Crystallization Kinetics of an Equimolar Liquid Crystalline Mixture and Its Components
Abstract
1. Introduction
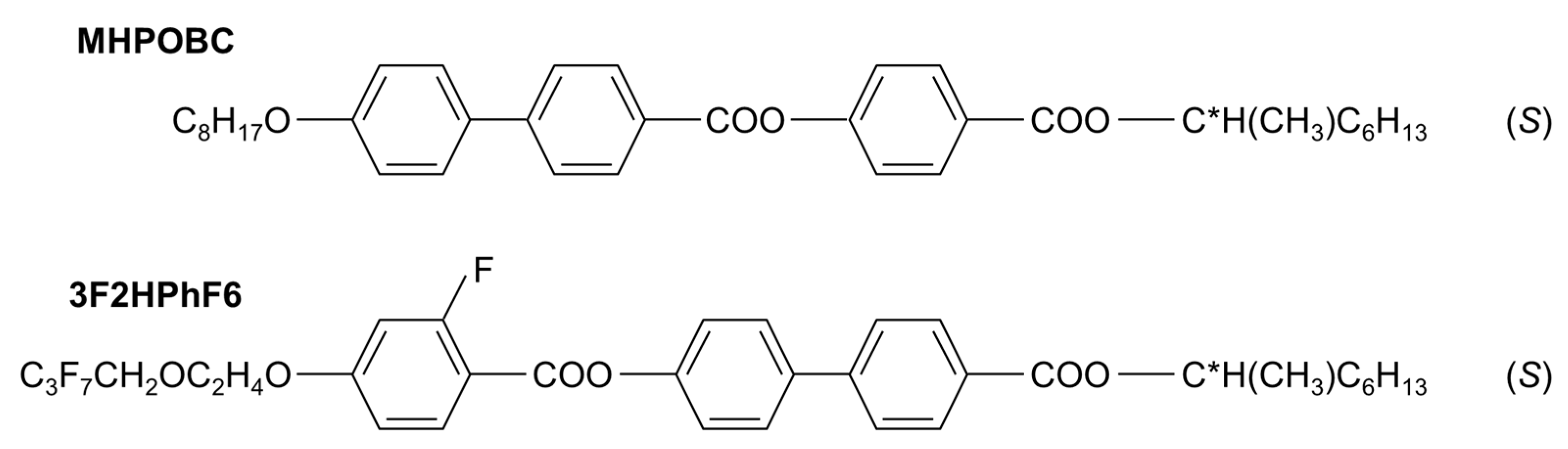
2. Materials and Methods
3. Results and Discussion
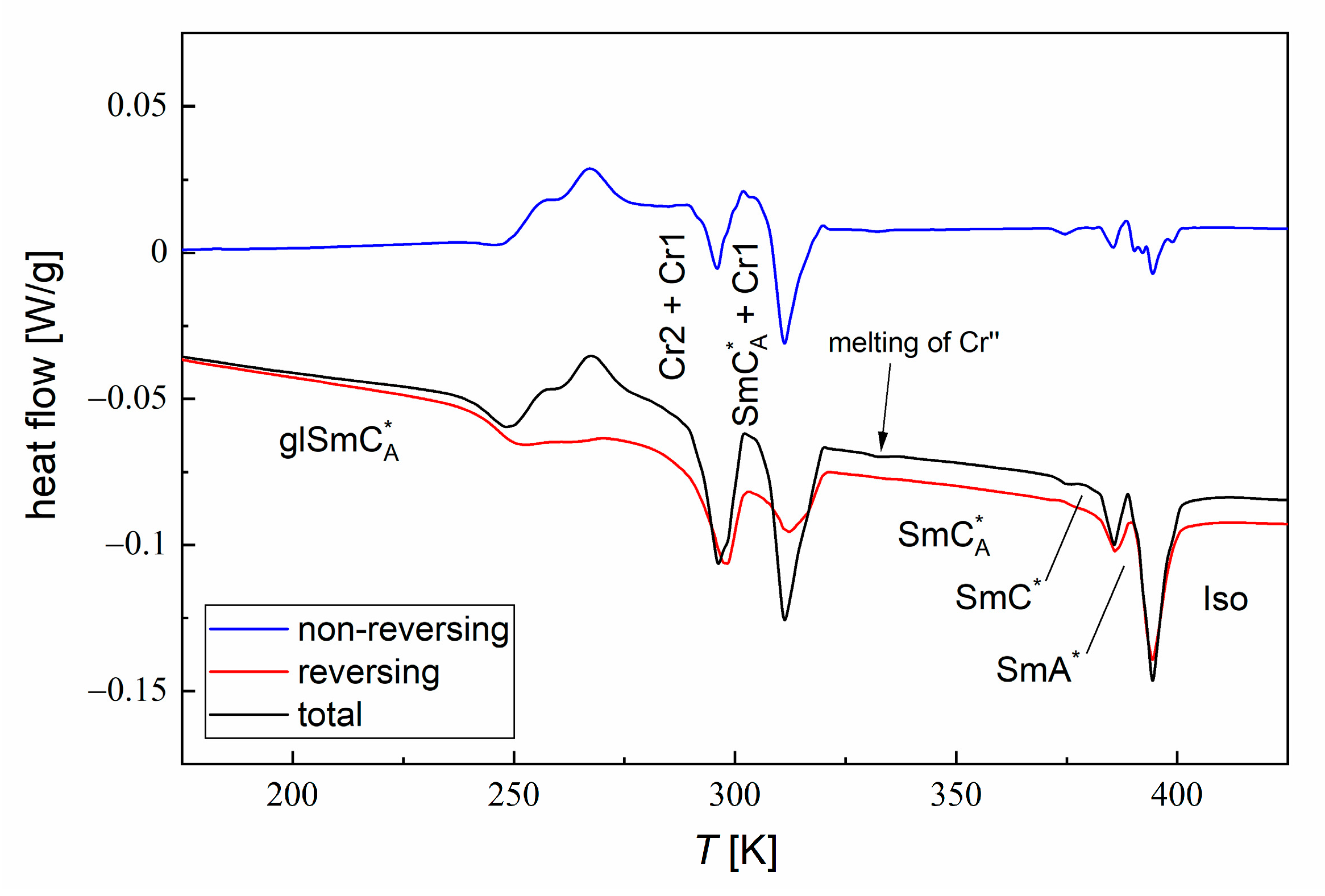
3.1. Phase Sequence
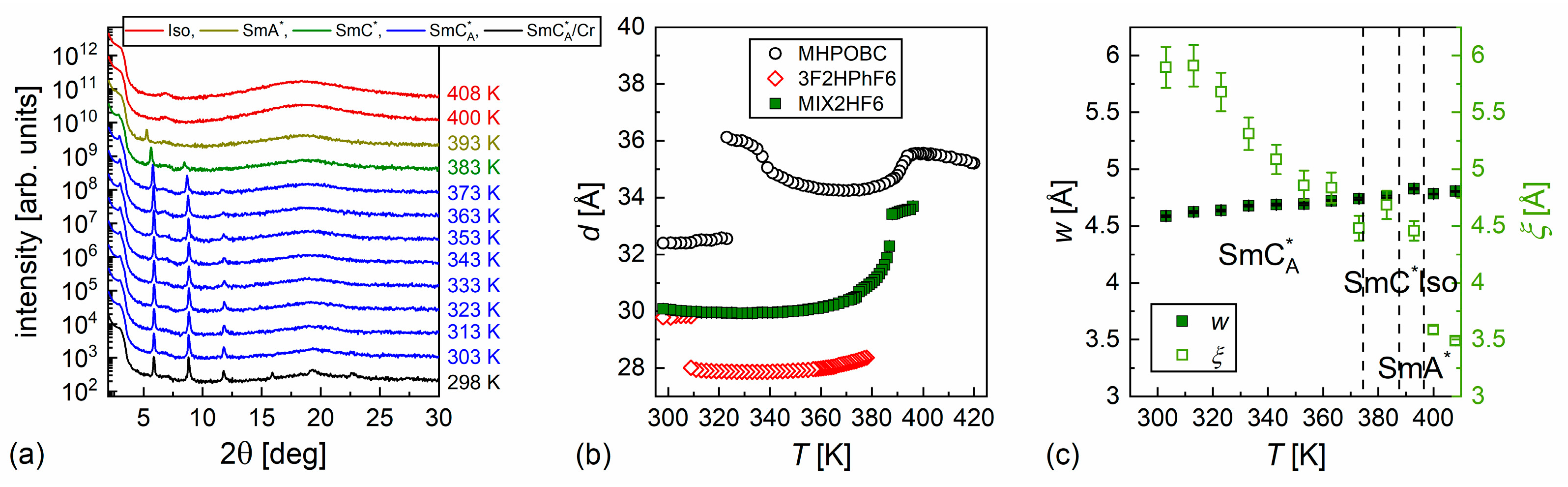
3.2. Structure of Smectic Phases
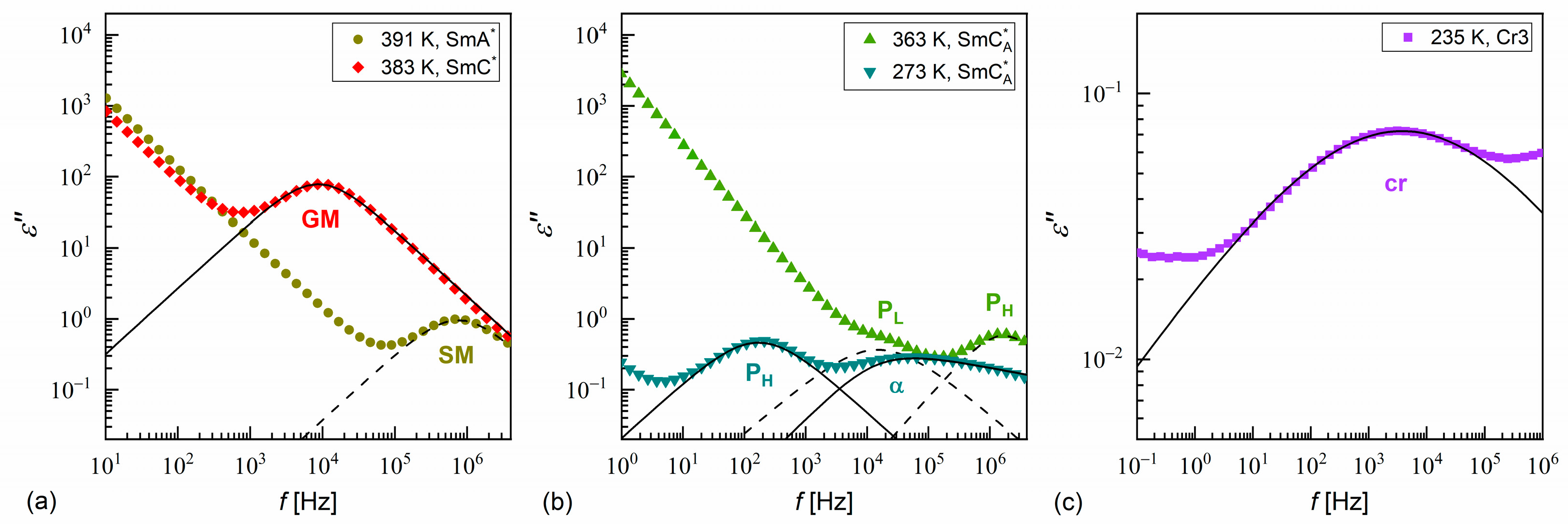
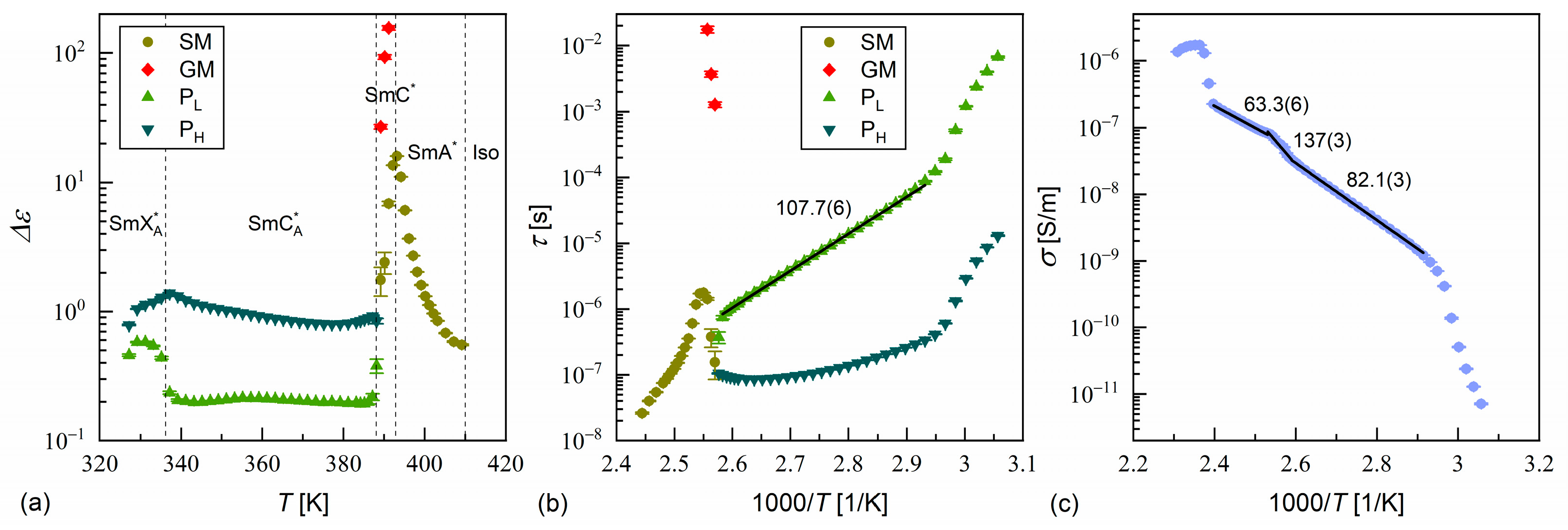
