Bacterial Nanocellulose Grafted in Yeast Films: The Influence of Plasticizer and Emulsifier Concentration on Film Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Antimicrobial Activity of CLEO Per Se and in Yeast Films
2.2.2. SCOBY Cultivation
2.2.3. Bacterial Nanocellulose Purification
2.2.4. Yeast Film Functionalized with CLEO and Bacterial Nanocellulose
2.2.5. Imagining Evaluation
2.2.6. Characterization of Y-CNC and Yc Films
2.2.7. FT-IR
2.2.8. GS/MS Analysis of Volatiles of CLEO and Y-CNCs
2.2.9. Mechanical Testing of Yeast Films
2.2.10. Statistical Analysis
3. Results and Discussions
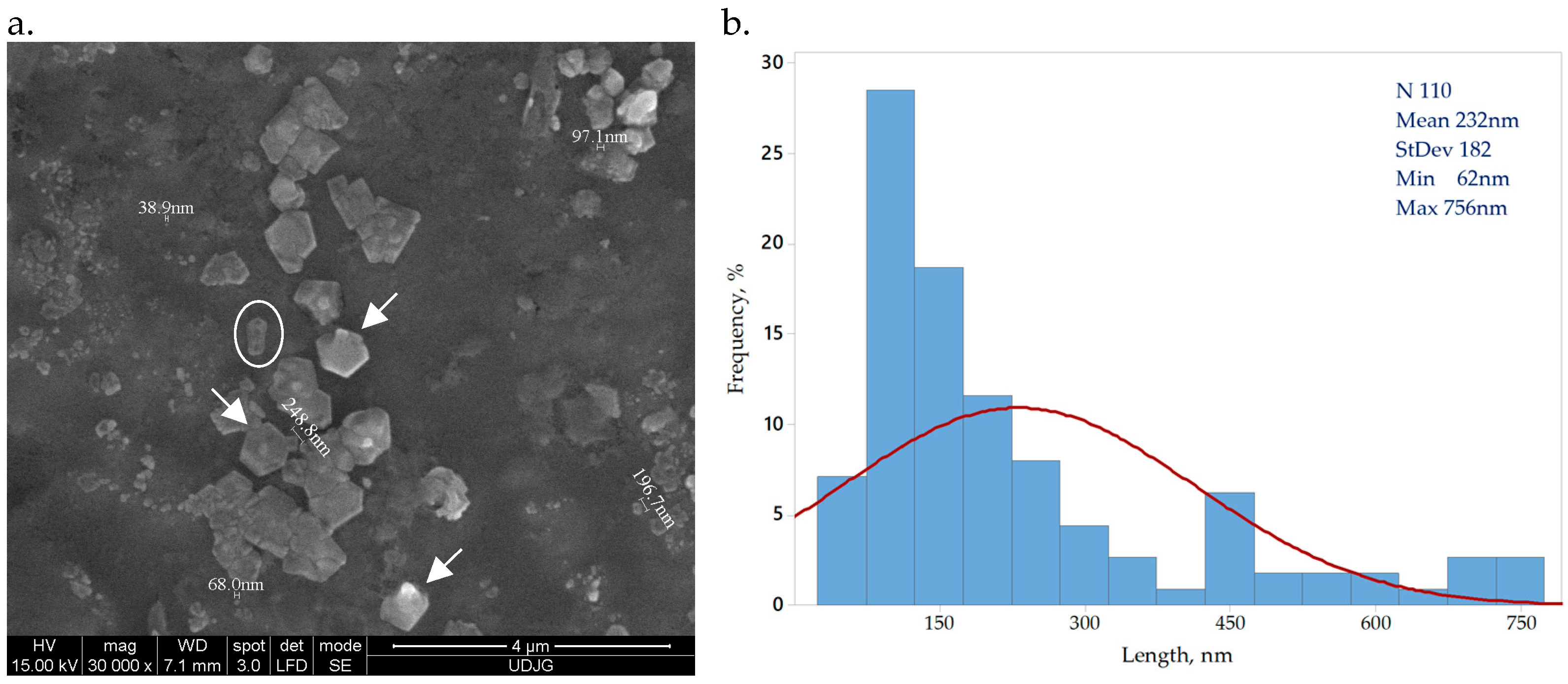
3.1. SEM Imaging
3.2. Characterization of Y-CNC and Yc Films
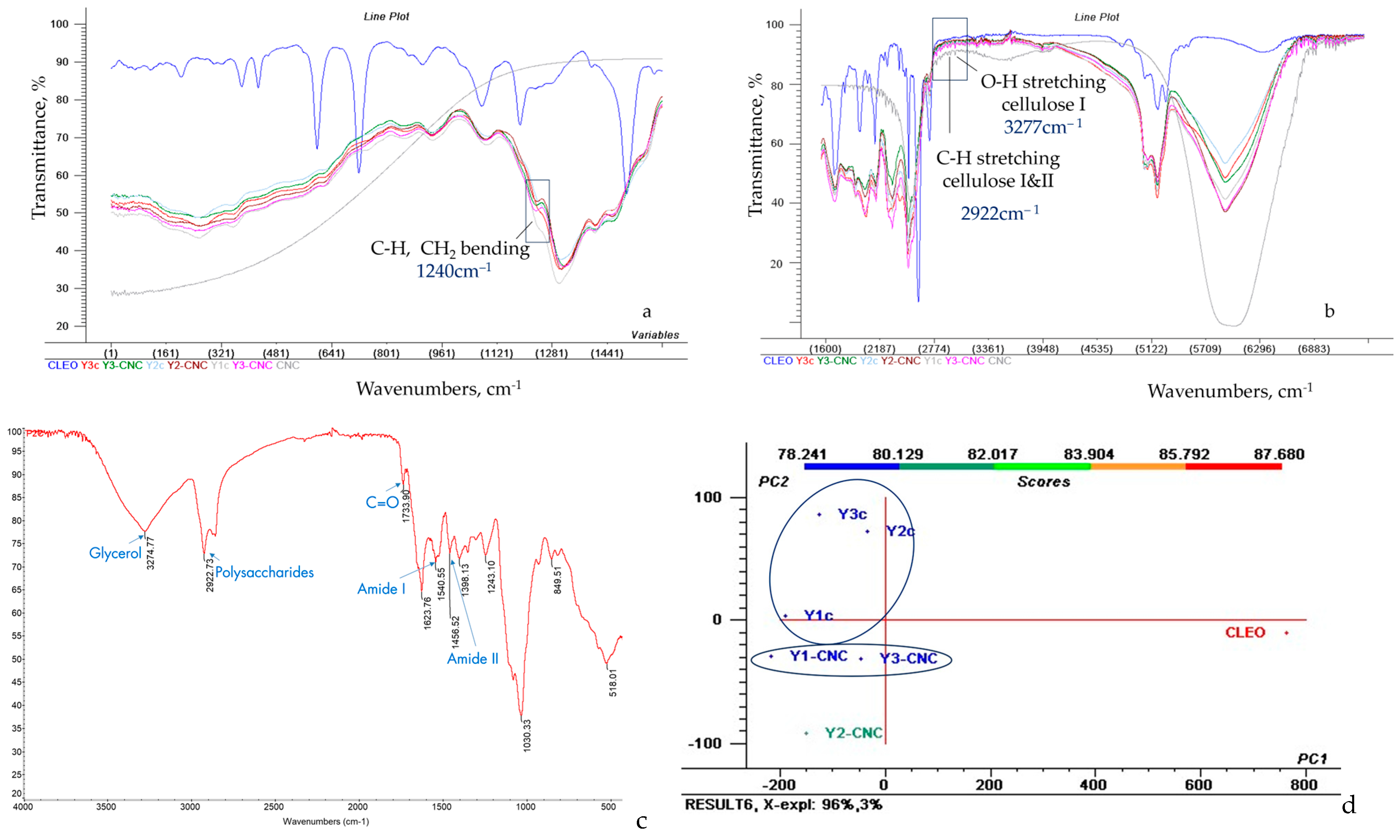
3.3. FT-IR Analysis
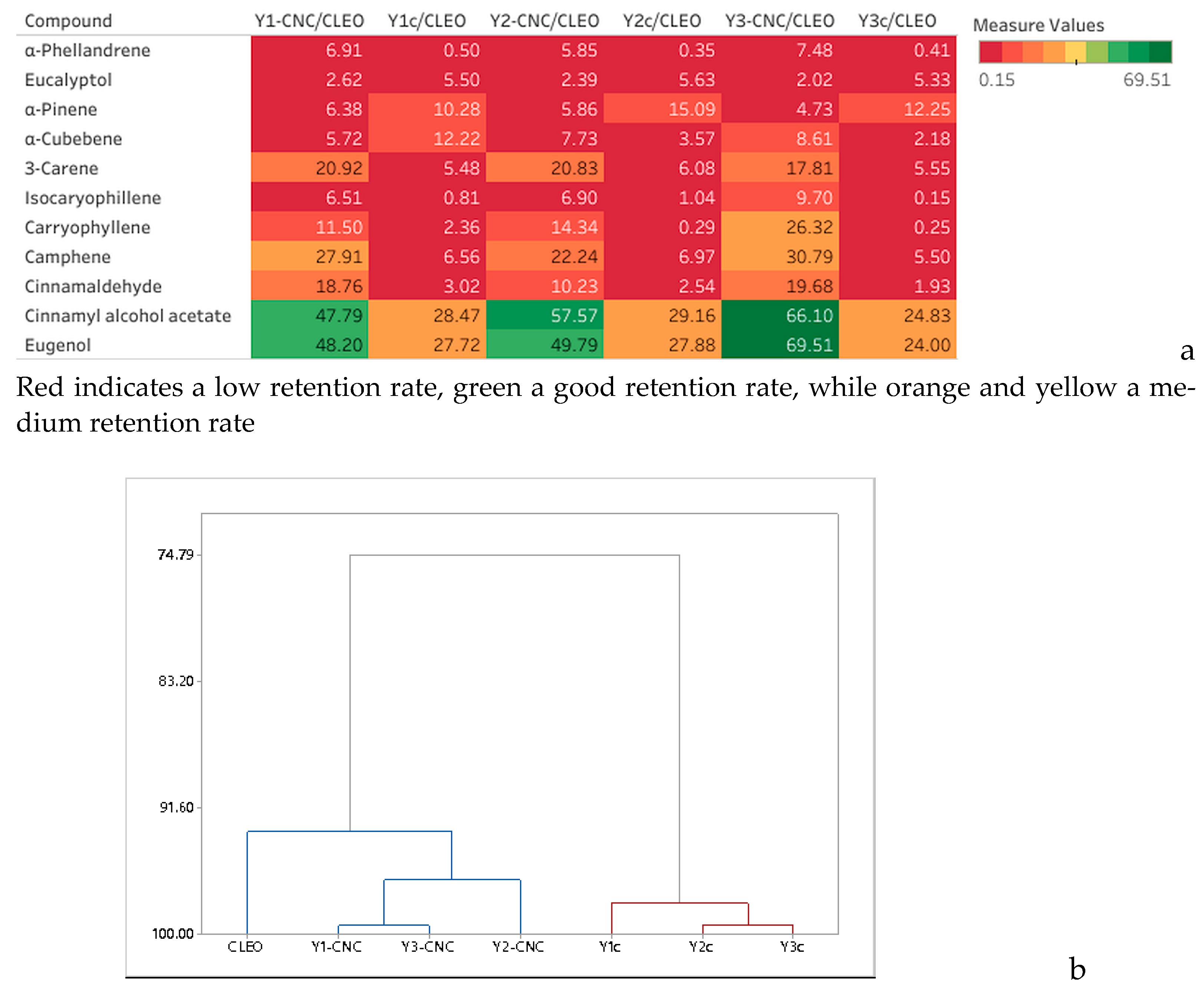
3.4. Analysis of Volatiles of CLEO and Y-CNCs by SPME GC/MS
3.5. Antimicrobial Activity of Y-CNC and Yc Against Spoilage Microorganisms and Pathogens
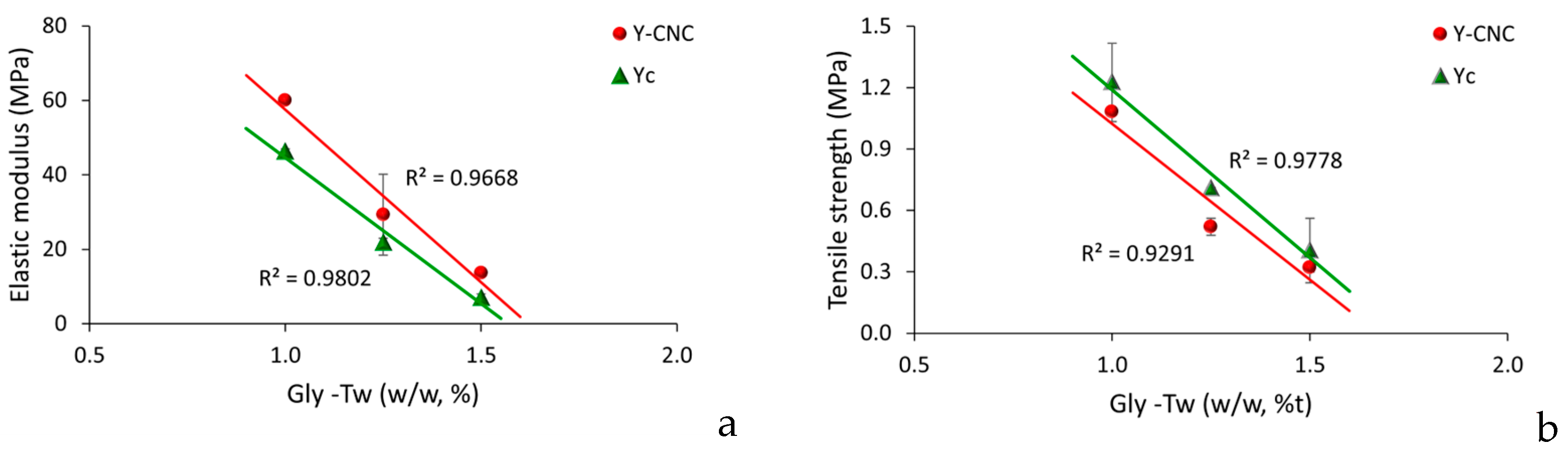
3.6. Mechanical Testing of Yeast Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassoun, A.; Boukid, F.; Ozogul, F.; Aït-Kaddour, A.; Soriano, J.M.; Lorenzo, J.M.; Perestrelo, R.; Galanakis, C.M.; Bono, G.; Bouyahya, A.; et al. Creating new opportunities for sustainable food packaging through dimensions of industry 4.0: New insights into the food waste perspective. Trends Food Sci. Technol. 2023, 142, 104238. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Noriega, E.; Tanja, F.; Bjørn, R.; Rotabakk, T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef]
- Hussain, S.; Akhter, R.; Maktedar, S.S. Advancements in sustainable food packaging: From eco-friendly materials to innovative technologies. Sustain. Food Technol. 2024, 2, 1297–1364. [Google Scholar] [CrossRef]
