Enhancing Soft Tissue Differentiation with Different Dual-Energy CT Systems: A Phantom Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Phantom
2.2. CT Scanners and Acquisition Protocols
- The Siemens Somatom Confidence scanner is a single source-detector system that performs the dual-energy acquisition by two sequential scans of the entire volume at 80 and 140 kVp. The advantage of this approach is that no hardware modification is required. This scanner is equipped with an ultrafast ceramic (UFC) detector [30];
- The Siemens Somatom Force DECT is equipped with a dual-source–dual-detector configuration. This configuration enables optimization of the separation of the low- and high-energy spectra (80 or 100 kV and 140 kVp or 150 kVp generally), with also the addition of tin filters. This scanner is equipped with the Stellar Detector, which reduces the electronic noise during image acquisition [31,32];
- The Siemens Somatom Definition Edge uses the split-filter technology, achieving spectral separation by using two filters of tin and gold. Also, this scanner is equipped with the Stellar detector;
- The GE Revolution GSI scanner is based on the concept of rapid kVp-switching. This technology is realized due to a high performance generator capable of very rapid transitions in tube potential (between 80 and 140 kVp) and a fast-sampling scintillator detector with very low afterglow (Gemstone [6]).
2.3. Image Analysis and Statistics
3. Results
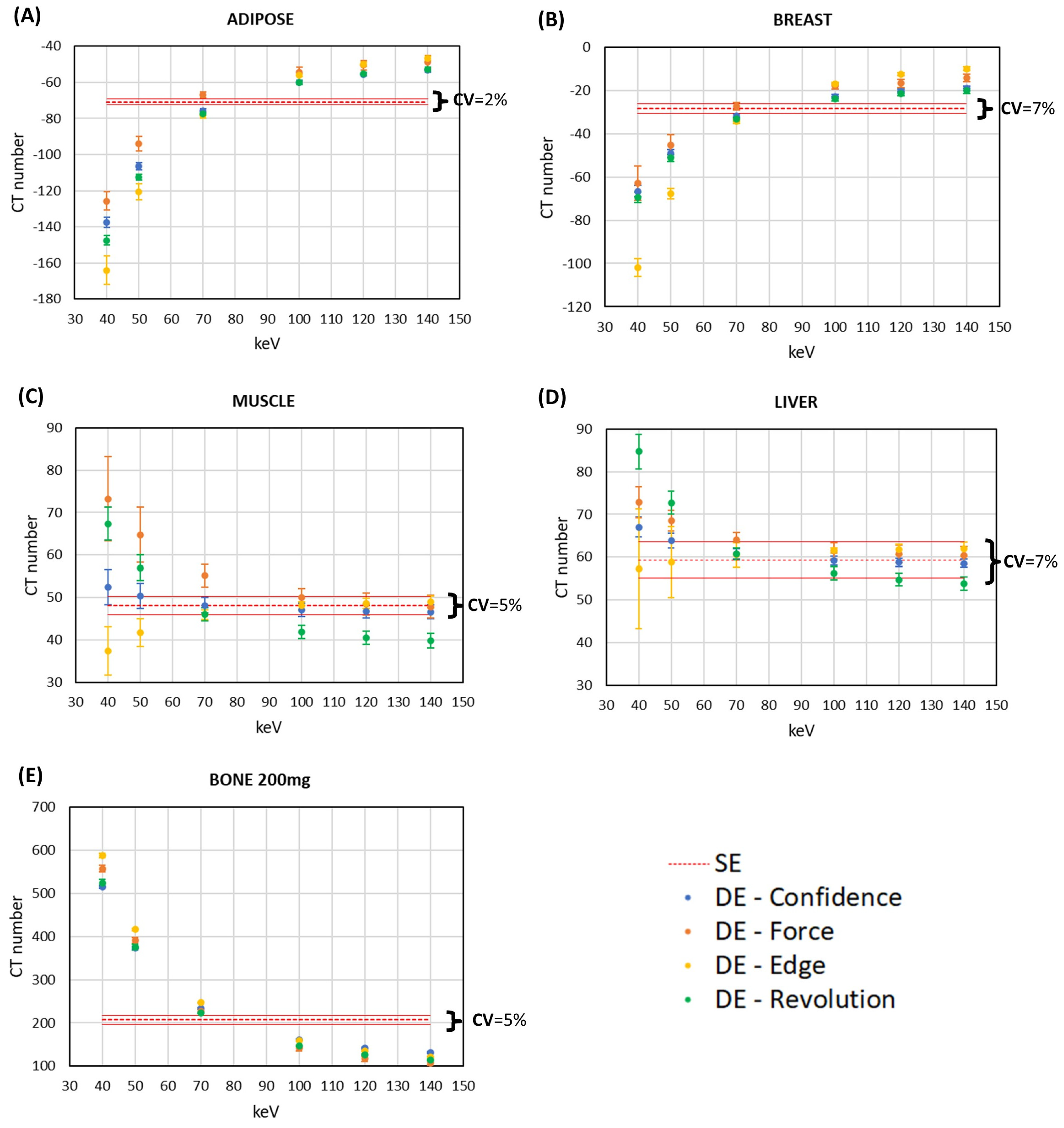
3.1. CT Number Accuracy
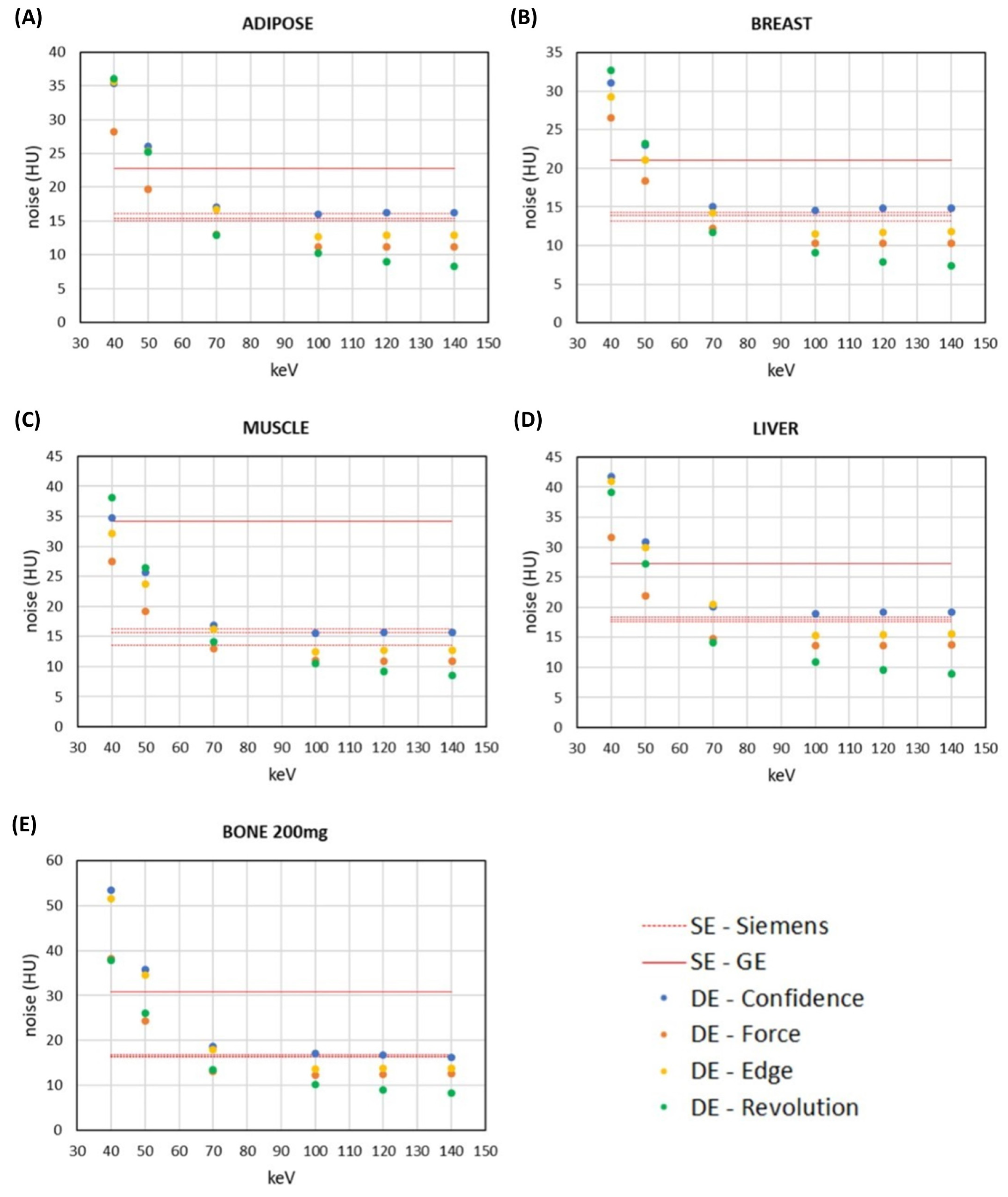
3.2. Comparison of Signal and Noise between DECT and SECT Images within the Same CT Scanner
3.3. Discrimination of Soft Tissues with DECT Images and SECT Images at Low Energy Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Alvarez, R.E.; Macovski, A. Energy-selective reconstructions in X-ray computerised tomography. Phys. Med. Biol. 1976, 21, 733–744. [Google Scholar] [CrossRef]
- Johnson, T.R.C.; Krauß, B.; Sedlmair, M.; Grasruck, M.; Bruder, H.; Morhard, D.; Fink, C.; Weckbach, S.; Lenhard, M.; Schmidt, B.; et al. Material differentiation by dual energy CT: Initial experience. Eur. Radiol. 2007, 17, 1510–1517. [Google Scholar] [CrossRef]
- Johnson, T.R.C. Dual-Energy CT: General Principles. Am. J. Roentgenol. 2012, 199, S3–S8. [Google Scholar] [CrossRef]
- Borges, A.P.; Antunes, C.; Curvo-Semedo, L. Pros and Cons of Dual-Energy CT Systems: “One Does Not Fit All”. Tomography 2023, 9, 195–216. [Google Scholar] [CrossRef]
- Chandra, N.; Langan, D.A. Gemstone Detector: Dual Energy Imaging via Fast kVp Switching. In Dual Energy CT in Clinical Practice; Johnson, T., Fink, C., Schönberg, S.O., Reiser, M.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 35–41. [Google Scholar] [CrossRef]