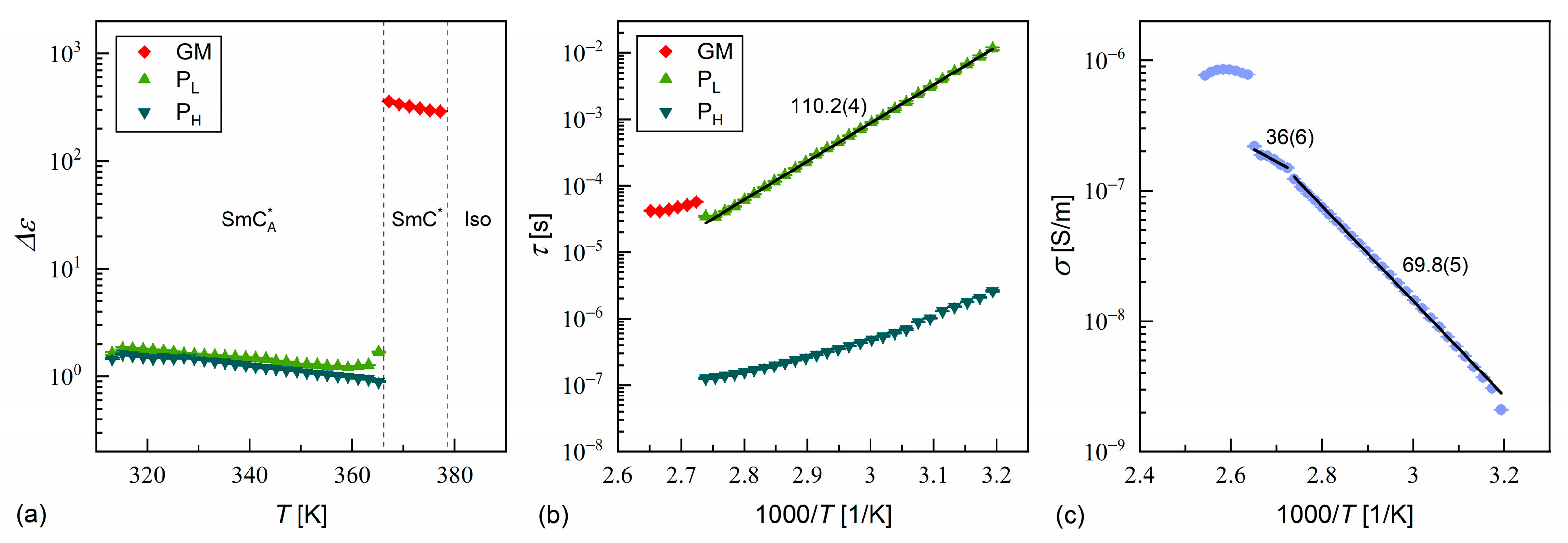
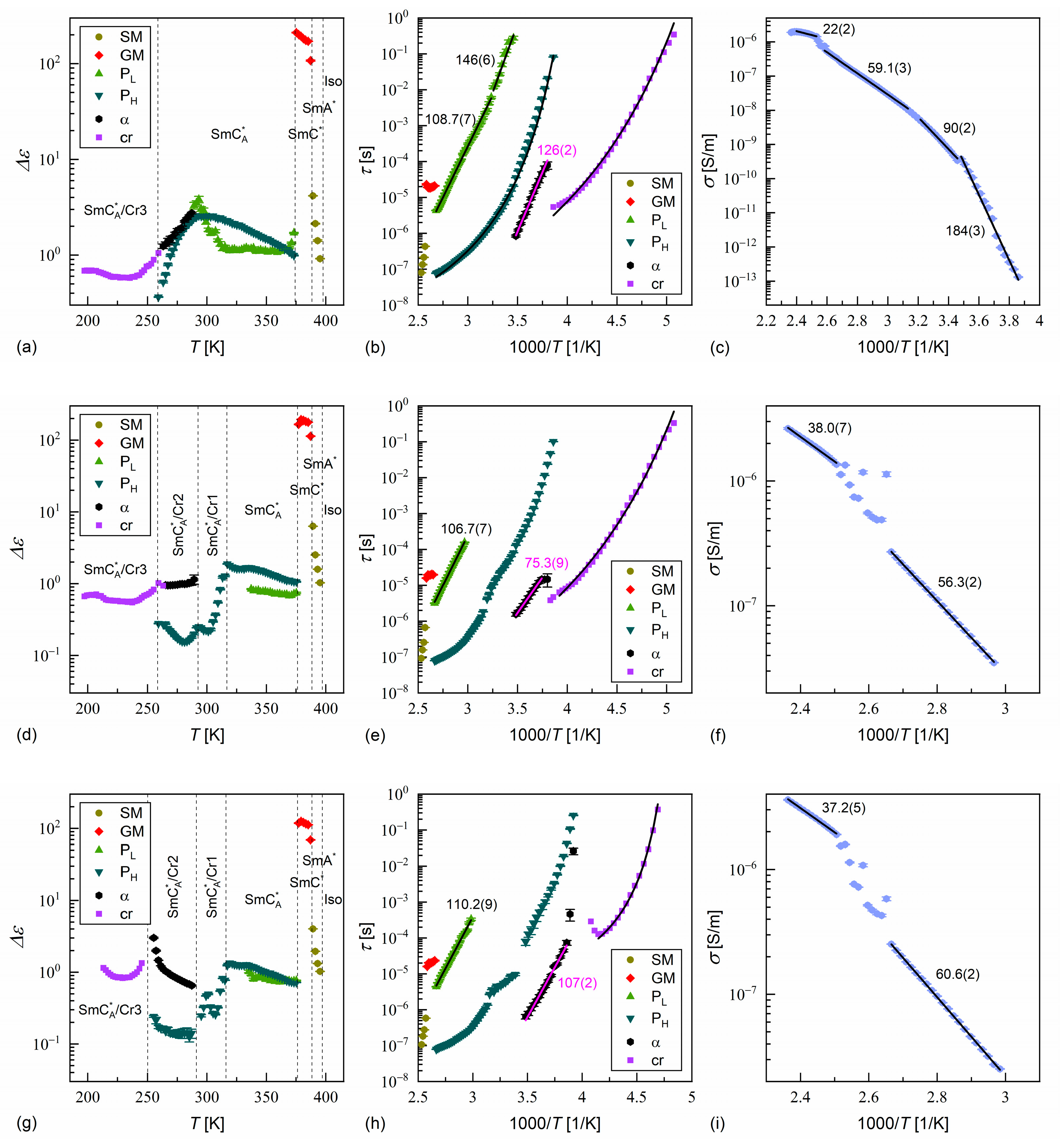
3.3. Dielectric Relaxation Processes
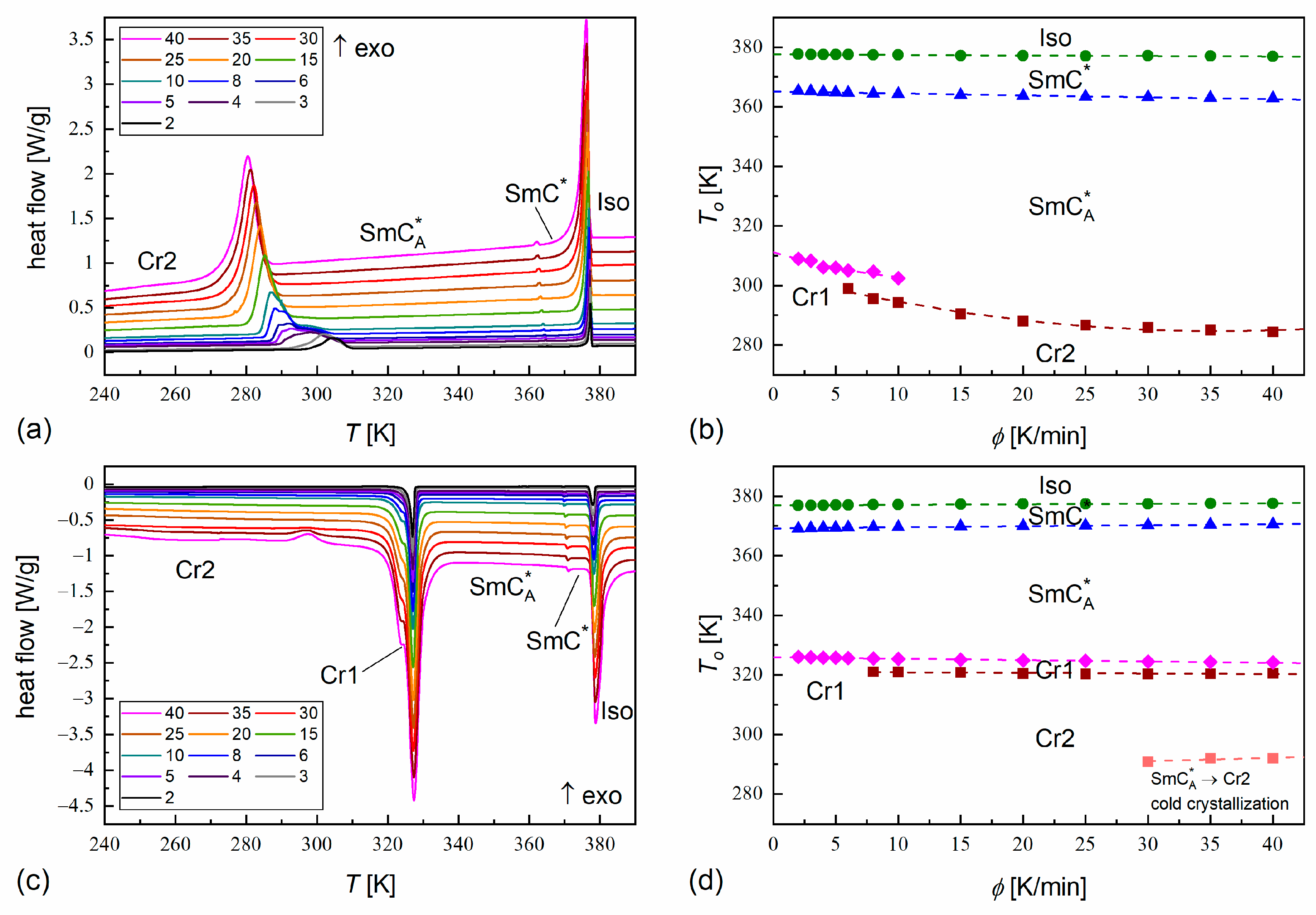
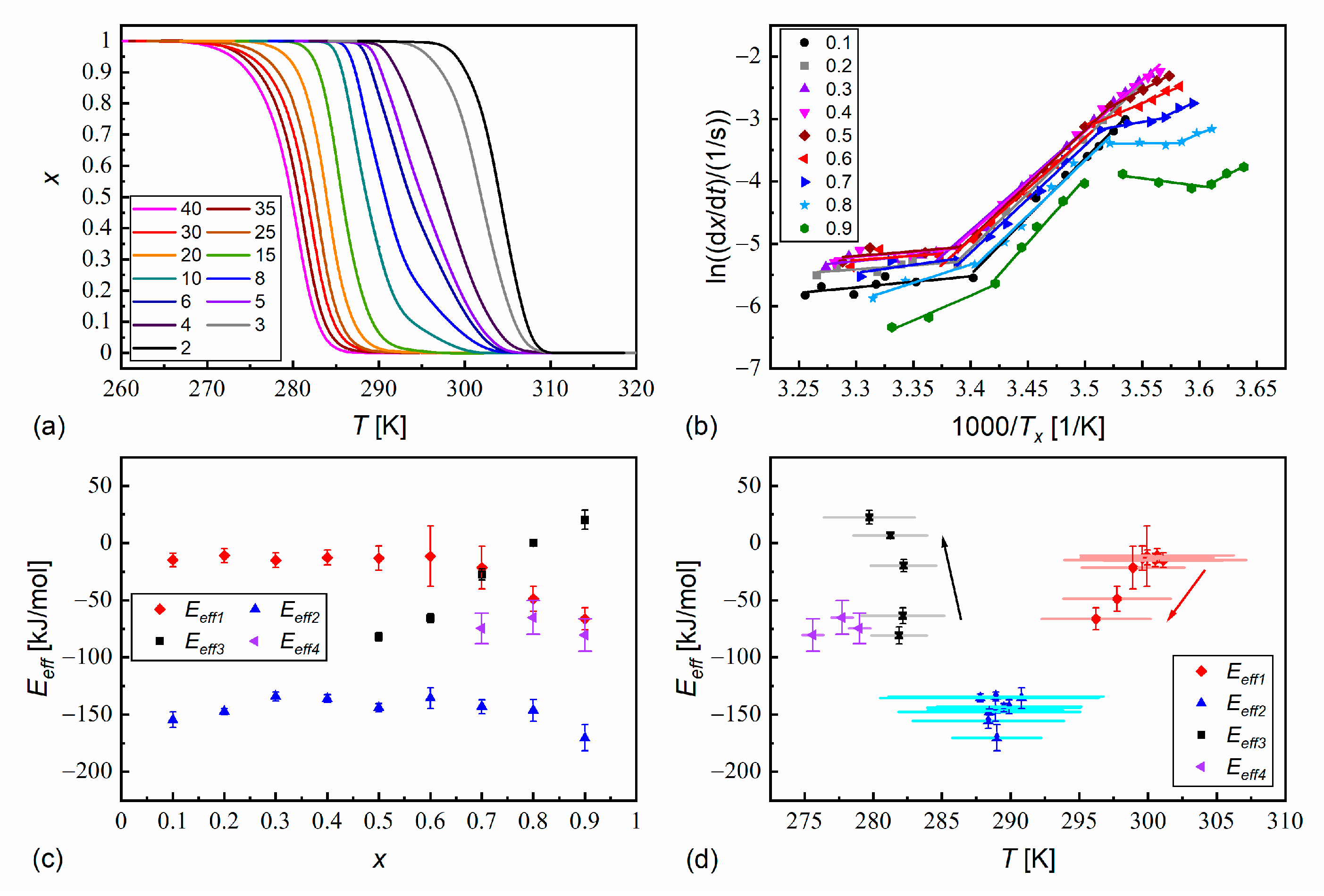
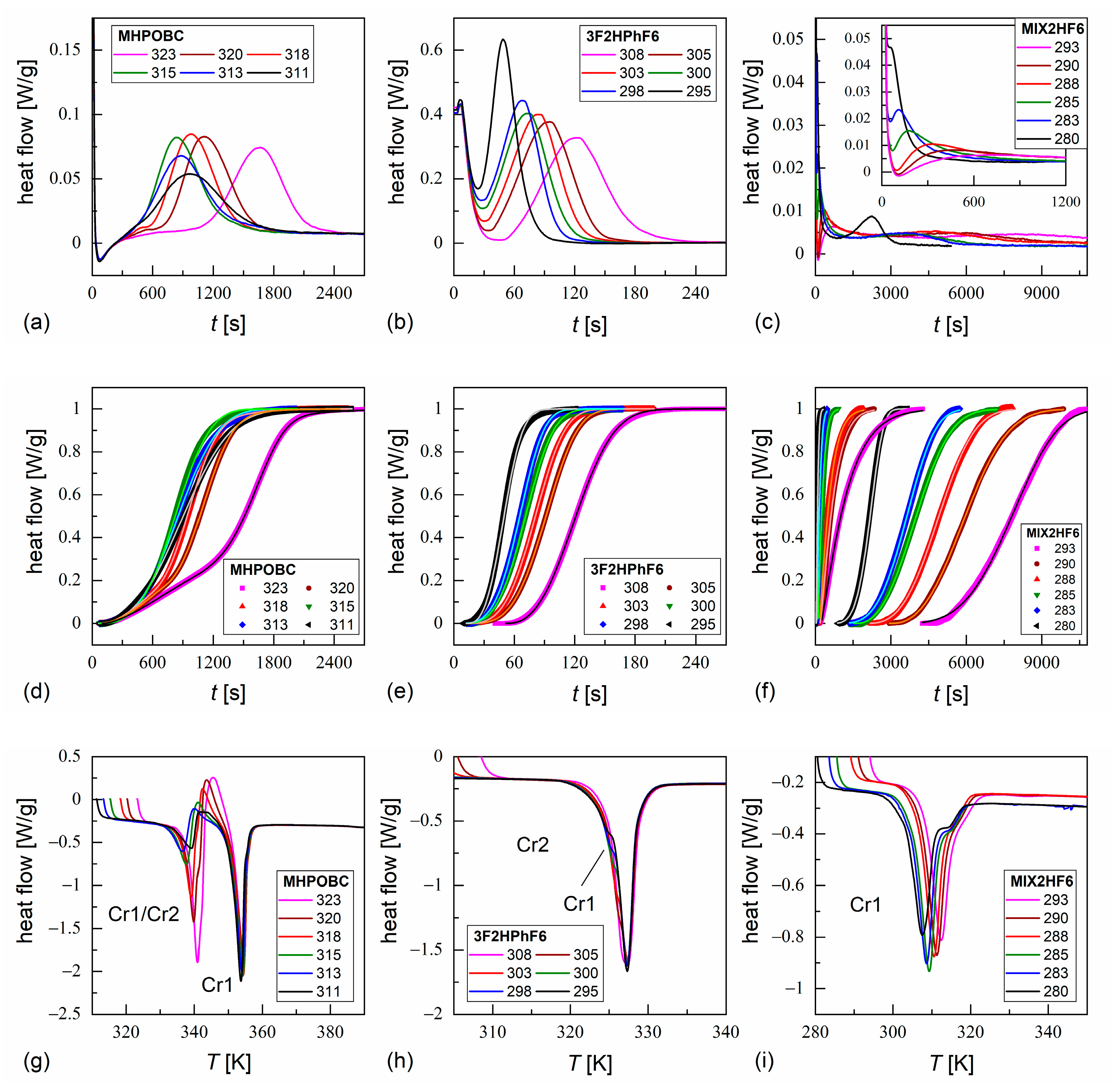
3.4. Non-Isothermal Crystallization
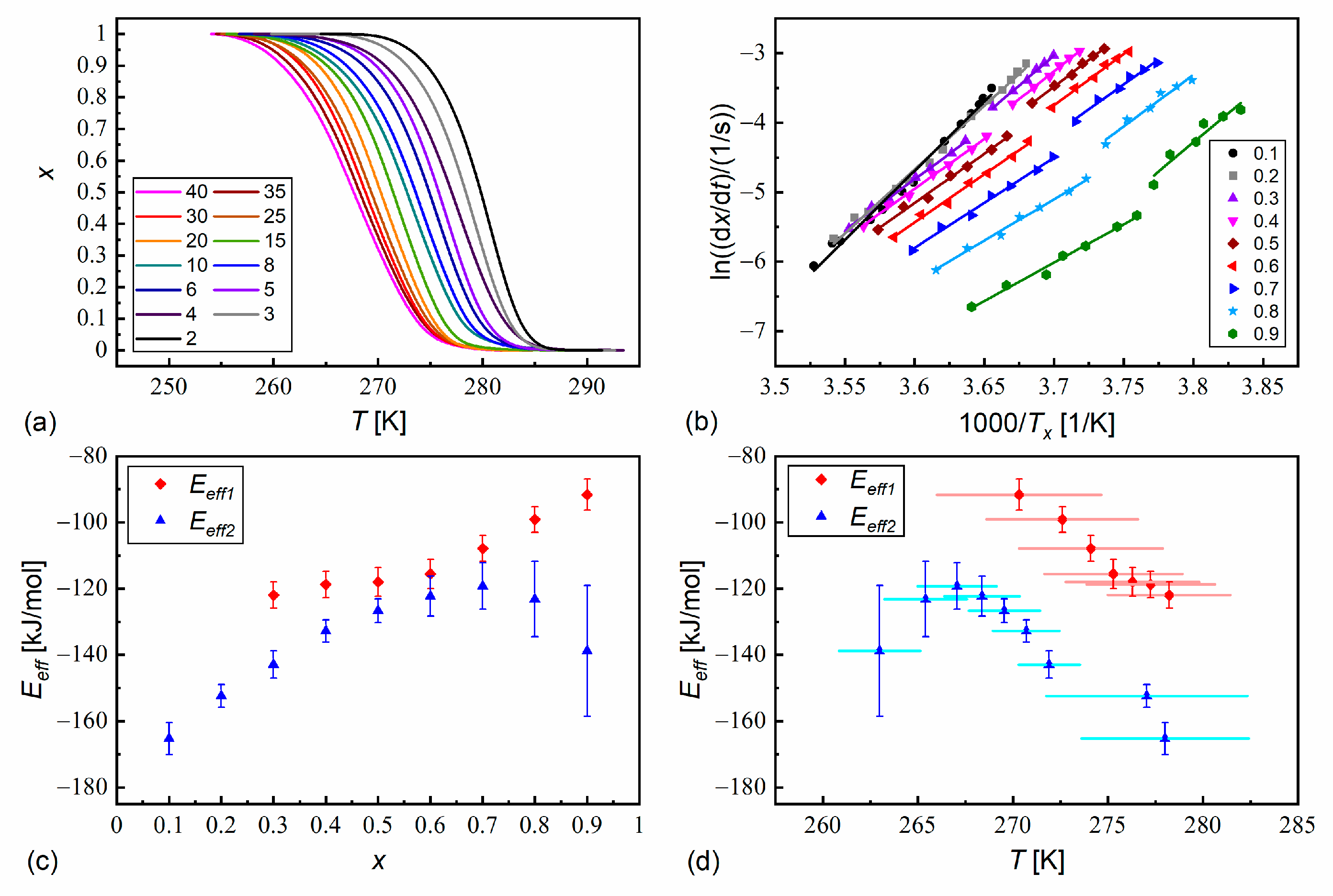
3.5. Isothermal Crystallization
4. Summary and Conclusions
- According to the DSC and XRD results, the investigated MHPOBC sample exhibits