Investigating the Corrosion Inhibition Mechanisms of Alkanolammonium Salts: A Case Study with Ethylethanolammonium 4-Nitrobenzoate on Carbon Steel in Saline Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Corrosion Inhibitor Solutions
2.2. Electrochemical Analysis
Open Circuit Potential (OCP)
2.3. Potentiodynamic Polarization (PDP) Testing
2.4. Electrochemical Impedance (EIS) Experiments
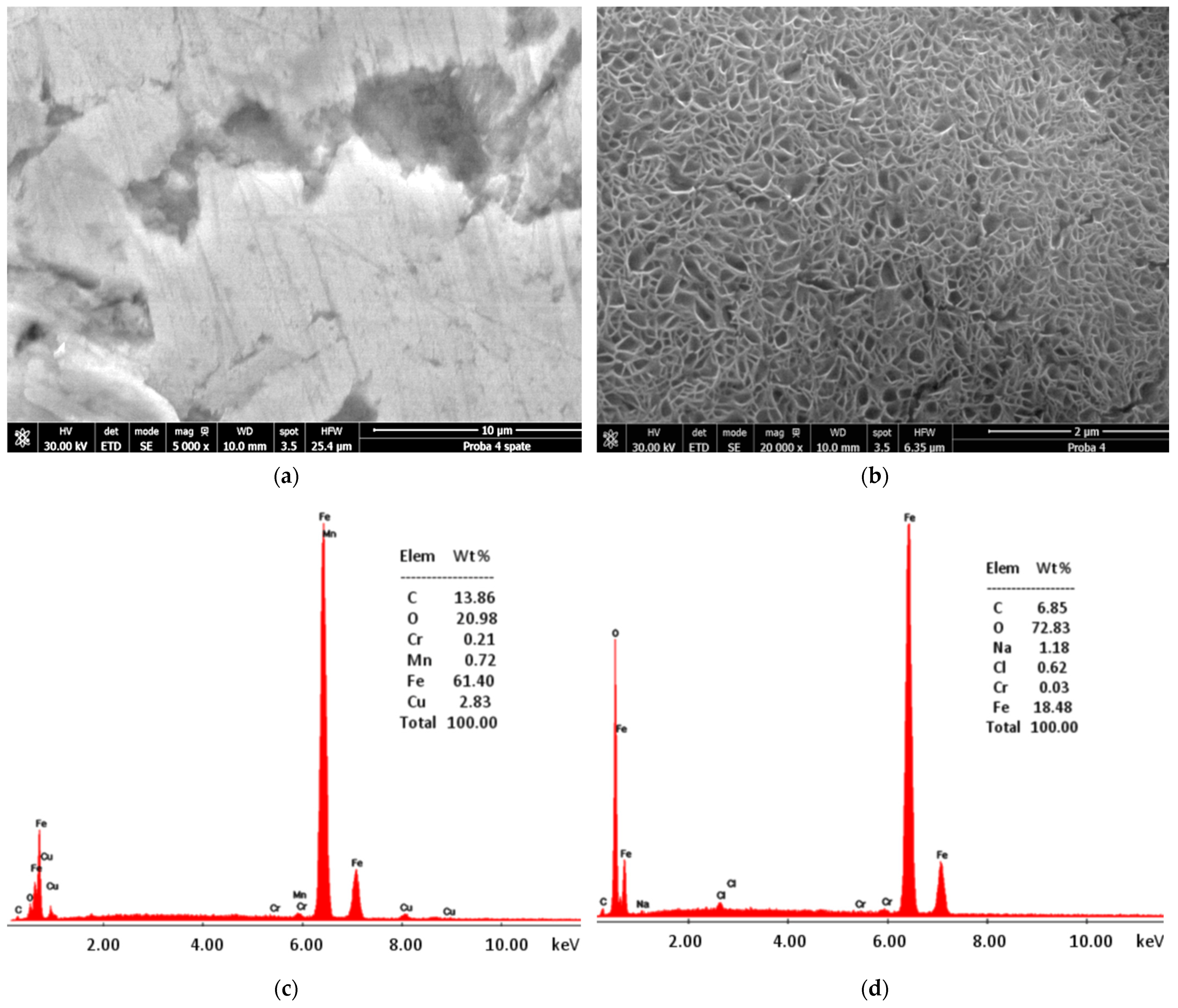
2.5. Electrode Surface Morphology Analysis
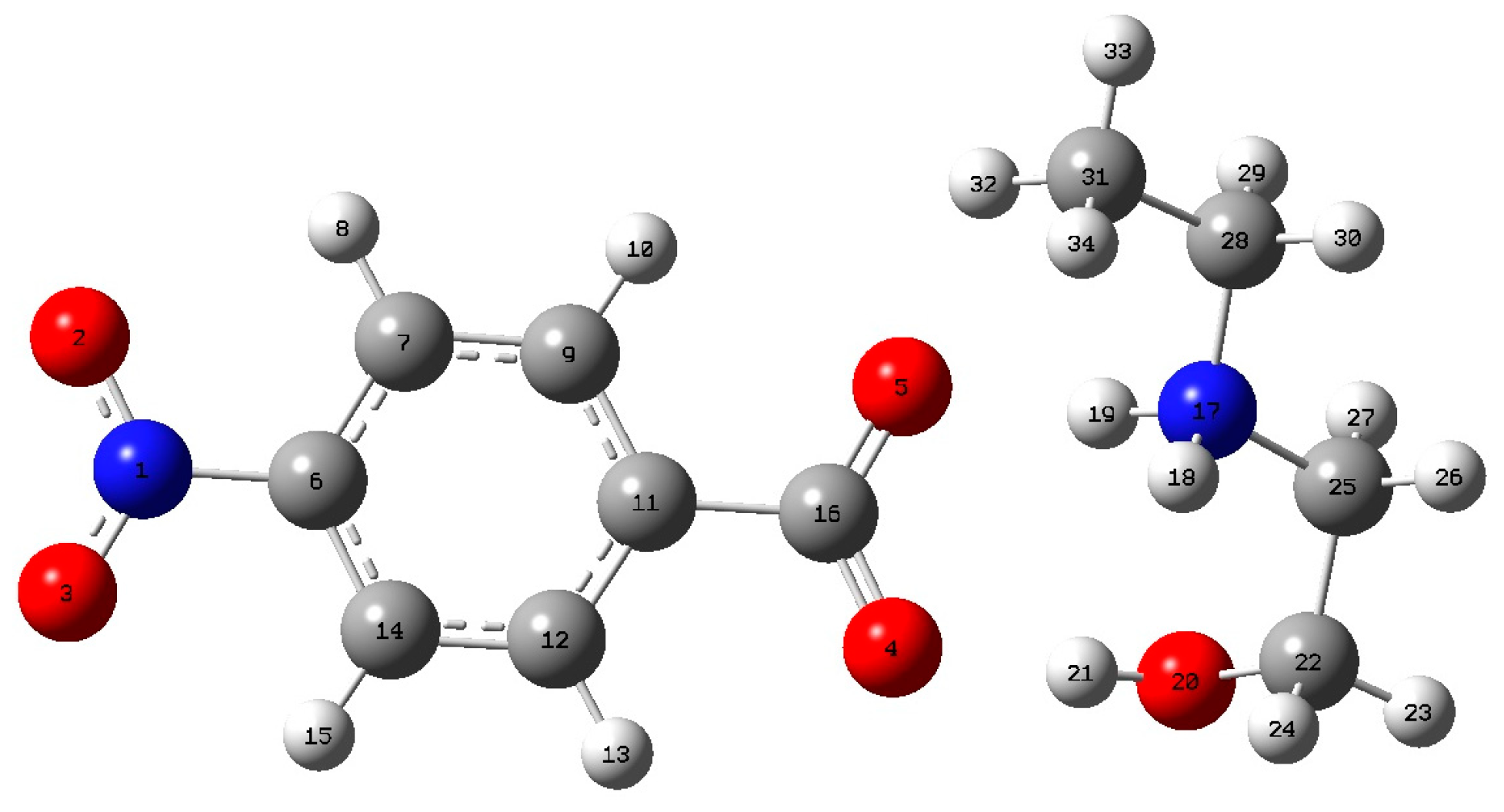
2.6. Computational Methods
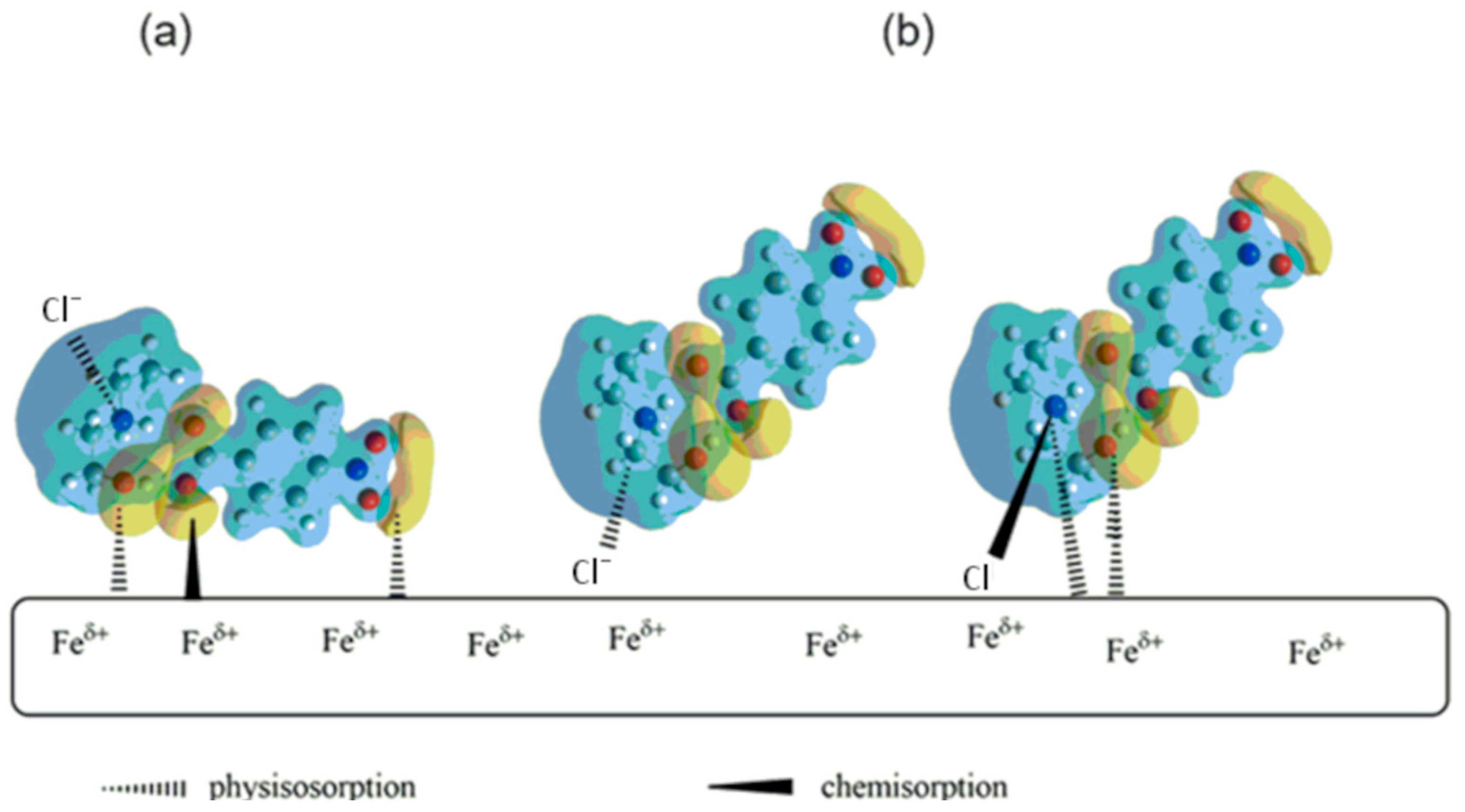
3. Results and Discussions
3.1. Electrochemical Analysis
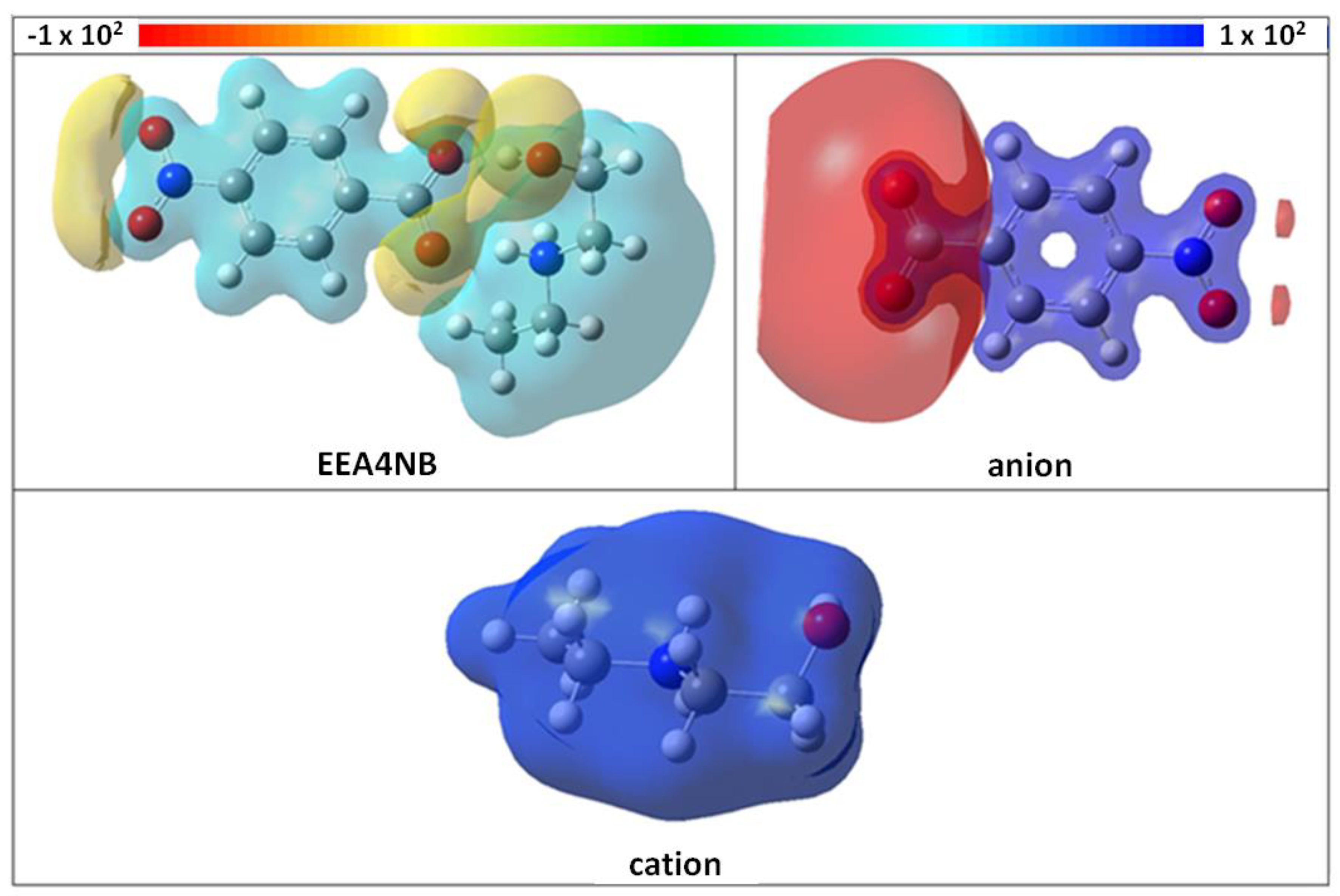
3.2. Computational Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, X.; Pan, X.; Yuan, S.; Du, S.; Zhang, C.; Liu, Y. Corrosion inhibition of mild steel in simulated seawater solution by a green eco-friendly mixture of glucomannan (GL) and bisquaternary ammonium salt (BQAS). Corros. Sci. 2017, 125, 139–151. [Google Scholar] [CrossRef]
- Alcántara, J.; Fuente, D.; Chico, B.; Simancas, J.; Díaz, I.; Morcillo, M. Marine Atmospheric Corrosion of Carbon Steel: A Review. Materials 2017, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Mazumde, M. A J. Global Impact of Corrosion: Occurrence, Cost and Mitigation, Glob. J. Eng. Sci. 2020, 5, 1–5. [Google Scholar] [CrossRef]
- Sherif, E.-S.M.; Almajid, A.A.; Khalil, A.K.; Junaedi, H.; Latief, F.H. Electrochemical studies on the corrosion behavior of API X-65 pipeline steel in chloride solutions. Int. J. Electrochem. Sci. 2013, 8, 9360–9370. [Google Scholar] [CrossRef]
- Ma, I.A.W.; Ammar, S.; Kumar, S.S.A.; Ramesh, K.; Ramesh, S. A Concise Review on Corrosion Inhibitors: Types, Mechanisms and Electrochemical Evaluation Studies. J. Coat. Technol. Res. 2022, 19, 241–268. [Google Scholar] [CrossRef]
- Hassan, H.H.; Abdelghani, E.; Amin, M.A. Inhibition of mild steel corrosion in hydrochloric acid solution by triazole derivatives: Part I. Polarization and EIS studies. Electrochim. Acta. 2007, 52, 6359–6366. [Google Scholar] [CrossRef]