- Goodsitt, M.M.; Christodoulou, E.G.; Larson, S.C. Accuracies of the synthesized monochromatic CT numbers and effective atomic numbers obtained with a rapid kVp switching dual energy CT scanner. Med. Phys. 2011, 38, 2222–2232. [Google Scholar] [CrossRef]
- Euler, A.; Parakh, A.; Falkowski, A.L.; Manneck, S.; Dashti, D.; Krauss, B.; Szucs-Farkas, Z.; Schindera, S.T. Initial Results of a Single-Source Dual-Energy Computed Tomography Technique Using a Split-Filter: Assessment of Image Quality, Radiation Dose, and Accuracy of Dual-Energy Applications in an In Vitro and In Vivo Study. Investig. Radiol. 2016, 51, 491–498. [Google Scholar] [CrossRef]
- Kaemmerer, N.; Brand, M.; Hammon, M.; May, M.; Wuest, W.; Krauss, B.; Uder, M.; Lell, M.M. Dual-Energy Computed Tomography Angiography of the Head and Neck With Single-Source Computed Tomography: A New Technical (Split Filter) Approach for Bone Removal. Investig. Radiol. 2016, 51, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Petersilka, M.; Bruder, H.; Krauss, B.; Stierstorfer, K.; Flohr, T.G. Technical principles of dual source CT. Eur. J. Radiol. 2008, 68, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Flohr, T.G.; McCollough, C.H.; Bruder, H.; Petersilka, M.; Gruber, K.; Süβ, C.; Grasruck, M.; Stierstorfer, K.; Krauss, B.; Raupach, R.; et al. First performance evaluation of a dual-source CT (DSCT) system. Eur. Radiol. 2006, 16, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Almeida, I.P.; Schyns, L.E.J.R.; Öllers, M.C.; Van Elmpt, W.; Parodi, K.; Landry, G.; Verhaegen, F. Dual-energy CT quantitative imaging: A comparison study between twin-beam and dual-source CT scanners. Med. Phys. 2017, 44, 171–179. [Google Scholar] [CrossRef]
- Jacobsen, M.C.; Schellingerhout, D.; Wood, C.A.; Tamm, E.P.; Godoy, M.C.; Sun, J.; Cody, D.D. Intermanufacturer Comparison of Dual-Energy CT Iodine Quantification and Monochromatic Attenuation: A Phantom Study. Radiology 2018, 287, 224–234. [Google Scholar] [CrossRef]
- Li, B.; Pomerleau, M.; Gupta, A.; Soto, J.A.; Anderson, S.W. Accuracy of Dual-Energy CT Virtual Unenhanced and Material-Specific Images: A Phantom Study. Am. J. Roentgenol. 2020, 215, 1146–1154. [Google Scholar] [CrossRef]
- Ohira, S.; Karino, T.; Ueda, Y.; Nitta, Y.; Kanayama, N.; Miyazaki, M.; Koizumi, M.; Teshima, T. How Well Does Dual-energy CT with Fast Kilovoltage Switching Quantify CT Number and Iodine and Calcium Concentrations? Acad. Radiol. 2018, 25, 519–528. [Google Scholar] [CrossRef]
