Is the Artificial Pollination of Walnut Trees with Drones Able to Minimize the Presence of Xanthomonas arboricola pv. juglandis? A Review
Abstract
:1. Introduction
2. Walnut Blight Prevention
Walnut Blight and Conditions
3. Walnut Buds: Bloom and Pollination Events—Cross-Pollination
4. Artificial Pollination—Artificial Pollination Technology
- Crop yield enhancement: In agriculture, artificial pollination is sometimes employed to increase crop yields. This can be particularly important for crops where natural pollination may be insufficient [31].
- Control of pollination: Artificial pollination allows for precise control over the pollination process. This is useful in hybrid seed production, where specific traits can be selected [32].
- Overcoming pollination challenges: Some crops may face challenges with natural pollination due to factors like low insect activity, unfavorable weather conditions, i.e., wind, or geographic isolation. Artificial pollination can overcome these challenges [33].
- Biotechnological research: In scientific research, artificial pollination can be used to study plant genetics, breeding, and other aspects of plant biology [34].
- Drones: Unmanned aerial vehicles (UAVs), or drones, equipped with special devices can be used for pollination. These devices may carry pollen and release it over crops, i.e., walnut trees, or mimic the actions of bees and other pollinators [35].
- Robotic pollinators: Small robots designed to mimic the behaviors of natural pollinators can navigate through crops, transferring pollen between flowers. These robots are often equipped with cameras and sensors to identify and locate flowers [36].
- Spraying devices: Some systems involve the use of sprayers to disperse pollen over crops. These devices can be mounted on tractors or other vehicles, releasing pollen in a controlled manner [37].
- Electrostatic pollination: This method uses an electrostatic charge to adhere pollen to flowers. The charged pollen is attracted to the stigma, increasing the chances of successful pollination [38].
- Vibrational devices: Certain crops respond well to vibrational stimulation, which can be achieved through devices that vibrate the flowers, causing the release of pollen [39].
- Artificial flowers: In controlled environments like greenhouses, artificial flowers containing pollen can be placed strategically to enhance pollination [40].
- Automated pollination systems: Some systems use automated robotic arms or other mechanical devices to transfer pollen between flowers. These systems can be programmed to work efficiently and quickly [41].
- Design and fabrication of quadrotor componentsFor the quadrotor to be able to carry and distribute pollen over trees, the quadrotor components needed to be selected correctly, i.e., the body size was chosen based on the pollen tank. The objective of the quadrotor was to pollinate, so the tank was equipped with a nozzle with four holes to allow pollen to fall uniformly. It was recommended that the propulsion system use a motor with a lower current. The electronic device contained various sensors, such as a compass, a global positioning system (GPS), a barometer, and connections for the flight control system [35].
- Modeling and ControlComputational fluid dynamic (CFD) software was used to simulate the airflow beneath the UAV. This simulation assisted in identifying the pollen streams under the robot so that the released pollen could be directed toward the target trees. The quadrotor controller was designed and simulated in MATLAB to ensure system stability [35].
- Pollination of walnut treesDifferent mixtures of pollen diluted with talcum powder were distributed over various groups of trees using the quadrotor. The results achieved on the trees were measured after a few months to evaluate the benefits of the proposed system [35].
5. Machine Learning and Pollination
- Predictive modeling: ML algorithms can analyze historical data on pollination success, environmental conditions, crop yields, and phenology to create predictive models. These models can help to predict optimal times for pollination, considering factors such as weather patterns, bloom asynchrony, and the availability of pollinators [52,53,54].
- Automated monitoring: ML-powered monitoring systems can analyze images or sensor data to track the health and development of crops. This real-time monitoring allows for the early detection of issues related to pollination, such as low pollination rates or the presence of pests that could affect pollinators [55,56].
- Optimizing pollination strategies: Machine learning algorithms can optimize artificial pollination strategies based on a variety of factors [57,58]. These include the type of crop, environmental conditions, and the efficiency of different pollination methods [59,60]. This can lead to more targeted and effective pollination efforts [61].
- Identification of pollinator behavior: ML can be used to analyze the behavior of natural pollinators, such as bees, by processing video footage or sensor data. Understanding pollinator behavior can provide insight into their preferences, movement patterns, and efficiency in pollinating specific crops [62,63].
- Genetic analysis: Machine learning techniques can analyze genetic data related to plant characteristics, including traits associated with pollination [64,65]. This information can contribute to breeding programs aimed at developing crops that are more resilient to environmental challenges and more compatible with artificial pollination methods [66,67].
- Data integration: Machine learning excels at integrating and analyzing large datasets from various sources. In the context of pollination, this can include data on weather conditions, soil quality, plant health, and more. The integrated data can provide a comprehensive understanding of the factors influencing pollination success [70,71,72].
6. Walnut Pollination: Model to Prevent or Reduce the Risk of the Walnut Blight Disease (Our View)
State of the Art Flowchart
- 1.1.
- Identify spring bud break, leaf emergence, and anthesis growth stages of the walnut cultivars.
- 1.2.