- Bleoanca, I.; Turtoi, M. Innovative Packaging Solutions. In Trends in Fish Processing Technologies, 1st ed.; Borda, D., Nicolau, A., Raspor, P., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 187–216. [Google Scholar]
- Khin, M.N.; Ahammed, S.; Kamal, M.M.; Saqib, M.N.; Liu, F.; Zhong, F. Investigating next-generation edible packaging: Protein-based films and coatings for delivering active compounds. Food Hydrocoll. Health 2024, 6, 100182. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, R.; Li, N.; Yang, X.; Lin, D. Nanocellulose: An amazing nanomaterial with diverse applications in food science. Carbohydr. Polym. 2023, 304, 120497. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.A.G.S.; Dourado, F.; Gama, M.; Poças, F. Nanocellulose Bio-Based Composites for Food Packaging. Nanomaterials 2020, 10, 2041. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Lappa, I.K.; Manikas, A.C.; Pastore Carbone, M.G.; Natsia, A.; Kachrimanidou, V.; Kopsahelis, N. Grafting bacterial cellulose nanowhiskers into whey protein/essential oil film composites: Effect on structure, essential oil release and antibacterial properties of films. Food Hydrocoll. 2023, 147 Pt A, 109374. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Salvay, A.G.; de la Osa, O.; Wagner, J.R. Characterization of thermal, mechanical and hydration properties of novel films based on Saccharomyces cerevisiae biomass. Innov. Food Sci. Emerg. Technol. 2018, 48, 240–247. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Salvay, A.G. Water Vapour Transport in Biopolymeric Materials: Effects of Thickness and Water Vapour Pressure Gradient on Yeast Biomass-Based Films. J. Polym. Environ. 2022, 30, 2976–2989. [Google Scholar] [CrossRef]
