Constructing Enhanced Composite Solid-State Electrolytes with Sb/Nb Co-Doped LLZO and PVDF-HFP
Abstract
:1. Introduction
2. Materials and Methods
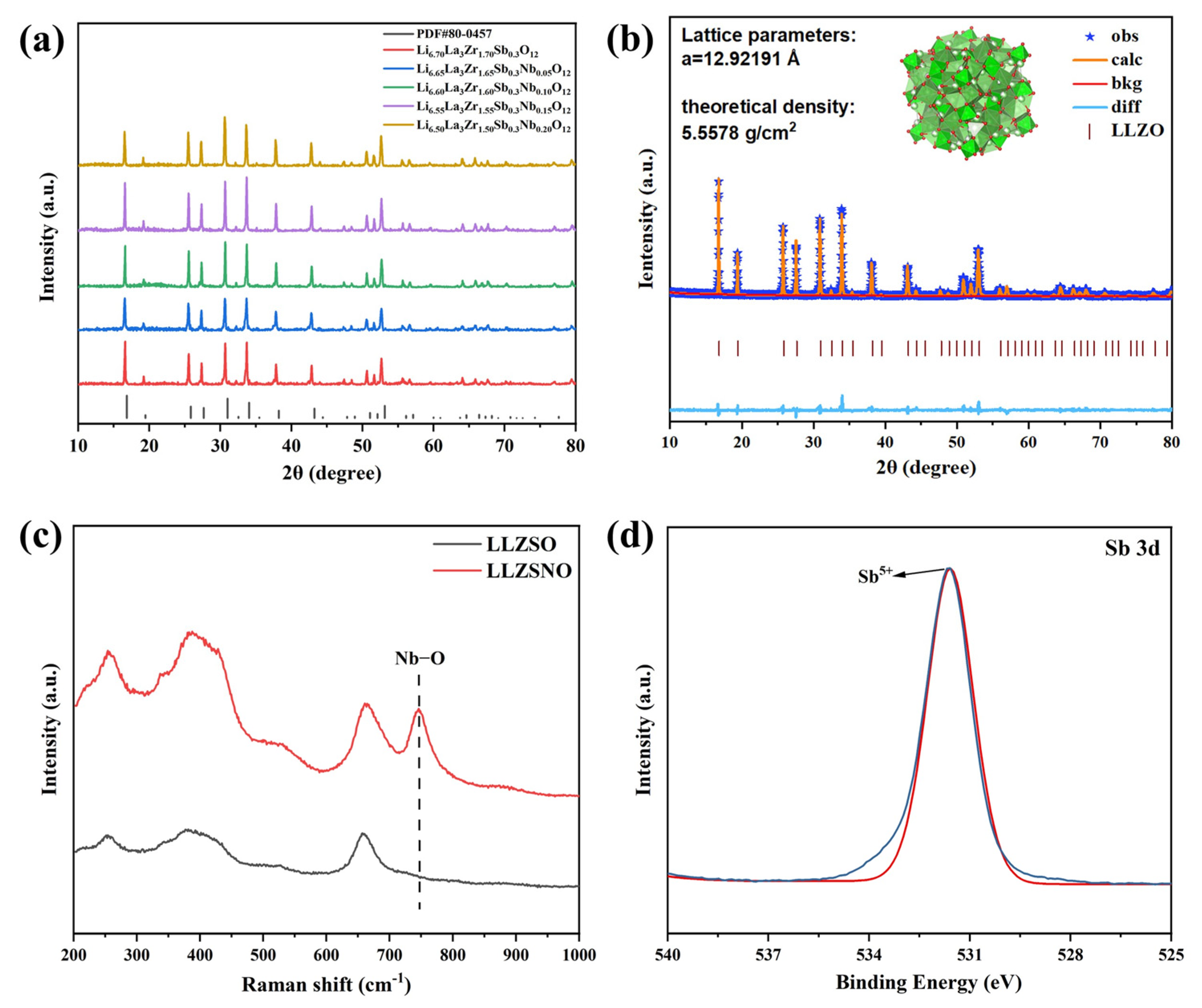
2.1. Preparation of LLZSNO
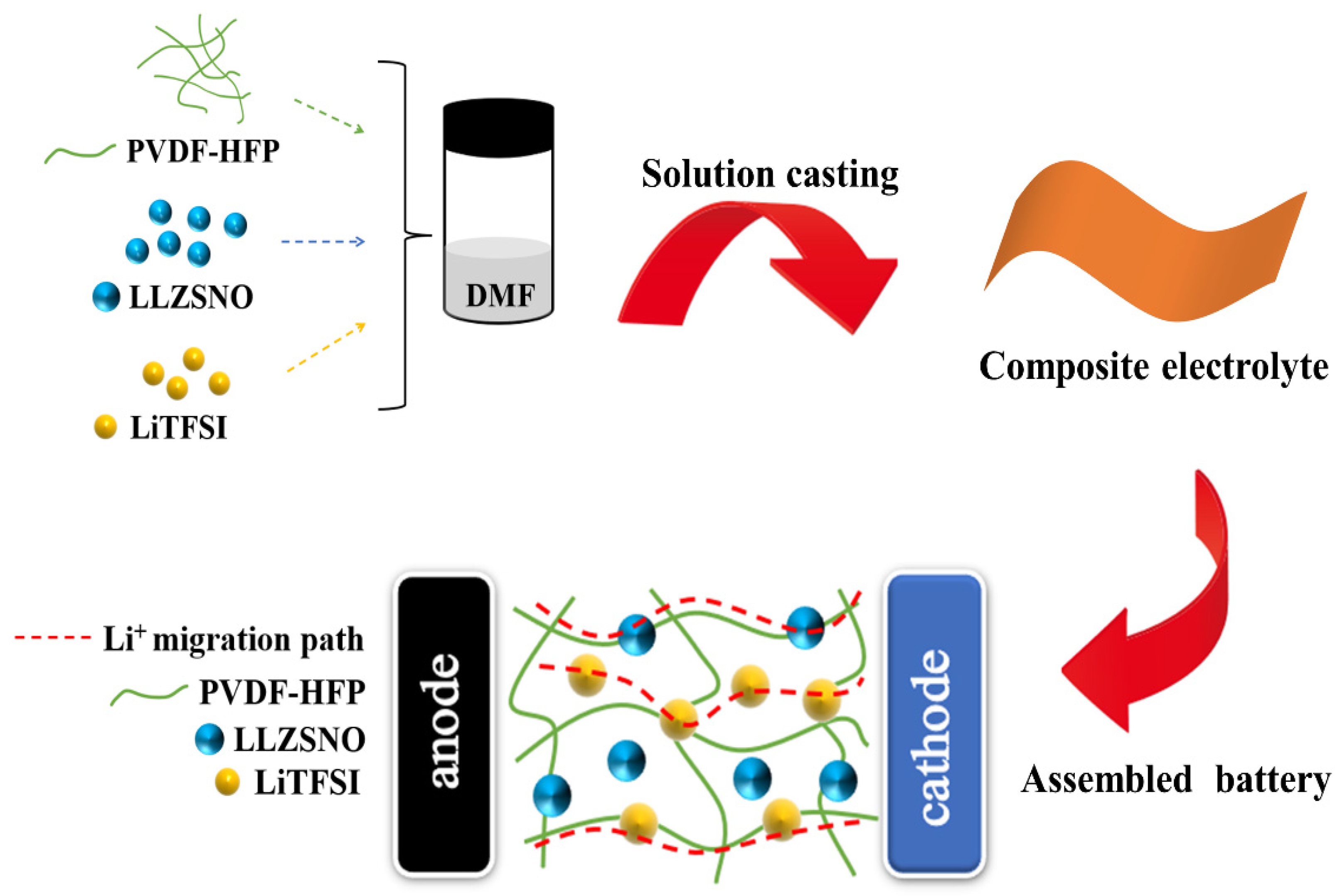
2.2. Preparation of Composite Solid Electrolyte Membranes
2.3. Battery Assembly
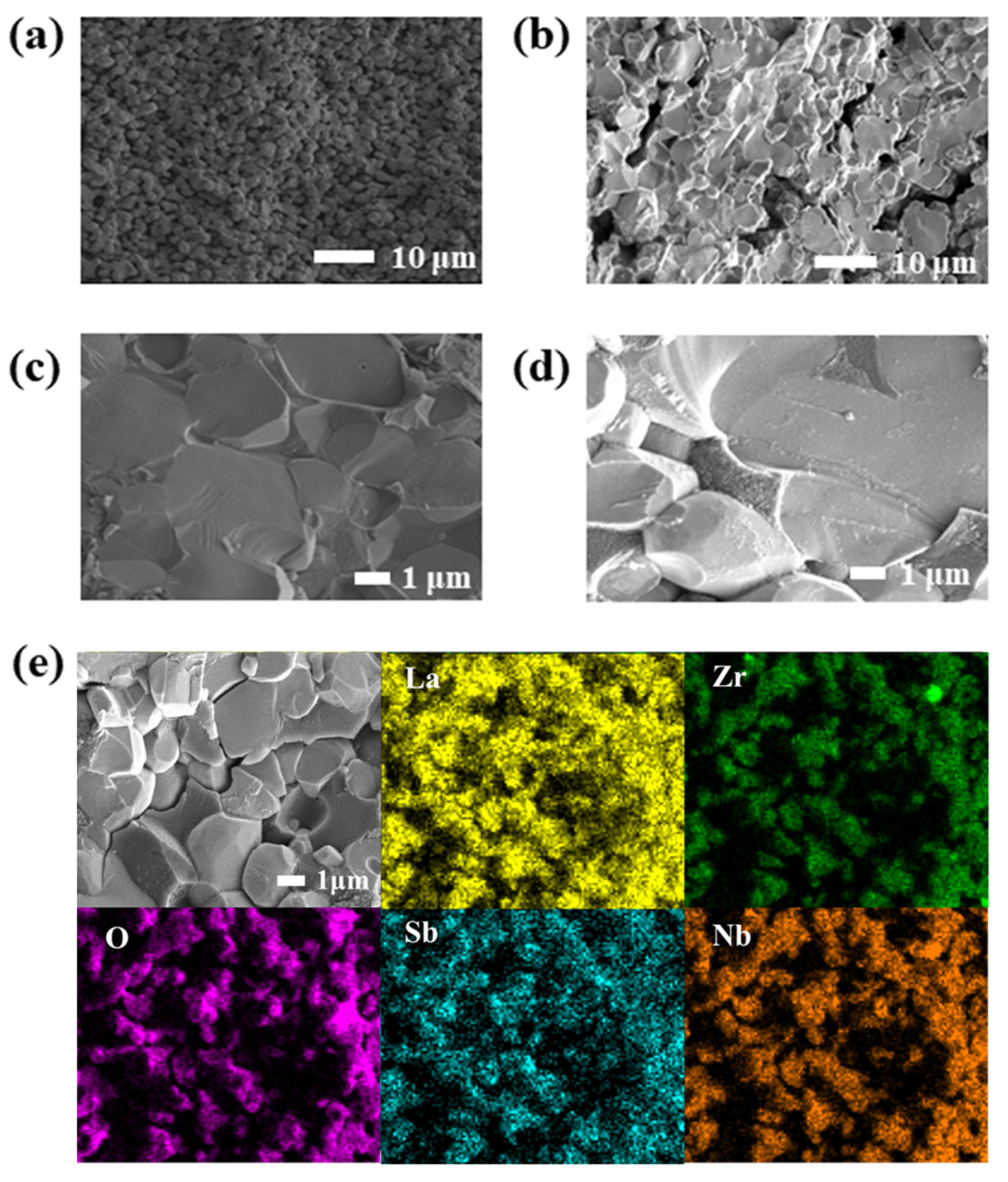
2.4. Characterization
2.5. Determination of the Relative Density of Ceramics
2.6. Electrochemical Measurement
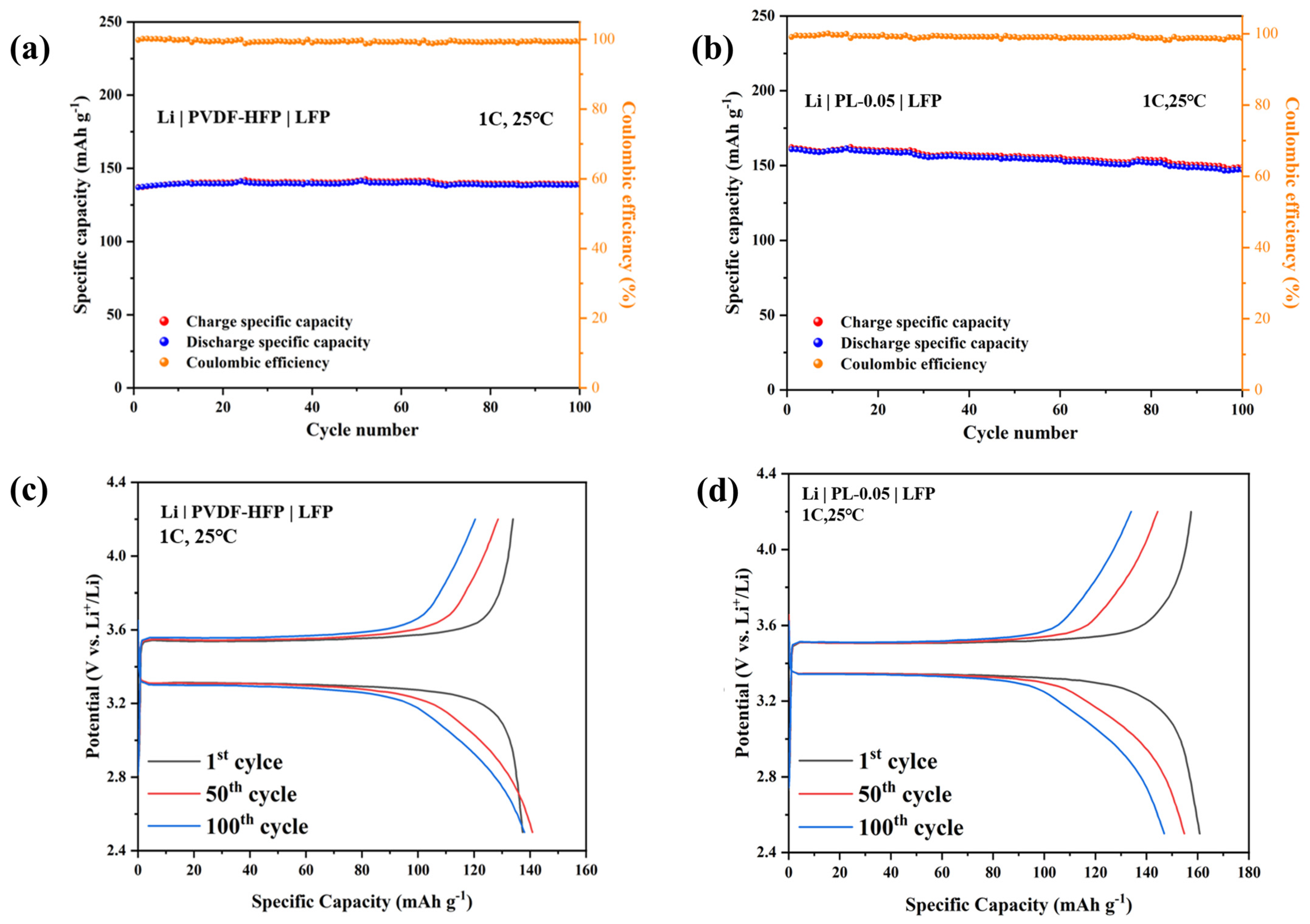
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, J.; Deng, W.; Shuai, H.; Li, S.; Xu, Z.; Li, J.; Hou, H.; Peng, H.; Zou, G. Garnet solid electrolyte for advanced all-solid-state Li batteries. Adv. Energy Mater. 2021, 11, 2000648. [Google Scholar] [CrossRef]
- Kravchyk, K.V.; Okur, F.; Kovalenko, M.V. Break-even analysis of all-solid-state batteries with Li-garnet solid electrolytes. ACS Energy Lett. 2021, 6, 2202–2207. [Google Scholar] [CrossRef]
- Liu, K.; Li, X.; Cai, J.; Yang, Z.; Chen, Z.; Key, B.; Zhang, Z.; Dzwiniel, T.L.; Liao, C. Design of high-voltage stable hybrid electrolyte with an ultrahigh Li transference number. ACS Energy Lett. 2021, 6, 1315–1323. [Google Scholar] [CrossRef]