- Understand how plants grow and develop during bud break, anthesis, and pollination.
- 1.3.
- Understand protandrous and protogynous mechanisms prior to pollen shedding.
- 2.1.
- Identify the “core” pollen microbiota.
- 2.2.
- Compare bacterial abundance and diversity between walnut cultivars.
- 2.3.
- Assess the impact of the pollination type on the variability of the flower pollen microbiota.
- 2.4.
- Estimate the role of X. arboricola pv. juglandis in stigma exposed to contaminated pollen.
- 3.1.
- Check reservoir cultivars, i.e., Chandler, for the presence of inoculum.
- 3.2.
- Check for conditions that encourage the disease to spread, such as moisture, and especially the combined action of wind and rain.
- 3.3.
- Check for air-borne inoculum when catkins open.
- 3.4.
- Check for pistil-late flowers.
- 3.5.
- Collect and store uninfected pollen.
- 4.1.
- Set genetic algorithm for ‘mutation’, ‘selection’, and ‘recombination’ based on pollen–microbe interaction.
- 4.2.
- Use metaheuristic algorithms, which include differential evolution.
- 4.3.
- Use the appropriate flower pollination algorithm that is suitable and inspired by the process of pollination.
- 5.1.
- Quadrotor (pollinating drone) to carry pollen grains.
- 5.2.
- Pollinating drone to make an ideal delivery system, landing on the pistil of a flower to result in pollination.
- 5.3.
- Drone technique would need some refinement in localization, mapping, and control.
- 5.4.
- Metaheuristic optimization algorithm to find the best (feasible) solution out of all possible solutions to the pollination optimization problem.
- 6.1.
- Analyze historical data on pollination success, environmental conditions, and crop yields (machine learning).
- 6.2.
- Tune flower pollination algorithm parameters (formulating the above steps, mainly steps 1 and 2, due to climate change).
- 6.3.
- Start the process from step 3 and improve the equipment at steps 4 and 5.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polito, V.S.; Pinney, K.; Weinbaum, S.; Aradhya, M.K.; Dangl, J.; Yanknin, Y.; Grant, J.A. Walnut pollination dynamics: Pollen flow in walnut orchards. Acta Hortic. 2005, 705, 465–472. [Google Scholar] [CrossRef]
- Ark, P.A. Further evidence of pollen dissemination of walnut blight. Phytopathology 1944, 34, 329–334. [Google Scholar]
- Kałużna, M.; Fischer-Le Saux, M.; Pothier, J.F.; Jacques, M.A.; Obradović, A.; Tavares, F.; Stefani, E. Xanthomonas arboricola pv. juglandis and pv. corylina: Brothers or distant relatives? Genetic clues, epidemiology, and insights for disease management. Mol. Plant Pathol. 2021, 22, 1481–1499. [Google Scholar] [CrossRef]
- Mulrean, E.N.; Schroth, M.N. Ecology of Xanthomonas campestris pv. juglandis on Persian (English) Walnuts. Phytopathology 1982, 72, 434–438. [Google Scholar] [CrossRef]