- Bamberg, F.; Dierks, A.; Nikolaou, K.; Reiser, M.F.; Becker, C.R.; Johnson, T.R.C. Metal artifact reduction by dual energy computed tomography using monoenergetic extrapolation. Eur. Radiol. 2011, 21, 1424–1429. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, Y.E.; Luo, S.; Shi, H.; Li, L.; Zheng, L.; Zhang, L.J.; Lu, G. Monoenergetic Imaging of Dual-energy CT Reduces Artifacts from Implanted Metal Orthopedic Devices in Patients with Factures. Acad. Radiol. 2011, 18, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Jinzaki, M.; Tanami, Y.; Ueno, A.; Yamada, M.; Kuribayashi, S. Virtual Monochromatic Spectral Imaging with Fast Kilovoltage Switching: Improved Image Quality as Compared with That Obtained with Conventional 120-kVp CT. Radiology 2011, 259, 257–262. [Google Scholar] [CrossRef]
- Pomerantz, S.R.; Kamalian, S.; Zhang, D.; Gupta, R.; Rapalino, O.; Sahani, D.V.; Lev, M.H. Virtual Monochromatic Reconstruction of Dual-Energy Unenhanced Head CT at 65–75 keV Maximizes Image Quality Compared with Conventional Polychromatic CT. Radiology 2013, 266, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, J.L.; Nöske, E.-M.; Kraft, J.; Burck, I.; Wagenblast, J.; Eckardt, A.; Frellesen, C.; Kerl, J.M.; Bauer, R.W.; Bodelle, B.; et al. Virtual Monoenergetic Dual-Energy Computed Tomography: Optimization of Kiloelectron Volt Settings in Head and Neck Cancer. Investig. Radiol. 2014, 49, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Böning, G.; Feldhaus, F.; Adelt, S.; Kahn, J.; Fehrenbach, U.; Streitparth, F. Clinical routine use of virtual monochromatic datasets based on spectral CT in patients with hypervascularized abdominal tumors—Evaluation of effectiveness and efficiency. Acta Radiol. 2019, 60, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Van Ommen, F.; Kauw, F.; Bennink, E.; Heit, J.J.; Wolman, D.N.; Dankbaar, J.W.; de Jong, H.W.; Wintermark, M. Image Quality of Virtual Monochromatic Reconstructions of Noncontrast CT on a Dual-Source CT Scanner in Adult Patients. Acad. Radiol. 2021, 28, e323–e330. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, K.N.; Guimarães, L.S.; Murthy, N.S.; Black, D.F.; Bongartz, T.; Manek, N.J.; Leng, S.; Fletcher, J.G.; McCollough, C.H. Identification of Intraarticular and Periarticular Uric Acid Crystals with Dual-Energy CT: Initial Evaluation. Radiology 2011, 261, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Van Elmpt, W.; Landry, G.; Das, M.; Verhaegen, F. Dual energy CT in radiotherapy: Current applications and future outlook. Radiother. Oncol. 2016, 119, 137–144. [Google Scholar] [CrossRef]
