Enhancing the Photocatalytic Activity of Immobilized TiO2 Using Laser-Micropatterned Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Synthesis of Photocatalysts and Coating of Substrates
2.3. Laser Microstructuring of Silicon Substrates
2.4. Characterization of Photocatalytic Surfaces
2.5. Photocatalytic Device
Selection of Photocatalytic Device Setup
2.6. Photocatalytic Degradation Experiments
Calculation of Kinetic Rate Constants
- C0: initial concentration of atrazine (μmol L−1);
- C: concentration of atrazine (μmol L−1);
- t: reaction time (min);
- kobs: observed rate constant (min−1).
2.7. Analytical Methods Used to Monitor Atrazine Degradation
3. Results and Discussion
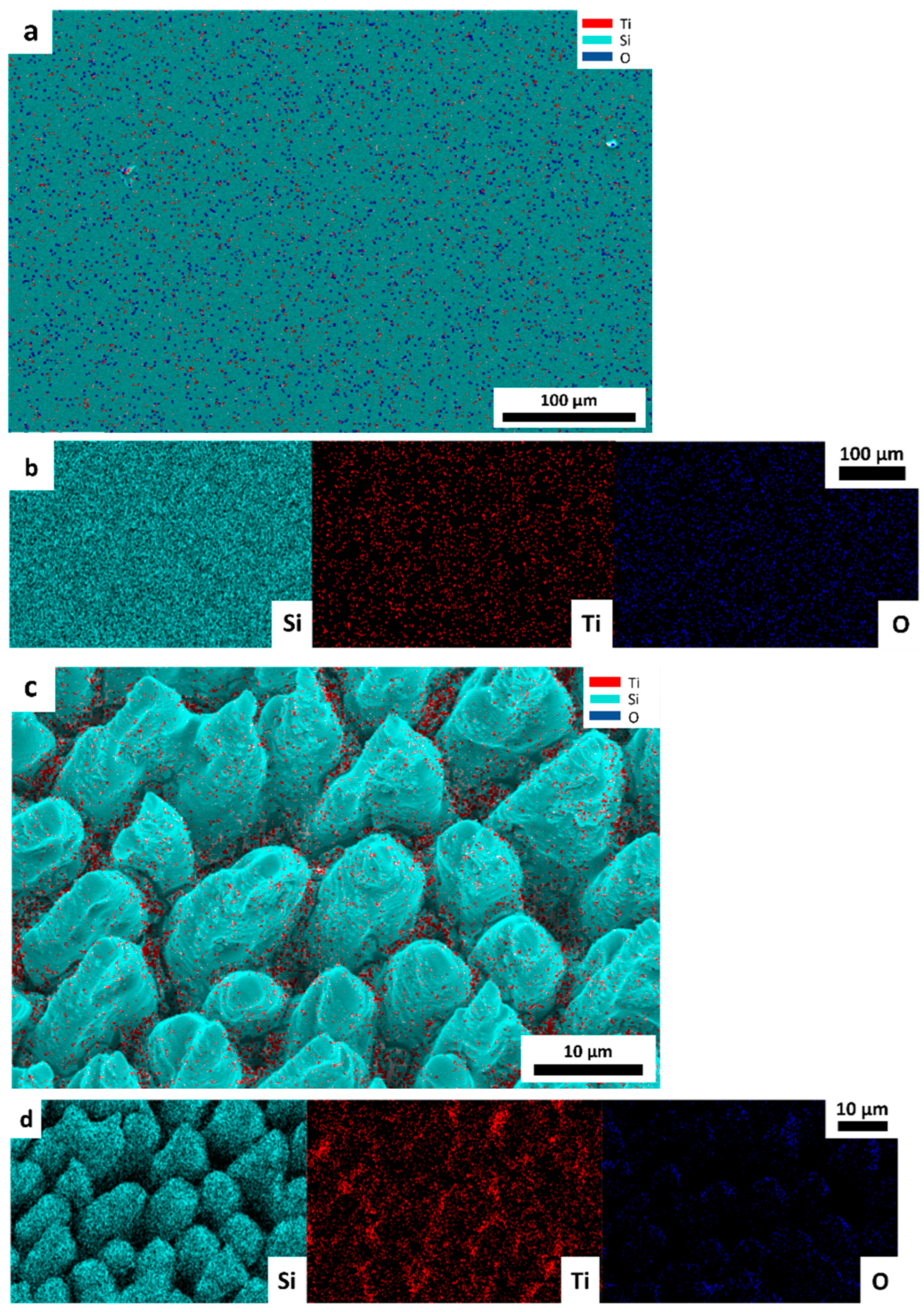
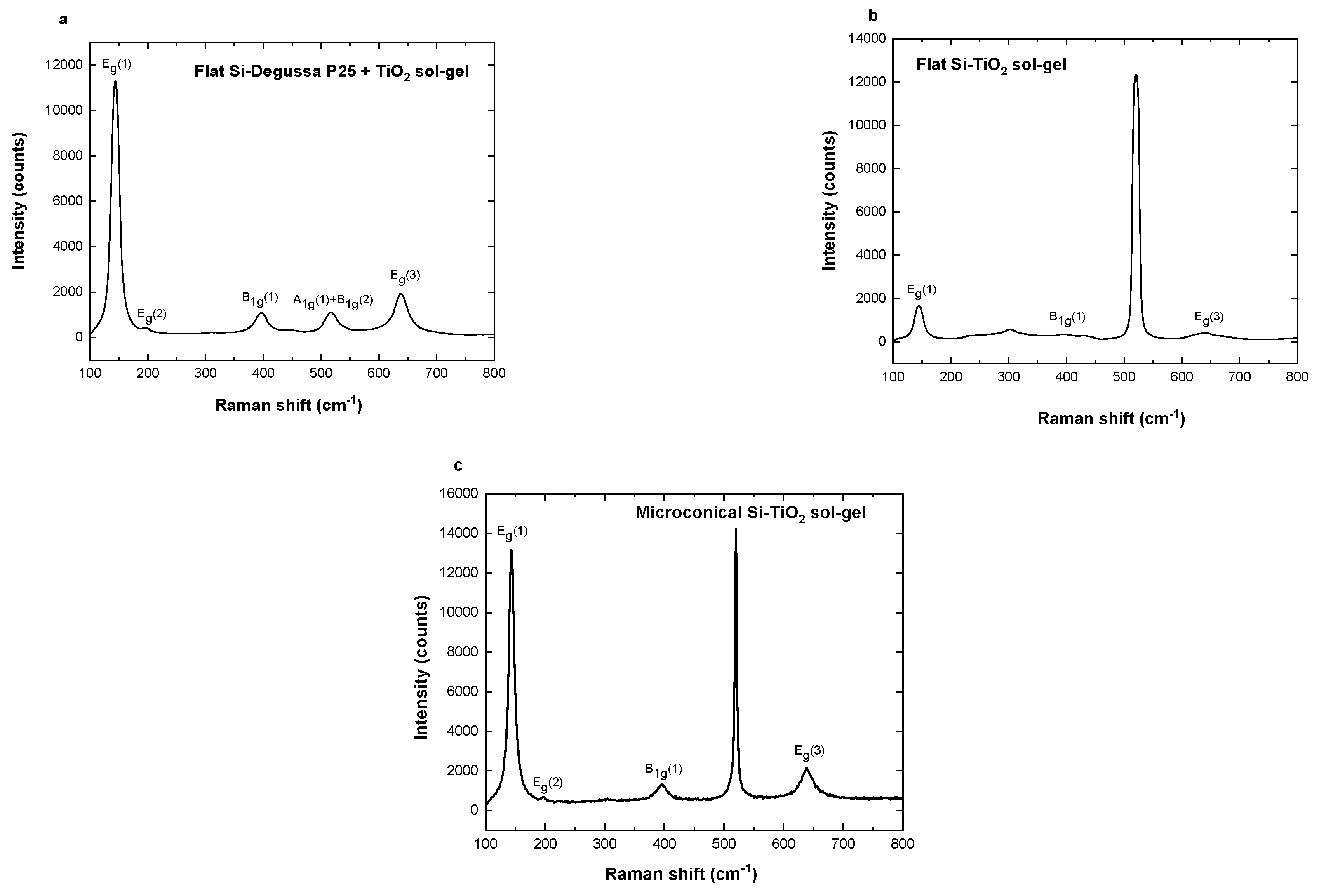
3.1. Morphological, Optical, and Structural Properties of Photocatalytic Surfaces
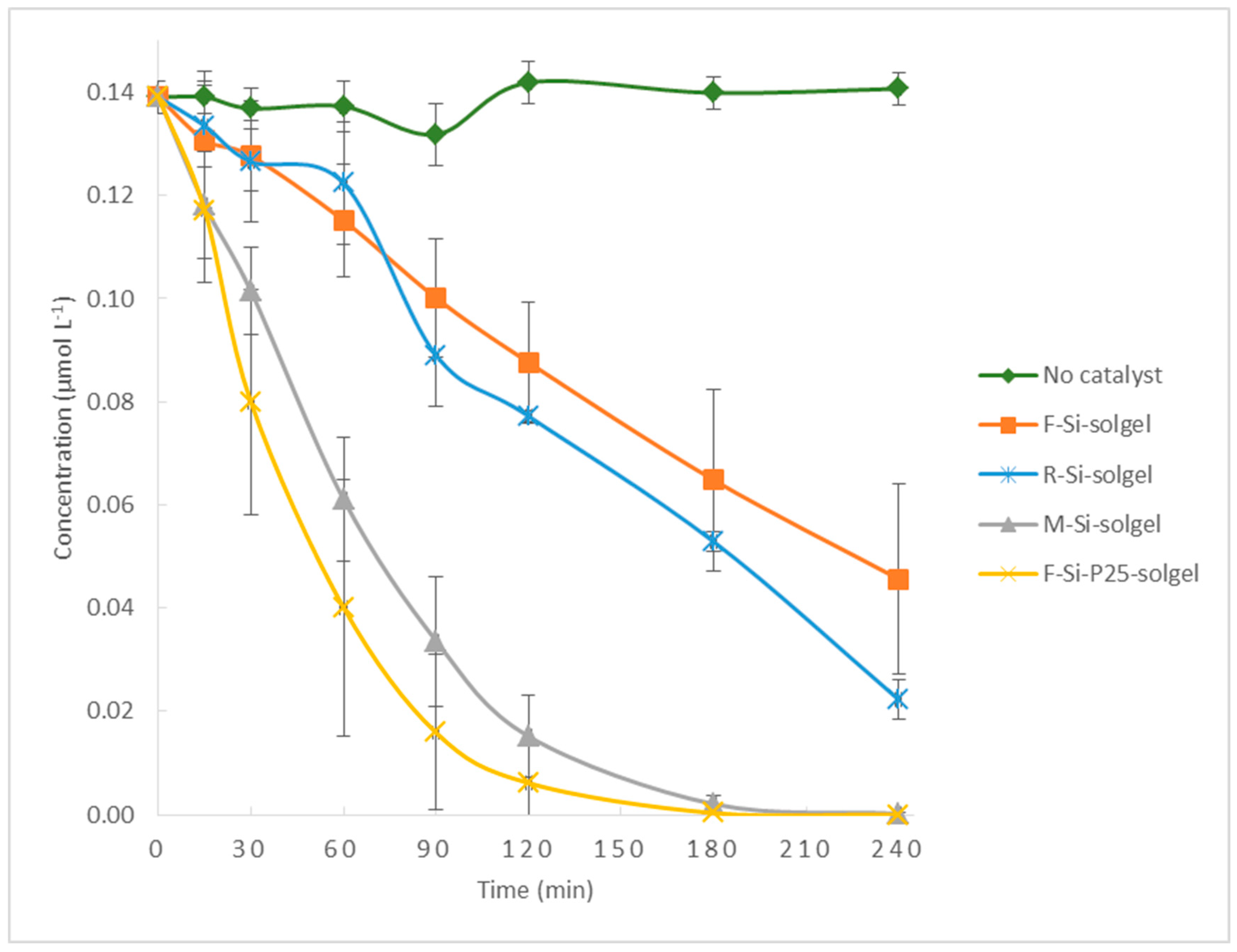
3.2. Photocatalytic Activity of Different Surfaces
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Manassero, A.; Satuf, M.L.; Alfano, O.M. Photocatalytic reactors with suspended and immobilized TiO2: Comparative efficiency evaluation. Chem. Eng. J. 2017, 326, 29–36. [Google Scholar] [CrossRef]
- Liu, N.; Ming, J.; Sharma, A.; Sun, X.; Kawazoe, N.; Chen, G.; Yang, Y. Sustainable photocatalytic disinfection of four representative pathogenic bacteria isolated from real water environment by immobilized TiO2-based composite and its mechanism. Chem. Eng. J. 2021, 426, 131217. [Google Scholar] [CrossRef]
- Kennedy, A.J.; McQueen, A.D.; Ballentine, M.L.; May, L.R.; Fernando, B.M.; Das, A.; Klaus, K.L.; Williams, C.B.; Bortner, M.J. Degradation of microcystin algal toxin by 3D printable polymer immobilized photocatalytic TiO2. Chem. Eng. J. 2023, 455, 140866. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Appunni, S.; Gopalram, K. Immobilized TiO2/chitosan beads for photocatalytic degradation of 2,4-dichlorophenoxyacetic acid. Int. J. Biol. Macromol. 2020, 161, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Décsiné Gombos, E.; Krakkó, D.; Záray, G.; Illés, Á.; Dóbé, S.; Szegedi, Á. Laponite immobilized TiO2 catalysts for photocatalytic degradation of phenols. J. Photochem. Photobiol. A Chem. 2020, 387, 112045. [Google Scholar] [CrossRef]