- Lang, M.D.; Evans, K. Epidemiology and status of walnut blight in Australia. J. Plant Pathol. 2010, 92, 49–56. [Google Scholar]
- Giovanardi, D.; Bonneau, S.; Gironde, S.; Saux, M.F.; Manceau, C.; Stefani, E. Morphological and genotypic features of Xanthomonas arboricola pv. juglandis populations from walnut groves in Romagna region, Italy. Eur. J. Plant Pathol. 2016, 145, 1–16. [Google Scholar] [CrossRef]
- Tontou, R.; Giovanardi, D.; Stefani, E. Pollen as a possible pathway for the dissemination of Pseudomonas syringae pv. actinide and bacterial canker of kiwifruit. Phytopathol. Mediterr. 2014, 53, 333–339. [Google Scholar] [CrossRef]
- Van der Zwet, T.; Bell, R.L. Survival of Erwinia amylovora on apple and pear pollen. Acta Hortic. 1992, 338, 111–112. [Google Scholar] [CrossRef]
- Garcin, A.; El-Maataoui, M.; Tichadou, S.; Prunet, J.P.; Ginibre, T.; Penet, C. Walnut blight, new knowledge for an old disease: Summary of research (1995–2000). Infos-Ctifl 2001, 171, 27–30. [Google Scholar]
- Giovanardi, D.; Dallai, D.; Stefani, E. Population features of Xanthomonas arboricola pv. juglandis and epidemiology of walnut blight in Romagna (Italy). In Proceedings of the Petria 13th Congress of the Mediterranean Phytopathological Union, Rome, Italy, 20–25 June 2010; Volume 20, pp. 96–97. [Google Scholar]
- Matsumoto, D.; Shimizu, S.; Shimazaki, A.; Ito, K.; Taira, S. Effects of Self-pollen Contamination in Artificial Pollination on Fruit Set of ‘Fuji Murasaki’ Akebia trifoliata. Hortic. J. 2022, 91, 431–436. [Google Scholar] [CrossRef]
- Everett, K.; Cohen, D.; Pushparajah, I.; Vergara, M.; Curtis, C.; Larsen, N.; Jia, Y. Heat treatments to kill Pseudomonas syringae pv actinidiae on contaminated pollen. N. Z. Plant Prot. 2012, 65, 8–18. [Google Scholar] [CrossRef]
- Dingley, A.; Anwar, S.; Kristiansen, P.; Warwick, N.W.; Wang, C.; Sindel, B.M.; Cazzonelli, C.I. Precision Pollination Strategies for Advancing Horticultural Tomato Crop Production. Agronomy 2022, 12, 518. [Google Scholar] [CrossRef]
- Hiraguri, T.; Kimura, T.; Endo, K.; Ohya, T.; Takanashi, T.; Shimizu, H. Shape classification technology of pollinated tomato flowers for robotic implementation. Sci. Rep. 2023, 13, 2159. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Molina, J.; Rodríguez, F.; Moreno, J.; Sánchez-Hermosilla, J.; Giménez, A. Robotics in greenhouses. Scoping review. Comput. Electron. Agric. 2024, 219, 108750. [Google Scholar] [CrossRef]
- Gudowska, A.; Cwajna, A.; Marjańska, E.; Moroń, D. Pollinators enhance the production of a superior strawberry—A global review and meta-analysis. Agric. Ecosyst. Environ. 2024, 362, 108815. [Google Scholar] [CrossRef]
