Abstract
A new generation of food packaging materials, centered on green solutions, is currently being developed in labs basing these materials on underused secondary industrial food by-products which have the ability to reduce the amount of petroleum-based packaging generated in order to minimize environmental harm and food by-products while ensuring food quality and safety. This study presents a sustainable biopolymeric combination based on bacterial nanocellulose grafted in yeast films, its potential to improve matrices properties, and the influence of plasticizer and emulsifier concentrations on mechanical properties, volatile fingerprint, and antimicrobial activity of films. Yeast films with 1.00% glycerol and 1.00% Tween 80, functionalized with a 2.00% mixture of cinnamon–lavender essential oils and with 2.00% bacterial cellulose produced from SCOBY presented improved mechanical properties compared to the control and exerted antipathogenic activity against Listeria monocytogens, Staphylococcus aureus, and Escherichia coli. Yeast films with grafted bacterial cellulose could be a sustainable food packaging solution for future applications.
1. Introduction
The worldwide environmental concern impacts every aspect of our lives, food packaging included. Due to the rapid growth of the global population and changing lifestyle, food packaging significance increased exponentially together with the innovation pressure for developing ecofriendly solutions. Thus, a new generation of packaging materials developed from underused secondary industrial food by-products is in the pipeline striving to achieve high functionality, eco-efficiency, and, moreover, sustainability. The next generation of food packaging should be based on green solutions (UN’s Sustainable Development Goal 12) and be able to interplay reduction in the amount of petroleum-based packaging to minimize environmental harm, food waste, and food by-products with food quality and safety [1].
Among easily renewable raw materials and/or secondary food industry products, several possibilities were studied recently to evaluate the potential of sustainable food packaging based on biodegradable polymers such as polylactic acid, microbial polyesters, or bacterial cellulose [2,3].
Edible coatings or films are sustainable food packaging solutions which can be obtained from vegetal or animal polysaccharides, proteins, lipids, or their mix resulting in composite packaging materials [4]. Their ability to act as carriers for bioactive substances, like antimicrobials, antioxidants, probiotics, or nutraceuticals, and compatibility with food matrix renders them a wide array of food applications. The encapsulation of bioactive substances ensures their protection from environmental factors like pH or temperature during processing, or their prolonged release during food storage or consumption, depending on the application [5]. Nevertheless, biopolymeric packaging applications from vegetal or animal resources are hindered by the limitations of these resources and by several properties that need improvement in order to reduce the gap between the properties of petroleum-based and biodegradable food packaging materials. For example, the improvement of biopolymer-based food packaging’s poor mechanical resistance, high permeability to water vapors or oxygen, would speed up their industrial applications [6]. For example, low density polyethylene has tensile strength ranging 8–25 MPa and water vapor permeability of 0.004–0.006 g mm/m2∙d·kPa [7,8], while for whey protein films, for example, the respective values are approx. 4 MPa and 5–7 g·mm/d·m2·kPa [8] and for yeast cell films 0.7–3.1 MPa [9] and 0.47–27.7 × 10−10 g∙s-1∙m−1∙Pa−1 [10].
To surpass these limitations, grafting compounds into a biopolymeric matrix could be a promising improvement strategy. Sugarcane bagasse and other lignocellulosic materials have been used as reinforcing fillers in whey protein films [11]; bio composite active packaging based on chitosan and β-cyclodextrin [12] and other bio composite matrices [13] confer a significant impact primarily on mechanical strength and water vapor permeability. However, vegetal cellulose has the disadvantage of low thermal stability [8].
One of the most promising sources of biopolymers is microbial biomass which could meet the promise of cheap applications based on circular economy principles, using agricultural and food waste for the production of resilient food packaging while enabling the food packaging industry to decouple from fossil stocks and return nutrients to the soil. Edible films and coatings can be obtained from both the baker or brewing yeast belonging to the same genus, Saccharomyces. However, the mechanical properties and the water vapor permeability of these materials still needs to be improved, for example, for yeast cell films tensile strength of 0.7–3.1 MPa [9] and 0.47–27.7 10−10 g∙s−1∙m−1∙Pa−1 [10] were reported. Thus, cellulose grafting using bacterial sources could contribute to exceeding these limitations, providing polymers with excellent mechanical properties, increased surface area and aspect ratio, and low coefficients of thermal expansion while being a good candidate for crosslinking or grafting [6].
Traditionally the symbiotic culture of bacteria and yeast (SCOBY) is used to obtain a fermented drink based on black or green tea, known as Kombucha, rich in antioxidants, phenols, glucuronic acid, and B complex vitamins [14]. The fermentation of the sweetened tea is ensured by the microbial consortium included in an exogenous cellulosic matrix, produced by the acetic bacteria in the consortium, which provides protection to all species of acetic bacteria (Komagataeibacter, Gluconobacter, and Acetobacter), probiotic lactic bacteria (Lactobacillus, Bifidobacterium) and yeasts resistant to osmotic stress (Brettanomyces, Zygosaccharomyces, Saccharomyces) that develop in symbiosis [15], forming simultaneously with fermentation three-dimensional layers in the form of a hydrogel [16]. Since the SCOBY cell biomass is formed in quantities that overpasses what is needed to obtain the traditionally fermented drink, an unused surplus of cellulosic membrane results. The high economic potential for valorization of bacterial cellulose compared to vegetable cellulose is due to its high chemical purity, as bacterial cellulose is not accompanied by hemicellulose, pectin, or lignin. Microbial cellulose is characterized by high biodegradability and high biocompatibility with food products, properties characteristic of cellulosic materials (high mechanical resistance, good chemical stability), high crystallinity (over 60%), low density (1.6 g∙cm−3) [17], easier purification compared to the polluting and expensive methods applied to vegetable cellulose, as well as the regeneration at low costs and in a relatively short time compared to the regeneration of vegetable cellulose [18,19,20]. The greatly simplified structure of bacterial cellulose compared to the three-dimensional structure of plant cellulose, where cellulose microfibrils are surrounded by hemicellulose and lignin, increases the accessibility of bacterial cellulose.
This study evaluates the potential of bacterial nanocellulose from SCOBY grafted in yeast films to improve the film properties and study the influence of concentrations of glycerol as plasticizer and emulsifier Tween 80 on mechanical properties, volatile fingerprint, and antimicrobial activity of films. To the best of authors’ knowledge, this is the first study to evaluate the possibility of combining these two microbial resources in order to obtain a biopolymeric film.
2. Materials and Methods
2.1. Materials
Commercial fresh baker yeast from Belbake, Lidl, Romania was used for yeast films. Glycerol (Honeywall, Charlotte, NC, USA), Tween 80 (Sigma Aldrich, St. Louis, MO, USA), cinnamon essential oil and lavender essential oil (CLEO) were purchased from AromaZone, Cabrières d’Avignon, France. SCOBY was kindly offered by TehnIA-BioAliment Research Platform, Galati, Romania. Helium (99.96% purity) used for GC/MS was provided by Linde Gaz SRL, Galati, Romania. In addition, 2,2-diphenyl-2-picrylhydrazyl (DPPH) and 2-octanol (97%) from Sigma Aldrich, Darmstadt, Germany were used for evaluation of yeast film properties. Antimicrobial activity of yeast films was tested against Geotrichum candidum MIUG M166, Rhodotorula glutinis MIUG D164 from MIUG collection, Dunarea de Jos University of Galati, Romania, Bacillus cereus ATCC 10876, and antipathogenic activity against Gram-positive Listeria monocytogenes Scott A and Staphylococcus aureus ATCC 25923, as well as against Gram-negative Escherichia coli ATCC 25922. L. monocytogenes was cultivated on non-selective media, Brain Heart Infusion, Oxoid, Hampshire, UK), S. aureus, and E. coli were cultivated on Muller–Hinton (Scharlau, Barcelona, Spain), B. cereus was cultivated on Plate Count Agar (PCA) (NutriSelect® Plus, Sigma Aldrich, St. Louis, MO, USA), and R. glutinis and G. candidum were cultivated on malt extract broth/agar.
2.2. Methods
2.2.1. Antimicrobial Activity of CLEO Per Se and in Yeast Films
Concentrations ranging 1–10% CLEO were used to determine minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum fungicidal concentration (MFC) [21,22] against the following test microorganisms: Geotrichum candidum, Rhodotorula glutinis, Bacillus cereus, Listeria monocytogenes, Staphylococcus aureus, and Escherichia coli. Following, 2.00% CLEO was used to functionalize yeast films.