- Lu, W.; Xue, M.; Zhang, C. Modified Li7La3Zr2O12 (LLZO) and LLZO-polymer composites for solid-state lithium batteries. Energy Storage Mater. 2021, 39, 108–129. [Google Scholar] [CrossRef]
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. R Rep. 2019, 136, 27–46. [Google Scholar] [CrossRef]
- Khan, K.; Hanif, M.B.; Xin, H.; Hussain, A.; Ali, H.G.; Fu, B.; Fang, Z.; Motola, M.; Xu, Z.; Wu, M. PEO-Based solid composite polymer electrolyte for high capacity retention all-solid-state lithium metal battery. Small 2024, 20, 2305772. [Google Scholar] [CrossRef] [PubMed]
- Kravchyk, K.V.; Kovalenko, M.V. Perspective on design and technical challenges of Li-garnet solid-state batteries. Sci. Technol. Adv. Mater. 2022, 23, 41–48. [Google Scholar] [CrossRef]
- Sanjuán, M.L.; Orera, A.; Sobrados, I.; Fuentes, A.F.; Sanz, J. Structural transition in orthorhombic Li5−xHxLa3Nb2O12 garnets induced by a concerted lithium and proton diffusion mechanism. J. Mater. Chem. A 2018, 6, 2708–2720. [Google Scholar] [CrossRef]
- Wu, J.; Liu, S.; Han, F.; Yao, X.; Wang, C. Lithium/sulfide all-solid-state batteries using sulfide electrolytes. Adv. Mater. 2021, 33, 2000751. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.; Zhou, Z.; Wang, K.; Zhai, W.; Li, Y.; Wang, J.; Wei, M. The stabilizing of 1T-MoS2 for all-solid-state lithium-ion batteries. Batteries 2022, 9, 26. [Google Scholar] [CrossRef]