- Abdallah, M.; AL Jahdaly, B.A.; Salem, M.M.; Fawzy, A.; Abdel Fattah, A.A. Pitting corrosion of nickel alloys and stainless steel in chloride solutions and its inhibition using some inorganic compounds. J. Mater. Environ. Sci. 2017, 8, 2599–2607. [Google Scholar]
- Humira, A.; Ashish, K. Understanding functional group effect on corrosion inhibition efficiency of selected organic compounds. J. Mol. Liq. 2021, 344, 117755. [Google Scholar] [CrossRef]
- Elsener, B. Corrosion inhibitors for reinforced concrete—An EFC state of the art report. In Corrosion of Reinforcement in Concrete: Monitoring, Prevention and Rehabilitation Techniques; EFC Series; Woodhead Publishing: Cambridge, UK, 2007; pp. 170–184. [Google Scholar]
- Zomorodian, A.; Behnood, A. Review of Corrosion Inhibitors in Reinforced Concrete: Conventional and Green Materials. Buildings 2023, 13, 1170. [Google Scholar] [CrossRef]
- Aslam, R.; Serdaroglu, G.; Zehra, S.; Verma, D.K.; Aslam, J.; Guo, L.; Verma, C.; Ebenso, E.E.; Quraishi, M.A. Corrosion inhibition of steel using different families of organic compounds: Past and present progress. J. Mol. Liq. 2022, 348, 118373. [Google Scholar] [CrossRef]
- Hassoune, M.; Bezzar, A.; Sail, L.; Ghomari, F. Corrosion inhibition of carbon steel by N,N′-Dimethylaminoethanol in simulated concrete pore solution contaminated with NaCl. J Adhes Sci Technol. 2018, 32, 68–90. [Google Scholar] [CrossRef]
- Alvarez, L.X.; Troconis de Rincón, O.; Escribano, J.; Rincon, T.; Brendy, C. Organic compounds as corrosion inhibitors for reinforced concrete: A review. Corros. Rev. 2023, 41, 617–634. [Google Scholar] [CrossRef]
- Wombacher, F.; Maeder, U.; Marazzani, B. Aminoalcohol based mixed corrosion inhibitors. Cem. Concr. Compos. 2004, 26, 209–216. [Google Scholar] [CrossRef]
- Tang, Z. A Review of Corrosion Inhibitors for Rust Preventative Fluids. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100759. [Google Scholar] [CrossRef]