- Sukyai, P.; Anongjanya, P.; Bunyahwuthakul, N.; Kongsin, K.; Harnkarnsujarit, N.; Sukatta, U.; Sothornvit, R.; Chollakup, R. Effect of cellulose nanocrystals from sugarcane bagasse on whey protein isolate-based films. Food Res. Int. 2018, 107, 528–535. [Google Scholar] [CrossRef]
- Adel, A.M.; Ibrahim, A.A.; El-Shafei, A.M.; Al-Shemy, M.T. Inclusion complex of clove oil with chitosan/β-cyclodextrin citrate/oxidized nanocellulose biocomposite for active food packaging. Food Packag. Shelf Life 2019, 20, 100307. [Google Scholar] [CrossRef]
- Noremylia, M.B.; Hassan, M.Z.; Ismail, Z. Recent advancement in isolation, processing, characterization and applications of emerging nanocellulose: A review. Int. J. Biol. Macromol. 2022, 206, 954–976. [Google Scholar] [CrossRef]
- Baschali, A.; Tsakalidou, E.; Kyriacou, A.; Karavasiloglou, N.; Matalas, A.L. Traditional Low-Alcoholic and Non-Alcoholic Fermented Beverages Consumed in European Countries: A Neglected Food Group. Nutr. Res. Rev. 2017, 30, 1–24. [Google Scholar] [CrossRef]
- Bystrom, C. Biomimetically Improved Materials Comprising Microfibrillated Cellulose; Umeå University: Umeå, Sweden, 2021. [Google Scholar]
- Pihurov, M.; Păcularu- Burada, B.; Cotârleţ, M.; Vasile, M.A.; Bahrim, G.E. Novel Insights for Metabiotics Production by Using Artisanal Probiotic Cultures. Microorganisms 2021, 9, 2184. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, C.; Brozena, A.H.; Zhu, J.Y.; Xu, L.; Driemeier, C.; Dai, J.; Rojas, O.J.; Isogai, A.; Wågberg, L.; et al. Developing Fibrillated Cellulose as a Sustainable Technological Material. Nature 2021, 590, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Ballesteros, B.E.; Guevara-Morales, A.; Martín-Martínez, E.S.; Figueroa-López, U.; Vieyra, H. Composite of Polylactic Acid and Microcellulose from Kombucha Membranes. e-Polymers 2021, 20, 015–026. [Google Scholar] [CrossRef]