- Rice, C.R.; McDonald, S.T.; Shi, Y.; Gan, H.; Lee, W.S.; Chen, Y.; Wang, Z. Perception, Path Planning, and Flight Control for a Drone-Enabled Autonomous Pollination System. Robotics 2022, 11, 144. [Google Scholar] [CrossRef]
- Yamada, N.; Hiraguri, T.; Shimizu, H.; Kimura, T.; Shimada, T.; Shibasaki, A.; Takemura, Y. Drone Flight Experiment using RTK Positioning for Pear Pollination. In Proceedings of the 2023 International Conference on Consumer Electronics—Taiwan (ICCE-Taiwan), PingTung, Taiwan, 17–19 July 2023; pp. 655–656. [Google Scholar] [CrossRef]
- Chevallier, A.; Bray, O.; Prunet, J.P.; Giraud, M. Factors influencing walnut blight symptoms emergence and development. Acta Hortic. 2010, 861, 473–478. [Google Scholar] [CrossRef]
- Vagelas, I.; Rumbos, C.I.; Tsiantos, J.A. Variation in disease development among persian walnut cultivars, selections and crosses when inoculated with Xanthomonas arboricola pv. juglandis in Greece. J. Plant Pathol. 2012, 94, 57–61. [Google Scholar] [CrossRef]
- Moragrega, C.; Llorente, I. Effects of leaf wetness duration, temperature, and host phenological stage on infection of walnut by Xanthomonas arboricola pv. juglandis. Plants 2023, 12, 2800. [Google Scholar] [CrossRef]
- Lindow, S.; Olson, W.; Buchner, R. Colonization of dormant walnut buds by Xanthomonas arboricola pv. juglandis is predictive of subsequent disease. Phytopathology 2014, 104, 1163–1174. [Google Scholar] [CrossRef]
- Buchner, R.P.; Gilles, C.; Olson, W.H.; Adaskaveg, J.E.; Lindow, S.E.; Koutsoukis, R. Spray timing and materials for walnut blight (Xanthomonas campestris pv. juglandis, Xanthomonas arboricola pv. juglandis) control in northern California USA. Acta Hortic. 2010, 861, 457–464. [Google Scholar] [CrossRef]
- Adaskaveg, J.E.; Förster, H.; Thompson, D.; Enns, J.; Connell, J.; Buchner, R. Epidemiology and management of walnut blight. Walnut Res. Rep. 2009, 241–257. [Google Scholar]
- Miller, P.W.; Bollen, W.B. Walnut Bacteriosis and Its Control; Agricultural Experiment, Station Technical Bulletin 9; United States Department of Agriculture Bureau of Plant Industry, Soils and Agricultural Engineering, Oregon State College: Corvallis, OR, USA, 1946. [Google Scholar]
- Franchi, G.G.; Piotto, B.; Nepi, M.; Baskin, C.C.; Baskin, J.M.; Pacini, E. Pollen and seed desiccation tolerance in relation to degree of developmental arrest, dispersal, and survival. J. Exp. Bot. 2011, 62, 5267–5281. [Google Scholar] [CrossRef] [PubMed]
- Donati, I.; Cellini, A.; Sangiorgio, D.; Vanneste, J.; Scortichini, M.; Balestra, G.; Spinelli, F. Pseudomonas syringae pv. actinidiae: Ecology, infection dynamics and disease epidemiology. Microb. Ecol. 2020, 80, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Farina, W.M.; Arenas, A.; Díaz, P.C.; Susic Martin, C.; Corriale, M.J. In-hive learning of specific mimic odours as a tool to enhance honey bee foraging and pollination activities in pear and apple crops. Sci. Rep. 2022, 12, 20510. [Google Scholar] [CrossRef] [PubMed]