the SmA*, SmCA*, and hexatic SmXA* phases. At least one smectic phase between SmA* and SmCA* is visible in a narrow temperature range in the DSC thermograms. Based on the BDS spectra, the phase sequence is probably SmA* → SmCα* → SmC* → SmCγ* → SmCA* → SmXA*.
- The equimolar mixture MIX2HF6 shows the SmA*, SmC*, and SmCA* phases. The experimental results do not indicate the presence of SmCα*, SmCγ*, and SmXA*.
- While the pure components crystallize easily during cooling, MIX2HF6 shows only partial crystallization, and the remaining supercooled SmCA* phase forms glass at = 241.5 K. The glass softening during heating occurs at a slightly higher temperature of ca. 245 K. The energy released during cold crystallization upon slow heating of a quickly cooled sample, as obtained by the modulated-temperature DSC method, equals 3.8 kJ/mol (6.1 J/g).
- The melt crystallization kinetics is usually controlled by the nucleation rate for the pure components (effective activation energy < 0), although in certain conditions, the diffusion rate has a dominant contribution to the overall crystallization kinetics ( > 0). Meanwhile, the melt crystallization of MIX2HF6 is controlled by the nucleation rate in all the tested conditions. MIX2HF6 crystallizes at lower temperatures and a lower rate than the pure compounds.