- Rakshit, A.; Suresh, C.A. Immobilization. In Photocatalysis; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Ali, I.; Suhail, M.; Alothman, Z.A.; Alwarthan, A. Recent advances in syntheses, properties and applications of TiO2 nanostructures. RSC Adv. 2018, 8, 30125–30147. [Google Scholar] [CrossRef]
- Iwamura, S.; Motohashi, S.; Mukai, S.R. Development of an efficient CVD technique to prepare TiO2/porous-carbon nanocomposites for high rate lithium-ion capacitors. RSC Adv. 2020, 10, 38196–38204. [Google Scholar] [CrossRef] [PubMed]
- Moslah, C.; Kandyla, M.; Mousdis, G.A.; Petropoulou, G.; Ksibi, M. Photocatalytic Properties of Titanium Dioxide Thin Films Doped with Noble Metals (Ag, Au, Pd, and Pt). Phys. Status Solidi (A) Appl. Mater. Sci. 2018, 215, 1800023. [Google Scholar] [CrossRef]
- Miao, H.; Hu, X.; Fan, J.; Li, C.; Sun, Q.; Hao, Y.; Zhang, G.; Bai, J.; Hou, X. Hydrothermal synthesis of TiO2 nanostructure films and their photoelectrochemical properties. Appl. Surf. Sci. 2015, 358, 418–424. [Google Scholar] [CrossRef]
- El Habra, N.; Visentin, F.; Russo, F.; Galenda, A.; Famengo, A.; Rancan, M.; Losurdo, M.; Armelao, L. Supported MOCVD TiO2 Thin Films Grown on Modified Stainless Steel Mesh for Sensing Applications. Nanomaterials 2023, 13, 2678. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Yuasa, H.; Okoshi, M. TiO2 thin films prepared by PLD for photocatalytic applications. Appl. Surf. Sci. 2002, 197–198, 393–397. [Google Scholar] [CrossRef]
- Obregón, S.; Rodríguez-González, V. Photocatalytic TiO2 thin films and coatings prepared by sol–gel processing: A brief review. J. Sol Gel Sci. Technol. 2022, 102, 125–141. [Google Scholar] [CrossRef]
- Nanditha, P.P.; Suji, S.K. TiO2 as a Photocatalysts for Wastewater Treatment—A Review. In Proceedings of the SSRN International Conference on Emerging Trends in Engineering, Yukthi, Kozhikode, India, 10–12 April 2023. [Google Scholar]
- Mao, H.; Zhang, F.; Du, M.; Dai, L.; Qian, Y.; Pang, H. Review on synthesis of porous TiO2-based catalysts for energy conversion systems. Ceram. Int. 2021, 47, 25177–25200. [Google Scholar] [CrossRef]
