine. Biomacromolecules 2016, 17, 788–797. [Google Scholar] [CrossRef]
- Singh, J.; Nayak, P. pH-Responsive Polymers for Drug Delivery: Trends and Opportunities. J. Polym. Sci. 2023, 61, 2828–2850. [Google Scholar] [CrossRef]
- Lamprecht, A.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y. pH-Sensitive Microsphere Delivery Increases Oral Bioavailability of Calcitonin. J. Control. Release 2004, 98, 1–9. [Google Scholar] [CrossRef]
- Ahmad, N.; Mohd Amin, M.C.I.; Ismail, I.; Buang, F. Enhancement of Oral Insulin Bioavailability: In Vitro and in Vivo Assessment of Nanoporous Stimuli-Responsive Hydrogel Microparticles. Expert Opin. Drug Deliv. 2016, 13, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Berthold, A.; Cremer, K.; Kreuter, J. Preparation and Characterization of Chitosan Microspheres as Drug Carrier for Prednisolone Sodium Phosphate as Model for Anti-Inflammatory Drugs. J. Control. Release 1996, 39, 17–25. [Google Scholar] [CrossRef]
- Baghersad, M.H.; Jamshidi, S.; Habibi, A.; Salimi, A. Synthesis, Characterization, and in Vitro Evaluation of Super Paramagnetic Nanoparticles Grafted with PAMPS for Controlled Delivery of Cationic Drugs. ChemistrySelect 2019, 4, 810–815. [Google Scholar] [CrossRef]
- Mun, G.A.; Nurkeeva, Z.S.; Khutoryanskiy, V.V.; Sarybayeva, G.S.; Dubolazov, A.V. PH-Effects in the Complex Formation of Polymers I. Interaction of Poly(Acrylic Acid) with Poly(Acrylamide). Eur. Polym. J. 2003, 39, 1687–1691. [Google Scholar] [CrossRef]
- Sharma, S.; Shukla, P.; Misra, A.; Mishra, P. Interfacial and Colloidal Properties of Emulsified Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 149–172. [Google Scholar] [CrossRef]
- Agrawal, G.; Agrawal, R. Stimuli-Responsive Microgels and Microgel-Based Systems: Advances in the Exploitation of Microgel Colloidal Properties and Their Interfacial Activity. Polymers 2018, 10, 418. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Dagdelen, S.; Marcisz, K.; Waleka-Bargiel, E.; Stojek, Z.; Karbarz, M. Redox-Degradable Microgel Based on Poly(Acrylic Acid) as Drug-Carrier with Very High Drug-Loading Capacity and Decreased Toxicity against Healthy Cells. Polym. Degrad. Stab. 2021, 190, 109652. [Google Scholar] [CrossRef]
- Ghorbaniazar, P.; Sepehrianazar, A.; Eskandani, M.; Nabi-Meibodi, M.; Kouhsoltani, M.; Hamishehkar, H. Preparation of Poly Acrylic Acid-Poly Acrylamide Composite Nanogels by Radiation Technique. Adv. Pharm. Bull. 2015, 5, 269–275. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Park, J.-S.; Kim, E.J.; Jeong, S.-I.; Lee, J.Y.; Lim, Y.-M. Preparation of Radiation Cross-Linked Poly(Acrylic Acid) Hydrogel Containing Metronidazole with Enhanced Antibacterial Activity. Int. J. Mol. Sci. 2019, 21, 187. [Google Scholar] [CrossRef]
- Ersoz, M.; Allahverdiyev, A. Investigation of Polyacrylic Acid Toxicity in Human Breast Cancer (MCF-7) and Mouse Fibroblast (L-929) Cell Lines. EuroBiotech J. 2021, 5, 123–129. [Google Scholar] [CrossRef]





| Sample Designation | Initial Monomer Feed | IPN MGs Composition | |
|---|---|---|---|
| PAA/PAAM IPN MGs | AA [mol. %] | AAM [mol. %] | * |
| PC100 | 100 | 0 | 1 |
| PC75 | 75 | 25 | 0.77 |
| PC67 | 67 | 33 | 0.69 |
| PC50 | 50 | 50 | 0.53 |
| PC33 | 33 | 67 | 0.35 |
| PC25 | 25 | 75 | 0.27 |
| PC0 | 0 | 100 | 0 |
| Sample | dn/dc (mL/g) | Mw (kg/mol) | Ð (Mw/Mn) | Rg ** (nm) | Rh (nm) | ρ (Rg/Rh) |
|---|---|---|---|---|---|---|
| PC0 | 0.142 | 48,700 | 1.02 | 261 | 168 | 1.55 |
| PC25 | 0.140 | 50,900 | 1.02 | 289 | 203 | 1.42 |
| PC33 | 0.136 | 6940 | 3.47 | 198 | 280 | 0.71 |
| PC50 | 0.132 | 7560 | 3.37 | 233 | 294 | 0.79 |
| PC66 | 0.129 | 7530 | 1.33 | 175 | 272 | 0.64 |
| PC75 * | 0.128 | 15,900 | 1.42 | 421 | 244 | 1.73 |
| PC100 | 0.126 | 503,000 | 4.51 | 400 | 243 | 1.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simeonov, M.; Shestakova, P.; Boye, S.; Lederer, A.; Vassileva, E. Smart Poly(acrylic acid)/Poly(acrylamide) Microgels with Interpenetrating Polymer Network Structure. Appl. Sci. 2024, 14, 11562. https://doi.org/10.3390/app142411562
Simeonov M, Shestakova P, Boye S, Lederer A, Vassileva E. Smart Poly(acrylic acid)/Poly(acrylamide) Microgels with Interpenetrating Polymer Network Structure. Applied Sciences. 2024; 14(24):11562. https://doi.org/10.3390/app142411562
Chicago/Turabian StyleSimeonov, Marin, Pavletta Shestakova, Susanne Boye, Albena Lederer, and Elena Vassileva. 2024. "Smart Poly(acrylic acid)/Poly(acrylamide) Microgels with Interpenetrating Polymer Network Structure" Applied Sciences 14, no. 24: 11562. https://doi.org/10.3390/app142411562
APA StyleSimeonov, M., Shestakova, P., Boye, S., Lederer, A., & Vassileva, E. (2024). Smart Poly(acrylic acid)/Poly(acrylamide) Microgels with Interpenetrating Polymer Network Structure. Applied Sciences, 14(24), 11562. https://doi.org/10.3390/app142411562