- Ormellese, M.; Lazzari, L.; Goidanich, S.; Fumagalli, G.; Brenna, A. A Study of Organic Substances as Inhibitors for Chloride-Induced Corrosion in Concrete. Corros. Sci. 2009, 51, 2959–2968. [Google Scholar] [CrossRef]
- Ormellese, M.; Bolzoni, F.; Lazzari, L.; Brenna, A.; Pedeferri, M. Organic Substances as Inhibitors for Chloride-Induced Corrosion in Reinforced Concrete. Mater. Corros. 2011, 62, 170–177. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Quraishi, M.A. Substituents effect on corrosion inhibition performance of organic compounds in aggressive ionic solutions: A review. J. Mol. Liq. 2018, 251, 100–118. [Google Scholar] [CrossRef]
- Ali Fathima Sabirneeza, A.; Geethanjali, R.; Subhashini, S. Polymeric Corrosion Inhibitors for Iron and Its Alloys: A Review. Chem. Eng. Commun. 2015, 202, 232–244. [Google Scholar] [CrossRef]
- Moschona, A.; Plesu, N.; Mezei, G.; Thomas, A.G.; Demadis, K.D. Corrosion protection of carbon steel by tetraphosphonates of systematically different molecular size. Corros. Sci. 2018, 145, 135–150. [Google Scholar] [CrossRef]
- Visa, A.; Plesu, N.; Maranescu, B.; Ilia, G.; Borota, A.; Crisan, L. Combined experimental and theoretical insights into the corrosion inhibition activity on carbon steel iron of phosphonic acids. Molecules 2021, 26, 135. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, A.; Berdimurodov, E.; Verma, C.; Verma, D.K.; Berdimuradov, K.; Quraishi, M.A.; Aliev, N. Constructing efficacy: A novel perspective on organic corrosion inhibitors and interfacial interactions. Chem. Pap. 2023. [Google Scholar] [CrossRef]
- Crisan, M.; Vlase, G.; Szerb, E.I.; Vlase, T. Thermal and kinetics studies of primary, secondary and tertiary alkanolammonium salts of 4-nitrobenzoic acid. J. Therm. Anal. Calorim. 2018, 132, 1409–1418. [Google Scholar] [CrossRef]