- Pedroncelli, A.; Puopolo, G. This tree is on fire: A review on the ecology of Erwinia amylovora, the causal agent of fire blight disease. J. Plant Pathol. 2023. [Google Scholar] [CrossRef]
- Broussard, M.A.; Coates, M.; Martinsen, P. Artificial pollination technologies: A review. Agronomy 2023, 13, 1351. [Google Scholar] [CrossRef]
- Castro, H.; Siopa, C.; Casais, V.; Castro, M.; Loureiro, J.; Gaspar, H.; Castro, S. Pollination as a key management tool in crop production: Kiwifruit orchards as a study case. Sci. Hort. 2021, 290, 110533. [Google Scholar] [CrossRef]
- Wurz, A.; Grass, I.; Tscharntke, T. Hand pollination of global crops—A systematic review. Basic Appl. Ecol. 2021, 56, 299–321. [Google Scholar] [CrossRef]
- Shimizu, H. Advanced technologies for pollination in plant factories. In Plant Factory Using Artificial Light; Elsevier: Amsterdam, The Netherlands, 2019; pp. 185–192. [Google Scholar] [CrossRef]
- Frachon, L.; Stirling, S.; Schiestl, F.P.; Dudareva, N. Combining biotechnology and evolution for understanding the mechanisms of pollinator attraction. Curr. Opin. Biotechnol. 2021, 70, 213–219. [Google Scholar] [CrossRef]
- Mazinani, M.; Zarafshan, P.; Dehghani, M.; Vahdati, K.; Etezadi, H. Design and analysis of an aerial pollination system for walnut trees. Biosyst. Eng. 2023, 225, 83–98. [Google Scholar] [CrossRef]
- Potts, S.G.; Neumann, P.; Vaissière, B.E.; Vereecken, N.J. Robotic bees for crop pollination: Why drones cannot replace biodiversity. Sci. Total Environ. 2018, 642, 665–667. [Google Scholar] [CrossRef]
- Chen, P.; Douzals, J.; Lan, Y.; Cotteux, E.; Delpuech, X.; Pouxviel, G.; Zhan, Y. Characteristics of unmanned aerial spraying systems and related spray drift: A review. Front. Plant Sci. 2022, 13, 870956. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Lan, Y.; Wang, L.; Lu, X.; Yan, K.; Deng, J.; Zeng, W. Development and prospect of UAV-based aerial electrostatic spray technology in China. Appl. Sci. 2021, 11, 4071. [Google Scholar] [CrossRef]
- Ge, C.; Dunno, K.D.; Singh, M.; Yuan, L.; Lu, L. Development of a drone’s vibration, shock, and atmospheric profiles. Appl. Sci. 2021, 11, 5176. [Google Scholar] [CrossRef]
- Russell, A.L.; Papaj, D.R. Artificial pollen dispensing flowers and feeders for bee behaviour experiments. J. Pollinat. Ecol. 2016, 18, 13–22. [Google Scholar] [CrossRef]
- Li, K.; Huo, Y.; Liu, Y.; Shi, Y.; He, Z.; Cui, Y. Design of a lightweight robotic arm for kiwifruit pollination. Comput. Electron. Agric. 2022, 198, 107114. [Google Scholar] [CrossRef]
- Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2009, 84, 523–538. [Google Scholar] [CrossRef]