- Kruis, M.F. Improving radiation physics, tumor visualisation, and treatment quantification in radiotherapy with spectral or dual-energy CT. J. Appl. Clin. Med. Phys. 2022, 23, e13468. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.; Wohlfahrt, P.; Hofmann, C.; Möhler, C.; Menkel, S.; Tschiche, M.; Krause, M.; Troost, E.G.; Enghardt, W.; Richter, C. Reduction of clinical safety margins in proton therapy enabled by the clinical implementation of dual-energy CT for direct stopping-power prediction. Radiother. Oncol. 2022, 166, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Phan, C.M.; Leidecker, C.; Brady, T.J.; Hirsch, J.A.; Nogueira, R.G.; Yoo, A.J. Evaluation of Dual-Energy CT for Differentiating Intracerebral Hemorrhage from Iodinated Contrast Material Staining. Radiology 2010, 257, 205–211. [Google Scholar] [CrossRef]
- Virarkar, M.K.; Vulasala, S.S.R.; Gupta, A.V.; Gopireddy, D.; Kumar, S.; Hernandez, M.; Lall, C.; Bhosale, P. Virtual Non-contrast Imaging in The Abdomen and The Pelvis: An Overview. Semin. Ultrasound CT MRI 2022, 43, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Sun Nuclear. CIRS. Electron Density Phantom, Model 062M. Available online: https://www.cirsinc.com/products/radiation-therapy/electron-density-phantom/ (accessed on 19 July 2023).
- Siemens Healthcare. Ultra Fast Ceramic. Available online: https://www.siemens-healthineers.com/it/computed-tomography/technologies-innovations/ufc-ultra-fast-ceramic (accessed on 19 July 2023).
- Ulzheimer, S.; Freund, J. The Stellar detector. Siemens Whitepaper. 2016. Available online: https://cdn0.scrvt.com/39b415fb07de4d9656c7b516d8e2d907/1800000003369873/c3565f4372f7/Computed-Tomography_Machine-Technology_Stellar-detector_Whitepaper_1800000003369873.pdf (accessed on 19 July 2023).
- Christe, A. CT dose and image quality in the last three scanner generations. World J. Radiol. 2013, 5, 421. [Google Scholar] [CrossRef]
- IAEA Human Health. International Atomic Energy Agency, Quality Assurance Programme for Computed Tomography: Diagnostic and Therapy Applications, IAEA Human Health Series No. 19, IAEA, Vienna 2012. Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/Pub1557_web.pdf (accessed on 24 October 2023).
- Grant, K.; Raupach, R. SAFIRE: Sinogram Affirmed Iterative Reconstruction. Siemens Whitepaper. 2012. Available online: https://cdn0.scrvt.com/39b415fb07de4d9656c7b516d8e2d907/1800000000306520/d80046026fd1/ct_SAFIRE_White_Paper_1800000000306520.pdf (accessed on 19 July 2023).
- Ramirez Giraldo, J.C.; Grant, K.; Raupach, R. ADMIRE: Advanced Modeled Iterative Reconstruction. Siemens Whitepaper. 2018. Available online: https://cdn0.scrvt.com/39b415fb07de4d9656c7b516d8e2d907/1800000005678345/c5bb3dd343ed/CT_Whitepaper_ADMIRE_1800000005678345.pdf (accessed on 19 July 2023).