Disk diffusion method was used for evaluation of antimicrobial activity of grafted bacterial cellulose (Y-CNC films), functionalized with 2.00% CLEO compared to control Yc samples, without bacterial cellulose. Inoculum was prepared from single colony cultivated overnight in stationary conditions, in liquid media, at 27 °C for fungi and yeast and 37 °C for bacteria. Film disks with diameter of approx. 15 mm were placed on culture media inoculated with approx. 106 CFU/mL test microorganism. After incubation at optimum temperature for each test microorganism inhibition diameters were measured with digital caliper. Independent duplicate films were measured, and results were reported as average ± standard deviation.
2.2.2. SCOBY Cultivation
SCOBY was cultivated according to a method adapted [23,24] by using an inoculum of 3.00% SCOBY and 0.20% Kombucha fermented tea on a sterile medium prepared from 0.50% (w/w) black tea leaves infused for 5 min and 5.00% (w/w) sucrose. The cultivation was performed under static conditions, at 22 ± 1 °C for 21 days.
2.2.3. Bacterial Nanocellulose Purification
SCOBY pellicles were thoroughly washed with distilled water and further treated with 1.0 M NaOH under continuous stirring at 500 rpm, 80 °C, for 1 h to remove tea leaves and metabolites and to inactivate microbial cells. Alkaline-treated cellulose pellicles were rinsed with filter water until washing water reached pH 7. The pellicles were grinded into 1–2 mm pieces with a grinder, lyophilized (−30 °C for 24 h), and stored in a dry, dark place until further use [25]. The lyophilized bacterial pellicles (BP) underwent acid hydrolysis according to an adapted method of [26] the following: 1.00% (w/v) BP in 60% (w/w) H2SO4 stirred at 5000 rpm, 45 °C, for 1 h. The acid hydrolysis was stopped by a 10-fold dilution with ultrapure water. To precipitate the bacterial cellulose (BC) crystals, a centrifugation at 10,000 rpm for 15 min was performed (Hettich Micro200R, Tuttlingen, Germany). The separated BC crystals were weighed and resuspended in ultrapure water to 2.00% (w/w) after pH adjustment to approx. 7 with 0.1 M NaOH. The yield of BC was 33.44% based on d.w. The BC solution was further sonicated for 30 min at 35% amplitude (15 s on, 15 s off) 20 kHz, 500 W, in order to obtain bacterial nanocellulose (CNC) following an adapted methodology presented by [27].
2.2.4. Yeast Film Functionalized with CLEO and Bacterial Nanocellulose
Yeast film emulsion was prepared from 15% (w/w) yeast BelBake in ultrapure water (Ultra-pure Water System, SMART N, Heal Force, Shanghai, China) sonicated at 30% amplitude, 20 kHz, 500 W, pulsation on 15 s, off 15 s, with a sonication titanium tip probe of 13 mm (VCX 500, Sonics Vibra Cell, Newtown, CT, USA). Sonication energy released at the end of the treatment was 125 kJ. A thermal crosslinking of yeast proteins was further performed at 92°C for 30 min [28] followed by cooling in an iced bath. The yeast film solution was plasticized with 1.00%, 1.25%, and 1.50% (w/w) glycerol, emulsified with Tween 80 1.00%, 1.25%, and 1.50% (w/w) and further functionalized with 2.00% (w/w) cinnamon and lavender 1:1 mix of essential oils (CLEO) (AromaZone, France) based on previous lab experiments and 2% (w/w) bacterial nanocellulose. The film forming emulsions were homogenized at 12,000 rpm with UltraTurrax T18 (IKA, Staufen, Germany) to stabilize the emulsions. Degassing was performed under vacuum, followed by the casting of film forming emulsions and drying at 22 ± 1 °C and 50% RH. The following samples were conditioned in a desiccator at 50% RH for a minimum of 72 h before testing them: Y1-CNC (1.00% glycerol, 1.00% Tween), Y2-CNC (1.25% glycerol, 1.25% Tween), and Y3-CNC (1.50% glycerol, 1.50% Tween); control samples were obtained the same, but without CNC (Y1c, Y2c and Y3c). Duplicate samples were obtained and average values were reported.
2.2.5. Imagining Evaluation
Scanning Electron Microscopy (SEM) was utilized for analysis of the bacterial cellulose structure, as well as for evaluating the yeast film with grafted CNC in terms of surface homogeneity (top-view images) and microstructure (cross-section images). Sputter Coater system (SPI Supplies, West Chester, PA, USA) was used to vacuum coating a 7 nm gold layer on the CNC, Y-CNC, and Yc samples to confer them conductivity. SEM images were acquired with Quanta 200 microscope (Thermo Fisher Scientific, Waltham, MA, USA), under low vacuum, at 15 kV beam accelerating voltage, with different magnification, depending on the samples: 30,000× for CNC, 5000× for film surface and cross-section. Particle distribution was performed with Image J software 1.54, NIH, USA [29].
2.2.6. Characterization of Y-CNC and Yc Films
Both control films without CNC (Yc) and films with grafted bacterial nanocellulose (Y-CNC) were physically characterized: thickness was determined to the nearest 0.0001 mm with an electronic micrometer (Digimatic Micrometer, Mitutoyo, Japan) by 10 different measurements in different areas in two independent films; preconditioned film disks of approx. 4 cm diameter were used to determine the free water (aw) in films with Fast lab water activity meter (GBX, Loire, France); dry matter was determined in duplicate samples with moisture analyzer RADWAG MA50.R, Crewe, UK. The CIEL*ab color parameters L* (luminosity), a* (green-red), and b* (blue-yellow) of yeast films were determined with Minolta colorimeter CR410, Tokyo, Japan (light source D65, observation angle 2°).
Total color change was calculated with Equation 1 using results from two independent experiments.
L*, a, b refers to color values for Y-CNC samples, Lc*, ac, bc refer to values for Yc samples.
Y-CNCs’ total color change was compared to the control Yc without CNC and interpreted according to the scale used by [30].
The Y-CNC and Yc placed on either a white or black background were measured to determining opacity films with Equation (2), where Lb represent film’s luminosity measured on black background and Lw represents film’s luminosity measured on white background [31]:
2.2.7. FT-IR
Fourier Transformed Infra-Red spectra for CLEO, CNC, and grafted Y-CNC functionalized with CLEO were analyzed in the range 400–7000 cm−1 with a resolution of 4 cm−1, on Nicolet iS50 spectrometer with attenuated total reflection accessory, DTGS detector and KBr beamsplitter (Thermo Scientific, Waltham, MA, USA), in controlled temperature environment, at 21 ± 0.5 °C [32], in independent duplicate samples.
2.2.8. GS/MS Analysis of Volatiles of CLEO and Y-CNCs
The volatile compounds (VOCs) present in the essential oil mixture CLEO and in the Y films were analyzed with a Trace GC-MS Ultra, MS ITQ 900 (Thermo Scientific, Waltham, MA, USA) according to the method described in detail by [30]. The SPME DVB/CAR/PDMS fiber adsorbed the VOCs that further were desorbed into Gc/Ms injection port. The components were tentatively identified using the ions signature resulting from Ms and the NIST 08 library database provided by XcaliburTM 4.0 (Thermo Fisher Scientific). Moreover, a semi-quantitative estimation was applied [21,30,32] using the VOCs area relative to the internal standard concentration (5 μL 2-octanol 5 mmol) used to spike the samples.
Hierarchical clustering was applied with Minitab® 18.1 Statistical Software (Coventry, UK) to enable grouping the variables by their similarity in two main clusters. Similarity and distance values for the clusters were estimated allowing final grouping of variables.
2.2.9. Mechanical Testing of Yeast Films
The mechanical behavior of yeast films was investigated by tensile testing on a universal testing machine (M350-5AT Testometric, Rochdale, UK) at 22 ± 0.2 °C, 50 ± 0.1% RH [33]. Yest films of 1 × 10 cm2 and a gauge length of 40 mm were subjected to tensile testing at 5 mm∙ min−1. The mechanical properties such as using elastic modulus and (maximum) tensile strength were extracted from the engineering stress-strain curves recorded for each film.
2.2.10. Statistical Analysis
Results were presented as average between two independent data sets and standard deviation. Statistically significant differences (p < 0.05) between groups were determined with ANOVA (analysis of variance) and Tukey’s post hoc test, Minitab® 18.1 Statistical Software (Coventry, UK). Principal Component Analysis with Varimax rotation was performed to explain the total variance, having total variance reduction as cut-off criterion (Unscrambler 9.7, CAMO, Oslo, Norway).
