Use of Selected Plant Extracts in Controlling and Neutralizing Toxins and Sporozoites Associated with Necrotic Enteritis and Coccidiosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Clostridium Inoculum and Phytogenic Extract Preparation
2.3. Preparation of Green Tea Powder and Ginger Extracts Used
2.4. E. tenella Sporozoites Purification
2.5. E. tenella Sporozoites Killing Assay Using Phytochemicals
2.6. RNA Extraction and Quantitative Real-Time RT-PCR
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Data Analysis
3. Results
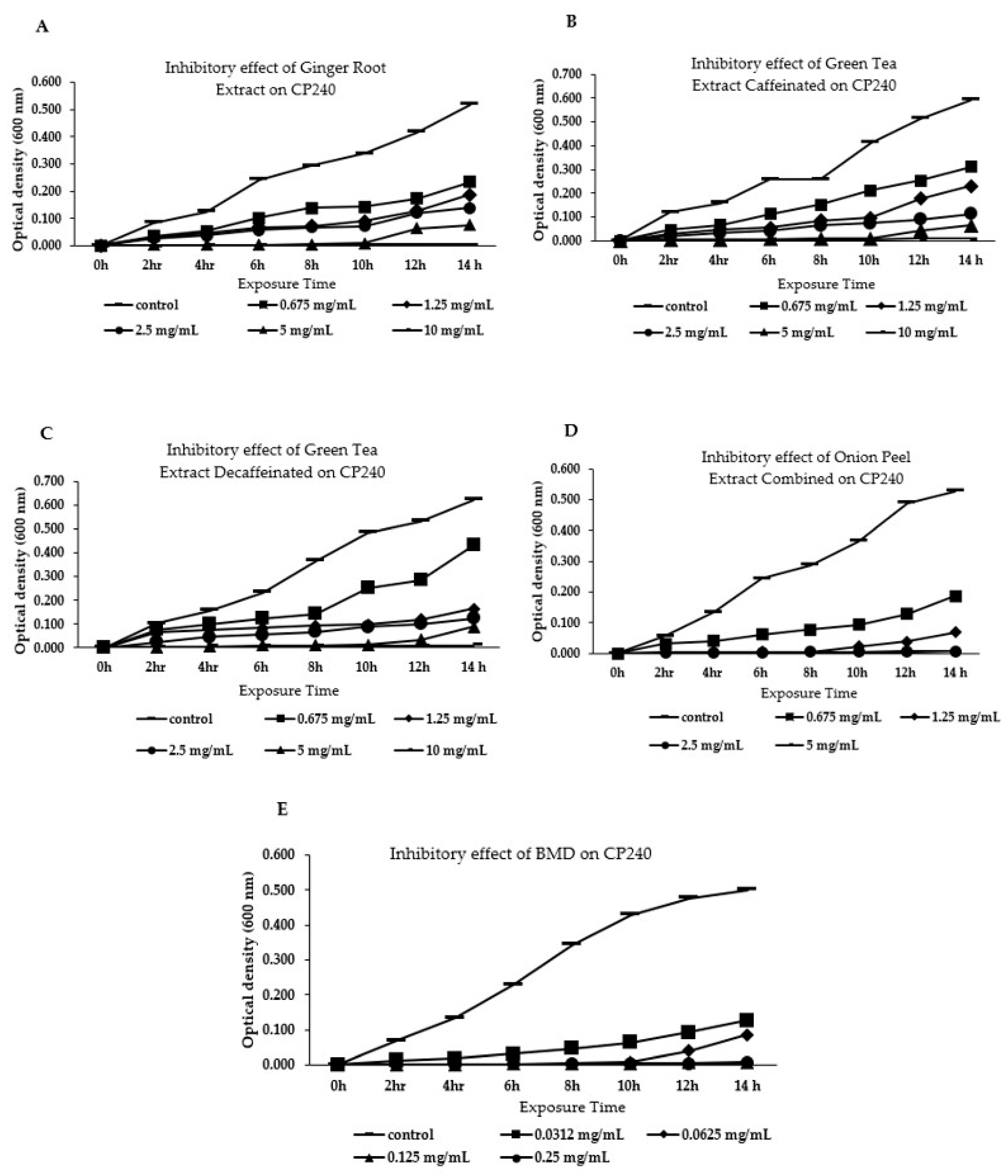
3.1. Effect of Different Phytogenic Extracts on Bacteria Growth Parameters
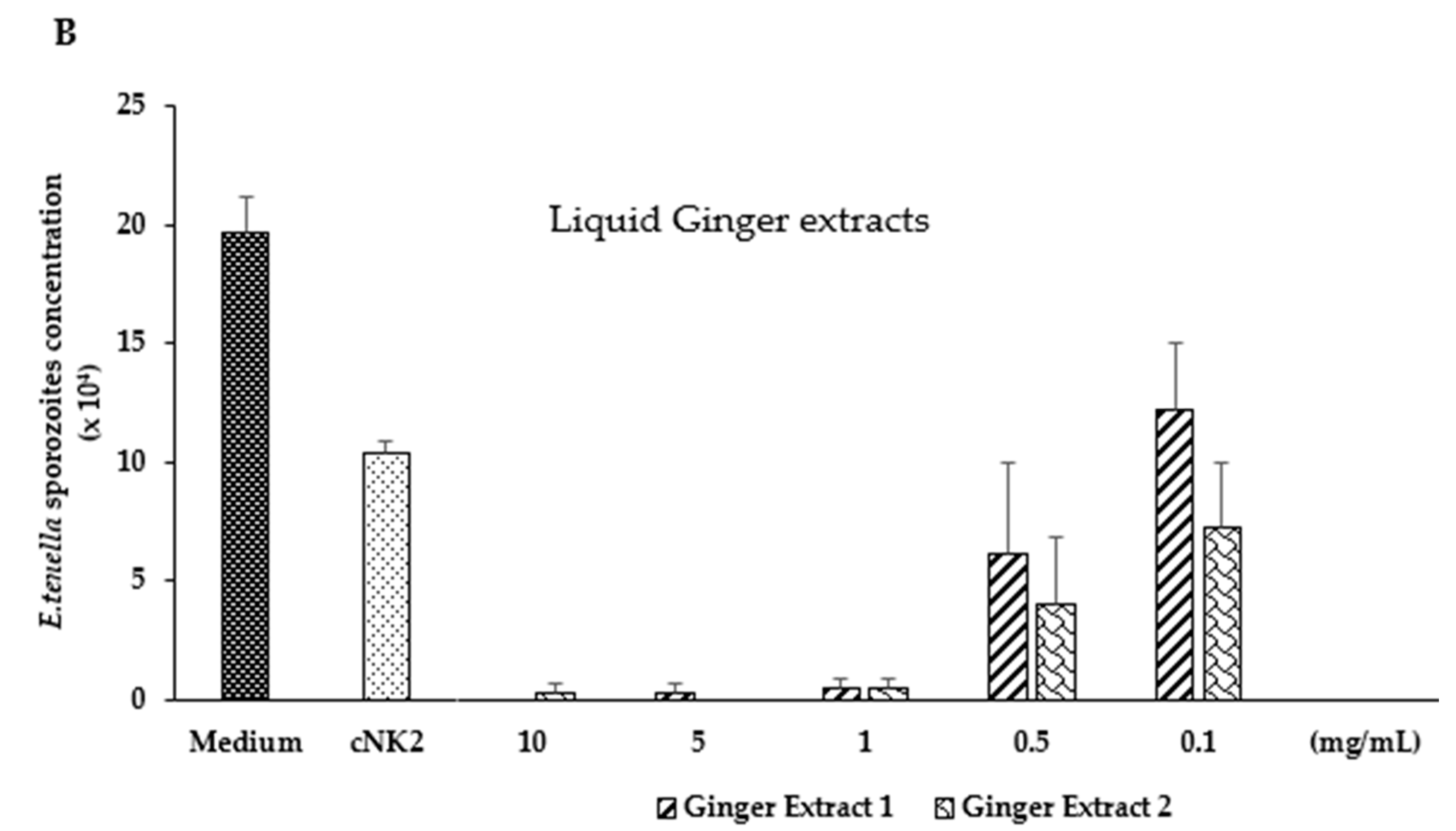
3.2. In Vitro Evaluation of Green Tea and Ginger Extracts on Killing Action on E. tenella Sporozoites
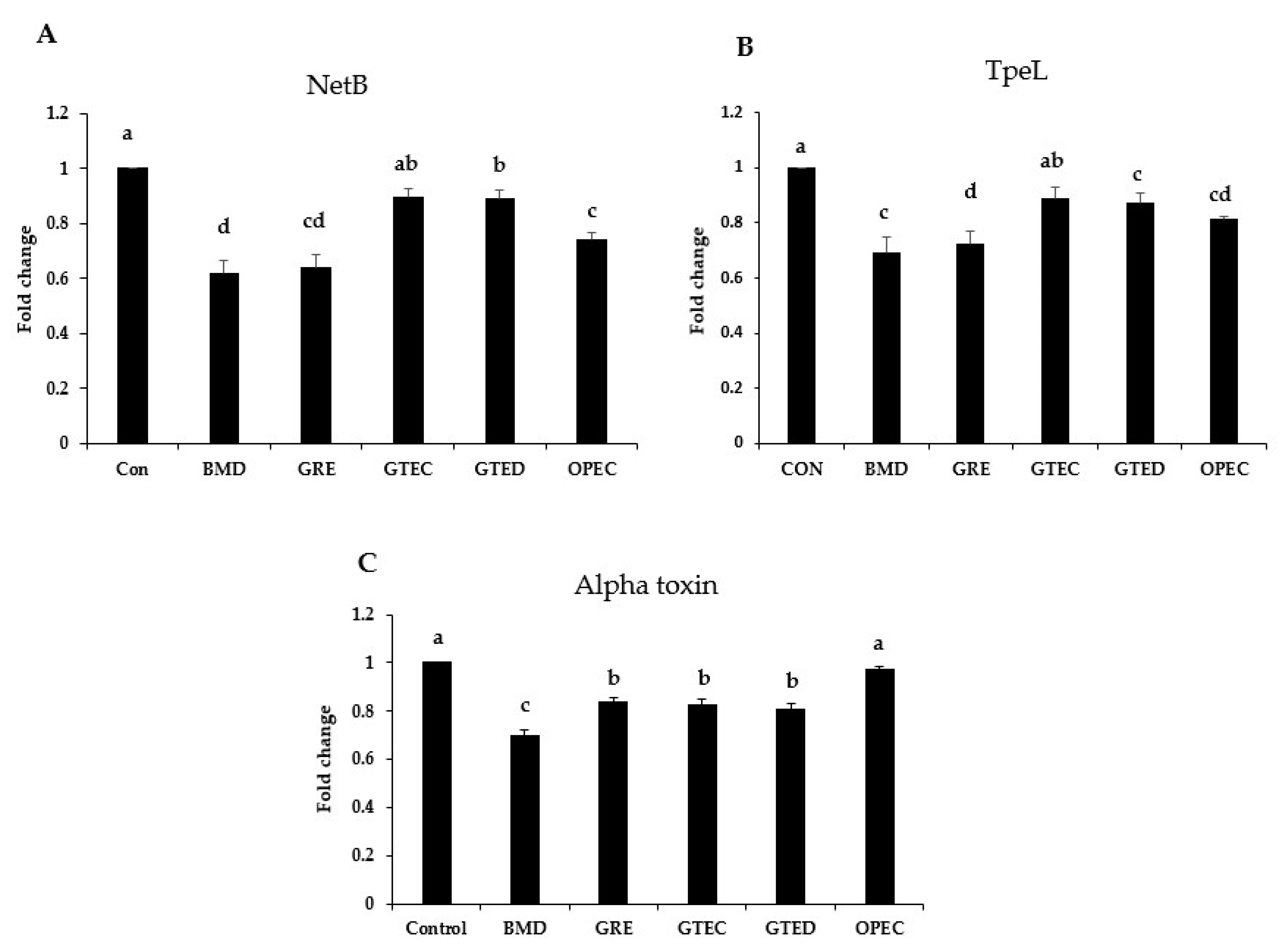
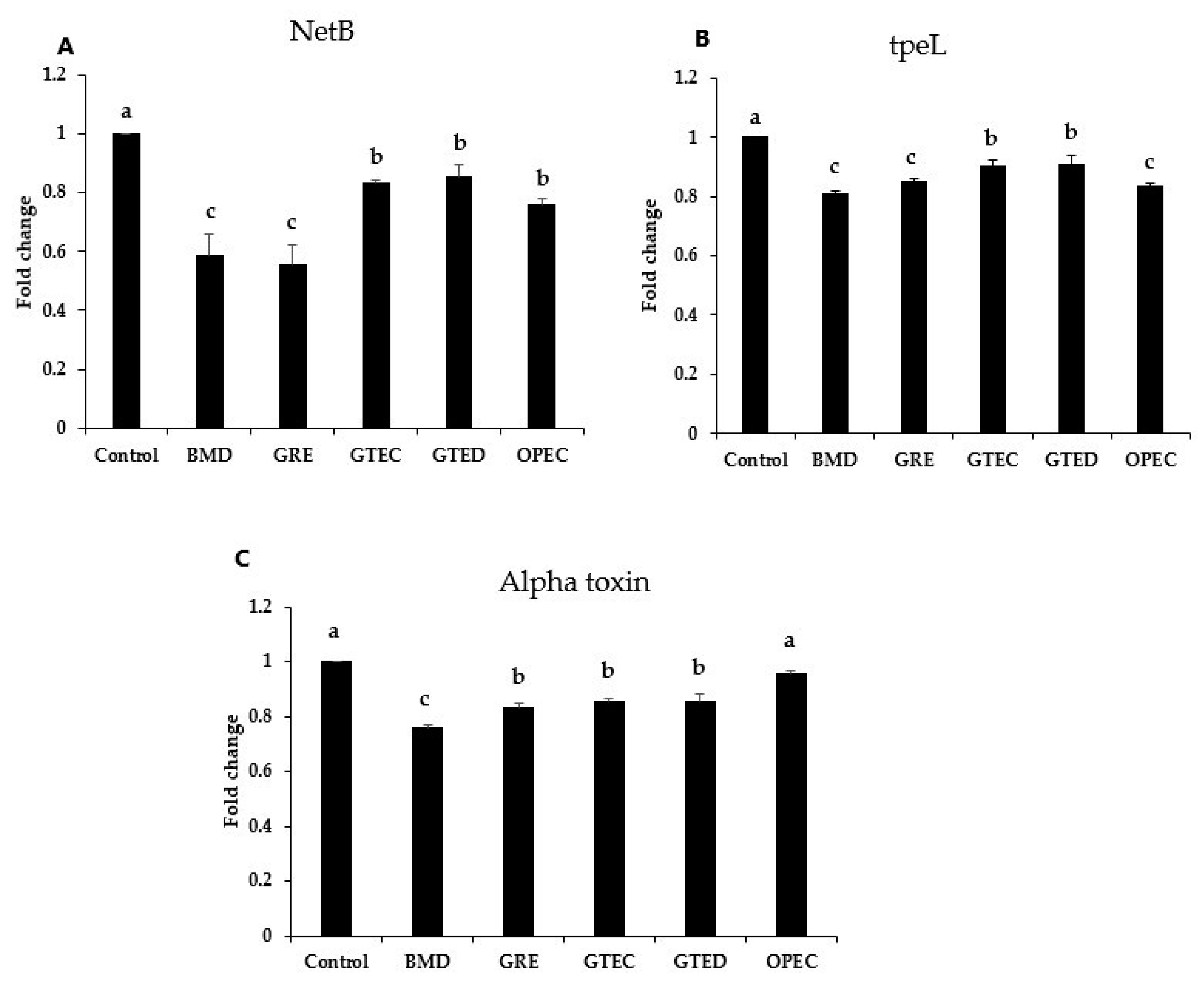
3.3. Effect of Phytogenic Extracts on C. perfringens Toxin Gene Expression
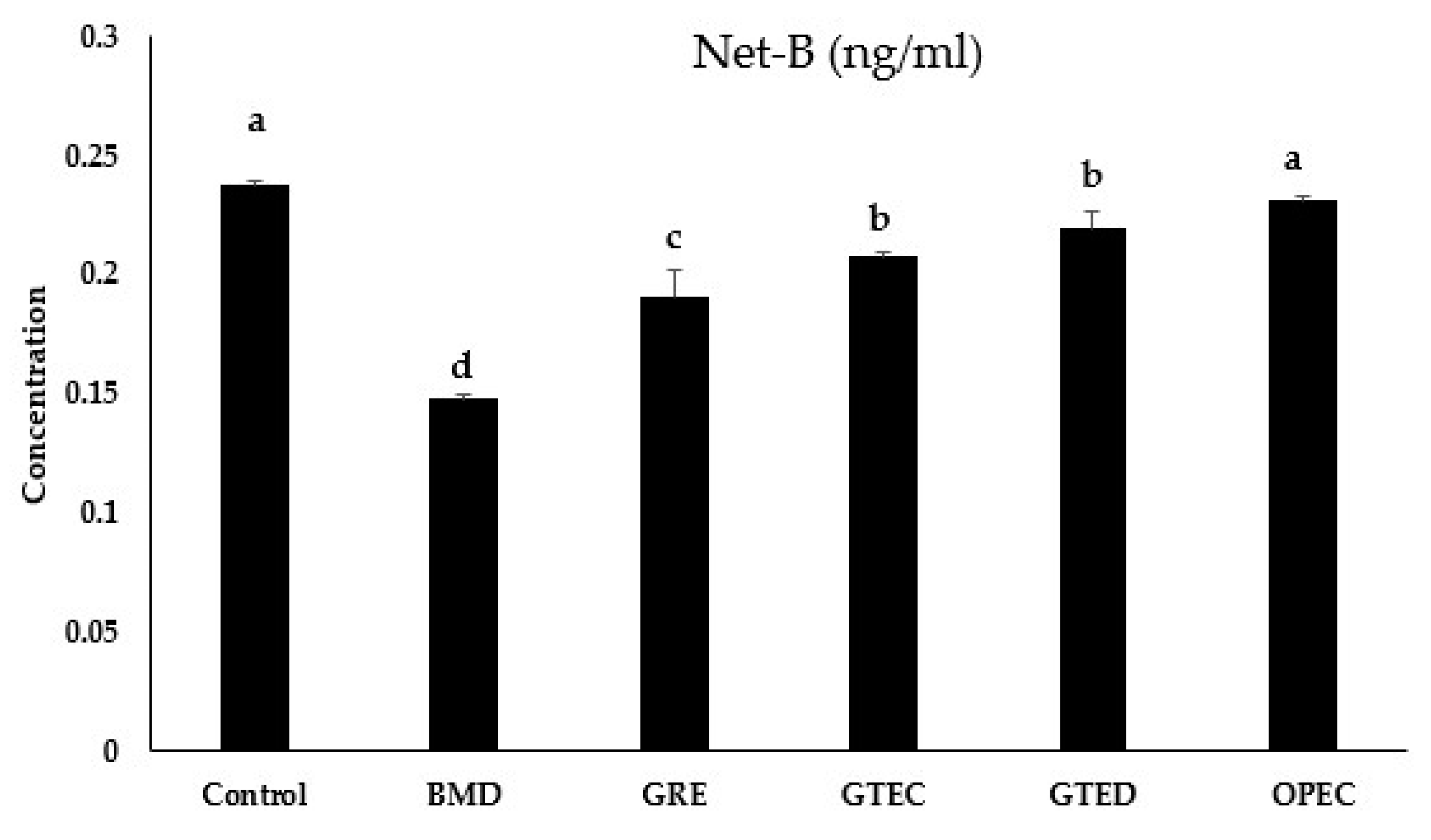