- Fan, J.; Yue, M.; Melnyk, R. Benefits of ASiR-V Reconstruction for Reducing Patient Radiation Dose and Preserving Diagnostic Quality in CT Exams. GE Whitepaper. n.d. 2014. Available online: https://www.gehealthcare.co.uk/-/media/6862ed3b10424182924e03a49f4a46d7.pdf (accessed on 19 July 2023).
- Geyer, L.L.; Schoepf, U.J.; Meinel, F.G.; Nance, J.W.; Bastarrika, G.; Leipsic, J.A.; Paul, N.S.; Rengo, M.; Laghi, A.; De Cecco, C.N. State of the Art: Iterative CT Reconstruction Techniques. Radiology 2015, 276, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Grant, K.L.; Flohr, T.G.; Krauss, B.; Sedlmair, M.; Thomas, C.; Schmidt, B. Assessment of an Advanced Image-Based Technique to Calculate Virtual Monoenergetic Computed Tomographic Images From a Dual-Energy Examination to Improve Contrast-To-Noise Ratio in Examinations Using Iodinated Contrast Media. Investig. Radiol. 2014, 49, 586–592. [Google Scholar] [CrossRef]
- D’Angelo, T.; Cicero, G.; Mazziotti, S.; Ascenti, G.; Albrecht, M.H.; Martin, S.S.; Othman, A.E.; Vogl, T.J.; Wichmann, J.L. Dual energy computed tomography virtual monoenergetic imaging: Technique and clinical applications. Br. J. Radiol. 2019, 92, 20180546. [Google Scholar] [CrossRef]
- NIST XCOM Database. 2023. Available online: https://physics.nist.gov/PhysRefData/Xcom/html/xcom1.html (accessed on 19 July 2023).
- Chai, T.; Draxler, R.R. Root mean square error (RMSE) or mean absolute error (MAE)?—Arguments against avoiding RMSE in the literature. Geosci. Model. Dev. 2014, 7, 1247–1250. [Google Scholar] [CrossRef]
- Koukou, V.; Martini, N.; Michail, C.; Sotiropoulou, P.; Fountzoula, C.; Kalyvas, N.; Kandarakis, I.; Nikiforidis, G.; Fountos, G. Dual Energy Method for Breast Imaging: A Simulation Study. Comput. Math. Methods Med. 2015, 2015, 574238. [Google Scholar] [CrossRef] [PubMed]
- Toia, G.V.; Mileto, A.; Wang, C.L.; Sahani, D.V. Quantitative dual-energy CT techniques in the abdomen. Abdom. Radiol. 2021, 47, 3003–3018. [Google Scholar] [CrossRef] [PubMed]
- Greffier, J.; Van Ngoc Ty, C.; Fitton, I.; Frandon, J.; Beregi, J.-P.; Dabli, D. Impact of Phantom Size on Low-Energy Virtual Monoenergetic Images of Three Dual-Energy CT Platforms. Diagnostics 2023, 13, 3039. [Google Scholar] [CrossRef]
- Papadakis, A.E.; Damilakis, J. Technical Note: Quality assessment of virtual monochromatic spectral images on a dual energy CT scanner. Phys. Med. 2021, 82, 114–121. [Google Scholar] [CrossRef]