3. Results and Discussions
3.1. SEM Imaging
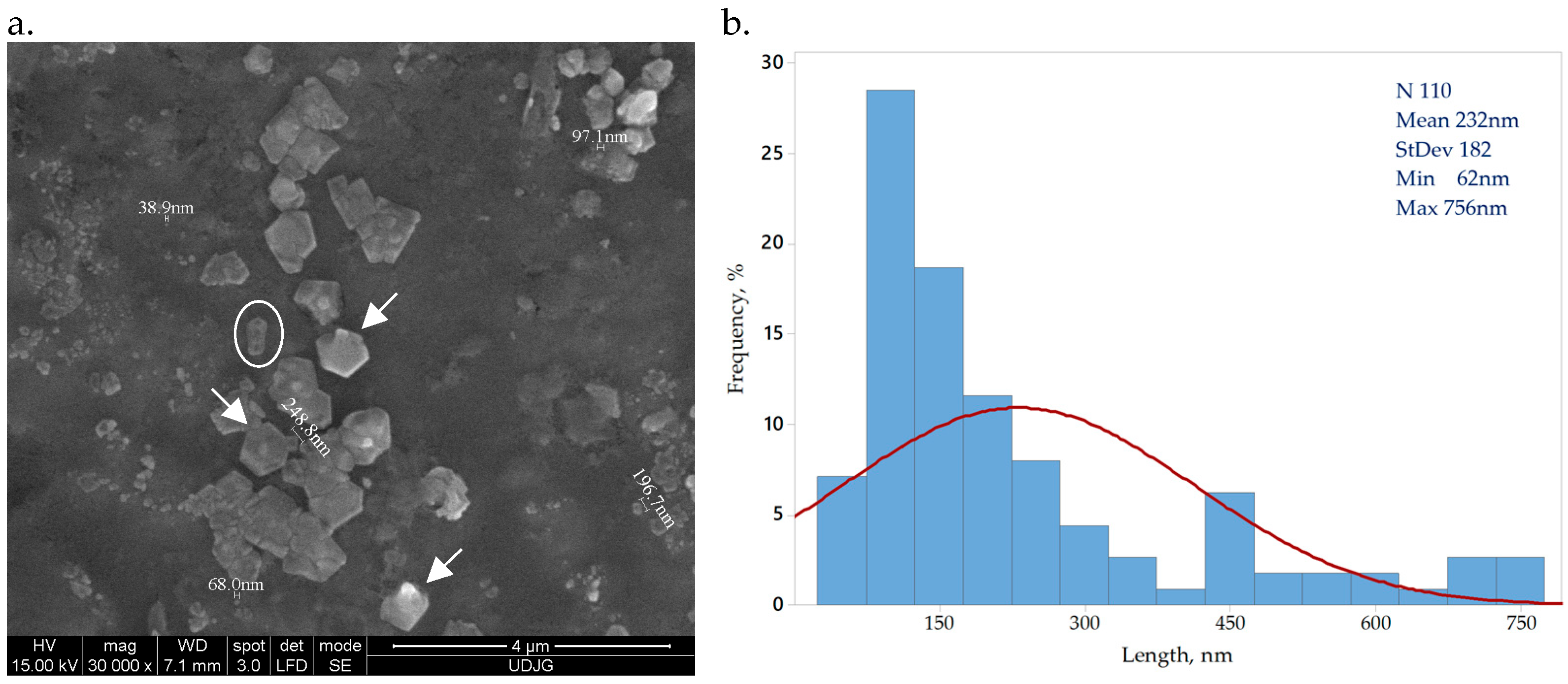
Bacterial cellulose obtained from SCOBY by acid hydrolysis presents a crystal shape, indicated by arrows in Figure 1a. The circled particle is a 3D rod-shape crystal of bacterial cellulose, a form previously reported by other research groups [34,35]. The Image J software analysis of 100 bacterial cellulose crystals indicated dimensions within nano scale, ranging between 62 and 756 nm, with an average 235 ± 188 nm Figure 1b. Similar reported values underline a dimension variation on the cellulose source and preparation method [34,35].
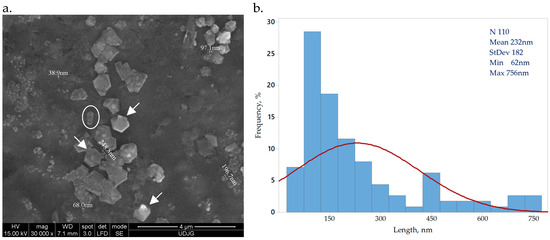
Figure 1.
Bacterial nanocellulose crystals (a) CNC magnified 30,000×; (b) CNC size distribution.
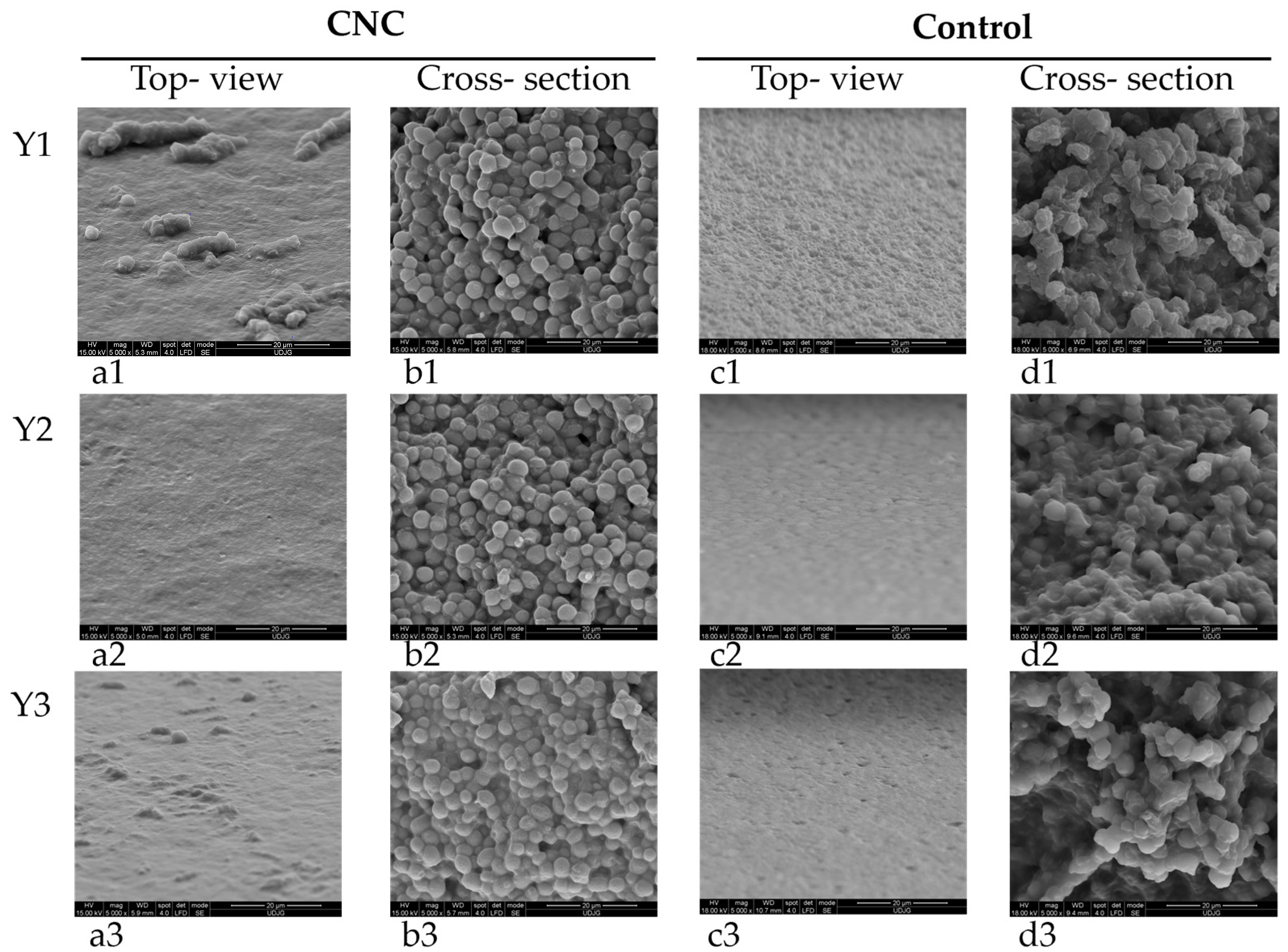
The evaluation of yeast film microstructures by SEM indicates, by top-view images, (Figure 2a) surfaces without pores or cracks but with protuberances that could be related to CNC presence. This fact correlates well with the surface roughness of these films compared to the control ones when touching the two types of films. In cross-section images (Figure 2b), yeast cells from film forming emulsion are easily recognizable, but nanocellulose particles also uniformly distributed onto each cell surface. The control films, without CNC, present a very uniform surface in top-view (Figure 2c) and in cross-section (Figure 2d) disrupted yeast cells due to sonication treatment, some with changed cellular volume. Similar modified morphology has been previously reported for sonicated yeast cells [36].