- Crisan, M.; Halip, L.; Bourosh, P.; Chicu, S.A.; Chumakov, Y. Synthesis, structure and toxicity evaluation of ethanolamine nitro/chloronitrobenzoates: A combined experimental and theoretical study. Chem. Cent. J. 2017, 11, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Croitor, L.; Crisan, M.; Vlase, G.; Vlase, T.; Bodnarescu, F.; Sumalan, R.; Petric, M.; Siminel, A.V.; Bourosh, P. Advances in new multicomponent crystal system: Structure, thermal kinetic analysis, photoluminescent, and biological activity investigations. J. Therm. Anal. Calorim. 2020, 142, 191–201. [Google Scholar] [CrossRef]
- Crisan, M.; Vlase, G.; Plesu, N.; Petric, M.; Croitor, L.; Kravstsov, V.; Chuamkov, Y.; Bourosh, P.; Vlase, T. Ethylethanolammonium 4-nitrobenzoate. J. Therm. Anal. Calorim. 2018, 134, 343–352. [Google Scholar] [CrossRef]
- Shahraki, M.; Dehdab, M.; Elmi, S. Theoretical studies on the corrosion inhibition performance of three amine derivatives on carbon steel: Molecular dynamics simulation and density functional theory approaches. J. Taiwan Inst. Chem. Eng. 2016, 62, 313–321. [Google Scholar] [CrossRef]
- Zarrouk, A.; Zarrok, H.; Salghi, R.; Hammouti, B.; Al-Deyab, S.S.; Touzani, R.; Bouachrine, M.; Warad, I.; Hadd, T.B. A Theoretical Investigation on the Corrosion Inhibition of Copper by Quinoxaline Derivatives in Nitric Acid Solution. Int. J. Electrochem. Sci. 2012, 7, 6353–6364. [Google Scholar] [CrossRef]
- Assad, H.; Thakur, A.; Sharma, A.K.; Kumar, A. Chapter 6—Density functional theory-based molecular modeling. In Computational Modelling and Simulations for Designing of Corrosion Inhibitors; Verma, D.K., Verma, C., Aslam, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 95–113. [Google Scholar] [CrossRef]
- Morell, C.; Grand, A.; Toro-Labbé, A. New Dual Descriptor for Chemical Reactivity. J. Phys. Chem. A 2005, 109, 205–212. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of ab initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Quantitative analysis of molecular surface based on improved Marching Tetrahedra algorithm. J. Mol. Graph. Model. 2012, 38, 314–323. [Google Scholar] [CrossRef]