- Zhang, H.; Du, G.; Lu, W.; Cheng, L.; Zhu, X.; Jiao, Z. Porous TiO2 hollow nanospheres: Synthesis, characterization and enhanced photocatalytic properties. CrystEngComm 2012, 14, 3793–3801. [Google Scholar] [CrossRef]
- Naik, A.P.; Mittal, H.; Wadi, V.K.S.; Sane, L.; Raj, A.S.S.; Alhassan, S.M.; Al Alili, A.R.; Bhosale, S.V.; Morajkar, P.P. Super porous TiO2 photocatalyst: Tailoring the agglomerate porosity into robust structural mesoporosity with enhanced surface area for efficient remediation of azo dye polluted waste water. J. Environ. Manag. 2020, 258, 110029. [Google Scholar] [CrossRef]
- Feng, X.; Pan, F.; Tran, B.Z.; Li, Y. Photocatalytic CO2 reduction on porous TiO2 synergistically promoted by atomic layer deposited MgO overcoating and photodeposited silver nanoparticles. Catal. Today 2020, 339, 328–336. [Google Scholar] [CrossRef]
- Ramasubbu, V.; Kumar, P.; Mothi, E.M.; Karuppasamy, K.; Kim, H.S.; Maiyalagan, T.; Shajan, X.S. Highly interconnected porous TiO2-Ni-MOF composite aerogel photoanodes for high power conversion efficiency in quasi-solid dye-sensitized solar cells. Appl. Surf. Sci. 2019, 496, 143646. [Google Scholar] [CrossRef]
- Tian, H.; Wang, S.; Zhang, C.; Veder, J.P.; Pan, J.; Jaroniec, M.; Wang, L.; Liu, J. Design and synthesis of porous ZnTiO3/TiO2 nanocages with heterojunctions for enhanced photocatalytic H2 production. J. Mater. Chem. A 2017, 5, 11615–11622. [Google Scholar] [CrossRef]
- Aleni, A.H.; Kretzschmar, N.; Jansson, A.; Ituarte, I.F.; St-Pierre, L. 3D printing of dense and porous TiO2 structures. Ceram. Int. 2020, 46, 16725–16732. [Google Scholar] [CrossRef]
- Wang, D.; Yu, R.; Chen, Y.; Kumada, N.; Kinomura, N.; Takano, M. Photocatalysis property of needle-like TiO2 prepared from a novel titanium glycolate precursor. Solid State Ion. 2004, 172, 101–104. [Google Scholar] [CrossRef]