- The melting temperature of MIX2HF6, = 308 K, is also lowered compared to 352 K for MHPOBC and 326 K for 3F2HPhF6. However, in some DSC thermograms of MIX2HF6, small fractions of the crystal phases with higher = 325 K and 328 K are observed. This is attributed to minor inhomogeneities in the mixture’s composition, which occur supposedly in the solid state. Nevertheless, the stability range of the antiferroelectric SmCA* phase in MIX2HF6 is broader than in its components.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meyer, R.B.; Liébert, L.; Strzelecki, L.; Keller, P. Ferroelectric liquid crystals. J. Phys. Lett. 1975, 36, 69–71. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Giesselmann, F. Current Topics in Smectic Liquid Crystal Research. ChemPhysChem 2006, 7, 20–45. [Google Scholar] [CrossRef] [PubMed]
- Rudquist, P. Smectic LCD Modes. In Handbook of Visual Display Technology; Chen, J., Cranton, W., Fihn, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Żurowska, M.; Dąbrowski, R.; Dziaduszek, J.; Garbat, K.; Filipowicz, M.; Tykarska, M.; Rejmer, W.; Czupryński, K.; Spadło, A.; Bennis, N.; et al. Influence of alkoxy chain length and fluorosubstitution on mesogenic and spectral properties of high tilted antiferroelectric esters. J. Mater. Chem. 2011, 21, 2144–2153. [Google Scholar] [CrossRef]
- Sato, H.; Hatano, J.; Tatemori, S.; Uehara, H.; Saito, S.; Saito, H.; Okabe, E. Antiferroelectricity of a Chiral Smectic Liquid Crystal Having Three Isolated Phenyl Rings in the Core. Mol. Cryst. Liq. Cryst. 1999, 328, 411–418. [Google Scholar] [CrossRef]
- Wu, S.L.; Chiang, C.T. V-shaped switching in binary mixtures of an achiral swallow-tailed material with the antiferroelectric liquid crystal (S)-MHPOBC. Liq. Cryst. 2002, 29, 39–45. [Google Scholar] [CrossRef]
- Kuczyński, W.; Goc, F.; Dardas, D.; Dąbrowski, R.; Hoffmann, J.; Stryła, B.; Małecki, J. Phase Transitions in a Liquid Crystal with Long-Range Dipole Order. Ferroelectrics 2002, 274, 83–100. [Google Scholar] [CrossRef]
- Piecek, W.; Raszewski, Z.; Perkowski, P.; Morawiak, P.; Żurowska, M.; Ziobro, D.; Kula, P.; Sun, X.W. Modification of High Tilted Antiferroelectric Mixture for Display Applications. Mol. Cryst. Liq. Cryst. 2009, 509, 336/[1078]–348/[1090]. [Google Scholar] [CrossRef]
- Rudzki, A.; Zalewski, S.; Suchodolska, B.; Czerwiec, J.; Ossowska-Chruściel, M.D.; Chruściel, J. Phase Behavior and Dynamics of Binary and Multicomponent Thioester Liquid Crystal Mixtures. J. Phys. Chem. B 2017, 121, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Seki, A.; Yoshio, M.; Mori, Y.; Funahashi, M. Ferroelectric Liquid-Crystalline Binary Mixtures Based on Achiral and Chiral Trifluoromethylphenylterthiophenes. ACS Appl. Mater. Interfaces 2020, 12, 53029–53038. [Google Scholar] [CrossRef]
- Dwivedi, A.; Dwivedi, S.; Pandey, M.B.; Dąbrowski, R.; Dhar, R. Collective molecular relaxations and electro-optical switching response of a wide room temperature antiferroelectric liquid crystal mixture. Opt. Mater. 2024, 149, 115016. [Google Scholar] [CrossRef]
- Tykarska, M.; Kurp, K.; Mironov, S.; Rychłowicz, N.; Karcz, J.; Dziaduszek, J.; Kula, P.; Stulov, S.; Bubnov, A. Tuning of self-organizing and electro-optical behaviour for orthoconic ferroelectric liquid crystal by non-chiral dopants. J. Mol. Liq. 2024, 409, 125426. [Google Scholar] [CrossRef]
- Górecka, E.; Pociecha, D.; Čepič, M.; Žekš, B.; Dąbrowski, R. Enantiomeric excess dependence of the phase diagram of antiferroelectric liquid crystals. Phys. Rev. E 2002, 65, 061703. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.; Barois, P.; Grelet, E.; Nallet, F.; Goodby, J.W.; Hird, M.; Micha, J.-S. Extension of the resonant scattering technique to liquid crystals without resonant element. Eur. Phys. J. E 2006, 20, 81–87. [Google Scholar] [CrossRef]
- Hirst, L.S.; Watson, S.J.; Gleeson, H.F.; Cluzeau, P.; Barois, P.; Pindak, R.; Pitney, J.; Cady, A.; Johnson, P.M.; Huang, C.C.; et al. Interlayer structures of the chiral smectic liquid crystal phases revealed by resonant x-ray scattering. Phys. Rev. E 2002, 65, 041705. [Google Scholar] [CrossRef] [PubMed]
- Chandani, A.D.L.; Ouchi, Y.; Takezoe, H.; Fukuda, A.; Terashima, K.; Furukawa, K.; Kishi, A. Novel Phases Exhibiting Tristable Switching. Jpn. J. Appl. Phys. 1989, 28, L1261–L1264. [Google Scholar] [CrossRef]
- Rozwadowski, T.; Massalska-Arodź, M.; Kolek, Ł.; Grzybowska, K.; Bąk, A.; Chłędowska, K. Kinetics of Cold Crystallization of 4-Cyano-3-fluorophenyl 4-Butylbenzoate (4CFPB) Glass Forming Liquid Crystal. I. Nonisothermal Process As Studied by Microscopic, Calorimetric, and Dielectric Methods. Cryst. Growth Des. 2015, 15, 2891–2900. [Google Scholar] [CrossRef]
- Sánchez, M.S.; Mathot, V.B.F.; Poel, G.V.; Ribelles, J.L.G. Effect of the Cooling Rate on the Nucleation Kinetics of Poly(l-Lactic Acid) and Its Influence on Morphology. Macromolecules 2007, 40, 7989–7997. [Google Scholar] [CrossRef]
- Blaabjerg, L.I.; Lindenberg, E.; Löbmann, K.; Grohganz, H.; Rades, T. Glass Forming Ability of Amorphous Drugs Investigated by Continuous Cooling and Isothermal Transformation. Mol. Pharmaceutics 2016, 13, 3318–3325. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H. Multifunctional glassy liquid crystals for photonics. J. Soc. Inf. Disp. 2004, 12, 205–211. [Google Scholar] [CrossRef]
- Philip Chen, H.M.; Katsis, D.; Chen, S.H. Deterministic Synthesis and Optical Properties of Glassy Chiral-Nematic Liquid Crystals. Chem. Mater. 2003, 15, 2534–2542. [Google Scholar] [CrossRef]
- Turunen, K.; Yazdani, M.R.; Santasalo-Aarnio, A.; Seppälä, A. Exceptional cold-crystallization kinetics of erythritol-polyelectrolyte enables long-term thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 230, 111273. [Google Scholar] [CrossRef]
- Ishikawa, T.; Honda, A.; Miyamura, K. Effects of alkyl chain length on the cold crystallization of Schiff-base nickel(II) complexes. CrystEngComm 2022, 24, 5900–5906. [Google Scholar] [CrossRef]
- Manna, S.K.; Sinha, A. Development of a Phenomenological Model On Surface Stabilized Ferroelectric Liquid Crystal Nanocomposite. IOSR J. Appl. Phys. 2012, 1, 33–38. [Google Scholar] [CrossRef]