- Li, M.; Zhu, W.; Zhang, P.; Chao, Y.; He, Q.; Yang, B.; Li, H.; Borisevich, A.; Dai, S. Graphene-Analogues Boron Nitride Nanosheets Confining Ionic Liquids: A High-Performance Quasi-Liquid Solid Electrolyte. Small 2016, 12, 3535–3542. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Takeiri, F.; Okamoto, K.; Saito, T.; Kamiyama, T.; Kuwabara, A.; Kobayashi, G. Electropositive Metal Doping into Lanthanum Hydride for H− Conducting Solid Electrolyte Use at Room Temperature. Adv. Energy Mater. 2023, 13, 2301993. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y. Garnet-type solid-state electrolytes: Materials, interfaces, and batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Xin, H.; Fu, B.; Hanif, M.B.; Li, P.; Beshiwork, B.A.; Fang, Z.; Motola, M.; Xu, Z.; Wu, M. Garnet/polymer solid electrolytes for high-performance solid-state lithium metal batteries: The role of amorphous Li2O2. J. Colloid Interface Sci. 2023, 642, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, S.Q.; Shen, L.; Liu, Q.; Ma, J.B.; Lv, W.; He, Y.B.; Yang, Q.H. Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-K.; Gwon, H.; Kim, H.; Yoon, G.; Shin, D.; Hong, J.; Jung, C.; Kim, J.-S. Unlocking the hidden chemical space in cubic-phase garnet solid electrolyte for efficient quasi-all-solid-state lithium batteries. Nat. Commun. 2022, 13, 7638. [Google Scholar] [CrossRef]