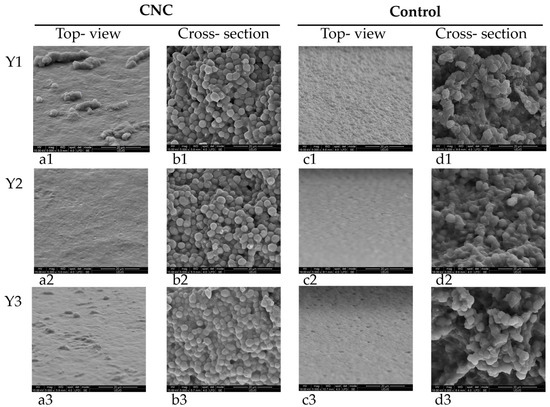
Figure 2.
Yeast films with grafted CNC (first two columns, a1–a3,b1–b3) (a1–a3). Top view 5000×, (b1–b3). Cross-section 5000× and control-without CNC (last two columns, c1–c3,d1–d3), (c1–c3). Top view 5000×, (d1–d3). Cross-section 5000×.
3.2. Characterization of Y-CNC and Yc Films
The thickness evaluation of yeast films indicates a uniform thickness between samples of approx. 0.20 mm, without significant differences (p > 0.05) (Table 1). Dry matter for Y-CNC films is significantly higher (p < 0.05) than that of control yeast films without CNC. The water activity of grafted CNC yeast films was significantly lower (p < 0.05) than those for control Yc films without bacterial cellulose, which could be explained by cellulose’ hydrophilicity, as other groups reported [37]. All CNC grafted yeast films presented visible differences in color when compared to the control samples, without CNC, according to the classification used by other researchers [30]. The film’s opacity evaluated by colorimetry was calculated based on luminosity measured on a white or black background. The grafted bacterial cellulose significantly increased the opacity of yeast films (p < 0.05) compared to the control samples, as other studies indicated [38], but the functionalized CNC films do not differ significantly (p > 0.05) of each other. Nevertheless, all Y-CNC films were see-through (Figure 3).

Table 1.
Physical properties of yeast films.

Figure 3.
Yeast films functionalized with 2.0% CLEO and 2.0% CNC from left to right: Y1-CNC, Y2-CNC, Y3-CNC.
3.3. FT-IR Analysis
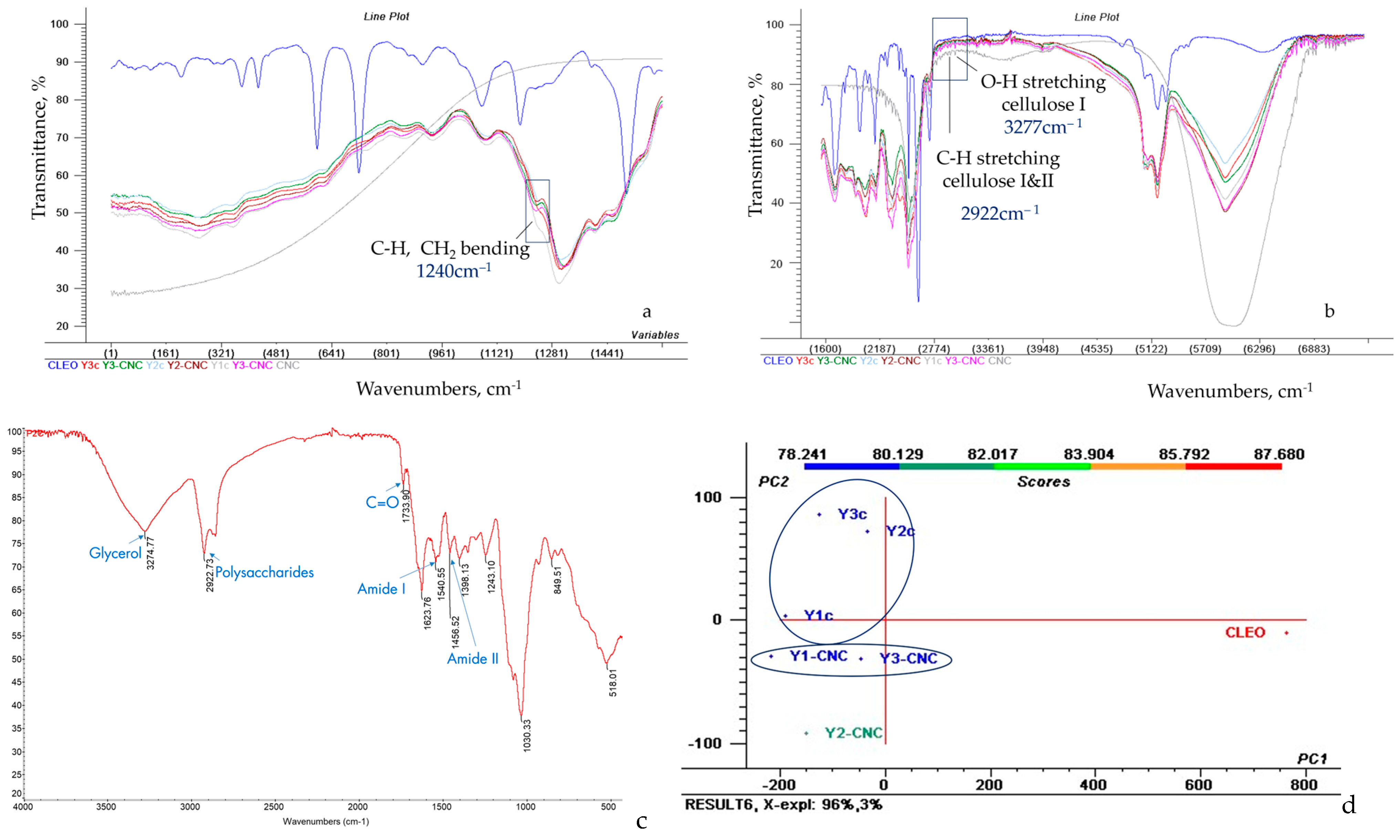
The Fourier Transformed Infrared Spectra of yeast films were divided for better visibility into 400–1600 cm−1 and 1600–7000 cm−1 (Figure 4a,b) and indicated the presence of functional groups characteristic for yeast, as previously reported by [39]: peaks at approx. 1034 cm−1 specific to C-O groups from polysaccharides, at approx. 1540 cm−1 indicating amide II (proteins, C-N stretching, and N-H bending), as well as at approx. 1623 cm−1 representing amide I (proteins, C=O stretching).
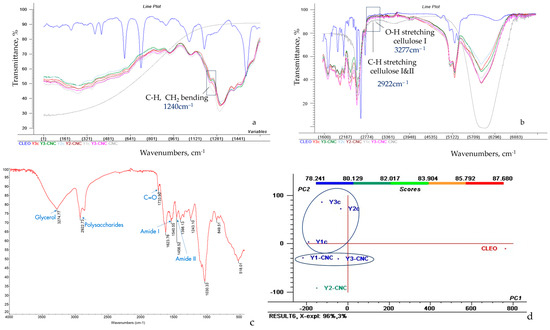
Figure 4.
FTIR spectra of CNC, CLEO and grafted CNC on yeast films, from 400 to 1600 cm−1 (a) and 1600–7000 cm−1 wavenumber (b), molecules demonstration in FTIR spectra of Y1-CNC (c), PCA scores and clusters (d).
The absorbance intensity for amide II in Y-CNC films decreased 1.12 times compared to amide I, which could be explained by the sensitivity of N-H groups from amide II to hydrogen bonding [39]. The influence of increasing concentrations of glycerol as plasticizer is related to a decreased intensity for amide I and II in yeast film samples. The influence of Tween as a peak at approx. 1733 cm−1 appears visible only in the spectra of Y3-CNC and Y3c, where it has the highest concentration of all samples (1.50%), while for the other two concentrations, samples with 1.25% or 1.00% Tween is less visible, neither in functionalized Y-CNC, nor in control Y films.
FTIR spectra of CNC (Figure 4a) indicates, in the group frequency region, the most intense absorption peak at 3277 cm−1 characteristic for O-H stretching of strong hydrogen bonds in cellulose I, at 2916 cm−1 for the C-H stretching, previously correlated to cellulose I and II [40]; analysis of the fingerprint region (Figure 4b) shows peaks at 1239 cm−1 corresponding to C-H and CH2 bending and at 1090 cm−1 due to C-O stretching [41].