- Refait, P.; Grolleau, A.-M.; Jeannin, M.; Rémazeilles, C.; Sabot, R. Corrosion of carbon steel in marine environments: Role of the corrosion product layer. Corros. Mater. Degrad. 2020, 1, 198–218. [Google Scholar] [CrossRef]
- Aslam, R.; Mobin, M.; Zehra, S.; Aslam, J. A comprehensive review of corrosion inhibitors employed to mitigate stainless steel corrosion in different environments. J. Mol. Liq. 2022, 364, 119992. [Google Scholar] [CrossRef]
- Ismail, N.A.; Moussa, A.M.; Kahraman, R.; Shakoor, R.A. Study on the corrosion behavior of polymeric nanocomposite coatings containing halloysite nanotubes loaded with multicomponent inhibitor. Arab. J. Chem. 2022, 15, 104107. [Google Scholar] [CrossRef]
- Madram, A.R.; Shokri, F.; Sovizi, M.R.; Kalhor, H. Aromatic Carboxylic Acids as Corrosion Inhibitors for Aluminium in Alkaline Solution. Port. Electrochim. Acta. 2016, 34, 395–405. [Google Scholar] [CrossRef]
- Ebenso, E.E. Synergistic effect of halide ions on the corrosion inhibition of aluminium in H2SO4 using 2-acetylphenothiazine, Mater. Chem. Phys. 2003, 79, 58–70. [Google Scholar] [CrossRef]
- Chowdhury, G.R.; Mitra, P.K. Effect of Induced Charge on the Passivity and Passivity Breakdown of 304LN Stainless Steels, Adv. Mater. Sci. Eng. 2011, 2011, 798262. [Google Scholar] [CrossRef]
- Schmitzhaus, T.E.; Ortega, M.R.; Schroeder, V.R.; Muller, I.L.; Mattedi, S.; de Fraga Malfatti, C. An amino-based protic ionic liquid as a corrosion inhibitor of mild steel in aqueous chloride solutions. Mater. Corros. 2020, 71, 1175–1193. [Google Scholar] [CrossRef]
- Liu, J.Z.; Zhao, D.; Cai, J.S.; Shi, L.; Liu, J.P. Arylaminoalcohols as corrosion inhibitors for carbon steel in chloride-contaminated simulated concrete pore solution. Int. J. Electochem. Sci. 2016, 11, 1135–1151. [Google Scholar] [CrossRef]