- Latif, H.; Azher, Z.; Shabbir, S.A.; Rasheed, S.; Pervaiz, E.; Sattar, A.; Imtiaz, A. A novel leaves and needles like TiO2 (LNT) electron transfer layer (ETL) as an alternative to meso-porous TiO2 electron transfer layer (ETL) in perovskite solar cell. Opt. Mater. 2020, 109, 110281. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Molhave, K.; Sun, H. Engineering the surface/interface structures of titanium dioxide micro and nano architectures towards environmental and electrochemical applications. Nanomaterials 2017, 7, 382. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.S.; Patil Girish, P.; Chavan Padmakar, G.; Sankapal Babasaheb, R. Vertically aligned TiO2 nanotubes: Highly stable electrochemical supercapacitor. J. Electroanal. Chem. 2016, 780, 197–200. [Google Scholar] [CrossRef]
- Amoako, G. Femtosecond Laser Structuring of Materials: A Review. Appl. Phys. Res. 2019, 11, 1. [Google Scholar] [CrossRef]
- Liu, X.; Coxon, P.R.; Peters, M.; Hoex, B.; Cole, J.M.; Fray, D.J. Black silicon: Fabrication methods, properties and solar energy applications. Energy Environ. Sci. 2014, 7, 3223–3263. [Google Scholar] [CrossRef]
- Lowndes, D.H.; Fowlkes, J.D.; Pedraza, A.J. Early stages of pulsed-laser growth of silicon microcolumns and microcones in air and SF6. Appl. Surf. Sci. 2000, 154–155, 647–658. [Google Scholar] [CrossRef]
- Pedraza, A.J.; Fowlkes, J.D.; Lowndes, D.H. Silicon microcolumn arrays grown by nanosecond pulsed-excimer laser irradiation. Appl. Phys. Lett. 1999, 74, 2322–2324. [Google Scholar] [CrossRef]
- Shen, M.Y.; Crouch, C.H.; Carey, J.E.; Mazur, E. Femtosecond laser-induced formation of submicrometer spikes on silicon in water. Appl. Phys. Lett. 2004, 85, 5694–5696. [Google Scholar] [CrossRef]
- Kanidi, M.; Dagkli, A.; Kelaidis, N.; Palles, D.; Aminalragia-Giamini, S.; Marquez-Velasco, J.; Colli, A.; Dimoulas, A.; Lidorikis, E.; Kandyla, M.; et al. Surface-Enhanced Raman Spectroscopy of Graphene Integrated in Plasmonic Silicon Platforms with Three-Dimensional Nanotopography. J. Phys. Chem. C 2019, 123, 3076–3087. [Google Scholar] [CrossRef]
- Tavera, T.; Perez, N.; Rodriguez, A.; Yurrita, P.; Olaizola, S.M.; Castano, E. Periodic patterning of silicon by direct nanosecond laser interference ablation. Appl. Surf. Sci. 2011, 258, 1175–1180. [Google Scholar] [CrossRef]