- Orduña-Malea, E.; Costas, R. Link-based approach to study scientific software usage: The case of VOSviewer. Scientometrics 2021, 126, 8153–8186. [Google Scholar] [CrossRef]
- Arruda, H.; Silva, É.R.; Lessa, M.; Proença, D.; Bartholo, R. VOSviewer and Bibliometrix. J. Med. Libr. Assoc. 2022, 110, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Vagelas, I.; Leontopoulos, S. A bibliometric analysis and a citation mapping process for the role of soil recycled organic matter and microbe interaction due to climate change using scopus database. Agric. Eng. 2023, 5, 581–610. [Google Scholar] [CrossRef]
- Lykas, C.; Vagelas, I. Innovations in agriculture for sustainable Agro-systems. Agronomy 2023, 13, 2309. [Google Scholar] [CrossRef]
- Mergos, P.E.; Yang, X. Flower pollination algorithm parameters tuning. Soft Comput. 2021, 25, 14429–14447. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, Y.; Qiao, S.; Huang, K. Flower pollination algorithm with bee pollinator for cluster analysis. Inf. Proc. Lett. 2016, 116, 1–14. [Google Scholar] [CrossRef]
- Ambika Manirajan, B.; Ratering, S.; Rusch, V.; Schwiertz, A.; Geissler-Plaum, R.; Cardinale, M.; Schnell, S. Bacterial microbiota associated with flower pollen is influenced by pollination type, and shows a high degree of diversity and species-specificity. Environ. Microbiol. 2016, 18, 5161–5174. [Google Scholar] [CrossRef] [PubMed]
- Akwasi, A.M.; Wei, X.; Duku, O.A. Observer controller-based structure for a modified flower pollination algorithm for wind power generation. Int. J. Autom. Control 2024, 18, 53–86. [Google Scholar] [CrossRef]
- Hemalatha, S.; Johny Renoald, A.; Banu, G.; Indirajith, K. Design and investigation of PV string/central architecture for bayesian fusion technique using grey wolf optimization and flower pollination optimized algorithm. Energy Convers. Manag. 2023, 286, 117078. [Google Scholar] [CrossRef]
- Campbell, D.R. Predicting plant reproductive success from models of competition for pollination. Oikos 1986, 47, 257–266. [Google Scholar] [CrossRef]
- Sáez, A.; di Virgilio, A.; Tiribelli, F.; Geslin, B. Simulation models to predict pollination success in apple orchards: A useful tool to test management practices. Apidologie 2018, 49, 551–561. [Google Scholar] [CrossRef]
- Stock, M.; Piot, N.; Vanbesien, S.; Meys, J.; Smagghe, G.; Baets, B.D. Pairwise learning for predicting pollination interactions based on traits and phylogeny. Ecol. Model. 2021, 451, 109508. [Google Scholar] [CrossRef]
- Pegoraro, L.; Hidalgo, O.; Leitch, I.J.; Pellicer, J.; Barlow, S.E. Automated video monitoring of insect pollinators in the field. Emerg. Top Life Sci. 2020, 4, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.N.; Rustia, D.J.; Yang, E.; Lin, T. Automated monitoring and analyses of honey bee pollen foraging behavior using a deep learning-based imaging system. Comput. Electron. Agric. 2021, 187, 106239. [Google Scholar] [CrossRef]