- Ismayilov, I.T.; Abd El-Lateef, H.M.; Abbasov, V.M.; Aliyeva, L.I.; Qasimov, E.E.; Efremenko, E.N.; Ismayilov, T.A.; Mamedxanova, S.A. Inhibition Effects Of Some Novel Surfactants Based On Corn Oil And Diethanolamine On Mild Steel Corrosion In Chloride Solutions Saturated With CO2. Int. J. Thin Film Sci. Tec. 2013, 2, 91–105. [Google Scholar] [CrossRef]
- Benzina Mechmeche, L.; Dhouibi, L.; Ben Ouezdou, M.; Triki, E.; Zucchi, F. Investigation of the early effectiveness of an amino-alcohol based corrosion inhibitor using simulated pore solutions and mortar specimens. Cement Concrete Comp. 2008, 30, 167–173. [Google Scholar] [CrossRef]
- Okeniyia, J.O.; Popoola, A.P.I.; Loto, C.A. Corrosion-inhibition and compressive-strength performance of Phyllanthus muellerianus and triethanolamine on steel-reinforced concrete immersed in saline/marine simulating-environment. Energy Procedia 2017, 119, 972–979. [Google Scholar] [CrossRef]
- Kahraman, R.; Umer, M.A.; Manzoor, U.; Abdullah, A.M.; Shahzad, K.; Sliem, M.H.; Shakoor, R.A.; Radwan, A.B. Electrochemical and thermodynamic study on the corrosion performance of API X120 steel in 3.5% NaCl solution. Sci. Rep. 2020, 10, 4314. [Google Scholar] [CrossRef]
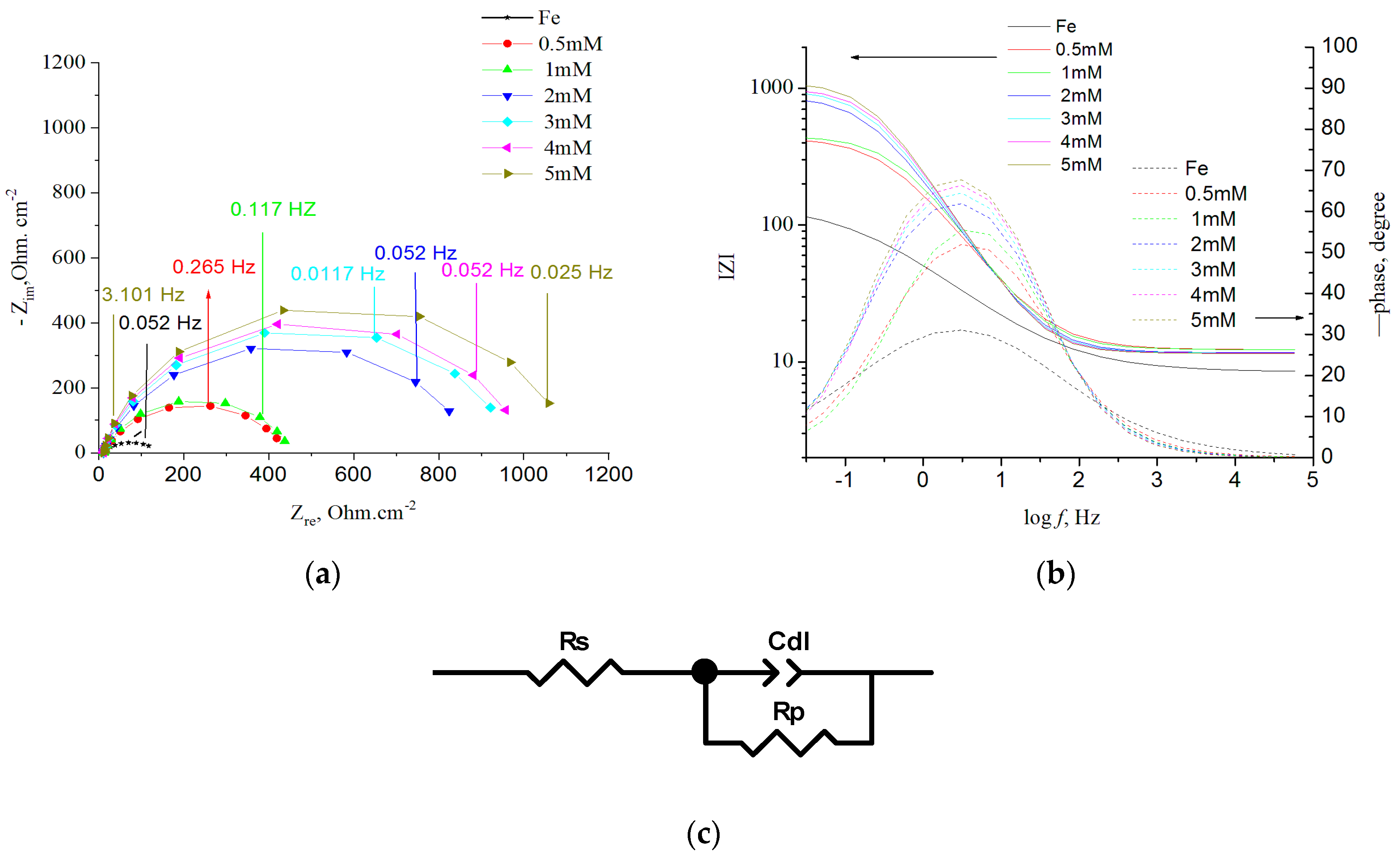
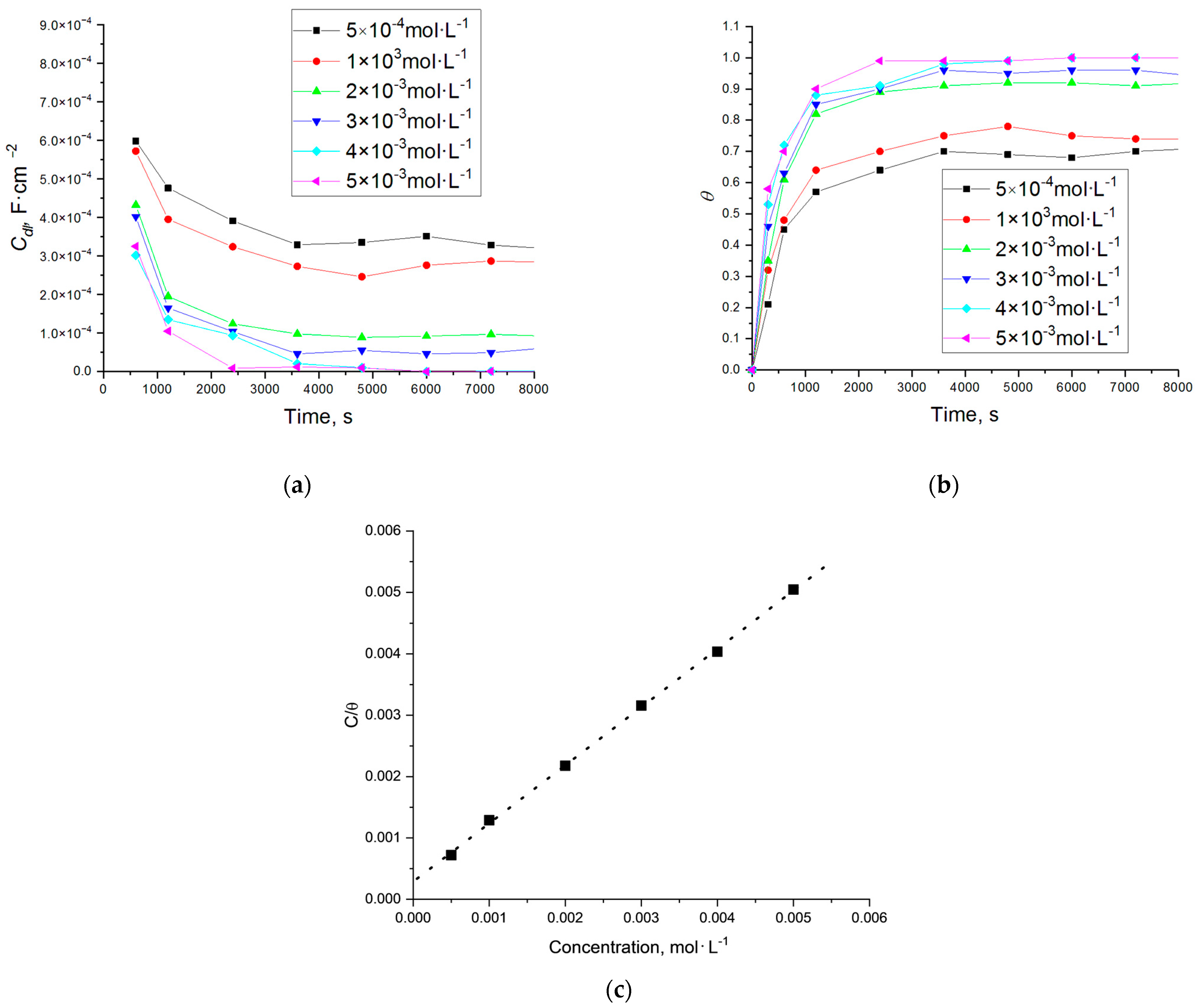

| Cinh, mM/SD * | Rs, Ω · cm2 | Rct, Ω · cm2 | Cdl-T, F · cm−2/sφ−1 | CPEdl-P, (φ) | IE, % |
|---|---|---|---|---|---|
| 0 | 11.92 ± 0.21 | 36 ± 12 | 7.93 × 10−3 ± 0.19 × 10−4 | 0.80 ± 0.03 | |
| 0.5 | 12.3 ± 0.82 | 430 ± 9 | 1.05 × 10−4 ± 1.03 × 10−4 | 0.76 ± 0.11 | 86.73 ± 2.01 |
| 1 | 12.3 ± 0.11 | 440 ± 9 | 9.05 × 10−4 ± 8.01 × 10−6 | 0.81 ± 0.07 | 88.60 ± 0.24 |
| 2 | 11.69 ± 0.41 | 872 ± 6 | 5.80 × 10−4 ± 0.39 × 10−5 | 0.82 ± 0.05 | 92.70 ± 1.27 |
| 3 | 11.62 ± 1.12 | 967 ± 11 | 3.80 × 10−4 ± 0.72 × 10−5 | 0.85 ± 0.04 | 95.22 ± 1.56 |
| 4 | 11.6 ± 0.98 | 993 ± 12 | 2.80 × 10−4 ± 0.03 × 10−4 | 0.87 ± 0.05 | 96.48 ± 2.11 |
| 5 | 11.53 ± 0.91 | 1130 ± 26 | 2.70 × 10−4 ± 0.08 × 10−4 | 0.88 ± 0.05 | 96.60 ± 5.03 |
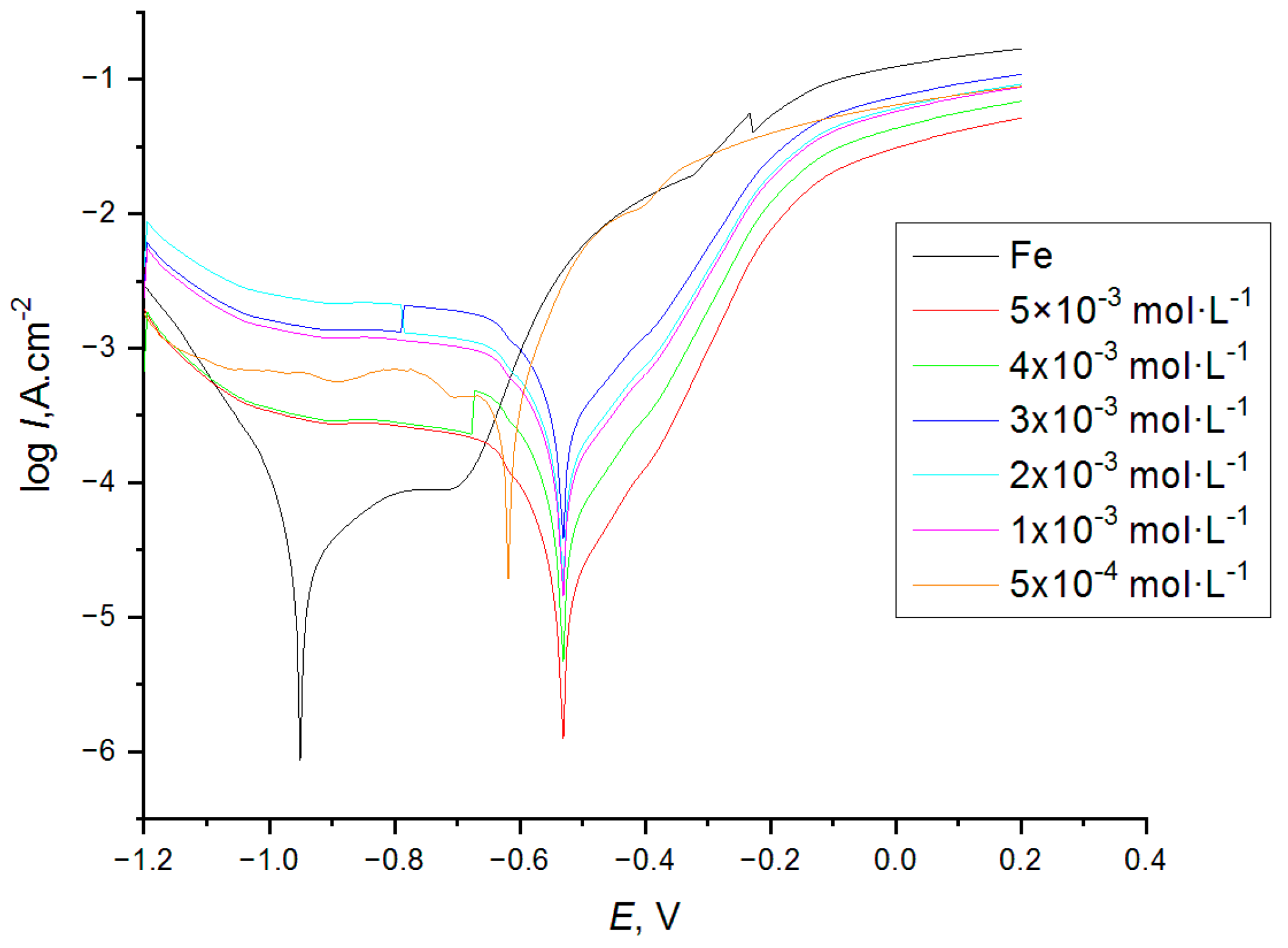