The analysis of CNC-grafted yeast films spectra (Figure 4b) indicates peaks at 3277 cm−1 distinctive for O-H stretching in cellulose I (intensities of 69.392% in Y1-CNC, 69.093% in Y2-CNC, 74.244% in Y3-CNC), at 2922 cm−1 corresponding to C-H stretching from cellulose I and II (intensities of 74.304% in Y1-CNC, 75.049% in Y2-CNC, 73.721% in Y3-CNC) and at approx. 1240 cm−1 related to C-H and CH2 bending (intensities of 70.887% in Y1-CNC, 73.330% in Y2-CNC, 72.881% in Y3-CNC), suggesting the successful incorporation of CNC in all three yeast films.
The spectra of CLEO mixture reveals peaks specific for both lavender and cinnamon, as the 848 cm−1 characteristic for monoterpenes or the one at 1733 cm−1, indicating C-O presence, which can be related to cinnamaldehyde, a cinnamon marker [42]; peaks which can be identified in the current yeast films (Y-CNCs). Moreover, cynamaldehyde can act as a crosslinker in developing 3D polymeric networks, and its presence in Y-CNC spectra can only be justified by the presence of grafted CNC [8].
PCA analysis (Figure 4c) indicates a clustering of grafted Y-CNC films in the third quadrant (Q III) while control yeast films Yc are positioned in the second quadrant (QII). A distinctive cluster is solely represented by CLEO in the fourth quadrant (QIV). Nevertheless, Y2-CNC is the most different of the other two grafted CNC yeast films. Y2-CNC and Y3-CNC samples are the closest ones to CLEO on abscissa, demonstrating a better retention of the total volatile compounds in comparison with Y1-CNC and control samples. The distinct cluster of the yeast samples grafted with CNC positioned in QIII, in comparison with control present in QII also demonstrate that CNC has significantly changed the film structure, contributing to the formation of new structural links that vibrates different from control, thus it is expected to present different properties. While the total variance was reduced by PCA rotation, 96% of variance is explained by PC1 and 3% is explained by PC2.
3.4. Analysis of Volatiles of CLEO and Y-CNCs by SPME GC/MS
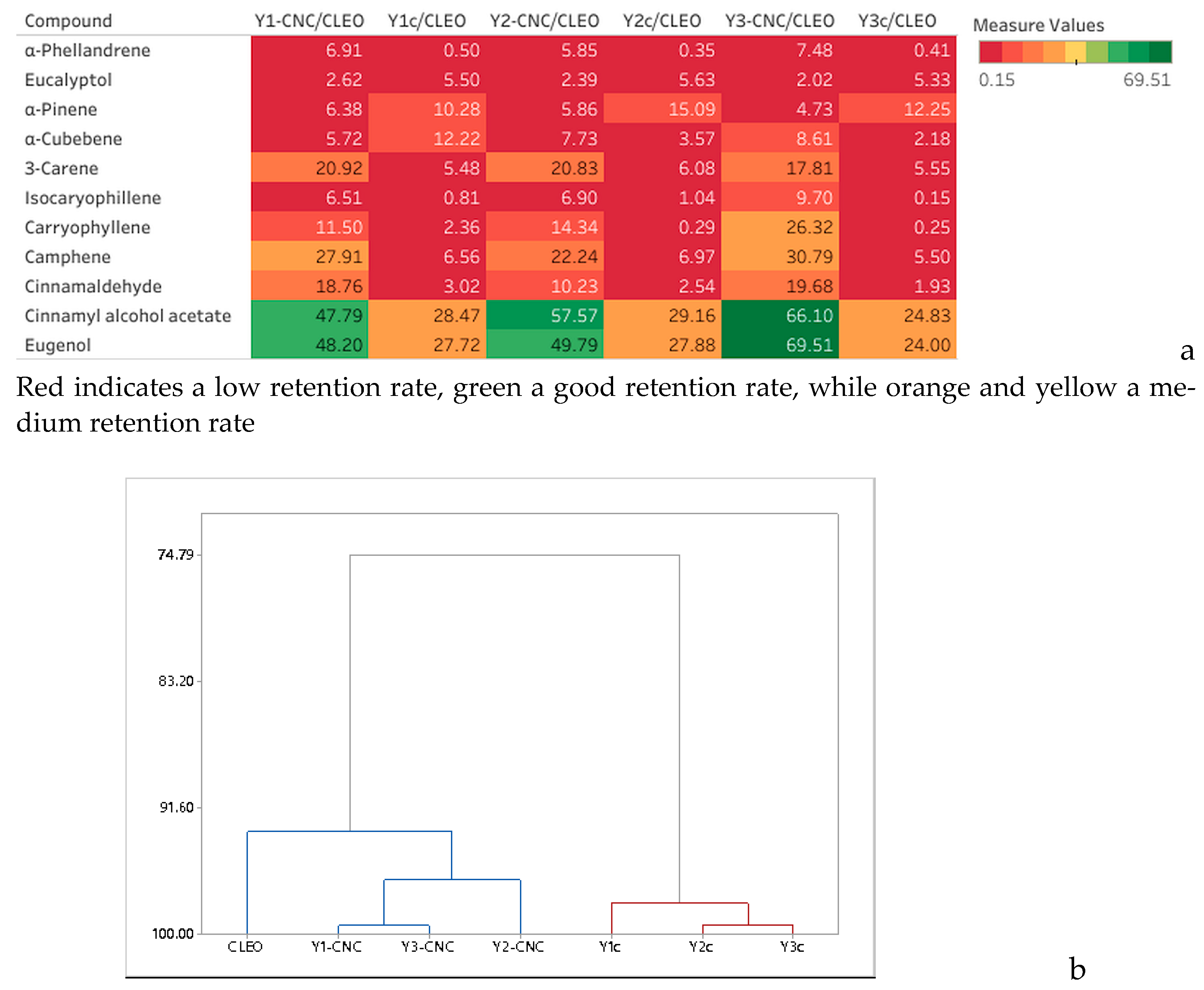
The composition of the CLEO mixture essential oil is presented in Table 2. The most abundant constituents of VOCs in the CLEO mixture is cinnamaldehyde, representing almost a quarter of the total VOCs and iso-caryophyllene representing ~15.5% of the volatiles, followed by α-Pinene, α-Phellandrene, and Eugenol. These VOCs originating either from lavender or cinnamon essential oil were reported also by other researchers [43,44,45,46]. The 10 identified compounds represent approx. 70% of the total mixture components. All the VOCs were further traced in the Y films containing CNC but also in the control films. When comparing the total VOC area of Y-CNC with Yc it can be noticed a 4.61–5.89-fold higher volatile retention for all the films containing cellulose, thus demonstrating a better retention capacity in comparison with films with no cellulose (Table 3). The hierarchical clustering graph (Figure 5a) also shows a strong correlation between Y-CNC and CLEO with 93.15 similarity coefficients while all the Yc films are placed in an independent cluster with 98.0 similarity coefficients.

Table 2.
GC/MS SPME fingerprint of CLEO mixture indicating the % of the 10 most abundant VOCs tentatively identified compounds.

Table 3.
The relative concentration (μg octanol/mg) of tentatively identified VOCs present in the yeast films determined by SPME GC/MS.
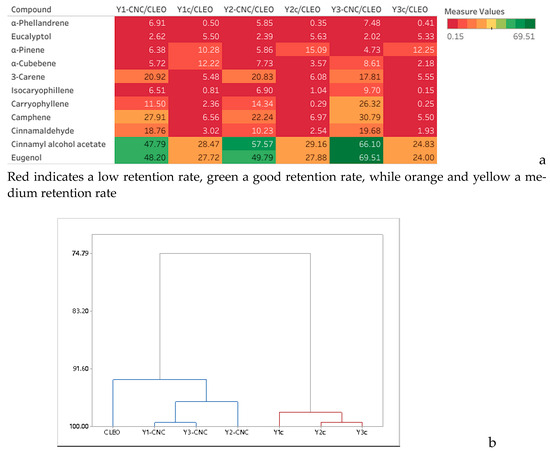
Figure 5.
Heat map showing the retention rate of VOCs (%) by the yeast films in comparison with CLEO (a) and dendogram (b).
Also, the dendrogram in Figure 5b shows correlations between Y1-CNC and Y3-CNC while in the case of control Y2c is closer from Y3c. The best retention capacity of VOCs displayed by the Y-CNC films was registered for Y3-CNC. However, when looking at each volatile component, there are just a few significant differences registered (p < 0.05), for example when comparing Y1-CNC and Y3-CNC with Y2-CNC for cinnamaldehyde and eugenol. These compounds were also reported to present high antimicrobial activity [47,48].
The heatmap (Figure 5a) compares the retention rates in relation with CLEO concentration and shows that for the highly volatile compounds eluted in the first half of the chromatogram the retention rate is relatively low for Y-CNC/CLEO and almost insignificant for Ycs given the red color of the heatmap. In the second half the retention rates tend to obtain better for Y-CNCs in comparison with control (Ycs) and the last two eluted components demonstrated the highest retention rates that varied from 47.99% in Y1-CNC to almost 68.00% in Y3-CNC in comparison with control samples where the retention was fair ranging from 24.41% in Y3c to 28.52 in Y2c.