- Dima, S.-O.; Panaitescu, D.-M.; Orban, C.; Ghiurea, M.; Doncea, S.-M.; Fierascu, R.C.; Nistor, C.L.; Alexandrescu, E.; Nicolae, C.-A.; Trică, B.; et al. Bacterial Nanocellulose from Side-Streams of Kombucha Beverages Production: Preparation and Physical-Chemical Properties. Polymers 2017, 9, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, P.; Singh, P.P.; Vo, D.-V.N.; Kumar, D.; Balasubramanian, K.; Mubayi, A.; Srivastava, A.; Sharma, A. Bacterial Nanocellulose: Green Polymer Materials for High Performance Energy Storage Applications. J. Environ. Chem. Eng. 2022, 10, 108176. [Google Scholar] [CrossRef]
- Bleoanca, I.; Saje, K.; Mihalcea, L.; Oniciuc, E.A.; Smole- Mozina, S.; Nicolau, A.N.; Borda, D. Contribution of high pressure and thyme extract to control Listeria monocytogenes in fresh cheese—A hurdle approach. Innov. Food Sci. Emerg. Technol. 2016, 38 Pt A, 7–14. [Google Scholar] [CrossRef]
- Dediu Botezatu, A.; Apetrei, R.M.; Costea, I.F.; Barbu, V.; Grigore-Gurgu, L.; Botez, F.; Dinica, R.M.; Furdui, B.; Cârâc, G. Synthesis and characterization of novel chitosan derivatives (containing dipyridinium quaternary salts) with antimicrobial potential. Carbohydr. Res. 2023, 534, 108964. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current challenges, applications and future perspectives of SCOBY cellulose of Kombucha fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Soares, M.G.; de Lima, M.; Reolon Schmidt, V.C. Technological aspects of kombucha, its applications and the symbiotic culture (SCOBY), and extraction of compounds of interest: A literature review. Trends Food Sci. Technol. 2021, 110, 539–550. [Google Scholar] [CrossRef]
- Gedarawatte, S.T.G.; Ravensdale, J.T.; Al-Salami, H.; Dykes, G.A.; Coorey, R. Antimicrobial Efficacy of Nisin-Loaded Bacterial Cellulose Nanocrystals against Selected Meat Spoilage Lactic Acid Bacteria. Carbohydr. Polym. 2021, 251, 117096. [Google Scholar] [CrossRef]