- DeVetter, L.W.; Chabert, S.; Milbrath, M.O.; Mallinger, R.E.; Walters, J.; Isaacs, R.; Galinato, S.P.; Kogan, C.J.; Brouwer, K.; Melathopoulos, A.; et al. Toward evidence-based decision support systems to optimize pollination and yields in highbush blueberry. Front. Sustain. Food Syst. 2022, 6, 1006201. [Google Scholar] [CrossRef]
- Menzel, C.M. Fruit set is moderately dependent on insect pollinators in strawberry and is limited by the availability of pollen under natural open conditions. J. Hortic. Sci. Biotechnol. 2023, 98, 685–714. [Google Scholar] [CrossRef]
- Yang, M.; Lyu, H.; Zhao, Y.; Sun, Y.; Pan, H.; Sun, Q.; Chen, J.; Qiang, B.; Yang, H. Delivery of pollen to forsythia flower pistils autonomously and precisely using a robot arm. Comput. Electron. Agric. 2023, 214, 108274. [Google Scholar] [CrossRef]
- Hiraguri, T.; Shimizu, H.; Kimura, T.; Matsuda, T.; Maruta, K.; Takemura, Y.; Ohya, T.; Takanashi, T. Autonomous drone-based pollination system using AI classifier to replace bees for greenhouse tomato cultivation. IEEE Access 2023, 11, 99352–99364. [Google Scholar] [CrossRef]
- Cong, W.; Dupont, Y.L.; Søegaard, K.; Eriksen, J. Optimizing yield and flower resources for pollinators in intensively managed multi-species grasslands. Agric. Ecosyst. Environ. 2020, 302, 107062. [Google Scholar] [CrossRef]
- Wajnberg, E.; Tel-Zur, N.; Shapira, I.; Lebber, Y.; Lev-Yadun, S.; Zurgil, U.; Reisman-Berman, O.; Keasar, T. Pollinator behavior drives sexual specializations in the hermaphrodite flowers of a heterodichogamous tree. Front. Plant Sci. 2019, 10, 1315. [Google Scholar] [CrossRef]
- Knauer, A.C.; Kokko, H.; Schiestl, F.P. Pollinator behaviour and resource limitation maintain honest floral signalling. Funct. Ecol. 2021, 35, 2536–2549. [Google Scholar] [CrossRef]
- Yuan, Y.; Byers, K.J.; Bradshaw, H.D. The genetic control of flower-pollinator specificity. Curr. Opin. Plant Biol. 2013, 16, 422–428. [Google Scholar] [CrossRef]
- Rose, J.P.; Sytsma, K.J. Complex interactions underlie the correlated evolution of floral traits and their association with pollinators in a clade with diverse pollination systems. Evolution 2021, 75, 1431–1449. [Google Scholar] [CrossRef]
- Feigs, J.T.; Holzhauer, S.I.; Huang, S.; Brunet, J.; Diekmann, M.; Hedwall, P.; Kramp, K.; Naaf, T. Pollinator movement activity influences genetic diversity and differentiation of spatially isolated populations of clonal forest herbs. Front. Ecol. Evol. 2022, 10, 908258. [Google Scholar] [CrossRef]
- Opedal, Ø.H.; Pérez-Barrales, R.; Brito, V.L.; Muchhala, N.; Capó, M.; Dellinger, A.S. Pollen as the link between floral phenotype and fitness. Am. J. Bot. 2023, 110, e16200. [Google Scholar] [CrossRef]