- Goswami, N.; Indu, M.; Murugan, R.; Kant, R. Experimental corroboration of theory for impedance response of solid electrolytes: Doped cubic garnet LLZO. J. Electroanal. Chem. 2021, 897, 115611. [Google Scholar] [CrossRef]
- Neises, J.; Scheld, W.S.; Seok, A.-R.; Lobe, S.; Finsterbusch, M.; Uhlenbruck, S.; Schmechel, R.; Benson, N. Study of thermal material properties for Ta-and Al-substituted Li7La3Zr2O12 (LLZO) solid-state electrolyte in dependency of temperature and grain size. J. Mater. Chem. A 2022, 10, 12177–12186. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.-S.; Miara, L.; Wang, Y.; Jung, S.-K.; Park, S.Y.; Song, Z.; Kim, H.; Badding, M.; Chang, J. High-energy and durable lithium metal batteries using garnet-type solid electrolytes with tailored lithium-metal compatibility. Nat. Commun. 2022, 13, 1883. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Hou, G.; Chen, Q.; Li, D.; Li, K.; Yuan, Q.; Wang, J.; Ci, L. Sheet-like garnet structure design for upgrading PEO-based electrolyte. Chem. Eng. J. 2022, 429, 132343. [Google Scholar] [CrossRef]