- Trtica, M.S.; Gakovic, B.M.; Radak, B.B.; Batani, D.; Desai, T.; Bussoli, M. Periodic surface structures on crystalline silicon created by 532 nm picosecond Nd:YAG laser pulses. Appl. Surf. Sci. 2007, 254, 1377–1381. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, T.; Zhang, P.; Zhao, Y.; Li, S. Review application of nanostructured black silicon. Nanoscale Res. Lett. 2018, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Z.; Wu, P.; Wu, S.; Hu, Y.; Zhu, W.; Li, J.; Wu, D.; Li, X.; Chu, J. One-step facile fabrication of controllable microcone and micromolar silicon arrays with tunable wettability by liquid-assisted femtosecond laser irradiation. RSC Adv. 2016, 6, 37463–37471. [Google Scholar] [CrossRef]
- Kanidi, M.; Papagiannopoulos, A.; Matei, A.; Dinescu, M.; Pispas, S.; Kandyla, M. Functional surfaces of laser-microstructured silicon coated with thermoresponsive PS/PNIPAM polymer blends: Switching reversibly between hydrophilicity and hydrophobicity. Appl. Surf. Sci. 2020, 527, 146841. [Google Scholar] [CrossRef]
- Park, J.K.; Yang, Z.; Kim, S. Black Silicon/Elastomer Composite Surface with Switchable Wettability and Adhesion between Lotus and Rose Petal Effects by Mechanical Strain. ACS Appl. Mater. Interfaces 2017, 9, 33333–33340. [Google Scholar] [CrossRef]
- Gao, X.; Guo, Z. Biomimetic Superhydrophobic Surfaces with Transition Metals and Their Oxides: A Review. J. Bionic Eng. 2017, 14, 401–439. [Google Scholar] [CrossRef]
- Hazell, G.; May, P.W.; Taylor, P.; Nobbs, A.H.; Welch, C.C.; Su, B. Studies of black silicon and black diamond as materials for antibacterial surfaces. Biomater. Sci. 2018, 6, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- EN 17120:2019; Photocatalysis—Water purification—Performance of Photocatalytic Materials by Measurement of Phenol Degradation. CEN: Brussels, Belgium, 2019.
- ISO 10676:2010; Test Method for Water Purification Performance of Semiconducting Photocatalytic Materials by Measurement of Forming Ability of Active Oxygen. ISO: London, UK, 2010.
- ISO 10678:2010; Determination of Photocatalytic Activity of Surfaces in an Aqueous Medium by Degradation of Methylene Blue. ISO: London, UK, 2010.