- Noda, Y.; Goshima, S.; Kaga, T.; Ando, T.; Miyoshi, T.; Kawai, N.; Kawada, H.; Tanahashi, Y.; Matsuo, M. Virtual monochromatic image at lower energy level for assessing pancreatic ductal adenocarcinoma in fast kV-switching dual-energy CT. Clin. Radiol. 2020, 75, 320.e17–320.e23. [Google Scholar] [CrossRef]
- Charyyev, S.; Wang, T.; Lei, Y.; Ghavidel, B.; Beitler, J.J.; McDonald, M.; Curran, W.J.; Liu, T.; Zhou, J.; Yang, X. Learning-based synthetic dual energy CT imaging from single energy CT for stopping power ratio calculation in proton radiation therapy. Br. J. Radiol. 2022, 95, 20210644. [Google Scholar] [CrossRef]
- Noid, G.; Zhu, J.; Tai, A.; Mistry, N.; Schott, D.; Prah, D.; Paulson, E.; Schultz, C.; Li, X.A. Improving Structure Delineation for Radiation Therapy Planning Using Dual-Energy CT. Front. Oncol. 2020, 10, 1694. [Google Scholar] [CrossRef]
- Dedes, G.; Dickmann, J.; Niepel, K.; Wesp, P.; Johnson, R.P.; Pankuch, M.; Bashkirov, V.; Rit, S.; Volz, L.; Schulte, R.W.; et al. Experimental comparison of proton CT and dual energy x-ray CT for relative stopping power estimation in proton therapy. Phys. Med. Biol. 2019, 64, 165002. [Google Scholar] [CrossRef]
- Ehn, S.; Sellerer, T.; Muenzel, D.; Fingerle, A.A.; Kopp, F.; Duda, M.; Mei, K.; Renger, B.; Herzen, J.; Dangelmaier, J.; et al. Assessment of quantification accuracy and image quality of a full-body dual-layer spectral CT system. J. Appl. Clin. Med. Phys. 2018, 19, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Alius, C.; Serban, D.; Tribus, L.C.; Costea, D.O.; Cristea, B.M.; Serboiu, C.; Motofei, I.; Dascalu, A.M.; Velescu, B.; Tudor, C.; et al. When Not to Operate on Acute Cases—A Surgeon’s Perspective on Rapid Assessment of Emergency Abdominopelvic Computed Tomography. J. Imaging 2023, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Cicero, G.; Ascenti, G.; Albrecht, M.H.; Blandino, A.; Cavallaro, M.; D’Angelo, T.; Carerj, M.L.; Vogl, T.J.; Mazziotti, S. Extra-abdominal dual-energy CT applications: A comprehensive overview. Radiol. Med. 2020, 125, 384–397. [Google Scholar] [CrossRef] [PubMed]
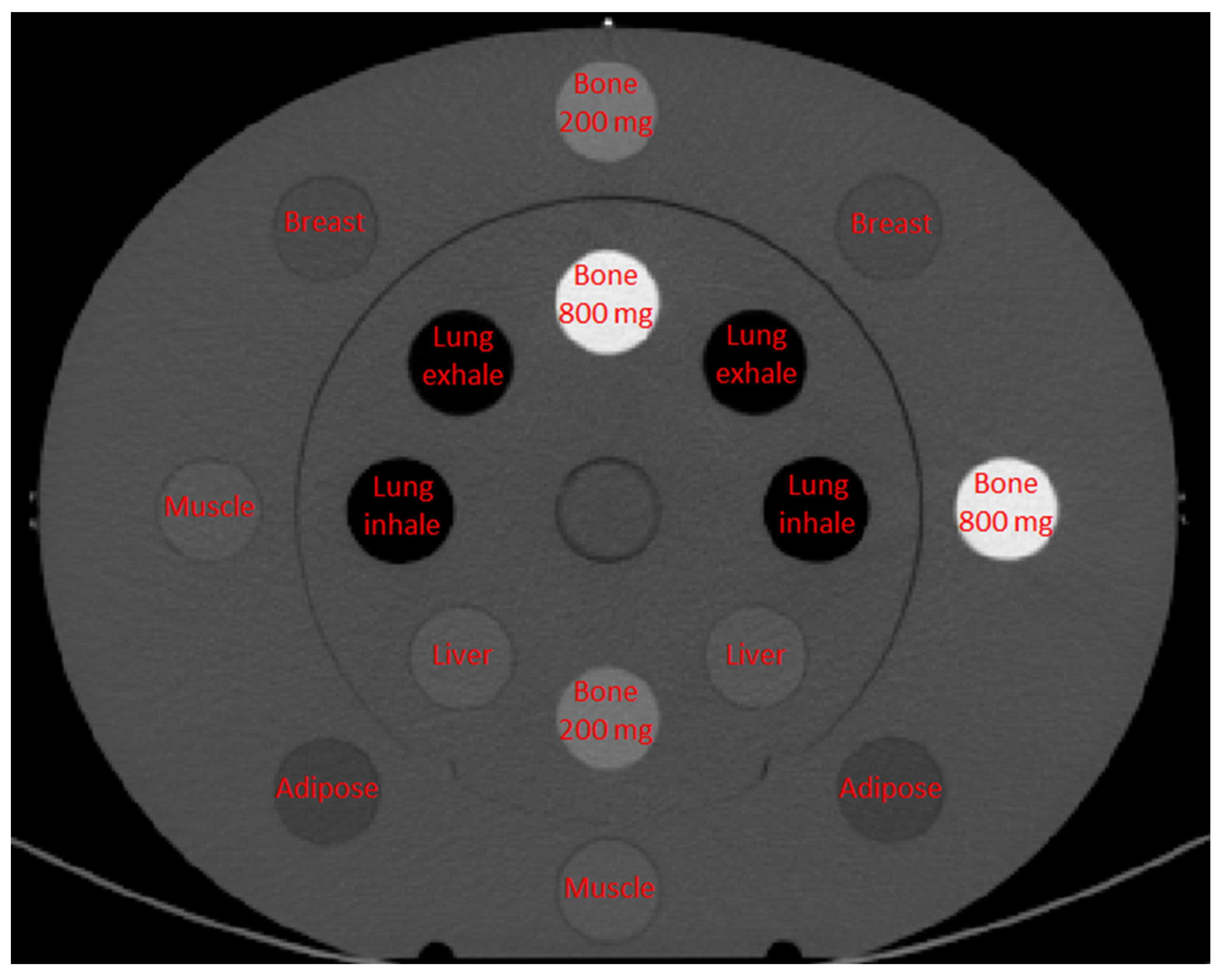
| SECT PROTOCOL | GE Revolution GSI | Siemens Confidence | Siemens Edge | Siemens Force |
|---|---|---|---|---|
| kVp | 120 | 120 | 120 | 120 |
| mAs | 380 | 380 | 380 | 380 |
| Rotation time (s) | 1 | 1 | 1 | 1 |
| Collimation (mm) | 20 × 1.25 | 16 × 1.2 | 32 × 1.2 | 96 × 0.6 |
| FOV (cm) | 50 | 50 | 50 | 50 |
| Pitch | 0.96 | 0.95 | 0.95 | 0.95 |
| Slice Thickness (mm) | 1.25 | 1.50 | 1.50 | 1.50 |
| Reconstruction algorithm (strength) | FBP, ASIR (30%) | FBP, SAFIRE (3) | FBP, ADMIRE (3) | FBP, ADMIRE (3) |
| Reconstruction kernel | Medium smooth | B30 | Br40 | Br40 |