- Vasconcelos, N.F.; Andrade Feitosa, J.P.; Portela da Gama, F.M.; Saraiva Morais, J.P.; Andrade, F.K.; Sá Moreira de Souza Filho, M.; de Freitas Rosa, M. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Perumal, A.B.; Nambiar, R.B.; Moses, J.A.; Anandharamakrishnan, C. Nanocellulose: Recent trends and applications in the food industry. Food Hydrocoll. 2022, 127, 107484. [Google Scholar] [CrossRef]
- Lago, A.; Delgado, J.F.; Rezzani, G.D.; Cottet, C.; Ramírez Tapias, Y.A.; Peltzer, M.A.; Salvay, A.G. Multi-Component Biodegradable Materials Based on Water Kefir Grains and Yeast Biomasses: Effect of the Mixing Ratio on the Properties of the Films. Polymers 2023, 15, 2594. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Bleoanca, I.; Lanciu, A.; Patrașcu, L.; Ceoromila, A.; Borda, D. Efficacy of two stabilizers in nanoemulsions with whey proteins and thyme essential oil as edible coatings for zucchini. Membranes 2022, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-García, M.; Calderón-Domínguez, G.; Chanona-Pérez, J.J.; Mendoza-Madrigal, A.G.; Di Pierro, P.; García-Almendárez, B.E.; Amaro-Reyes, A.; Regalado-González, C. Physical, Structural, Barrier, and Antifungal Characterization of Chitosan–Zein Edible Films with Added Essential Oils. Int. J. Mol. Sci. 2017, 18, 2370. [Google Scholar] [CrossRef]
- Dorofte, A.L.; Dima, C.; Bleoanca, I.; Aprodu, I.; Alexe, P.; Kharazmi, M.S.; Mahdi Jafari, S.; Dima, S.; Borda, D. Mechanism of β–cyclodextrin—Thyme nanocomplex formation and release: In silico behavior, structural and functional properties. Carbohydr. Polym. Technol. Appl. 2024, 7, 100422. [Google Scholar] [CrossRef]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: Montgomery, PA, USA, 1996.