- Lalik, S.; Stefańczyk, O.; Dardas, D.; Deptuch, A.; Yevchenko, T.; Ohkoshi, S.-i.; Marzec, M. Nanocomposites Based on Antiferroelectric Liquid Crystal (S)-MHPOBC Doping with Au Nanoparticles. Molecules 2022, 27, 3663. [Google Scholar] [CrossRef]
- Dardas, D.; Lalik, S.; Nowacka, Z.; Yevchenko, T.; Marzec, M. Electro-Optic Effect of Laser Photobleaching on Viscoelastic Properties of Chiral Liquid Crystals. Crystals 2023, 13, 164. [Google Scholar] [CrossRef]
- Lagerwall, J.P.; Scalia, G. The effects of carbon nanotubes on the clearing transition of the antiferroelectric liquid crystal MHPOBC. Ferroelectrics 2016, 495, 69–74. [Google Scholar] [CrossRef]
- Lisetski, L.; Bulavin, L.; Lebovka, N. Effects of Dispersed Carbon Nanotubes and Emerging Supramolecular Structures on Phase Transitions in Liquid Crystals: Physico-Chemical Aspects. Liquids 2023, 3, 246–277. [Google Scholar] [CrossRef]
- Ema, K.; Yao, H.; Kawamura, I.; Chan, T.; Garland, C.W. High-resolution calorimetric study of the antiferroelectric liquid crystals methylheptyloxycarbonylphenyl octyloxybiphenyl carboxylate and its octylcarbonylbiphenyl analog. Phys. Rev. E 1993, 47, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Betts, R.; Dierking, I. Machine learning classification of polar sub-phases in liquid crystal MHPOBC. Soft Matter 2023, 19, 7502–7512. [Google Scholar] [CrossRef] [PubMed]
- Vertogen, G.; de Jeu, W.H. Thermotropic Liquid Crystals. Fundamentals; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar] [CrossRef]
- Deptuch, A.; Marzec, M.; Jaworska-Gołąb, T.; Dziurka, M.; Hooper, J.; Srebro-Hooper, M.; Fryń, P.; Fitas, J.; Urbańska, M.; Tykarska, M. Influence of carbon chain length on physical properties of 3FmHPhF homologues. Liq. Cryst. 2019, 46, 2201–2212. [Google Scholar] [CrossRef]
- D’havé, K.; Rudquist, P.; Lagerwall, S.T.; Pauwels, H.; Drzewiński, W.; Dąbrowski, R. Solution of the dark state problem in antiferroelectric liquid crystal displays. Appl. Phys. Lett. 2000, 76, 3528–3530. [Google Scholar] [CrossRef]
- Dąbrowski, R.; Gąsowska, J.; Otón, J.; Piecek, W.; Przedmojski, J.; Tykarska, M. High tilted antiferroelectric liquid crystalline materials. Displays 2004, 25, 9–19. [Google Scholar] [CrossRef]
- Massalska-Arodź, M. On structural organisation and crystallisation/vitrification phenomena in low molecular weight glass-forming liquid crystals. Liq. Cryst. 2024, 51, 1073–1085. [Google Scholar] [CrossRef]
- Drzewicz, A.; Jasiurkowska-Delaporte, M.; Juszyńska-Gałązka, E.; Deptuch, A.; Gałązka, M.; Zając, W.; Drzewiński, W. On relaxation and vibrational dynamics in the thermodynamic states of a chiral smectogenic glass-former. Phys. Chem. Chem. Phys. 2022, 24, 4595–4612. [Google Scholar] [CrossRef]
- Deptuch, A.; Jasiurkowska-Delaporte, M.; Juszyńska-Gałązka, E.; Drzewicz, A.; Zając, W.; Urbańska, M. Molecular Dynamics and Kinetics of Isothermal Cold Crystallization in the Chiral Smectogenic 3F7FPhH6 Glassformer. Crystals 2021, 11, 1487. [Google Scholar] [CrossRef]
- Drzewicz, A. Insight Into Phase Situation and Kinetics of Cold- and Melt Crystallization Processes of Chiral Smectogenic Liquid Crystals; Institute of Nuclear Physics Polish Academy of Sciences: Kraków, Poland, 2023. [Google Scholar] [CrossRef]
- Osiecka, N.; Galewski, Z.; Massalska-Arodź, M. TOApy program for the thermooptical analysis of phase transitions. Thermochim. Acta 2017, 655, 106–111. [Google Scholar] [CrossRef]
- Bearden, J.A. X-Ray Wavelengths. Rev. Mod. Phys. 1967, 39, 78–124. [Google Scholar] [CrossRef]
- McCauley, J.W.; Newnham, R.E.; Gibbs, G.V. Crystal Structure Analysis of Synthetic Fluorophlogopite. Am. Mineral. 1973, 58, 249–254. [Google Scholar]
- Crystallography Open Database, Entry 9000302. Available online: https://www.crystallography.net/cod/9000302.html (accessed on 11 March 2024).
- Hubbard, C.R. National Bureau of Standards Certificate, Standard Reference Material 675, Low 2θ (Large d-Spacing) Standard for X-Ray Powder Diffraction. Available online: https://tsapps.nist.gov/srmext/certificates/675.pdf (accessed on 11 March 2024).
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Ghanbari, E.; Picken, S.J.; van Esch, J.H. Analysis of differential scanning calorimetry (DSC): Determining the transition temperatures, and enthalpy and heat capacity changes in multicomponent systems by analytical model fitting. J. Therm. Anal. Calorim. 2023, 148, 12393–12409. [Google Scholar] [CrossRef]
- Li, J.; Takezoe, H.; Fukuda, A. Novel Temperature Dependences of Helical Pitch in Ferroelectric and Antiferroelectric Chiral Smectic Liquid Crystals. Jpn. J. Appl. Phys. 1991, 30, 532–536. [Google Scholar] [CrossRef]
- Budai, J.; Pindak, R.; Davey, S.C.; Goodby, J.W. A structural investigation of the liquid crystal phases of 4-(2′-methylbutyl)phenyl 4′-n-octylbiphenyl-4-carboxylate. J. Phys. Lett. 1984, 45, 1053–1062. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane analysis of α-dispersions in some polymer systems. J. Polym. Sci. C: Polym. Symp. 1966, 14, 99–117. [Google Scholar] [CrossRef]
- Khened, S.M.; Krishna Prasad, S.; Shivkumar, B.; Sadashiva, B.K. Dielectric studies of Goldstone mode and soft mode in the vicinity of the A-C* transition. J. Phys. II France 1991, 1, 171–180. [Google Scholar] [CrossRef]
- Buivydas, M.; Gouda, F.; Andersson, G.; Lagerwall, S.T.; Stebler, B.; Bömelburg, J.; Heppke, G.; Gestblom, B. Collective and non-collective excitations in antiferroelectric and ferrielectric liquid crystals studied by dielectric relaxation spectroscopy and electro-optic measurements. Liq. Cryst. 1997, 23, 723–739. [Google Scholar] [CrossRef]
- Pandey, M.B.; Dhar, R.; Dąbrowski, R. Chiral Smectics: As Observed through Dielectric Spectroscopy. Mol. Cryst. Liq. Cryst. 2011, 541, 222/[460]–235/[473]. [Google Scholar] [CrossRef]
- Douali, R.; Legrand, C.; Laux, V.; Isaert, N.; Joly, G.; Nguyen, H.T. Correlation between dielectric and optical measurements in the smectic-Cα* phase. Phys. Rev. E 2004, 69, 031709. [Google Scholar] [CrossRef]
- Kolek, Ł.; Massalska-Arodź, M.; Adrjanowicz, K.; Rozwadowski, T.; Dychtoń, K.; Drajewicz, M.; Kula, P. Molecular dynamics and cold crystallization process in a liquid-crystalline substance with para-, ferro- and antiferro-electric phases as studied by dielectric spectroscopy and scanning calorimetry. J. Mol. Liq. 2020, 297, 111913. [Google Scholar] [CrossRef]
