Bioflocculant Producing Bacillus megaterium from Poultry Slaughterhouse Wastewater: Elucidation of Flocculation Efficacy and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Isolation
2.2. Bioflocculant Production
2.3. Flocculation Activity
- A = absorbance of control, and
- B = absorbance of sample.
2.4. Bioflocculant Extraction and Purification
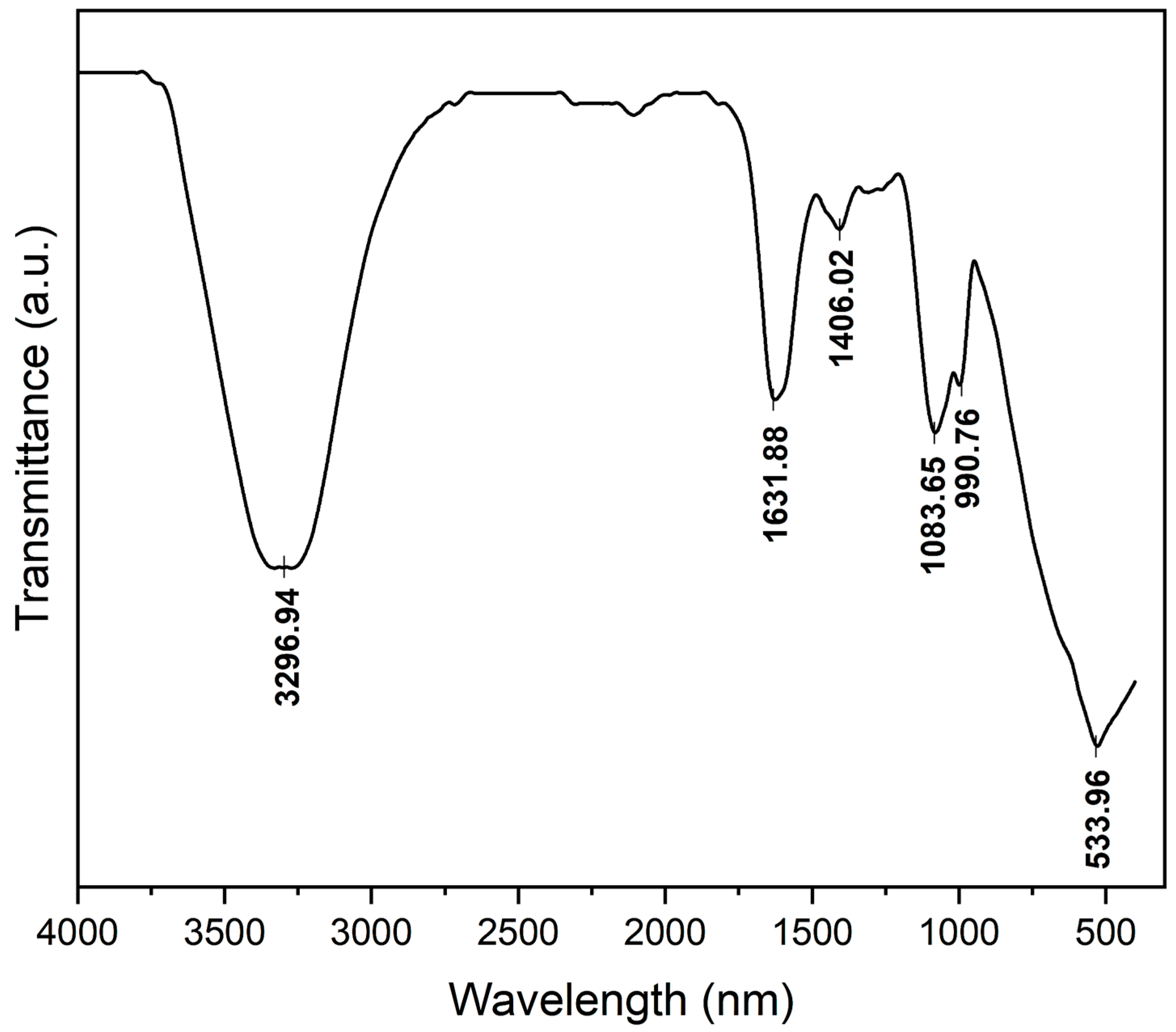
2.5. Characterization of Bioflocculant Produced by FTIR
2.6. Bioflocculants Stability Analysis
2.7. Response Surface Methodology (RSM) Experimental Design
2.8. Zeta Potential
- Kaolin clay before pH adjustments at various pHs,
- Kaolin clay suspension with CaCl2 at various pHs, and
- Kaolin clay suspension with CaCl2 and bioflocculants at various pHs and dosages.