- Mills, A.; Hill, C.; Robertson, P.K.J. Overview of the current ISO tests for photocatalytic materials. J. Photochem. Photobiol. A Chem. 2012, 237, 7–23. [Google Scholar] [CrossRef]
- Hiskia, A.; Ecke, M.; Troupis, A.; Kokorakis, A.; Hennig, H.; Papaconstantinou, E. Sonolytic, photolytic, and photocatalytic decomposition of atrazine in the presence of polyoxometalates. Environ. Sci. Technol. 2001, 35, 2358–2364. [Google Scholar] [CrossRef] [PubMed]
- Frysali, M.A.; Papoutsakis, L.; Kenanakis, G.; Anastasiadis Spiros, H. Functional Surfaces with Photocatalytic Behavior and Reversible Wettability: ZnO Coating on Silicon Spikes. J. Phys. Chem. C 2015, 119, 25401–25407. [Google Scholar] [CrossRef]
- Priyalakshmi Devi, K.; Goswami, P.; Chaturvedi, H. Fabrication of nanocrystalline TiO2 thin films using Sol-Gel spin coating technology and investigation of its structural, morphology and optical characteristics. Appl. Surf. Sci. 2022, 591, 153226. [Google Scholar] [CrossRef]
- Starinskiy, S.V.; Rodionov, A.A.; Shukhov, Y.G.; Safonov, A.I.; Maximovskiy, E.A.; Sulyaeva, V.S.; Bulgakov, A.V. Formation of periodic superhydrophilic microstructures by infrared nanosecond laser processing of single-crystal silicon. Appl. Surf. Sci. 2020, 512, 145753. [Google Scholar] [CrossRef]
- Parra, S.; Elena Stanca, S.; Guasaquillo, I.; Ravindranathan Thampi, K. Photocatalytic degradation of atrazine using suspended and supported TiO2. Appl. Catal. B Environ. 2004, 51, 107–116. [Google Scholar] [CrossRef]
- Ijadpanah-Saravy, H.; Safari, M.; Khodadadi-Darban, A.; Rezaei, A. Synthesis of Titanium Dioxide Nanoparticles for Photocatalytic Degradation of Cyanide in Wastewater. Anal. Lett. 2014, 47, 1772–1782. [Google Scholar] [CrossRef]
- Fan, Z.; Cui, D.; Zhang, Z.; Zhao, Z.; Chen, H.; Fan, Y.; Li, P.; Zhang, Z.; Xue, C.; Yan, S. Recent progress of black silicon: From fabrications to applications. Nanomaterials 2021, 11, 41. [Google Scholar] [CrossRef]