- Kolek, Ł.; Jasiurkowska-Delaporte, M.; Massalska-Arodź, M.; Szaj, W.; Rozwadowski, T. Mesomorphic and dynamic properties of 3F5BFBiHex antiferroelectric liquid crystal as reflected by polarized optical microscopy, differential scanning calorimetry and broadband dielectric spectroscopy. J. Mol. Liq. 2020, 320, 114338. [Google Scholar] [CrossRef]
- Böhmer, R.; Ngai, K.L.; Angell, C.A.; Plazek, D.J. Nonexponential relaxations in strong and fragile glass formers. J. Chem. Phys. 1993, 99, 4201–4209. [Google Scholar] [CrossRef]
- Wang, M.-L.; Angell, C.A. Response to “Comment on ‘Direct determination of the fragility indices of glassforming liquids by differential scanning calorimetry: Kinetic versus thermodynamic fragilities’” [J. Chem. Phys. 118, 10351 (2003)]. J. Chem. Phys. 2003, 118, 10353–10355. [Google Scholar] [CrossRef]
- Jones, K.J.; Kinshott, I.; Reading, M.; Lacey, A.A.; Nikolopoulos, C.; Pollock, H.M. The origin and interpretation of the signals of MTDSC. Thermochim. Acta 1997, 304–305, 187–199. [Google Scholar] [CrossRef]
- Wunderlich, B. A classification of molecules, phases, and transitions as recognized by thermal analysis. Thermochim. Acta 1999, 340–341, 37–52. [Google Scholar] [CrossRef]
- Puertas, R.; Rute, M.A.; Salud, J.; López, D.O.; Diez, S.; van Miltenburg, J.K.; Pardo, L.C.; Tamarit, J.L.; Barrio, M.; Pérez-Jubindo, M.A.; et al. Thermodynamic, crystallographic, and dielectric study of the nature of glass transitions in cyclo-octanol. Phys. Rev. B 2004, 69, 224202. [Google Scholar] [CrossRef]
- Osiecka, N.; Gałązka, M.; Marzec, M.; Zając, W.; Massalska-Arodź, M. Molecular Dynamic in Ethosuximide Glass Forming Pharmaceutical as Studied by Dielectric Relaxation Spectroscopy. J. Pharm. Sci. 2019, 108, 102–108. [Google Scholar] [CrossRef]
- Rozwadowski, T.; Massalska-Arodź, M.; Krawczyk, J.; Juszyńska-Gałązka, E. Molecular dynamics of 4-cyano-3-fluorophenyl 4-butylbenzoate (4CFPB) glass-forming liquid crystal in unidirectional silicon nanopores. Liq. Cryst. 2014, 41, 1073–1079. [Google Scholar] [CrossRef]
- Androulaki, K.; Chrissopoulou, K.; Prevosto, D.; Labardi, M.; Anastasiadis, S.H. Dynamics of Hyperbranched Polymers under Confinement: A Dielectric Relaxation Study. ACS Appl. Mater. Interfaces 2015, 7, 12387–12398. [Google Scholar] [CrossRef] [PubMed]
- Rozwadowski, T.; Yamamura, Y.; Saito, K. Interplay between Melt and Cold Crystallization in a Smectic Liquid Crystal, 4-Pentylphenyl 4-(trans-4-Pentylcyclohexyl)benzoate. Cryst. Growth Des. 2021, 21, 2777–2785. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of Peak Temperature With Heating Rate in Differential Thermal Analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Matusita, K.; Komatsu, T.; Yokota, R. Kinetics of non-isothermal crystallization process and activation energy for crystal growth in amorphous materials. J. Mater. Sci. 1984, 19, 291–296. [Google Scholar] [CrossRef]
- Augis, J.A.; Bennett, J.E. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J. Therm. Anal. 1978, 13, 283–292. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional Kinetic Analysis of Thermally Stimulated Processes in Polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Štrbac, G.R.; Jarić, S.; Lukić-Petrović, S.R.; Vigi, R.; Ćelić, N.; Štrbac, D.D. Isoconversional Analysis of Thermally Stimulated Effects in Cux(As2Se3)100−x Glasses. Acta Phys. Pol. A 2023, 143, 369–375. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avramov, I.; Avramova, K.; Rüssel, C. New method to analyze data on overall crystallization kinetics. Cryst. Growth Des. 2005, 285, 394–399. [Google Scholar] [CrossRef]
- Patki, R.; Mezghani, K.; Philips, P.J. Crystallization Kinetics of Polymers. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Kolodziejczyk, K.; Paluch, M.; Grzybowska, K.; Grzybowski, A.; Wojnarowska, Z.; Hawelek, L.; Ziolo, J.D. Relaxation Dynamics and Crystallization Study of Sildenafil in the Liquid and Glassy States. Mol. Pharm. 2013, 10, 2270–2282. [Google Scholar] [CrossRef]
- Koperwas, K.; Tu, W.; Affouard, F.; Adrjanowicz, K.; Kaskosz, F.; Paluch, M. Pressure Dependence of the Crystallization Rate for the S-Enantiomer and a Racemic Mixture of Ibuprofen. Cryst. Growth Des. 2021, 21, 7075–7086. [Google Scholar] [CrossRef]
- Velisaris, C.N.; Seferis, J.C. Crystallization kinetics of polyetheretherketone (peek) matrices. Polym. Eng. Sci. 1986, 26, 1574–1581. [Google Scholar] [CrossRef]




| Sample | SmXA* | SmCA* | SmC* | SmA* | Iso | ||||
|---|---|---|---|---|---|---|---|---|---|
| cooling | |||||||||
| MHPOBC | ● | 339 337.3 1.5 | ● | 394 a 386.6 >0.1 | ● | 394 a 388.3 >0.1 | ● | 424 418.2 6.0 | ● |
| 3F2HPhF6 | - | ● | 366 365.1 >0.1 | ● | - | 380 377.6 6.8 | ● | ||
| MIX2HF6 | - | ● | 377 373.6 >0.1 | ● | 388 386.9 1.1 | ● | 394 395.9 4.6 | ● | |
| heating | |||||||||
| MHPOBC | - | ● | 394 a 385.0 >0.1 | ● | 394 a 389.3 >0.1 | ● | 424 416.6 5.9 | ● | |
| 3F2HPhF6 | - | ● | 371 369.2 >0.1 | ● | - | 380 376.9 6.8 | ● | ||
| MIX2HF6 | - | ● | 377 375.6 >0.1 | ● | 388 386.2 0.9 | ● | 394 395.9 4.8 | ● | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deptuch, A.; Paliga, A.; Drzewicz, A.; Piwowarczyk, M.; Urbańska, M.; Juszyńska-Gałązka, E. Crystallization Kinetics of an Equimolar Liquid Crystalline Mixture and Its Components. Appl. Sci. 2024, 14, 11701. https://doi.org/10.3390/app142411701
Deptuch A, Paliga A, Drzewicz A, Piwowarczyk M, Urbańska M, Juszyńska-Gałązka E. Crystallization Kinetics of an Equimolar Liquid Crystalline Mixture and Its Components. Applied Sciences. 2024; 14(24):11701. https://doi.org/10.3390/app142411701
Chicago/Turabian StyleDeptuch, Aleksandra, Anna Paliga, Anna Drzewicz, Marcin Piwowarczyk, Magdalena Urbańska, and Ewa Juszyńska-Gałązka. 2024. "Crystallization Kinetics of an Equimolar Liquid Crystalline Mixture and Its Components" Applied Sciences 14, no. 24: 11701. https://doi.org/10.3390/app142411701
APA StyleDeptuch, A., Paliga, A., Drzewicz, A., Piwowarczyk, M., Urbańska, M., & Juszyńska-Gałązka, E. (2024). Crystallization Kinetics of an Equimolar Liquid Crystalline Mixture and Its Components. Applied Sciences, 14(24), 11701. https://doi.org/10.3390/app142411701