3.5. Antimicrobial Activity of Y-CNC and Yc Against Spoilage Microorganisms and Pathogens
The MIC and MFC values for tested spoilage microorganisms were 1% (w/w) CLEO, while MBC was 2% (w/w) CLEO for B. cereus, L. monocytogenes, S. aureus and E. coli. Based on these results the yeast films were functionalized with 2% CLEO.
The evaluation of antimicrobial activity of control films with 2.00% CLEO against spoilage microflora indicated inhibition ranging between 27.99 ± 1.97 mm against Rhodotorula glutinis, 23.60 ± 0.68 mm against Geotrichum candidum, and 15.29 ± 3.17 mm against Bacillus cereus (Table 4). On the contrary, disk diffusion assay of antimicrobial activity of control films functionalized with 2.00% CLEO against the tested food borne pathogens demonstrated that they failed to exert any inhibition. A similar result was previously reported for essential oil included in films matrices, limiting their antimicrobial efficacy (e.g., whey protein isolate film functionalized with 7–9% sage EO [49]).

Table 4.
Antimicrobial activity of yeast films. Results are average ± SD of two independent replicates, expressed in mm. nd—not detected.
Grafting CNC in yeast films improved approx. 2 times their antimicrobial activity against all tested spoilage microflora, inhibition diameters reaching approx. 62.85 ± 0.45 mm against R. glutinis, 55.55 ± 0.35 mm against G. candidum, and 33.49 ± 3.12 mm against B. cereus. Moreover, the grafted CNC yeast films exerted antibacterial activity against all three tested pathogens, ranging 11–13 mm, significantly different (p < 0.05) from the antimicrobial activity against spoilage microorganisms. These findings were confirmed by [8] who reported an increase up to 80% in antimicrobial activity of whey protein films with 10% grafted bacterial cellulose, functionalized with oregano essential oil against food borne pathogens such as Staphylococcus aureus, Listeria monocytogenes, Salmonella enteritidis, and Pseudomonas aeruginosa, with higher inhibition against Gram-positive bacteria [8]. A plausible explanation for this spectacular increase in antibacterial activity could be connected to the altered morphology of cellulose functionalized films, with increased retention capacity due to surface irregularities and release profile of essential oils, hypothesis which is sustained by the GC/MS volatile fingerprint of the yeast films.
The antibacterial activity of eugenol, present in CLEO from cinnamon essential oil and in functionalized CLEO yeast film as indicated by GC/MS analysis, was previously reported against L. monocytogenes. Its antibacterial activity is based on cell membrane alteration, inhibition of ATP-ase and of motility, antibiofilm formation through quorum sensing activity, and inhibition of extracellular polymeric substances production [26,50]. The antifungal activity of cinnamon essential oil presented by [51] was mostly due to phenylpropene, with its conjugated double chemical bond, inhibiting spoilage yeast and fungi. Moreover, the presence in CLEO of lavender’s eucalyptol identified by GC/MS in functionalized yeast films is known to contribute with antibacterial effect, most probably through cell wall changes [38].
3.6. Mechanical Testing of Yeast Films
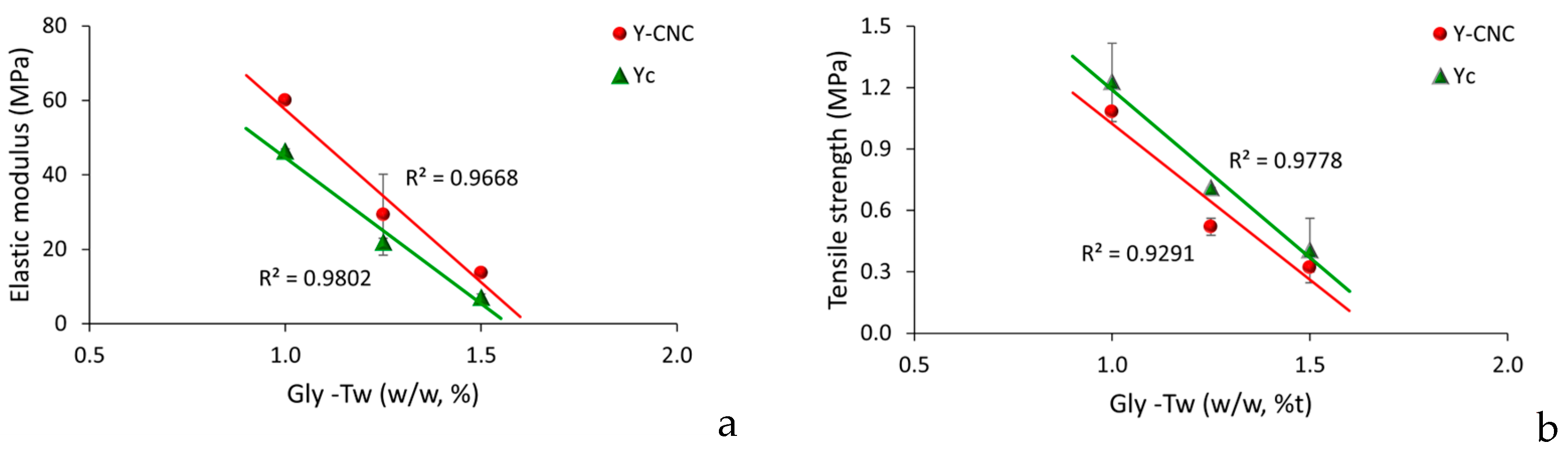
The evaluation of mechanical properties took into consideration the simultaneous influence of two variables in yeast films, increasing concentrations of plasticizers and emulsifier and grafted bacterial cellulose (Figure 6). Increasing concentrations of glycerol as plasticizer and Tween as emulsifier in yeast film forming solutions influenced the rigidity of both functionalized yeast films with bacterial cellulose and in the control samples, without CNC (Figure 6a). In general, the higher the plasticizer concentration, the lower the rigidity, the films being more flexible. A possible explanation for this effect could be the reduced formation of intermolecular van der Waals forces and increased formation of intermolecular bond between polymeric matrix and plasticizer, as indicated by [52].
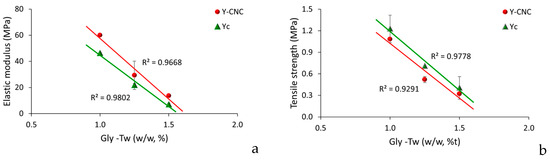
Figure 6.
Mechanical properties of yeast films (a). Elastic modulus, (b). Tensile strength.
Grafting bacterial nanocellulose into yeast film increased elastic modulus compared to the control samples without CNC (Figure 6a), which could probably be determined by an improved continuity within biopolymeric matrix, producing a mobility reduction in particles, as reported by [53] for polyvinyl alcohol/chitosan films reinforced with 4% (w/w) bacterial cellulose.
Tensile strengths of both control and Y-CNC films presented a linear decrease with increasing concentration of the plasticizer (Figure 6b), which correlates to their role in reducing intermolecular forces, previously reported by [17,52].
As SEM analysis of grafted CNC yeast film surfaces indicated, the presence of irregularities on their surface made them rougher compared to the control ones, which was indicated to be often correlated to lower tensile strengths values in films [8]. Similar tensile strengths range values were reported by Papadaki’s research group for 7% whey protein films functionalized with oregano oil and bacterial cellulose nanowiskers (2–10% w/w based on protein amount), of approx. 1.7 ± 0.2 MPa. Other similar results were reported by [54,55].
4. Conclusions
The potential of grafting bacterial nanocellulose in yeast films functionalized with a mixture of cinnamon and lavender essential oil was evaluated in this study, as well as the influence of different concentrations of plasticizer, glycerol, and emulsifier, Tween 80, on mechanical properties, volatile fingerprint and the antimicrobial activity of films.
Bacterial cellulose nanocrystals were obtained from Kombucha’s SCOBY valorization, with dimensions ranging between 62 and 756 nm, with an average of 235 ± 188 nm.
Films based on yeast biomass with concentrations ranging 1.00–1.50% of glycerol as plasticizer and Tween as surfactant were obtained, functionalized with CLEO and bacterial nanocellulose, and further characterized. Grafted bacterial cellulose to yeast films were uniformly distributed in films, determined rougher films surfaces, with irregularities, indicated by SEM analysis. The grafted bacterial cellulose significantly increased the opacity of yeast films (p < 0.05) compared to the control samples but are still see-through. FT-IR spectra confirmed successful incorporation of bacterial cellulose in all three yeast films. Volatile fingerprint of CLEO mix indicated cinnamaldehyde as most abundant constituent of total VOC, almost 25% and iso-caryophyllene representing ~15.5% of the volatiles, but also α-Pinene, α-Phellandrene, and Eugenol, which have antimicrobial activity. The VOCs from CLEO were identified in CNC yeast films, induced strong antimicrobial activity against spoilage microorgansims, as R. glutinis, G. candidum and B. cereus, but also significant antipathogen activity against L. monocytogenes, S. aureus, and E. coli.