2.9. Bonding Type Determination
3. Results and Discussion
3.1. Identification and Characterization of the Microbes
3.2. Characterization of the Produced Bioflocculant
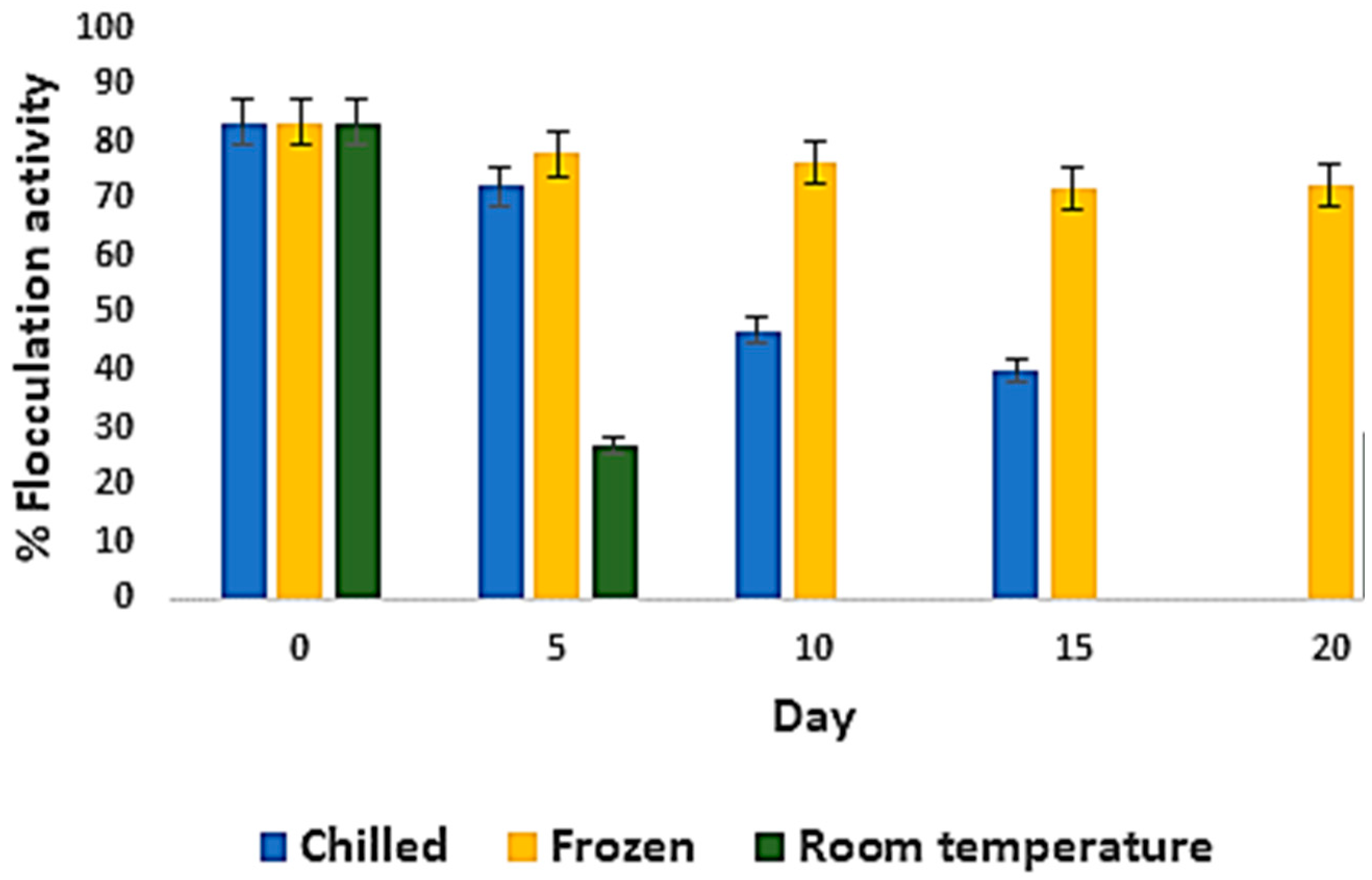
3.3. Effect of Storage Conditions
3.4. Zeta Potential
| Conditions | Zeta Potential (mV) | |||
|---|---|---|---|---|
| pH | Bioflocculant (% v/v) | Kaolin | Kaolin/CaCl2 | Kaolin/CaCl2/Bioflocculants |
| 2.96 | 2 | −19.8 | −4.64 | −16.9 |
| 4 | 1 | −31.7 | −11.1 | −20.4 |
| 4 | 3 | −21.6 | ||
| 6.5 | 0.59 | −41.2 | −20.3 | −21.0 |
| 6.5 | 2 | −22.6 | ||
| 6.5 | 3.41 | −21.4 | ||
| 9 | 1 | −44.0 | −17.9 | −21.1 |
| 9 | 3 | −20.6 | ||
| 10.04 | 2 | −48.3 | −19.7 | −21.2 |
3.5. Confirmation of Flocculation Mechanism through Bonding Type Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, X.; Li, Y.; Tang, X.; Jiang, J.; He, Q.; Xiong, Z.; Zheng, H. Biopolymer-based flocculants: A review of recent technologies. Environ. Sci. Pollut. Res. 2021, 28, 46934–46963. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Dong, Z.; Sun, D.; Dong, Q.; Wang, S.; Zhu, J.; Liu, C. Production of bioflocculant using feather waste as nitrogen source and its use in recycling of straw ash-washing wastewater with low-density and high pH property. Chemosphere 2020, 252, 126495. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, R.A.; Auta, S.H.; Jagaba, A. Laboratory guided production of bioflocculant by microorganisms isolated from water stored in clay pot. In Proceedings of the 3rd International Annual Symposium of the Biotechnology Society of Nigeria South-East Zone, Abakaliki, Nigeria, 15–16 February 2023. [Google Scholar]
- Bakar, S.N.H.A.; Hasan, H.A.; Abdullah, S.R.S.; Kasan, N.A.; Muhamad, M.H.; Kurniawan, S.B. A review of the production process of bacteria-based polymeric flocculants. J. Water Process Eng. 2021, 40, 101915. [Google Scholar] [CrossRef]
- Selepe, T.N.; Akanbi, R.; Maliehe, T.S.; Moganedi, K.; Masoko, P. Flocculating activity of a bioflocculant from Bacillus megaterium BMBF in treatment of domestic and coal mine wastewater. Appl. Sci. 2022, 12, 8312. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Ekundayo, T.C.; Okoh, A.I. Global research trends on bioflocculant potentials in wastewater remediation from 1990 to 2019 using a bibliometric approach. Lett. Appl. Microbiol. 2020, 71, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liang, J.; Liu, Y.; Zhang, Y.; Ma, P.; Pan, Z.; Jiang, W. Production of a bioflocculant from Enterobacter sp. P3 using brewery wastewater as substrate and its application in fracturing flowback water treatment. Environ. Sci. Pollut. Res. 2020, 27, 18242–18253. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, J.N.; Wan Dagang, W.R.Z. Culture optimization for production and characterization of bioflocculant by Aspergillus flavus grown on chicken viscera hydrolysate. World J. Microbiol. Biotechnol. 2019, 35, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gosai, H.G.; Narolkar, S. Isolation, Characterization and Optimization of Bioflocculant Producing Bacteria from the Aquaculture Ponds. J. Emerg. Technol. Innov. Res. 2022, 1–9. [Google Scholar]
- Pagliaro, M.; Ciriminna, R.; Yusuf, M.; Eskandarinezhad, S.; Wani, I.A.; Ghahremani, M.; Nezhad, Z.R. Application of nanocellulose composites in the environmental engineering as a catalyst, flocculants, and energy storages: A review. J. Compos. Compd. 2021, 3, 114–128. [Google Scholar] [CrossRef]
- Artifon, W.; Cesca, K.; de Andrade, C.J.; de Souza, A.A.U.; de Oliveira, D. Dyestuffs from textile industry wastewaters: Trends and gaps in the use of bioflocculants. Process Biochem. 2021, 111, 181–190. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, Y.; Yang, J.; Chen, P.; Sun, Y.; Wang, M.; Ma, Y. A bioflocculant from Corynebacterium glutamicum and its application in acid mine wastewater treatment. Front. Bioeng. Biotechnol. 2023, 11, 1136473. [Google Scholar] [CrossRef] [PubMed]
- De Nardi, I.R.; Fuzi, T.P.; Del Nery, V. Performance evaluation and operating strategies of dissolved-air flotation system treating poultry slaughterhouse wastewater. Resour. Conserv. Recycl. 2008, 52, 533–544. [Google Scholar] [CrossRef]
- Del Nery, V.; Damianovic, M.H.Z.; Moura, R.B.; Pozzi, E.; Pires, E.C.; Foresti, E. Poultry slaughterhouse wastewater treatment plant for high quality effluent. Water Sci. Technol. 2016, 73, 309–316. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Othman, A.R.; Purwanti, I.F.; Imron, M.F.; Ahmad, A.; Hasan, H.A. Isolation and characterisation of bioflocculant-producing bacteria from aquaculture effluent and its performance in treating high turbid water. J. Water Process Eng. 2021, 42, 102194. [Google Scholar] [CrossRef]
- Humudat, Y.R.; Al-Naseri, S.K.; Kadhim, S.A. Production of highly efficient bacterial flocculant in water treatment. Int. J. Adv. Res. 2014, 2, 297–301. [Google Scholar]
- Mukandi, M. Modelling of a Bioflocculant Supported Dissolved Air Flotation System for Fats Oil and Grease Laden Wastewater Pretreatment. Master’s Thesis, Cape Peninsula University of Technology, Cape Town, South Africa, 2017. [Google Scholar]