| CTDIvol (mGy) | 34 | 31 | 30 | 35 |
| DECT PROTOCOL | GE Revolution GSI | Siemens Confidence | Siemens Edge | Siemens Force |
| DECT technique | kVp switching | sequential scan | split filter | dual source |
| kVp | 80–140 | 80–140 | 120 | 80–140 |
| Added filter | No | No | Au, Sn | No |
| mAs | 600 | 600–142 | 1450 | 600–109 |
| Rotation time (s) | 1 | 1 | 1 | 1 |
| Collimation (mm) | 20 × 1.25 | 16 × 1.2 | 64 × 0.6 | 96 × 0.6 |
| FOV (cm) | 50 | 50 | 50 | 50 |
| Pitch | 0.96 | 0.95 | 0.25 | 0.95 |
| Slice Thickness (mm) | 1.25 | 1.50 | 1.00 | 1.50 |
| Reconstruction algorithm (strength) | FBP, ASIR (30%) | FBP, SAFIRE (3) | FBP, ADMIRE (3) | FBP, ADMIRE (3) |
| Reconstruction kernel | Medium smooth | D30 | Qr40 | Qr40 |
| VMI energy | 40–140 keV | 40–140 keV | 40–140 keV | 40–140 keV |
| CTDIvol (mGy) | 36 | 31 | 31 | 32 |
| Tissue and Energy | RMSE (HU) | |||
|---|---|---|---|---|
| Siemens Confidence | Siemens Force | Siemens Edge | GE Revolution GSI | |
| Adipose | ||||
| 40 keV | 14.7 | 26.7 | 14.3 | 5.2 |
| 50 keV | 4.7 | 17.2 | 10.9 | 2.5 |
| 70 keV | 1.3 | 8.6 | 3.2 | 2.2 |
| 100 keV | 1.3 | 5.5 | 3.2 | 1.5 |
| 120 keV | 1.0 | 5.4 | 5.3 | 1.1 |
| 140 keV | 1.1 | 5.4 | 6.0 | 1.1 |
| Breast | ||||
| 40 keV | 21.9 | 26.6 | 14.1 | 19.0 |
| 50 keV | 13.1 | 17.5 | 6.3 | 10.9 |
| 70 keV | 8.6 | 13.0 | 6.0 | 7.0 |
| 100 keV | 7.3 | 11.9 | 12.9 | 6.3 |
| 120 keV | 7.3 | 11.0 | 15.2 | 6.3 |
| 140 keV | 7.1 | 12.1 | 16.1 | 6.0 |
| Muscle | ||||
| 40 keV | 4.4 | 21.7 | 17.5 | 14.0 |
| 50 keV | 2.9 | 15.8 | 9.2 | 7.3 |
| 70 keV | 3.5 | 10.4 | 1.4 | 1.8 |
| 100 keV | 4.1 | 7.1 | 4.8 | 2.1 |
| 120 keV | 4.3 | 6.3 | 6.0 | 2.7 |
| 140 keV | 3.8 | 5.4 | 6.0 | 3.8 |
| Liver | ||||
| 40 keV | 5.1 | 10.9 | 14.8 | 22.5 |
| 50 keV | 5.0 | 9.6 | 8.2 | 13.8 |
| 70 keV | 6.3 | 9.6 | 6.6 | 6.3 |
| 100 keV | 6.5 | 8.9 | 8.8 | 3.8 |
| 120 keV | 6.5 | 8.7 | 9.6 | 2.8 |
| 140 keV | 5.9 | 7.9 | 9.4 | 1.9 |
| Bone 200 mg | ||||
| 40 keV | 170.8 | 128.9 | 97.8 | 161.4 |
| 50 keV | 89.6 | 72.5 | 47.1 | 87.2 |
| 70 keV | 31.6 | 38.8 | 19.1 | 42.4 |
| 100 keV | 13.9 | 34.0 | 16.9 | 29.1 |
| 120 keV | 10.8 | 36.4 | 18.0 | 27.1 |
| 140 keV | 10.4 | 34.7 | 19.9 | 27.7 |
| Tissue Comparison | DECT Level vs. SECT Images | Mean Difference in HU (95% CI) | |||
|---|---|---|---|---|---|
| Siemens Confidence | Siemens Edge | Siemens Force | GE Revolution GSI | ||
| Adipose vs. Breast | 40 keV | 29.7 (28.4; 30.9) | 14.4 (11.5; 17.2) | 22.2 (19.4; 25.1) | 36.0 (35.1; 37.0) |
| 50 keV | 16.1 (15.2; 17.0) | 5.7 (4.0; 7.5) | 8.4 (6.4; 10.5) | 19.2 (18.6; 19.9) | |