| Cinh, mM/SD * | Ecorr, V | Jcorr, A·cm−2 | -βc, V·decade−1 | βa, V·decade−1 | Rp, ohm·cm−2 | CR mm·Year−1 |
|---|---|---|---|---|---|---|
| 0 | −0.930 ± −0.06 | 8.54 × 10−4 ± 1.33 × 10−6 | 0.320 ± 0.025 | 0.162 ± 0.012 | 38 ± 2 | 0.942 ± 0.042 |
| 0.5 | −0.612 ± −0.011 | 6.81 × 10−4 ± 0.82 × 10−6 | 0.225 ± 0.003 | 0.280 ± 0.007 | 380 ± 7 | 0.812 ± 0.007 |
| 1 | −0.524 ± −0.013 | 6.10 × 10−5 ± 0.41 × 10−6 | 0.122 ± 0.073 | 0.095 ± 0.016 | 439 ± 6 | 0.750 ± 0.021 |
| 2 | −0.532 ± −0.009 | 5.84 × 10−5 ± 1.87 × 10−7 | 0.114 ± 0.009 | 0.088 ± 0.014 | 866 ± 6 | 0.421 ± 0.003 |
| 3 | −0.514 ± −0.021 | 5.21 × 10−5 ± 0.92 × 10−7 | 0.114 ± 0.014 | 0.031 ± 0.002 | 977 ± 11 | 0.408 ± 0.004 |
| 4 | −0.520 ± −0.019 | 3.83 × 10−5 ± 1.05 × 10−7 | 0.105 ± 0.023 | 0.042 ± 0.010 | 988 ± 7 | 0.402 ± 0.008 |
| 5 | −0.518 ± −0.013 | 1.45 × 10−5 ± 1.16 × 10−7 | 0.114 ± 0.017 | 0.031 ± 0.004 | 1062 ± 17 | 0.215 ± 0.011 |
| Inhibitor | EHOMO, eV | ELUMO, eV | Egap, eV | p, Bohr3 | µ, Debye | χ, eV | η, eV | ∆N |
|---|---|---|---|---|---|---|---|---|
| EEA4NB | −9.41 | −1.2 | 8.21 | 221.57 | 16.715 | 6.033 | 2.9449 | 0.164 |
| anion | −8.591 | −1.093 | 7.498 | 148.04 | 11.753 | 4.794 | 1.798 | 0.613 |
| cation | −10.695 | 0.992 | 11.687 | 69.9 | 4.225 | 5.039 | 3.801 | 0.258 |
| Atoms | EEA4NB | Anion |
|---|---|---|
| N1 | 0.0502 | 0.1435 |
| O2 | −0.1393 | 0.2014 |
| O3 | −0.1396 | 0.2013 |
| O4 | −0.4833 | −0.3275 |
| O5 | −0.3895 | −0.3013 |
| C6 | −0.2217 | 0.0051 |
| C7 | −0.0601 | 0.0384 |
| C9 | −0.0952 | 0.0051 |
| C11 | −0.2104 | 0.0551 |
| C12 | −0.0939 | 0.0057 |
| C14 | −0.0603 | 0.0386 |
| C16 | −0.0843 | −0.1018 |
| cation | ||
| N17 | −0.0062 | 0.0563 |
| O20 | −0.0217 | −0.4216 |
| C22 | −0.0031 | −0.0581 |
| C25 | −0.0010 | −0.0322 |
| C28 | −0.0005 | 0.0215 |
| C31 | −0.0006 | 0.0083 |
| Inhibitor/Inhibitor Concentration | Medium | Jcorr µA/cm2 | IE, % | Adsoption Isotherm | Ref. |
|---|---|---|---|---|---|
| Ammonium salt of the sulfated fatty acid diethanolamine amide (AS)/50 ppm | 1% NaCl | 31 | 92.0 | Langmuir | [42] |