- Maliehe, T.S.; Basson, A.K.; Dlamini, N.G. Removal of pollutants in mine wastewater by a non-cytotoxic polymeric bioflocculant from Alcaligenes faecalis HCB2. Int. J. Environ. Res. Public Health 2019, 16, 4001. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zou, J.; Shao, Z.; Zhang, J.; Liu, Z.; Yu, Z. Characteristics and flocculating mechanism of a novel bioflocculant HBF-3 produced by deep-sea bacterium mutant Halomonas sp. V3a’. World J. Microbiol. Biotechnol. 2010, 26, 1135–1141. [Google Scholar] [CrossRef]
- Blackwood, K.S.; Turenne, C.Y.; Harmsen, D.; Kabani, A.M. Reassessment of sequence-based targets for identification of Bacillus species. J. Clin. Microbiol. 2004, 42, 1626–1630. [Google Scholar] [CrossRef] [PubMed]
- Karthiga Devi, K.; Natarajan, K.A. Isolation and characterization of a bioflocculant from Bacillus megaterium for turbidity and arsenic removal. Min. Metall. Explor. 2015, 32, 222–229. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, Z.; Huang, X.; Du, X.; Wang, C.A.; Li, J.; Wang, L.; Xu, Q. Isolation, identification, and optimization of culture conditions of a bioflocculant-producing bacterium Bacillus megaterium SP1 and its application in aquaculture wastewater treatment. Biomed Res. Int. 2016. [Google Scholar] [CrossRef]
- Pu, L.; Zeng, Y.J.; Xu, P.; Li, F.Z.; Zong, M.H.; Yang, J.G.; Lou, W.Y. Using a novel polysaccharide BM2 produced by Bacillus megaterium strain PL8 as an efficient bioflocculant for wastewater treatment. Int. J. Biol. Macromol. 2020, 162, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.J.; Sun, M.; Sheng, G.P.; Li, Y.; Li, W.W.; Yao, R.S.; Yu, H.Q. Identification of key constituents and structure of the extracellular polymeric substances excreted by Bacillus megaterium TF10 for their flocculation capacity. Environ. Sci. Technol. 2011, 45, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Tawila, Z.M.A.; Ismail, S.; Amr, S.S.A.; Abou Elkhair, E.K. A novel efficient bioflocculant QZ-7 for the removal of heavy metals from industrial wastewater. RSC Adv. 2019, 9, 27825–27834. [Google Scholar] [CrossRef]
- Oyewole, O.A.; Jagaba, A.; Abdulhammed, A.A.; Yakubu, J.G.; Maude, A.M.; Abioye, O.P.; Adeniyi, O.D.; Egwim, E.C. Production and characterization of a bioflocculant produced by microorganisms isolated from earthen pond sludge. Bioresour. Technol. Rep. 2023, 22, 101492. [Google Scholar] [CrossRef]
- Artifon, W.; Mazur, L.P.; de Souza, A.A.U.; de Oliveira, D. Production of bioflocculants from spent brewer’s yeast and its application in the treatment of effluents with textile dyes. J. Water Process Eng. 2022, 49, 102997. [Google Scholar] [CrossRef]
- Zheng, Y.; Ye, Z.L.; Fang, X.L.; Li, Y.H.; Cai, W.M. Production and characteristics of a bioflocculant produced by Bacillus sp. F19. Bioresour. Technol. 2008, 99, 7686–7691. [Google Scholar] [CrossRef]