- Bonilla, M.R.; García Daza, F.A.; Ranque, P.; Aguesse, F.; Carrasco, J.; Akhmatskaya, E. Unveiling interfacial Li-ion dynamics in Li7La3Zr2O12/PEO (LiTFSI) composite polymer-ceramic solid electrolytes for all-solid-state lithium batteries. ACS Appl. Mater. Interfaces 2021, 13, 30653–30667. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.-Z.; He, H.; Nan, C.-W. Tailoring inorganic–polymer composites for the mass production of solid-state batteries. Nat. Rev. Mater. 2021, 6, 1003–1019. [Google Scholar] [CrossRef]
- Wu, N.; Chien, P.H.; Qian, Y.; Li, Y.; Xu, H.; Grundish, N.S.; Xu, B.; Jin, H.; Hu, Y.Y.; Yu, G. Enhanced surface interactions enable fast Li+ conduction in oxide/polymer composite electrolyte. Angew. Chem. Int. Ed. 2020, 59, 4131–4137. [Google Scholar] [CrossRef] [PubMed]
- Salimkhani, H.; Yurum, A.; Gursel, S.A. A glance at the influence of different dopant elements on Li7La3Zr2O12 garnets. Ionics 2021, 27, 3673–3698. [Google Scholar] [CrossRef]
- Liang, X.; Li, S.; Yang, G.; Wu, X.; Huang, D.; Ning, Y.; Luo, J.; Fang, Z. High lithium-ion conductivity in all-solid-state lithium batteries by Sb doping LLZO. Appl. Phys. A 2022, 128, 4. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Y.; Zhang, Q.; Zheng, Y.; Chen, H.; Guo, L. Effect of dual doping on the structure and performance of garnet-type Li7La3Zr2O12 ceramic electrolytes for solid-state lithium-ion batteries. Ceram. Int. 2019, 45, 17874–17883. [Google Scholar] [CrossRef]