3.4. Effect of Selected Phytogenic Extracts on Protein Concentration of NetB Toxin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, K.A.; Elbourne, L.D.; Tetu, S.G.; Melville, S.B.; Rood, J.I.; Paulsen, I.T. Genomic analyses of Clostridium perfringens isolates from five toxinotypes. Microbiol. Res. 2015, 166, 255–263. [Google Scholar] [CrossRef]
- Uzal, F.A.; Freedman, J.C.; Shrestha, A.; Theoret, J.R.; Garcia, J.; Awad, M.M.; Adams, V.; Moore, R.J.; Rood, J.I.; McClane, B.A. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol. 2014, 9, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Keyburn, A.L.; Boyce, J.D.; Vaz, P.; Bannam, T.L.; Ford, M.E.; Parker, D.; Di Rubbo, A.; Rood, J.I.; Moore, R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008, 4, e26. [Google Scholar] [CrossRef]
- Lee, K.W.; Lillehoj, H.S. Role of Clostridium perfringens necrotic enteritis B-like toxin in disease pathogenesis. Vaccines 2022, 10, 61. [Google Scholar] [CrossRef]
- Yang, W.Y.; Chou, C.H.; Wang, C. Characterization of toxin genes and quantitative analysis of netB in necrotic enteritis (NE)-producing and non-NE-producing Clostridium perfringens isolated from chickens. Anaerobe 2018, 54, 115–120. [Google Scholar] [CrossRef]
- Duff, A.F.; Vuong, C.N.; Searer, K.L.; Briggs, W.N.; Wilson, K.M.; Hargis, B.M.; Bergham, L.R.; Bielke, L.R. Preliminary studies on development of a novel subunit vaccine targeting Clostridium perfringens mucolytic enzymes for the control of necrotic enteritis in broilers. Poult. Sci. 2019, 98, 6319–6325. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.K.; Songer, J.G. Virulence of Clostridium perfringens in an experimental model of poultry necrotic enteritis. Vet. Microbiol. 2010, 142, 323–328. [Google Scholar] [CrossRef]
- Johnson, J.; Reid, W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016, 45, 275–281. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Alyemni, A.H.; Swilam, E.O.; Al-Ghadi, M. Comparative anticoccidial effect of some natural products against Eimeria spp. infection on performance traits, intestinal lesion and occyte number in broiler. Pak. J. Zool. 2017, 49, 1989–1995. [Google Scholar] [CrossRef]