| 70 keV | 2.8 (2.2; 3.4) | −2.6 (−3.5; −1.7) | −0.8 (−1.5; 0.0) | 1.9 (1.5; 2.4) | |
| Adipose vs. Muscle | 40 keV | 73.9 (72.5; 75.2) | 89.4 (87.6; 91.3) | 79.2 (75.9; 82.6) | 93.6 (92.3; 94.8) |
| 50 keV | 40.5 (39.5; 41.4) | 48.3 (47.1; 49.4) | 39.1 (37.1; 41.2) | 47.9 (47.0; 48.9) | |
| 70 keV | 7.8 (7.2; 8.3) | 8.0 (7.3; 8.7) | 2.9 (1.8; 3.9) | 1.8 (1.1; 2.5) | |
| Adipose vs. Liver | 40 keV | 75.3 (74.3; 76.2) | 97.4 (94.6; 100.2) | 67.6 (66.1; 69.2) | 96.5 (95.0; 98.0) |
| 50 keV | 41.1 (40.4; 41.8) | 55.3 (53.4; 57.2) | 31.6 (30.3; 32.9) | 49.7 (48.7; 50.6) | |
| 70 keV | 7.6 (7.2; 8.1) | 14.1 (13.0; 15.2) | 0.2 (−0.6; 1.0) | 2.3 (1.7; 2.9) | |
| Breast vs. Bone 200 mg | 40 keV | 349.3 (347.8; 350.7) | 471.3 (469.3; 473.4) | 376.9 (374.1; 379.7) | 348.0 (345.5; 350.5) |
| 50 keV | 189.9 (188.9; 191.0) | 265.1 (263.6; 266.6) | 192.9 (190.6; 195.1) | 181.3 (179.3; 183.3) | |
| 70 keV | 33.4 (32.7; 34.0) | 62.3 (61.0; 63.5) | 11.6 (9.7; 13.5) | 10.5 (9.7; 11.4) | |
| Muscle vs. Liver | 40 keV | 1.4 (0.1; 2.7) | 8.4 (5.5; 11.3) | −2.5 (−4.1; −0.9) | 2.9 (1.3; 4.5) |
| 50 keV | 0.7 (−0.3; 1.6) | 7.0 (4.9; 9.1) | −4.6 (−5.7; −3.6) | 1.7 (0.7; 2.7) | |
| 70 keV | −0.1 (−0.8; 0.5) | 6.1 (4.8; 7.3) | −2.7 (−3.5; −1.8) | 0.5 (−0.2; 1.2) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, P.; D’Alessio, A.; Pascuzzo, R.; Gallo, S.; Fumagalli, M.L.; Ortenzia, O.; Tenconi, C.; Cavatorta, C.; Pignoli, E.; Ghetti, C.; et al. Enhancing Soft Tissue Differentiation with Different Dual-Energy CT Systems: A Phantom Study. Appl. Sci. 2024, 14, 1724. https://doi.org/10.3390/app14051724
Gallo P, D’Alessio A, Pascuzzo R, Gallo S, Fumagalli ML, Ortenzia O, Tenconi C, Cavatorta C, Pignoli E, Ghetti C, et al. Enhancing Soft Tissue Differentiation with Different Dual-Energy CT Systems: A Phantom Study. Applied Sciences. 2024; 14(5):1724. https://doi.org/10.3390/app14051724
Chicago/Turabian StyleGallo, Pasqualina, Andrea D’Alessio, Riccardo Pascuzzo, Salvatore Gallo, Maria Luisa Fumagalli, Ornella Ortenzia, Chiara Tenconi, Claudia Cavatorta, Emanuele Pignoli, Caterina Ghetti, and et al. 2024. "Enhancing Soft Tissue Differentiation with Different Dual-Energy CT Systems: A Phantom Study" Applied Sciences 14, no. 5: 1724. https://doi.org/10.3390/app14051724
APA StyleGallo, P., D’Alessio, A., Pascuzzo, R., Gallo, S., Fumagalli, M. L., Ortenzia, O., Tenconi, C., Cavatorta, C., Pignoli, E., Ghetti, C., Bruzzone, M. G., & De Martin, E. (2024). Enhancing Soft Tissue Differentiation with Different Dual-Energy CT Systems: A Phantom Study. Applied Sciences, 14(5), 1724. https://doi.org/10.3390/app14051724