- Ma, K.; Chen, B.; Li, C.-X.; Thangadurai, V. Synthesis, structure, transport properties, electrochemical stability window, and lithium plating/stripping of Mg and Nb codoped Li7La3Zr2O12 garnet-type solid electrolytes. J. Phys. Chem. C 2022, 126, 7828–7840. [Google Scholar] [CrossRef]
- Huang, X.; Xiu, T.; Badding, M.E.; Wen, Z. Two-step sintering strategy to prepare dense Li-Garnet electrolyte ceramics with high Li+ conductivity. Ceram. Int. 2018, 44, 5660–5667. [Google Scholar] [CrossRef]
- Kim, Y.; Waluyo, I.; Hunt, A.; Yildiz, B. Avoiding CO2 improves thermal stability at the Interface of Li7La3Zr2O12 electrolyte with layered oxide cathodes. Adv. Energy Mater. 2022, 12, 2102741. [Google Scholar] [CrossRef]
- Hou, H.; Cheng, L.; Richardson, T.; Chen, G.; Doeff, M.; Zheng, R.; Russo, R.; Zorba, V. Three-dimensional elemental imaging of Li-ion solid-state electrolytes using fs-laser induced breakdown spectroscopy (LIBS). J. Anal. At. Spectrom. 2015, 30, 2295–2302. [Google Scholar] [CrossRef]
- Wang, R.; Liu, F.; Duan, J.; Ren, Y.; Li, M.; Cao, J. Enhanced electrochemical performance of Al-and Nb-codoped LLZO ceramic powder and its composite solid electrolyte. ACS Appl. Energy Mater. 2021, 4, 13912–13921. [Google Scholar] [CrossRef]