- Blue, C.E.C.; Jababu, Y.; Ibrahim, S.A.; Minor, R.C.; Williams, L.L.; Adetunji, A.O.; Ali, R.; Young, L.S.; Fasina, Y.O. Spray-Dried Plasma Promotes Broiler Chick Growth by Enhancing Immune Surveillance. Animal 2023, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.K.; Dalloul, R.A. Centennial Review: Recent developments in host-pathogen interactions during necrotic enteritis in poultry. Poult. Sci. 2021, 100, 101330. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, A.; Casey, T.; Franco, J.; Shah, D.; Fasina, Y. Proteomic Analysis of the Effect of Salmonella Challenge on Broiler Chicken. Molecules 2022, 27, 7277. [Google Scholar] [CrossRef] [PubMed]
- Babaei, S.; Rahimi, S.; Torshizi, M.A.K.; Tahmasebi, G.; Khaleghi Miran, S.N. Effects of propolis, royal jelly, honey and bee pollen on growth performance and immune system of Japanese quails. Vet. Res Vet. Q. 2016, 7, 13–20. [Google Scholar]
- Wickramasuriya, S.S.; Park, I.; Lee, K.; Lee, Y.; Kim, W.H.; Nam, H.; Lillehoj, H.S. Role of physiology, immunity, microbiota, and infectious diseases in the gut health of poultry. Vaccines 2022, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef] [PubMed]
- Dosu, G.; Obanla, T.; Zhang, S.; Sang, S.; Adetunji, A.O.; Fahrenholz, A.O.; Ferket, P.R.; Nagabhushanam, K.; Fasina, Y.O. Supplementation of ginger root extract into broiler chicken diet: Effects on growth performance and immunocompetence. Poult. Sci. 2023, 102, 102897. [Google Scholar] [CrossRef]
- Chandravanshi, A.; Naik, M.G.; Chandravanshi, P.; Rathore, S.S. Camellia sinensis (Green tea) as feed additive enhanced immune response and disease resistance of Cyprinus carpio (common carp) against Aeromonas hydrophila infection. J. Exp. Zool. 2020, 23, 1383–1390. [Google Scholar]
- Khaki, A.; Fathiazad, F.; Nouri, M.; Khaki, A.A.; Khamenehi, H.J.; Hamadeh, M. Evaluation of androgenic activity of Allium cepa on spermatogenesis in the rat. Folia Morphol. 2009, 68, 45–51. [Google Scholar]