- Channab, B.-E.; El Idrissi, A.; Essamlali, Y.; Zahouily, M. Nanocellulose: Structure, modification, biodegradation and applications in agriculture as slow/controlled release fertilizer, superabsorbent, and crop protection: A review. J. Environ. Manag. 2024, 352, 119928. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Sharma, A.; Ahlawat, V.; Bhattacharya, M.; Goswami, S. Process optimization for the production of cellulose nanocrystals from rice straw derived α-cellulose. Mater. Sci. Energy Technol. 2020, 3, 328–334. [Google Scholar] [CrossRef]
- Soro, A.B.; Oliveira, M.; O’Donnell, C.P.; Tiwari, B.K. Ultrasound assisted modulation of yeast growth and inactivation kinetics. Ultrason Sonochem. 2021, 80, 105819. [Google Scholar] [CrossRef]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef]
- Abral, H.; Pratama, A.B.; Handayani, D.; Mahardika, M.; Aminah, I.; Sandrawati, N.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. Antimicrobial Edible Film Prepared from Bacterial Cellulose Nanofibers/Starch/Chitosan for a Food Packaging Alternative. Int. J. Polym. Sci. 2021, 2021, 6641284. [Google Scholar] [CrossRef]
- Mihoubi, W.; Sahli, E.; Gargouri, A.; Amiel, C. FTIR spectroscopy of whole cells for the monitoring of yeast apoptosis mediated by p53 over-expression and its suppression by Nigella sativa extracts. PLoS ONE 2017, 12, e0180680. [Google Scholar] [CrossRef] [PubMed]
- Suryanto, H.; Muhajir, M.; Susilo, B.D.; Aji Pradana, Y.R.; Wijaya, H.W.; Ansari, A.S.; Yanuhar, U. Nanofibrillation of Bacterial Cellulose Using High-Pressure Homogenization and Its Films Characteristics. J. Renewable Mat. 2021, 9, 1717–1728. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Hao, S.; Huang, J. Preparation of All-Cellulose Composites Based on Controlled Dissolution Procedure. Starch-Stärke 2021, 73, 2000280. [Google Scholar] [CrossRef]
- Popa, R.M.; Fetea, F.; Socaciu, C. Attenuated Total Reflectance—Fourier Transform Infrared Spectroscopy Applied for the Evaluation of Essential Oils’ Pattern Recognition and Thermo-oxidative Stability: A Comparative Study. Stud. Univ. Babes-Bolyai Chem. 2021, 66, 33–50. [Google Scholar] [CrossRef]
- Qiao, Y.; Yu, Z.; Bai, L.; Li, H.; Zhang, S.; Liu, J.; Gao, Z.; Yang, X. Chemical composition of essential oils from Thymus mongolicus, Cinnamomum verum, and Origanum vulgare and their acaricidal effects on Haemaphysalis longicornis (Acari: Ixodidae). Ecotoxicol. Environ. Saf. 2021, 224, 112672. [Google Scholar] [CrossRef]
- Nunes, C.; Raposo, M.F.J.; Petronilho, S.; Machado, F.; Fulgêncio, R.; Gomes, M.H.; Evtuguin, D.V.; Rocha, S.; Coimbra, M. Cinnamomum burmannii decoction: A thickening and flavouring ingredient. LWT 2022, 153, 112428. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Sudha, T.; Darwish, N.H.E.; Chader, H.; Belkadi, A.; Rajabi, M.; Houche, A.; Benkebailli, F.; Oudjida, F.; Mousa, S.A. A New Eucalyptol-Rich Lavender (Lavandula stoechas L.) Essential Oil: Emerging Potential for Therapy against Inflammation and Cancer. Molecules 2020, 25, 3671. [Google Scholar] [CrossRef]
- Bouyahya, A.; Chamkhi, I.; El Menyiy, N.; El Moudden, H.; Harhar, H.; El Idrissi, Z.L.; Khouchlaa, A.; Jouadi, I.; El Baaboua, A.; Taha, D.; et al. Traditional use, phytochemistry, toxicology, and pharmacological properties of Lavandula dentata L.: A comprehensive review. S. Afr. J. Bot. 2023, 154, 67–87. [Google Scholar] [CrossRef]
- Gomes, C.; Moreira, R.G.; Castell-Perez, E. Poly (DL-lactide-co-glycolide) (PLGA) Nanoparticles with Entrapped trans-Cinnamaldehyde and Eugenol for Antimicrobial Delivery Applications. J. Food Sci. 2021, 76, N16–N24. [Google Scholar] [CrossRef]