- Gao, B.; Jalem, R.; Tian, H.K.; Tateyama, Y. Revealing atomic-scale ionic stability and transport around grain boundaries of garnet Li7La3Zr2O12 solid electrolyte. Adv. Energy Mater. 2022, 12, 2102151. [Google Scholar] [CrossRef]
- Chen, X.; Wang, T.; Lu, W.; Cao, T.; Xue, M.; Li, B.; Zhang, C. Synthesis of Ta and Ca doped Li7La3Zr2O12 solid-state electrolyte via simple solution method and its application in suppressing shuttle effect of Li-S battery. J. Alloys Compd. 2018, 744, 386–394. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Z.; Ciucci, F. Data mining of molecular dynamics data reveals Li diffusion characteristics in garnet Li7La3Zr2O12. Sci. Rep. 2017, 7, 40769. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Su, J.; Song, Z.; Xiu, T.; Jin, J.; Badding, M.E.; Wen, Z. Improvement of density and electrochemical performance of garnet-type Li7La3Zr2O12 for solid-state lithium metal batteries enabled by W and Ta co-doping strategy. Mater. Today Energy 2022, 27, 101034. [Google Scholar] [CrossRef]
- Liu, J.; Gao, X.; Hartley, G.O.; Rees, G.J.; Gong, C.; Richter, F.H.; Janek, J.; Xia, Y.; Robertson, A.W.; Johnson, L.R. The interface between Li6.5La3Zr1.5Ta0.5O12 and liquid electrolyte. Joule 2020, 4, 101–108. [Google Scholar] [CrossRef]
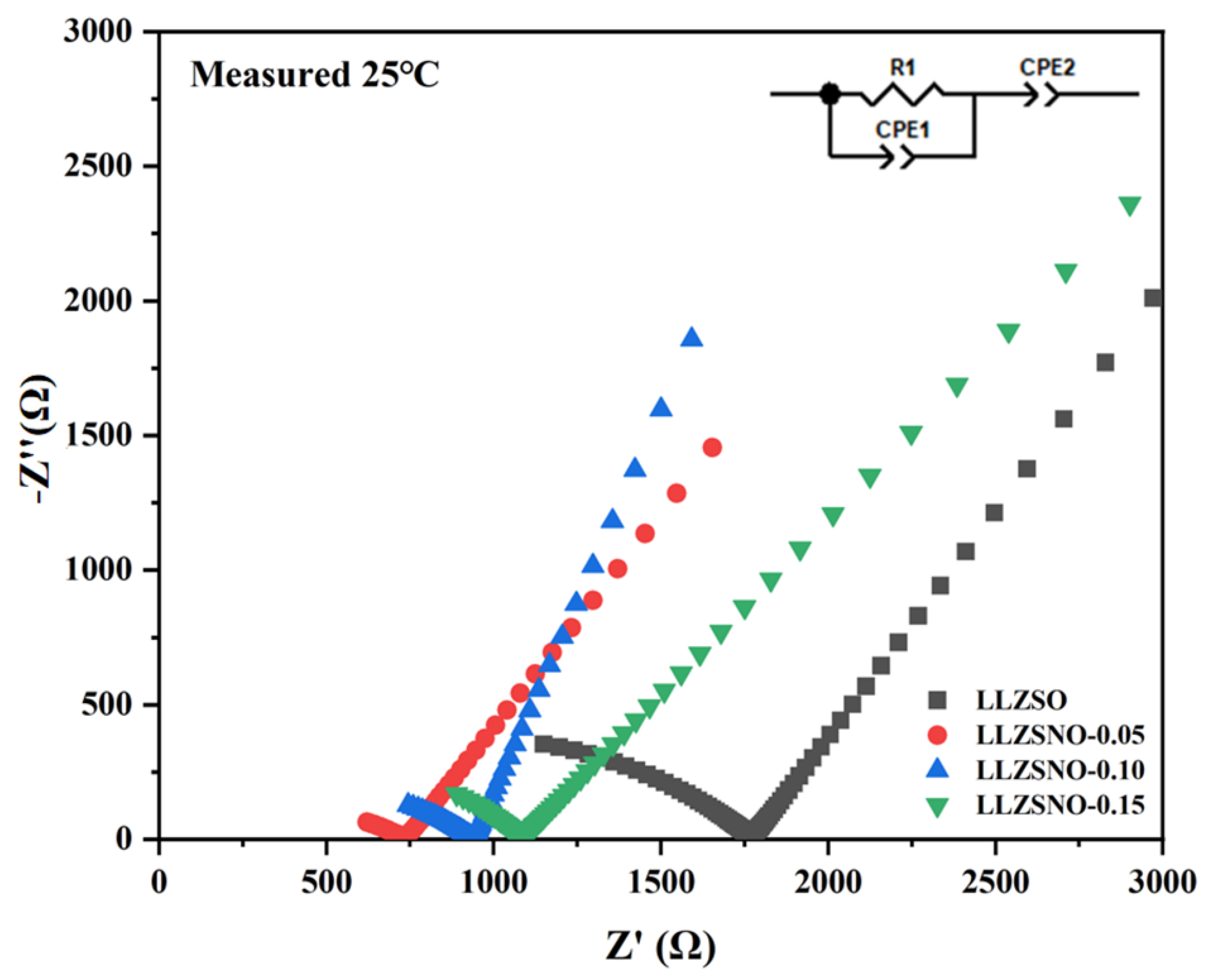