- Abu Tawila, Z.M.; Ismail, S.; Dadrasnia, A.; Usman, M.M. Production and characterization of a bioflocculant produced by Bacillus salmalaya 139SI-7 and its applications in wastewater treatment. Molecules 2018, 23, 2689. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, L.; Kiran, G.S.; Ramakrishna, S.; Cheng, K.Y.; Ramachandran, R.A.; Mathew, M.T.; Pruncu, C.I. Towards improving the corrosion resistance using a novel eco-friendly bioflocculant polymer produced from Bacillus sp. Mater. Today Commun. 2023, 35, 105438. [Google Scholar] [CrossRef]
- Maliehe, T.S.; Selepe, N.T.; Ntombela, G.; Simonis, J.; Basson, A.K.; Ngema, S.; Xaba, P.S.; Mpanza, F. Production and characteristics of bioflocculant TPT-1 from a consortium of Bacillus pumilus JX860616 and Alcaligenes faecalis HCB2. Afr. J. Microbiol. Res. 2016, 10, 1561–1575. [Google Scholar] [CrossRef]
- Yin, Y.J.; Tian, Z.M.; Tang, W.; Li, L.; Song, L.Y.; McElmurry, S.P. Production and characterization of high efficiency bioflocculant isolated from Klebsiella sp. ZZ-3. Bioresour. Technol. 2014, 171, 336–342. [Google Scholar] [CrossRef]
- Setlow, P. Germination of spores of Bacillus species: What we know and do not know. J. Bacteriol. 2014, 19, 1297–1305. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, L.; Zhuang, X.; Shi, J.; Wang, Y.; He, N.; Chang, Y.I. Flocculation Characterization of a Bioflocculant from Bacillus licheniformis. Ind. Eng. Chem. Res. 2015, 54, 2894–2901. [Google Scholar] [CrossRef]
- Guo, J.; Yu, J.; Xin, X.; Zou, C.; Cheng, Q.; Yang, H.; Nengzi, L. Characterization and flocculation mechanism of a bioflocculant from hydrolyzate of rice stover. Bioresour. Technol. 2015, 177, 393–397. [Google Scholar] [CrossRef]
- Yokoi, A.; Arima, T.; Hirose, J.; Hayashi, S.; Takasaki, Y. Flocculation properties of poly(γ-glutamic acid) produced by Bacillus subtilis. J. Ferment. Bioeng. 1996, 82, 84–87. [Google Scholar] [CrossRef]
- Agunbiade, M.; Oladipo, B.; Ademakinwa, A.N.; Awolusi, O.; Adesiyan, I.M.; Oyekola, O.; Ololade, O.; Ojo, A. Bioflocculant produced by Bacillus velezensis and its potential application in brewery wastewater treatment. Sci. Rep. 2022, 12, 10945. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cong, L.; Yuan, H.; Yang, J. The mechanism of kaolin clay flocculation by a cation-independent bioflocculant produced by Chryseobacterium daeguense W6. AIMS. Environ. Sci. 2015, 2, 169–179. [Google Scholar] [CrossRef]
- Xia, M.; Zhou, H.; Amanze, C.; Hu, L.; Shen, L.; Yu, R.; Liu, Y.; Chen, M.; Li, J.; Wu, X.; et al. A novel polysaccharides-based bioflocculant produced by Bacillus subtilis ZHX3 and its application in the treatment of multiple pollutants. Chemosphere 2022, 289, 133185. [Google Scholar] [CrossRef] [PubMed]