| Aminomethylene-phosphonate/4.9 mM | 3.5% NaCl, pH = ~3 | 4.03 | 87.99 | Langmuir | [20] |
| Amino-alcohol-based mixed inhibitor (Ferrogard 903)/1 mL/50 mL water | 0.5 M NaCl, pH = 12.1 | 12,000 | - | - | [43] |
| Phyllanthus muellerianus leaf extract (2 g) and triethanolamine (6 g)/(C6H15NO3: TEA) | 3.5 wt% NaCl | - | 98 | - | [44] |
| Aryl aminoalcohol/5 ppm | 0.3 M NaCl | 1.74 | 89.9 | Langmuir | [41] |
| N-methyl-2-hydroxyethylamine (M-2HEAOL) and bis-2-hidroxyethylamine (B-HEAOL)oleate/5 mmol/L | 0.01 M NaCl | 0.12 | 97 | Langmuir | [40] |
| Polyethyleneimine (PEI)/100 μmol L−1 | 3.5 % NaCl | 10.9 | 86.54 | Langmuir | [45] |
| EEA4NB | 3% NaCl | 0.145 | 96.6 | Langmuir | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crisan, M.; Muntean, C.; Chumakov, Y.; Plesu, N. Investigating the Corrosion Inhibition Mechanisms of Alkanolammonium Salts: A Case Study with Ethylethanolammonium 4-Nitrobenzoate on Carbon Steel in Saline Solution. Appl. Sci. 2024, 14, 1832. https://doi.org/10.3390/app14051832
Crisan M, Muntean C, Chumakov Y, Plesu N. Investigating the Corrosion Inhibition Mechanisms of Alkanolammonium Salts: A Case Study with Ethylethanolammonium 4-Nitrobenzoate on Carbon Steel in Saline Solution. Applied Sciences. 2024; 14(5):1832. https://doi.org/10.3390/app14051832
Chicago/Turabian StyleCrisan, Manuela, Cornelia Muntean, Yurii Chumakov, and Nicoleta Plesu. 2024. "Investigating the Corrosion Inhibition Mechanisms of Alkanolammonium Salts: A Case Study with Ethylethanolammonium 4-Nitrobenzoate on Carbon Steel in Saline Solution" Applied Sciences 14, no. 5: 1832. https://doi.org/10.3390/app14051832
APA StyleCrisan, M., Muntean, C., Chumakov, Y., & Plesu, N. (2024). Investigating the Corrosion Inhibition Mechanisms of Alkanolammonium Salts: A Case Study with Ethylethanolammonium 4-Nitrobenzoate on Carbon Steel in Saline Solution. Applied Sciences, 14(5), 1832. https://doi.org/10.3390/app14051832