Yeast films with 1.50% glycerol and 1.50% Tween 80 are more flexible and bacterial nanocellulose grafted into yeast film increases elastic modulus compared to the control samples without CNC.
The development and characterization of an innovative, sustainable packaging solution based on yeast biomass, functionalized with cinnamon and lavender essential oil mix and bacterial cellulose obtained from SCOBY indicated that it could be further optimized to fit different food applications as active packaging.
Author Contributions
Conceptualization, I.B. and D.B.; methodology, I.B., L.G.-G., A.C.C., F.S. and D.B.; software, D.B.; validation, D.B. and I.B.; formal analysis, I.B.; investigation A.C.C. and L.G.-G.; resources, I.B.; data curation, I.B.; writing—original draft preparation, I.B.; writing—review and editing, I.B. and D.B.; visualization, A.C.C. and I.B.; supervision, D.B., F.S. and C.F.; project administration, I.B.; funding acquisition, I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grant number RF 2486/31.05.2024 from Dunarea de Jos University of Galati, Romania.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Center MoRAS developed through Grant POSCCE ID 1815, cod SMIS 48,745 10.10.2024, (www.moras.ugal.ro).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hassoun, A.; Boukid, F.; Ozogul, F.; Aït-Kaddour, A.; Soriano, J.M.; Lorenzo, J.M.; Perestrelo, R.; Galanakis, C.M.; Bono, G.; Bouyahya, A.; et al. Creating new opportunities for sustainable food packaging through dimensions of industry 4.0: New insights into the food waste perspective. Trends Food Sci. Technol. 2023, 142, 104238. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Noriega, E.; Tanja, F.; Bjørn, R.; Rotabakk, T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef]
- Hussain, S.; Akhter, R.; Maktedar, S.S. Advancements in sustainable food packaging: From eco-friendly materials to innovative technologies. Sustain. Food Technol. 2024, 2, 1297–1364. [Google Scholar] [CrossRef]
- Bleoanca, I.; Turtoi, M. Innovative Packaging Solutions. In Trends in Fish Processing Technologies, 1st ed.; Borda, D., Nicolau, A., Raspor, P., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 187–216. [Google Scholar]
- Khin, M.N.; Ahammed, S.; Kamal, M.M.; Saqib, M.N.; Liu, F.; Zhong, F. Investigating next-generation edible packaging: Protein-based films and coatings for delivering active compounds. Food Hydrocoll. Health 2024, 6, 100182. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, R.; Li, N.; Yang, X.; Lin, D. Nanocellulose: An amazing nanomaterial with diverse applications in food science. Carbohydr. Polym. 2023, 304, 120497. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.A.G.S.; Dourado, F.; Gama, M.; Poças, F. Nanocellulose Bio-Based Composites for Food Packaging. Nanomaterials 2020, 10, 2041. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Lappa, I.K.; Manikas, A.C.; Pastore Carbone, M.G.; Natsia, A.; Kachrimanidou, V.; Kopsahelis, N. Grafting bacterial cellulose nanowhiskers into whey protein/essential oil film composites: Effect on structure, essential oil release and antibacterial properties of films. Food Hydrocoll. 2023, 147 Pt A, 109374. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Salvay, A.G.; de la Osa, O.; Wagner, J.R. Characterization of thermal, mechanical and hydration properties of novel films based on Saccharomyces cerevisiae biomass. Innov. Food Sci. Emerg. Technol. 2018, 48, 240–247. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Salvay, A.G. Water Vapour Transport in Biopolymeric Materials: Effects of Thickness and Water Vapour Pressure Gradient on Yeast Biomass-Based Films. J. Polym. Environ. 2022, 30, 2976–2989. [Google Scholar] [CrossRef]
- Sukyai, P.; Anongjanya, P.; Bunyahwuthakul, N.; Kongsin, K.; Harnkarnsujarit, N.; Sukatta, U.; Sothornvit, R.; Chollakup, R. Effect of cellulose nanocrystals from sugarcane bagasse on whey protein isolate-based films. Food Res. Int. 2018, 107, 528–535. [Google Scholar] [CrossRef]
- Adel, A.M.; Ibrahim, A.A.; El-Shafei, A.M.; Al-Shemy, M.T. Inclusion complex of clove oil with chitosan/β-cyclodextrin citrate/oxidized nanocellulose biocomposite for active food packaging. Food Packag. Shelf Life 2019, 20, 100307. [Google Scholar] [CrossRef]
- Noremylia, M.B.; Hassan, M.Z.; Ismail, Z. Recent advancement in isolation, processing, characterization and applications of emerging nanocellulose: A review. Int. J. Biol. Macromol. 2022, 206, 954–976. [Google Scholar] [CrossRef]
- Baschali, A.; Tsakalidou, E.; Kyriacou, A.; Karavasiloglou, N.; Matalas, A.L. Traditional Low-Alcoholic and Non-Alcoholic Fermented Beverages Consumed in European Countries: A Neglected Food Group. Nutr. Res. Rev. 2017, 30, 1–24. [Google Scholar] [CrossRef]
- Bystrom, C. Biomimetically Improved Materials Comprising Microfibrillated Cellulose; Umeå University: Umeå, Sweden, 2021. [Google Scholar]
- Pihurov, M.; Păcularu- Burada, B.; Cotârleţ, M.; Vasile, M.A.; Bahrim, G.E. Novel Insights for Metabiotics Production by Using Artisanal Probiotic Cultures. Microorganisms 2021, 9, 2184. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing Fibrillated Cellulose as a Sustainable Technological Material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Ballesteros, B.E.; Guevara-Morales, A.; Martín-Martínez, E.S.; Figueroa-López, U.; Vieyra, H. Composite of Polylactic Acid and Microcellulose from Kombucha Membranes. e-Polymers 2021, 20, 015–026. [Google Scholar] [CrossRef]
- Dima, S.-O.; Panaitescu, D.-M.; Orban, C.; Ghiurea, M.; Doncea, S.-M.; Fierascu, R.C.; Nistor, C.L.; Alexandrescu, E.; Nicolae, C.-A.; Trică, B.; et al. Bacterial Nanocellulose from Side-Streams of Kombucha Beverages Production: Preparation and Physical-Chemical Properties. Polymers 2017, 9, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, P.; Singh, P.P.; Vo, D.-V.N.; Kumar, D.; Balasubramanian, K.; Mubayi, A.; Srivastava, A.; Sharma, A. Bacterial Nanocellulose: Green Polymer Materials for High Performance Energy Storage Applications. J. Environ. Chem. Eng. 2022, 10, 108176. [Google Scholar] [CrossRef]
- Bleoanca, I.; Saje, K.; Mihalcea, L.; Oniciuc, E.A.; Smole- Mozina, S.; Nicolau, A.N.; Borda, D. Contribution of high pressure and thyme extract to control Listeria monocytogenes in fresh cheese—A hurdle approach. Innov. Food Sci. Emerg. Technol. 2016, 38 Pt A, 7–14. [Google Scholar] [CrossRef]
- Dediu Botezatu, A.; Apetrei, R.M.; Costea, I.F.; Barbu, V.; Grigore-Gurgu, L.; Botez, F.; Dinica, R.M.; Furdui, B.; Cârâc, G. Synthesis and characterization of novel chitosan derivatives (containing dipyridinium quaternary salts) with antimicrobial potential. Carbohydr. Res. 2023, 534, 108964. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current challenges, applications and future perspectives of SCOBY cellulose of Kombucha fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Soares, M.G.; de Lima, M.; Reolon Schmidt, V.C. Technological aspects of kombucha, its applications and the symbiotic culture (SCOBY), and extraction of compounds of interest: A literature review. Trends Food Sci. Technol. 2021, 110, 539–550. [Google Scholar] [CrossRef]
- Gedarawatte, S.T.G.; Ravensdale, J.T.; Al-Salami, H.; Dykes, G.A.; Coorey, R. Antimicrobial Efficacy of Nisin-Loaded Bacterial Cellulose Nanocrystals against Selected Meat Spoilage Lactic Acid Bacteria. Carbohydr. Polym. 2021, 251, 117096. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Andrade Feitosa, J.P.; Portela da Gama, F.M.; Saraiva Morais, J.P.; Andrade, F.K.; Sá Moreira de Souza Filho, M.; de Freitas Rosa, M. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Perumal, A.B.; Nambiar, R.B.; Moses, J.A.; Anandharamakrishnan, C. Nanocellulose: Recent trends and applications in the food industry. Food Hydrocoll. 2022, 127, 107484. [Google Scholar] [CrossRef]
- Lago, A.; Delgado, J.F.; Rezzani, G.D.; Cottet, C.; Ramírez Tapias, Y.A.; Peltzer, M.A.; Salvay, A.G. Multi-Component Biodegradable Materials Based on Water Kefir Grains and Yeast Biomasses: Effect of the Mixing Ratio on the Properties of the Films. Polymers 2023, 15, 2594. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Bleoanca, I.; Lanciu, A.; Patrașcu, L.; Ceoromila, A.; Borda, D. Efficacy of two stabilizers in nanoemulsions with whey proteins and thyme essential oil as edible coatings for zucchini. Membranes 2022, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-García, M.; Calderón-Domínguez, G.; Chanona-Pérez, J.J.; Mendoza-Madrigal, A.G.; Di Pierro, P.; García-Almendárez, B.E.; Amaro-Reyes, A.; Regalado-González, C. Physical, Structural, Barrier, and Antifungal Characterization of Chitosan–Zein Edible Films with Added Essential Oils. Int. J. Mol. Sci. 2017, 18, 2370. [Google Scholar] [CrossRef]
- Dorofte, A.L.; Dima, C.; Bleoanca, I.; Aprodu, I.; Alexe, P.; Kharazmi, M.S.; Mahdi Jafari, S.; Dima, S.; Borda, D. Mechanism of β–cyclodextrin—Thyme nanocomplex formation and release: In silico behavior, structural and functional properties. Carbohydr. Polym. Technol. Appl. 2024, 7, 100422. [Google Scholar] [CrossRef]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: Montgomery, PA, USA, 1996.