- Crouch, C.H.; Carey, J.E.; Warrender, J.M.; Aziz, M.J.; Mazur, E.; Genin, F.Y. Comparison of structure and properties of femtosecond and nanosecond laser-structured silicon. Appl. Phys. Lett. 2004, 84, 1850–1852. [Google Scholar] [CrossRef]
- Kotsifaki, D.G.; Kandyla, M.; Lagoudakis, P.G. Near-field enhanced optical tweezers utilizing femtosecond-laser nanostructured substrates. Appl. Phys. Lett. 2015, 107, 211111. [Google Scholar] [CrossRef]
- Znad, H.; Ang, M.H.; Tade, M.O. Ta/TiO2- and Nb/TiO2-mixed oxides as efficient solar photocatalysts: Preparation, characterization, and photocatalytic activity. Int. J. Photoenergy 2012, 2012, 548158. [Google Scholar] [CrossRef]
- Subramanian, A.; Wang, H.W. Effect of hydroxyl group attachment on TiO2 films for dye-sensitized solar cells. Appl. Surf. Sci. 2012, 258, 7833–7838. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Shao, L.; Chen, C.; Liu, G.; Yang, Y. Preparation and photocatalytic properties of nanometer TiO2 thin films by improved ultrasonic spray pyrolysis. Rare Met. 2011, 30, 233–237. [Google Scholar] [CrossRef]
- Meier, A.J.; Hintze, P.E.; Devor, R.; Maloney, P.; Mazyck, D.W.; Coutts, J.L.; Bauer, B.M.; Surma, J.; Shah, M.G. Visible-Light-Responsive Photocatalysis: Ag-Doped TiO2 Catalyst Development and Reactor Design Testing. In Proceedings of the 46th International Conference on Environmental Systems, Vienna, Austria, 10–14 July 2016. [Google Scholar]
- Parker, J.H.; Feldman, D.W.; Ashkin, M. Raman Scattering by Silicon and Germanium. Phys. Rev. 1967, 155, 712–714. [Google Scholar] [CrossRef]
- Parker, J.C.; Siegel, R.W. Raman Microprobe Study of Nanophase TiO2 and Oxidation-Induced Spectral Changes. J. Mater. Res. 1990, 5, 1246–1252. [Google Scholar] [CrossRef]
- Zhang, W.F.; He, Y.L.; Zhang, S.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Liang, S.; Dong, X.; Su, M.; Sun, H. Analysis of herbicide atrazine and its degradation products in cereals by ultra-performance liquid chromatography-mass spectrometry. Anal. Methods 2016, 8, 3599–3604. [Google Scholar] [CrossRef]