- Sanla-Ead, N.; Jangchud, A.; Chonhenchob, V.; Suppakul, P. Antimicrobial Activity of Cinnamaldehyde and Eugenol and Their Activity after Incorporation into Cellulose-based Packaging Films. Packag. Technol. Sci. 2012, 25, 7–17. [Google Scholar] [CrossRef]
- Fernandez-Pan, I.; Royo, M.; Mate, J.I. Antimicrobial activity of whey protein isolate edible films with essential oils against food spoilers and foodborne pathogens. J. Food Sci. 2012, 77, M383–M390. [Google Scholar] [CrossRef]
- Balasubramanian, B.; Xue, J.; Luo, Y.; Upadhyay, A. Eugenol nanoemulsion reduces Listeria monocytogenes biofilm by modulating motility, quorum sensing, and biofilm architecture. Front. Sustain. Food Syst. 2023, 7, 1272373. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Q.; Wang, Z.; Cao, H.; Zhang, D. Structure-activity relationships of cinnamaldehyde and eugenol derivatives against plant pathogenic fungi. Ind. Crops Prod. 2017, 97, 388–394. [Google Scholar] [CrossRef]
- Bang, Y.J.; Shankar, S.; Rhim, J.W. In situ synthesis of multi-functional gelatin/resorcinol/silver nanoparticles composite films. Food Packag. Shelf Life 2019, 22, 100399. [Google Scholar] [CrossRef]
- Choo, K.W.; Dhital, R.; Mao, L.; Lin, M.; Mustapha, A. Development of polyvinyl alcohol/chitosan/modified bacterial nanocellulose films incorporated with 4-hexylresorcinol for food packaging applications. Food Packag. Shelf Life 2021, 30, 100769. [Google Scholar] [CrossRef]
- Delgado, J.F.; de la Osa, O.; Salvay, A.G.; Cavallo, E.; Cerrutti, P.; Foresti, M.L.; Peltzer, M.A. Reinforcement of Yeast Biomass Films with Bacterial Cellulose and Rice Husk Cellulose Nanofibres. J. Polym. Environ. 2021, 29, 3242–3251. [Google Scholar] [CrossRef]
- Rana, H.; Garg, R.; Singh, N.; Goswami, S. Guargum/nanocellulose based novel crosslinked antimicrobial film with enhanced barrier and mechanical properties for food packaging. J. Environ. Chem. Eng. 2023, 11, 109254. [Google Scholar] [CrossRef]


| Yeast Film Samples | Thickness, mm | Dry Matter, % | aw | ∆E | Opacity, % |
|---|---|---|---|---|---|
| Y1-CNC | 0.19± 0.02 a | 83.448 ± 0.115 a | 0.305 ± 0.006 b | 22.55 ± 0.01 a | 59.64 ± 0.04 a |
| Y2-CNC | 0.20 ± 0.02 a | 83.505 ± 0.384 a | 0.314 ± 0.007 b | 19.87 ± 0.04 c | 59.10 ± 0.00 a |
| Y3-CNC | 0.19 ± 0.02 a | 83.571 ± 0.324 a | 0.443 ± 0.003 b | 21.93 ± 0.20 b | 60.67 ± 0.02 a |
| Y1c | 0.19 ± 0.02 a | 72.740 ± 0.071 b | 0.561 ± 0.090 a | 48.01 ± 0.05 b | |
| Y2c | 0.20 ± 0.02 a | 72.091 ± 0.145 b | 0.562 ± 0.080 a | 51.34 ± 0.04 b | |
| Y3c | 0.19 ± 0.02 a | 72.374 ±0.368 b | 0.589 ± 0.070 a | 52.52 ± 0.04 b |
| RT | Compound | Top Five Ions | % Total VOC |
|---|---|---|---|
| 18.06 | α-Phellandrene | 91; 93; 77; 65; 51 | 3.83 ± 0.11 |
| 20.47 | Eucalyptol | 119;91; 134; 115; 77 | 0.21 ± 0.01 |
| 21.19 | α-Pinene | 91; 93;77; 51; 121 | 4.79 ± 0.52 |
| 30.27 | α-Cubebene | 105; 161; 119; 91; 93 | 0.39 ± 0.08 |
| 33.61 | 3-Carene | 91; 93; 81;79; 77; | 13.52 ± 8.62 |
| 36.54 | Isocaryophillene | 91; 133; 120; 77; 105 | 15.5 ± 4.35 |
| 40.13 | Carryophyllene | 93; 133; 91; 47; 79 | 3.51 ± 0.89 |
| 41.82 | Camphene | 93; 121; 79; 91; 39 | 0.83 ± 0.09 |
| 57.14 | Cinnamaldehyde | 131; 103; 77; 132; 51 | 24.74 ± 7.87 |
| 61.36 | Cinnamyl alcohol acetate | 115; 133; 91; 176; 77 | 0.62 ± 0.25 |
| 61.82 | Eugenol | 164; 149; 131; 103; 91 | 2.47 ± 0.62 |
| RT | Compound | Y1-CNC | Y1c | Y2-CNC | Y2c | Y3-CNC | Y3c |
|---|---|---|---|---|---|---|---|
| 15.55 | α-Phellandrene | 8.3351 ± 0.8783 a* | 0.3717 ± 0.0375 b | 6.9914 ± 0.4999 a | 0.2646 ± 0.0134 b | 8.5200 ± 7.6635 a | 0.4448 ± 0.0164 b |