- Kijlstra, J. Double Layer Relaxation in Colloids. Doctoral Thesis, Wageningen University and Research, Wageningen, The Netherlands, 1992. [Google Scholar]
- Yukselen, Y.; Kaya, A. Zeta Potential of Kaolinite in the Presence of Alkali, Alkaline Earth and Hydrolyzable Metal Ions. Water Air Soil Pollut. 2003, 145, 155–168. [Google Scholar] [CrossRef]
- Gonzalez-Perez, A.; Hägg, K.; Duteil, F. Optimizing NOM Removal: Impact of Calcium Chloride. Sustainability 2021, 13, 6338. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O.; Aremu, O.S. Bioflocculant production and heavy metal sorption by metal resistant bacterial isolates from gold mining soil. Chemosphere 2019, 231, 113–120. [Google Scholar] [CrossRef]
- Mohamed Hatta, N.S. Physicochemical Characterisation and Flocculation Analysis of a Novel Cationic Chitosan-Like Bioflocculant from Citrobacter Youngae GTC 01314 to Improve Sludge Dewatering. Masters Thesis, Curtin University, Perth, Australia, 2021. [Google Scholar]
- Bisht, V.; Lal, B. Exploration of performance kinetics and mechanism of action of a potential novel bioflocculant BF-VB2 on clay and dye wastewater flocculation. Front. Microbiol. 2019, 10, 1288. [Google Scholar] [CrossRef] [PubMed]


| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 22.97 | 5 | 4.59 | 5.05 | 0.0281 | significant |
| A-pH | 4.18 | 1 | 4.18 | 4.59 | 0.0694 | |
| B-Biofloculant dosage | 0.2002 | 1 | 0.2002 | 0.2200 | 0.6533 | |
| AB | 0.7225 | 1 | 0.7225 | 0.7937 | 0.4026 | |
| A2 | 17.26 | 1 | 17.26 | 18.96 | 0.0033 | |
| B2 | 1.74 | 1 | 1.74 | 1.91 | 0.2094 | |
| Residual | 6.37 | 7 | 0.9103 | |||
| Lack of Fit | 6.37 | 3 | 2.12 | |||
| Pure Error | 0.0000 | 4 | 0.0000 | |||
| Corr. Total | 29.34 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukandi, M.R.; Basitere, M.; Ntwampe, S.K.O.; Chidi, B.S. Bioflocculant Producing Bacillus megaterium from Poultry Slaughterhouse Wastewater: Elucidation of Flocculation Efficacy and Mechanism. Appl. Sci. 2024, 14, 3031. https://doi.org/10.3390/app14073031
Mukandi MR, Basitere M, Ntwampe SKO, Chidi BS. Bioflocculant Producing Bacillus megaterium from Poultry Slaughterhouse Wastewater: Elucidation of Flocculation Efficacy and Mechanism. Applied Sciences. 2024; 14(7):3031. https://doi.org/10.3390/app14073031
Chicago/Turabian StyleMukandi, Melody Ruvimbo, Moses Basitere, Seteno Karabo Obed Ntwampe, and Boredi Silas Chidi. 2024. "Bioflocculant Producing Bacillus megaterium from Poultry Slaughterhouse Wastewater: Elucidation of Flocculation Efficacy and Mechanism" Applied Sciences 14, no. 7: 3031. https://doi.org/10.3390/app14073031