- Channab, B.-E.; El Idrissi, A.; Essamlali, Y.; Zahouily, M. Nanocellulose: Structure, modification, biodegradation and applications in agriculture as slow/controlled release fertilizer, superabsorbent, and crop protection: A review. J. Environ. Manag. 2024, 352, 119928. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Sharma, A.; Ahlawat, V.; Bhattacharya, M.; Goswami, S. Process optimization for the production of cellulose nanocrystals from rice straw derived α-cellulose. Mater. Sci. Energy Technol. 2020, 3, 328–334. [Google Scholar] [CrossRef]
- Soro, A.B.; Oliveira, M.; O’Donnell, C.P.; Tiwari, B.K. Ultrasound assisted modulation of yeast growth and inactivation kinetics. Ultrason Sonochem. 2021, 80, 105819. [Google Scholar] [CrossRef]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef]
- Abral, H.; Pratama, A.B.; Handayani, D.; Mahardika, M.; Aminah, I.; Sandrawati, N.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. Antimicrobial Edible Film Prepared from Bacterial Cellulose Nanofibers/Starch/Chitosan for a Food Packaging Alternative. Int. J. Polym. Sci. 2021, 2021, 6641284. [Google Scholar] [CrossRef]
- Mihoubi, W.; Sahli, E.; Gargouri, A.; Amiel, C. FTIR spectroscopy of whole cells for the monitoring of yeast apoptosis mediated by p53 over-expression and its suppression by Nigella sativa extracts. PLoS ONE 2017, 12, e0180680. [Google Scholar] [CrossRef] [PubMed]
- Suryanto, H.; Muhajir, M.; Susilo, B.D.; Aji Pradana, Y.R.; Wijaya, H.W.; Ansari, A.S.; Yanuhar, U. Nanofibrillation of Bacterial Cellulose Using High-Pressure Homogenization and Its Films Characteristics. J. Renewable Mat. 2021, 9, 1717–1728. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Hao, S.; Huang, J. Preparation of All-Cellulose Composites Based on Controlled Dissolution Procedure. Starch-Stärke 2021, 73, 2000280. [Google Scholar] [CrossRef]
- Popa, R.M.; Fetea, F.; Socaciu, C. Attenuated Total Reflectance—Fourier Transform Infrared Spectroscopy Applied for the Evaluation of Essential Oils’ Pattern Recognition and Thermo-oxidative Stability: A Comparative Study. Stud. Univ. Babes-Bolyai Chem. 2021, 66, 33–50. [Google Scholar] [CrossRef]
- Qiao, Y.; Yu, Z.; Bai, L.; Li, H.; Zhang, S.; Liu, J.; Gao, Z.; Yang, X. Chemical composition of essential oils from Thymus mongolicus, Cinnamomum verum, and Origanum vulgare and their acaricidal effects on Haemaphysalis longicornis (Acari: Ixodidae). Ecotoxicol. Environ. Saf. 2021, 224, 112672. [Google Scholar] [CrossRef]
- Nunes, C.; Raposo, M.F.J.; Petronilho, S.; Machado, F.; Fulgêncio, R.; Gomes, M.H.; Evtuguin, D.V.; Rocha, S.; Coimbra, M. Cinnamomum burmannii decoction: A thickening and flavouring ingredient. LWT 2022, 153, 112428. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Sudha, T.; Darwish, N.H.E.; Chader, H.; Belkadi, A.; Rajabi, M.; Houche, A.; Benkebailli, F.; Oudjida, F.; Mousa, S.A. A New Eucalyptol-Rich Lavender (Lavandula stoechas L.) Essential Oil: Emerging Potential for Therapy against Inflammation and Cancer. Molecules 2020, 25, 3671. [Google Scholar] [CrossRef]
- Bouyahya, A.; Chamkhi, I.; El Menyiy, N.; El Moudden, H.; Harhar, H.; El Idrissi, Z.L.; Khouchlaa, A.; Jouadi, I.; El Baaboua, A.; Taha, D.; et al. Traditional use, phytochemistry, toxicology, and pharmacological properties of Lavandula dentata L.: A comprehensive review. S. Afr. J. Bot. 2023, 154, 67–87. [Google Scholar] [CrossRef]
- Gomes, C.; Moreira, R.G.; Castell-Perez, E. Poly (DL-lactide-co-glycolide) (PLGA) Nanoparticles with Entrapped trans-Cinnamaldehyde and Eugenol for Antimicrobial Delivery Applications. J. Food Sci. 2021, 76, N16–N24. [Google Scholar] [CrossRef]
- Sanla-Ead, N.; Jangchud, A.; Chonhenchob, V.; Suppakul, P. Antimicrobial Activity of Cinnamaldehyde and Eugenol and Their Activity after Incorporation into Cellulose-based Packaging Films. Packag. Technol. Sci. 2012, 25, 7–17. [Google Scholar] [CrossRef]
- Fernandez-Pan, I.; Royo, M.; Mate, J.I. Antimicrobial activity of whey protein isolate edible films with essential oils against food spoilers and foodborne pathogens. J. Food Sci. 2012, 77, M383–M390. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Xue, J.; Luo, Y.; Upadhyay, A. Eugenol nanoemulsion reduces Listeria monocytogenes biofilm by modulating motility, quorum sensing, and biofilm architecture. Front. Sustain. Food Syst. 2023, 7, 1272373. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Q.; Wang, Z.; Cao, H.; Zhang, D. Structure-activity relationships of cinnamaldehyde and eugenol derivatives against plant pathogenic fungi. Ind. Crops Prod. 2017, 97, 388–394. [Google Scholar] [CrossRef]
- Bang, Y.J.; Shankar, S.; Rhim, J.W. In situ synthesis of multi-functional gelatin/resorcinol/silver nanoparticles composite films. Food Packag. Shelf Life 2019, 22, 100399. [Google Scholar] [CrossRef]
- Choo, K.W.; Dhital, R.; Mao, L.; Lin, M.; Mustapha, A. Development of polyvinyl alcohol/chitosan/modified bacterial nanocellulose films incorporated with 4-hexylresorcinol for food packaging applications. Food Packag. Shelf Life 2021, 30, 100769. [Google Scholar] [CrossRef]
- Delgado, J.F.; de la Osa, O.; Salvay, A.G.; Cavallo, E.; Cerrutti, P.; Foresti, M.L.; Peltzer, M.A. Reinforcement of Yeast Biomass Films with Bacterial Cellulose and Rice Husk Cellulose Nanofibres. J. Polym. Environ. 2021, 29, 3242–3251. [Google Scholar] [CrossRef]
- Rana, H.; Garg, R.; Singh, N.; Goswami, S. Guargum/nanocellulose based novel crosslinked antimicrobial film with enhanced barrier and mechanical properties for food packaging. J. Environ. Chem. Eng. 2023, 11, 109254. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).