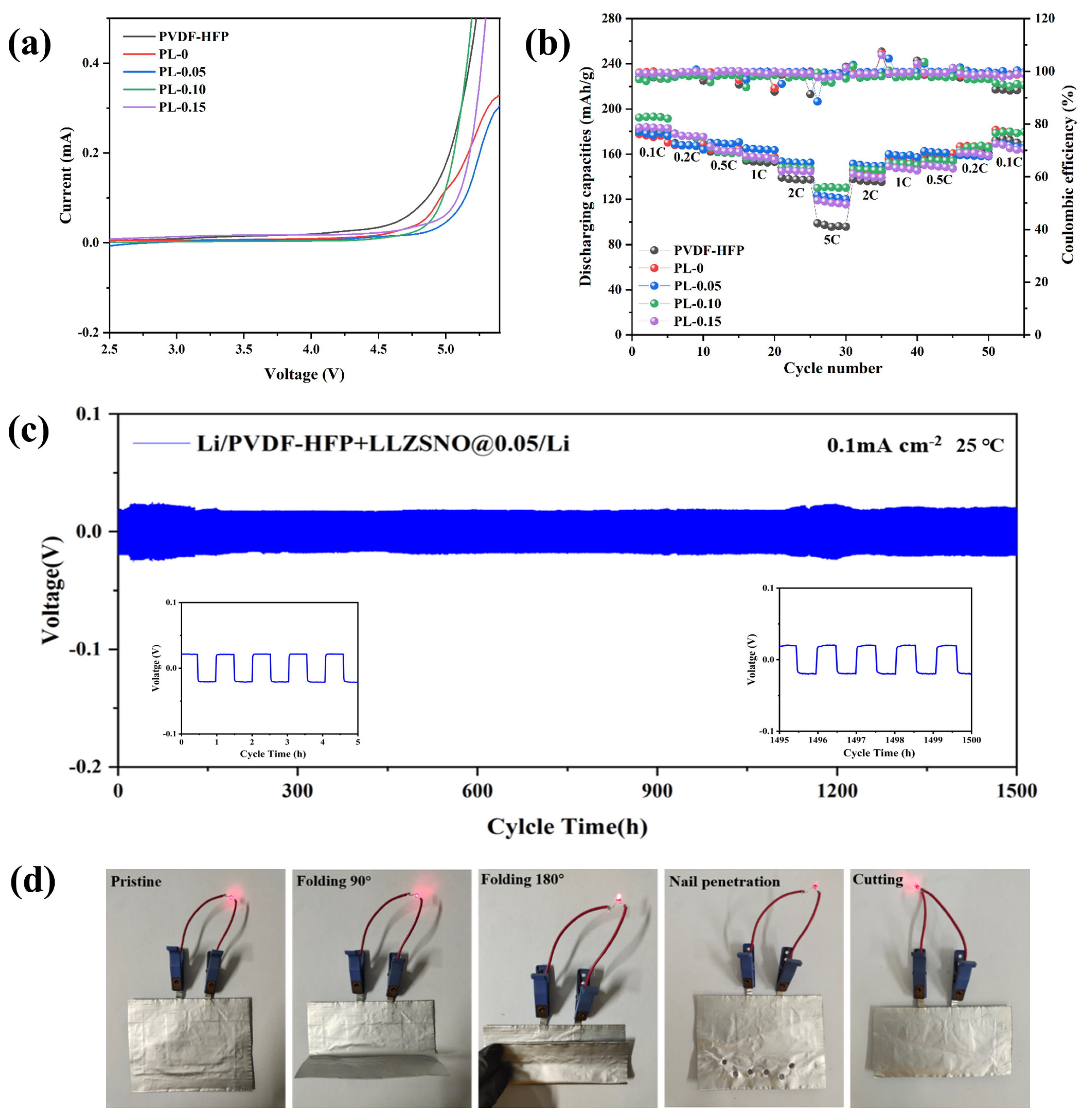
| Nb Content | Impedance (Ω) | Relative Density (%) | Ionic Conductivity (S/cm) |
|---|---|---|---|
| 0 | 1750 | 82.4 | 8.53 × 10−5 |
| 0.05 | 720 | 88.7 | 2.08 × 10−4 |
| 0.10 | 930 | 86.5 | 1.61 × 10−4 |
| 0.15 | 1080 | 87.2 | 1.38 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Liu, Y.; Tan, Y.; Chang, W.; Wu, J.; Wu, T.; Lai, C. Constructing Enhanced Composite Solid-State Electrolytes with Sb/Nb Co-Doped LLZO and PVDF-HFP. Appl. Sci. 2024, 14, 3115. https://doi.org/10.3390/app14073115
Cai J, Liu Y, Tan Y, Chang W, Wu J, Wu T, Lai C. Constructing Enhanced Composite Solid-State Electrolytes with Sb/Nb Co-Doped LLZO and PVDF-HFP. Applied Sciences. 2024; 14(7):3115. https://doi.org/10.3390/app14073115
Chicago/Turabian StyleCai, Jinhai, Yingjie Liu, Yingying Tan, Wanying Chang, Jingyi Wu, Tong Wu, and Chunyan Lai. 2024. "Constructing Enhanced Composite Solid-State Electrolytes with Sb/Nb Co-Doped LLZO and PVDF-HFP" Applied Sciences 14, no. 7: 3115. https://doi.org/10.3390/app14073115
APA StyleCai, J., Liu, Y., Tan, Y., Chang, W., Wu, J., Wu, T., & Lai, C. (2024). Constructing Enhanced Composite Solid-State Electrolytes with Sb/Nb Co-Doped LLZO and PVDF-HFP. Applied Sciences, 14(7), 3115. https://doi.org/10.3390/app14073115