| 20.99 | Eucalyptol | 10.6402 ± 0.4542 b,c,d | 13.7195 ± 1.0082 b,c | 9.6020 ± 1.7646 c,d | 14.2506 ± 0.4535 b | 7.7407 ± 3.1974 d | 19.4343 ± 0.5172 a |
| 21.09 | α-Pinene | 12.1140 ± 1.3180 b,c | 12.0064 ± 1.0205 b,c | 11.0502 ± 3.4526 c | 17.8724 ± 2.4968 a,b | 8.4957 ± 3.6956 c | 20.9208 ± 1.0006 a |
| 30.27 | α-Cubebene | 2.4883 ± 0.0571 a,b | 3.2684 ± 0.2763 a,b | 3.3364 ± 0.7767 a,b | 0.9676 ± 0.0004 a,b | 3.5411 ± 2.2334 a | 0.8522 ± 0.0774 c |
| 33.28 | 3-Carene | 280.8569 ± 3.5412 a | 45.2357 ± 4.1817 b | 277.3323 ± 42.9301 a | 50.9255 ± 0.4197 b | 225.9471 ± 84.4584 a | 67.0109 ± 2.5374 b |
| 36.54 | Isocaryophillene | 107.3567 ± 1.3888 a,b,c | 8.2026 ± 0.6888 c,d | 112.7899 ± 6.7610 a,b | 10.7145 ± 0.0350 b,c,d | 151.1714 ± 92.5792 a | 2.2533 ± 0.2731 d |
| 40.13 | Carryophyllene | 33.0730 ± 8.1522 a,b | 4.1729 ± 0.4353 b | 40.8924 ± 0.0594 a,b | 0.5121 ± 0.0150 b | 71.5447 ± 59.1497 a | 0.6523 ± 0.0234 b |
| 41.82 | Camphene | 26.0349 ± 0.2658 a | 3.7647 ± 0.1600 c | 20.5753 ± 4.6087 b | 4.0548 ± 0.3324 c | 27.1458 ± 1.0197 a | 4.6099 ± 0.3129 c |
| 57.14 | Cinnamaldehyde | 645.7746 ± 54.5487 a | 63.9653 ± 5.7683 c | 349.1368 ± 13.6337 b | 54.5912 ± 1.1929 c | 640.3287 ± 69.2735 a | 59.5939 ± 6.8278 c |
| 61.36 | Cinnamyl alcohol acetate | 43.9767 ± 5.9907 a | 16.1146 ± 1.2309 b | 52.5298 ± 6.1073 a | 16.7350 ± 0.7395 b | 57.4868 ± 10.1600 a | 20.5435 ± 1.8134 b |
| 61.82 | Eugenol | 151.4517 ± 0.2127 b | 53.5715 ± 4.0509 c | 155.1897 ± 17.9855 b | 54.6175 ± 9.2841 c | 206.4238 ± 30.7993 a | 67.7942 ± 5.7487 c |
| Yeast Film | Y1c | Y2c | Y3c | Y1-CNC | Y2-CNC | Y3-CNC |
|---|---|---|---|---|---|---|
| Test Microorganism | ||||||
| Bacillus cereus | 14.95 ± 0.35 c | 15.08 ± 0.43 c | 15.29 ± 3.17 c | 29.26 ± 1.04 c | 33.49 ± 3.12 c | 33.11 ± 1.56 c |
| Geotrichum candidum | 21.00 ± 0.30 b | 23.28 ± 0.46 b | 23.60 ± 0.67 b | 55.55 ± 0.35 b | 54.07 ± 0.51 b | 54.53 ± 0.45 b |
| Rhodotorula glutinis | 27.01 ± 0.57 a | 27.99 ± 1.97 a | 26.44 ± 0.38 a | 62.85 ± 0.41 a | 59.02 ± 0.66 a | 62.80 ± 0.45 a |
| Listeria monocytogenes | nd | nd | nd | 13.00 ± 0.00 d | 12.50 ± 0.05 d | 12.50 ± 0.05 d |
| Staphylococcus aureus | nd | nd | nd | 11.75 ± 0.03 d | 11.75 ± 0.03 d | 12.00 ± 0.00 d |
| Escherichia coli | nd | nd | nd | 10.25 ± 0.03 d | 11.00 ± 0.00 d | 11.00 ± 0.00 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bleoanca, I.; Grigore-Gurgu, L.; Ceoromila, A.C.; Borda, D.; Stan, F.; Fetecau, C. Bacterial Nanocellulose Grafted in Yeast Films: The Influence of Plasticizer and Emulsifier Concentration on Film Properties. Appl. Sci. 2024, 14, 12010. https://doi.org/10.3390/app142412010
Bleoanca I, Grigore-Gurgu L, Ceoromila AC, Borda D, Stan F, Fetecau C. Bacterial Nanocellulose Grafted in Yeast Films: The Influence of Plasticizer and Emulsifier Concentration on Film Properties. Applied Sciences. 2024; 14(24):12010. https://doi.org/10.3390/app142412010
Chicago/Turabian StyleBleoanca, Iulia, Leontina Grigore-Gurgu, Alina Cantaragiu Ceoromila, Daniela Borda, Felicia Stan, and Catalin Fetecau. 2024. "Bacterial Nanocellulose Grafted in Yeast Films: The Influence of Plasticizer and Emulsifier Concentration on Film Properties" Applied Sciences 14, no. 24: 12010. https://doi.org/10.3390/app142412010
APA StyleBleoanca, I., Grigore-Gurgu, L., Ceoromila, A. C., Borda, D., Stan, F., & Fetecau, C. (2024). Bacterial Nanocellulose Grafted in Yeast Films: The Influence of Plasticizer and Emulsifier Concentration on Film Properties. Applied Sciences, 14(24), 12010. https://doi.org/10.3390/app142412010