- Barons, M.J.; Shenvi, A. Where the bee sucks—A dynamic bayesian network approach to decision support for pollinator abundance strategies. arXiv 2022, arXiv:2212.03179. [Google Scholar] [CrossRef]
- Lonsdorf, E.V.; Kremen, C.; Ricketts, T.H.; Winfree, R.; Williams, N.M.; Greenleaf, S.S. Modelling pollination services across agricultural landscapes. Ann. Bot. 2009, 103, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.P.; Salim, J.A.; Trekels, M.; Groom, Q.J.; Parr, C.S.; Soares, F.M.; Agostini, K.; Saraiva, A.; Molloy, L.; Hodson, S.; et al. Plant-pollinator interaction data: A case study of the WorldFAIR project. Biodivers. Inf. Sci. Stand. 2022, 6, e94310. [Google Scholar] [CrossRef]
- Salim, J.A.; Zermoglio, P.F.; Drucker, D.P.; Soares, F.M.; Saraiva, A.M.; Agostini, K.; Freitas, L.; Wolowski, M.; Rech, A.R.; Maués, M.M.; et al. Plant-pollinator vocabulary—A contribution to interaction data standardization. Biodivers. Inf. Sci. Stand. 2021, 5, e75636. [Google Scholar] [CrossRef]
- Salim, J.A.; Saraiva, A.M.; Zermoglio, P.F.; Agostini, K.; Wolowski, M.; Drucker, D.P.; Soares, F.M.; Bergamo, P.J.; Varassin, I.G.; Freitas, L.; et al. Data standardization of plant–pollinator interactions. GigaScience 2022, 11, giac043. [Google Scholar] [CrossRef] [PubMed]
- Hulens, D.; Van Ranst, W.; Cao, Y.; Goedemé, T. Autonomous Visual Navigation for a Flower Pollination Drone. Machines 2022, 10, 364. [Google Scholar] [CrossRef]


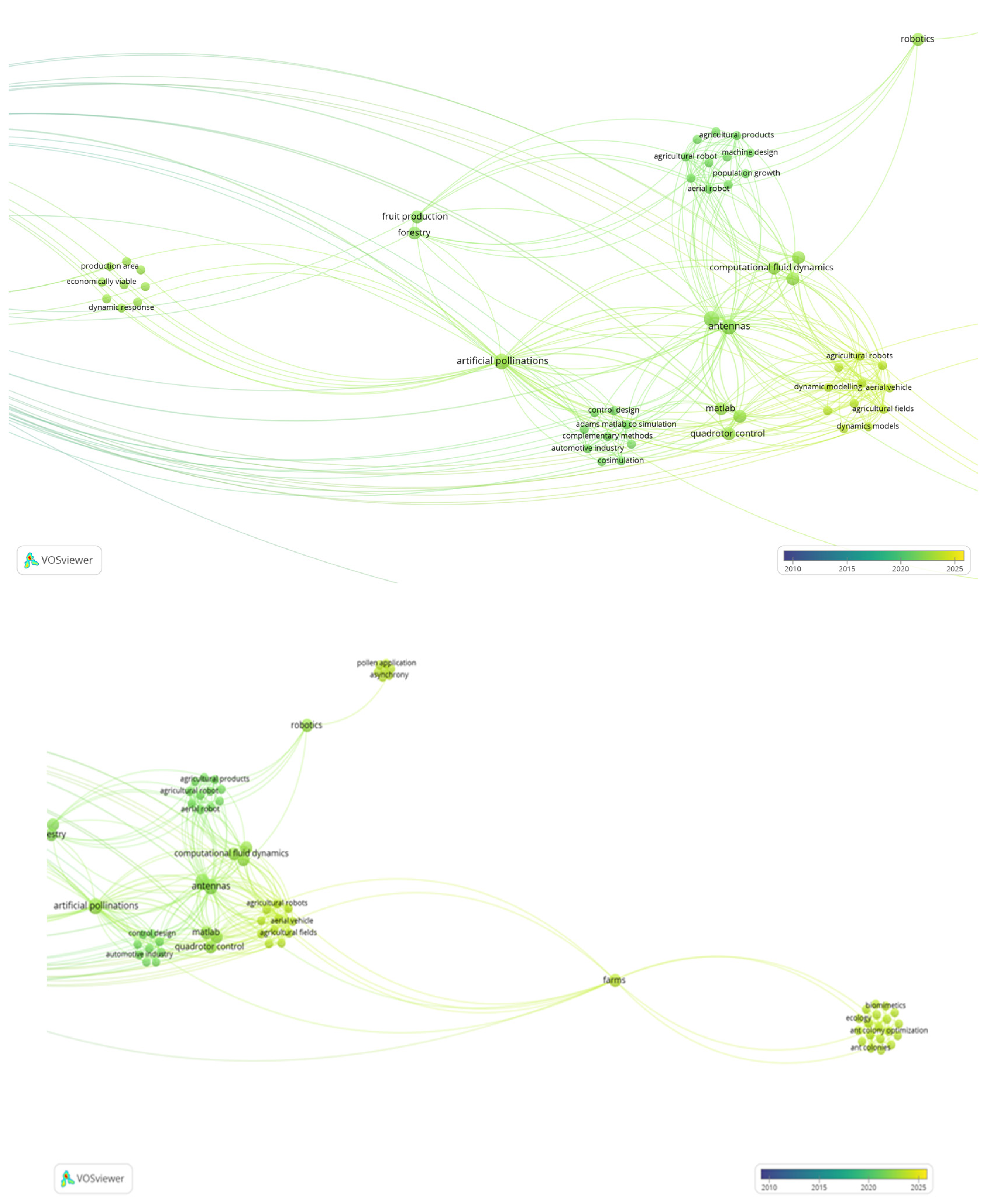
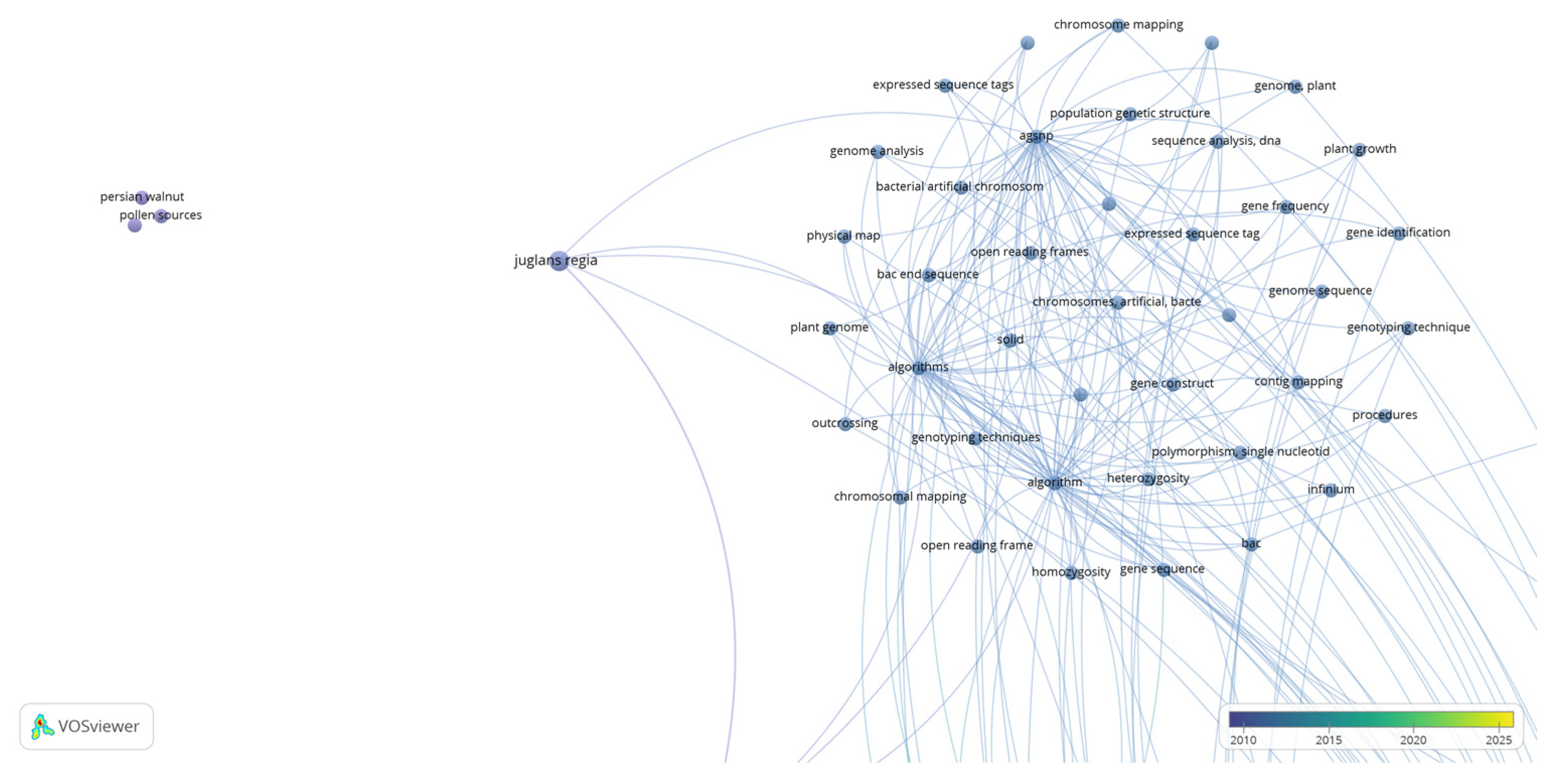
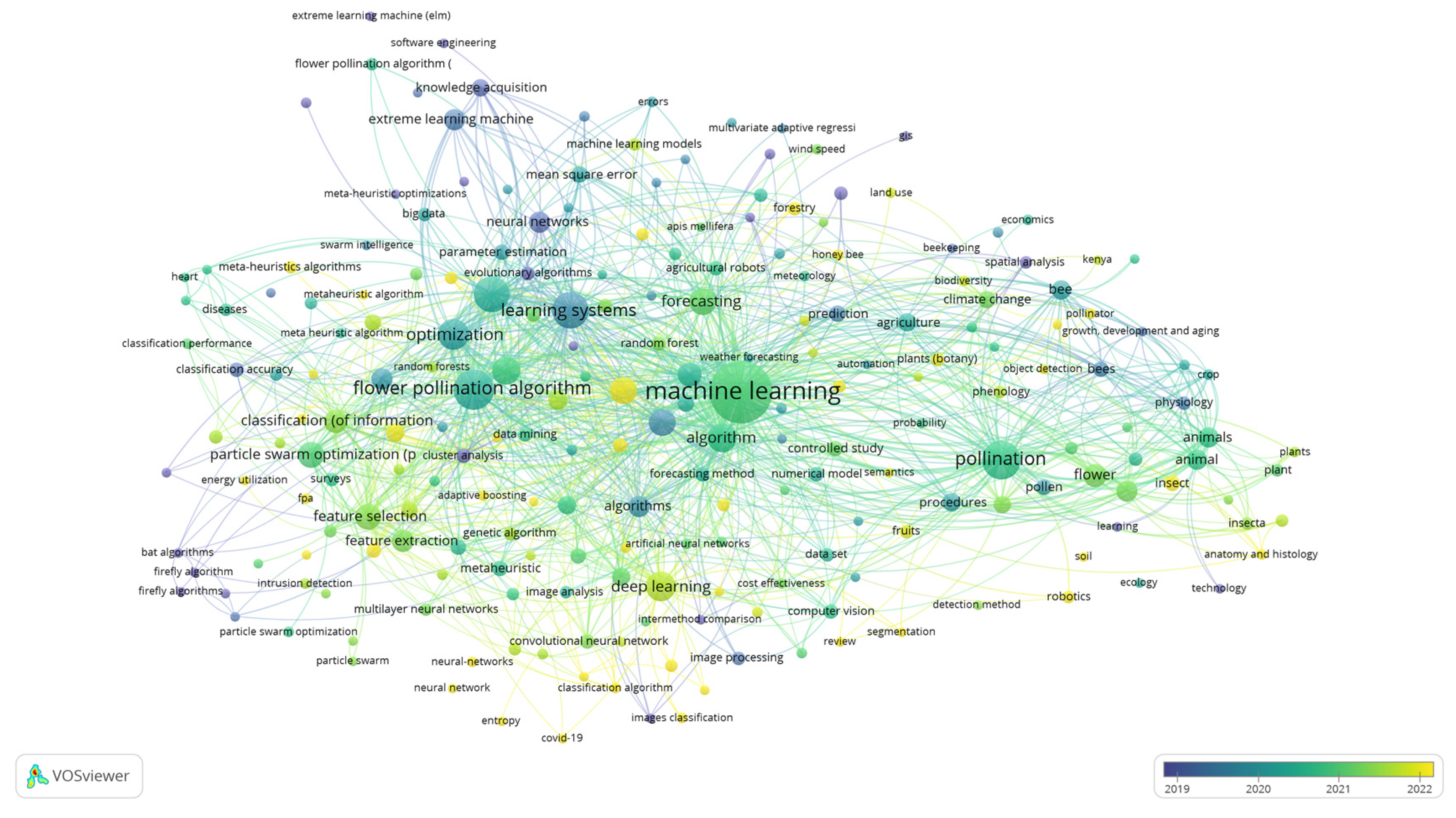

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manthos, I.; Sotiropoulos, T.; Vagelas, I. Is the Artificial Pollination of Walnut Trees with Drones Able to Minimize the Presence of Xanthomonas arboricola pv. juglandis? A Review. Appl. Sci. 2024, 14, 2732. https://doi.org/10.3390/app14072732
Manthos I, Sotiropoulos T, Vagelas I. Is the Artificial Pollination of Walnut Trees with Drones Able to Minimize the Presence of Xanthomonas arboricola pv. juglandis? A Review. Applied Sciences. 2024; 14(7):2732. https://doi.org/10.3390/app14072732
Chicago/Turabian StyleManthos, Ioannis, Thomas Sotiropoulos, and Ioannis Vagelas. 2024. "Is the Artificial Pollination of Walnut Trees with Drones Able to Minimize the Presence of Xanthomonas arboricola pv. juglandis? A Review" Applied Sciences 14, no. 7: 2732. https://doi.org/10.3390/app14072732
APA StyleManthos, I., Sotiropoulos, T., & Vagelas, I. (2024). Is the Artificial Pollination of Walnut Trees with Drones Able to Minimize the Presence of Xanthomonas arboricola pv. juglandis? A Review. Applied Sciences, 14(7), 2732. https://doi.org/10.3390/app14072732