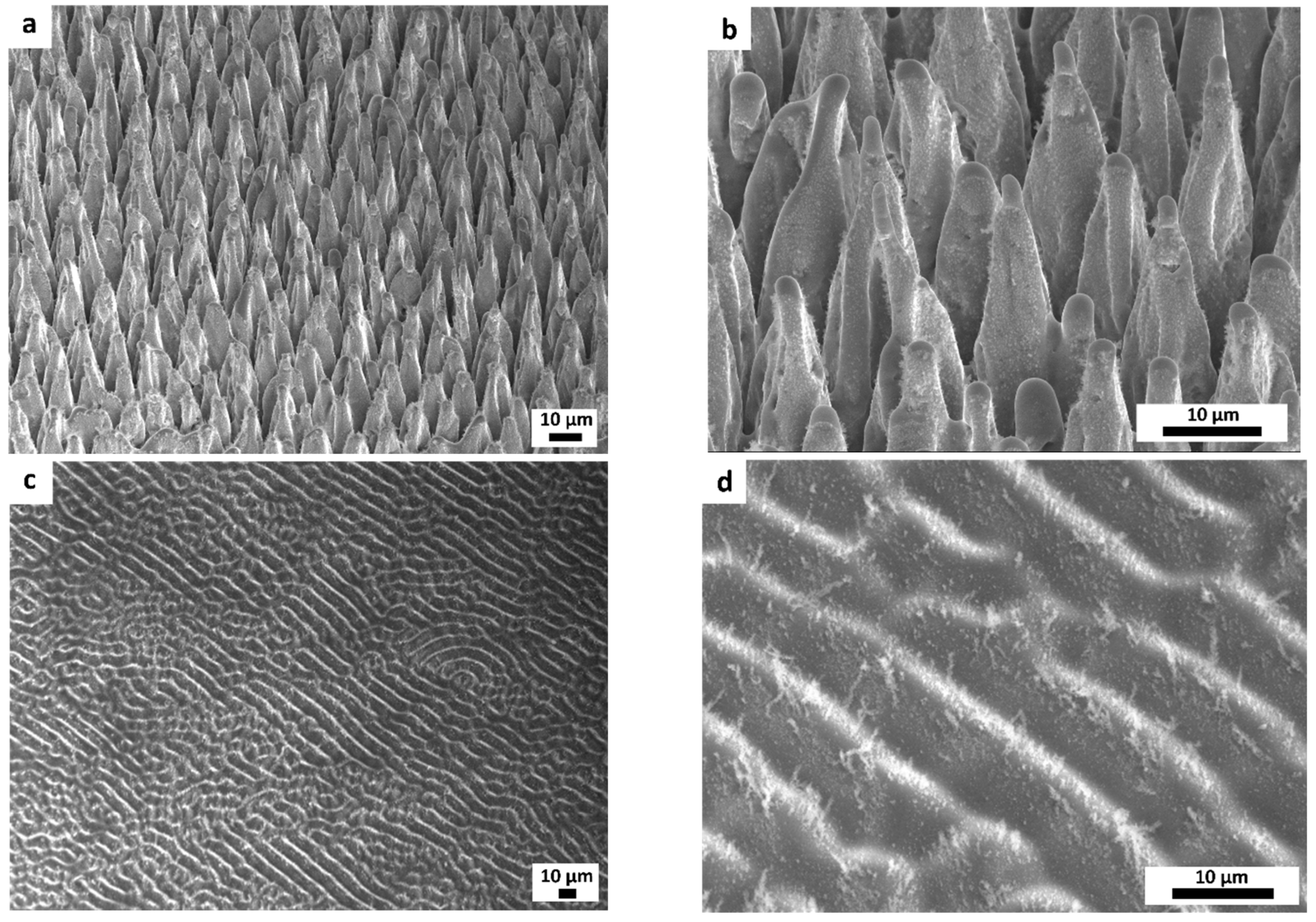
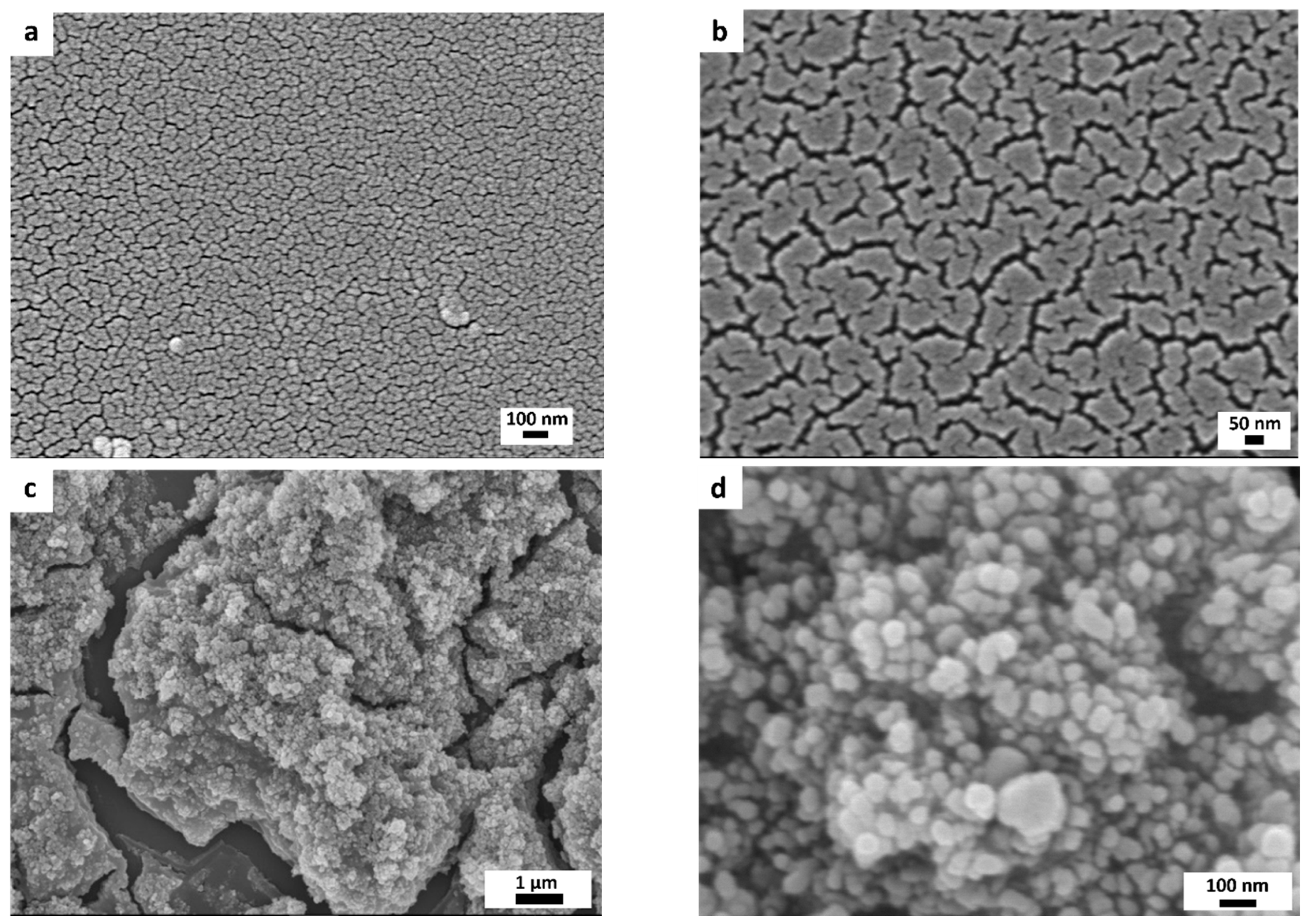

| Half-Height Diameter | Height | Distance between Neighboring Microcones |
|---|---|---|
| [μm] | [μm] | [μm] |
| 6.8 ± 0.2 | 29.0 ± 0.7 | 9.0 ± 0.4 |
| kobs (min−1) | ||||
|---|---|---|---|---|
| F-Si-solgel | F-Si-P25-solgel | R-Si-solgel | M-Si-solgel | |
| Experiment 1 | 5.1 × 10−3 | 39.1 × 10−3 | 7.8 × 10−3 | 22.5 × 10−3 |
| Experiment 2 | 3.9 × 10−3 | 43.6 × 10−3 | 6.8 × 10−3 | 18.9 × 10−3 |
| Experiment 3 | 3.6 × 10−3 | 40.7 × 10−3 | n.a. 2 | 21.3 × 10−3 |
| Experiment 4 | 8.8 × 10−3 | 17.5 × 10−3 | n.a. | 26.0 × 10−3 |
| Experiment 5 | 3.1 × 10−3 | 22.5 × 10−3 | n.a. | 31.7 × 10−3 |
| Mean value ± SD 1 | (4.9 ± 2.3) × 10−3 | (32.7 ± 11.8) × 10−3 | (7.3 ± 0.7) × 10−3 | (24.1 ± 5.0) × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakis, T.; Zervou, S.-K.; Triantis, T.M.; Christophoridis, C.; Bizani, E.; Starinskiy, S.V.; Koralli, P.; Mousdis, G.; Hiskia, A.; Kandyla, M. Enhancing the Photocatalytic Activity of Immobilized TiO2 Using Laser-Micropatterned Surfaces. Appl. Sci. 2024, 14, 3033. https://doi.org/10.3390/app14073033
Giannakis T, Zervou S-K, Triantis TM, Christophoridis C, Bizani E, Starinskiy SV, Koralli P, Mousdis G, Hiskia A, Kandyla M. Enhancing the Photocatalytic Activity of Immobilized TiO2 Using Laser-Micropatterned Surfaces. Applied Sciences. 2024; 14(7):3033. https://doi.org/10.3390/app14073033
Chicago/Turabian StyleGiannakis, Theodoros, Sevasti-Kiriaki Zervou, Theodoros M. Triantis, Christophoros Christophoridis, Erasmia Bizani, Sergey V. Starinskiy, Panagiota Koralli, Georgios Mousdis, Anastasia Hiskia, and Maria Kandyla. 2024. "Enhancing the Photocatalytic Activity of Immobilized TiO2 Using Laser-Micropatterned Surfaces" Applied Sciences 14, no. 7: 3033. https://doi.org/10.3390/app14073033