- Khaki, A.; Farnam, A.; Badie, A.D.; Nikniaz, H. Treatment effects of onion (Allium cepa) and ginger (Zingiber officinale) on sexual behavior of rat after inducing an antiepileptic drug (lamotrigine). Balkan Med. J. 2012, 29, 236–246. [Google Scholar] [CrossRef]
- Settle, T.; Leonard, S.S.; Falkenstein, E.; Fix, N.; Van Dyke, K.; Klandorf, H. Effects of a Phytogenic Feed Additive Versus an Antibiotic Feed Additive on Oxidative Stress in Broiler Chicks and a Possible Mechanism Determined by Electron Spin Resonance. Int. J. Poult. Sci. 2014, 13, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.I.; Jun, M.H.; Lillehoj, H.S.; Dalloul, R.A.; Kong, I.K.; Kim, S.; Min, W. Anticoccidial effect of green tea-based diets against Eimeria maxima. Vet. Parasitol. 2007, 144, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Kim, W.H.; Li, C.; Lillehoj, H.S. Detection of necrotic enteritis B–like toxin secreted by Clostridium perfringens using capture enzyme-linked immunosorbent assay. Avian Dis. 2020, 64, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.H.; Lillehoj, H.S.; Siragusa, G.R.; Bannerman, D.D.; Lillehoj, E.P. Antimicrobial activity of chicken NK-lysin against. Eimeria sporozoites. Avian Dis. 2008, 52, 302–305. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N.A. Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- Afsharmanesh, M.; Sadaghi, B. Effects of dietary alternatives (probiotic, green tea powder, and Kombucha tea) as antimicrobial growth promoters on growth, ileal nutrient digestibility, blood parameters, and immune response of broiler chickens. Comp. Clin. Pathol. 2014, 23, 717–724. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.; Al-Nasser, A.; Al-Surrayai, T.; Sultan, H.; Al-Attal, D.; Al-Kandari, R.; Al-Saleem, H.; Al-Holi, A.; Dashti, F. Effect of Ginger Powder on Production Performance, Antioxidant Status, Hematological Parameters, Digestibility, and Plasma Cholesterol Content in Broiler Chickens. Animal 2022, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.A. Plant extracts as natural modulators of gut microbiota community structure and functionality. Heliyon 2020, 6, e05474. [Google Scholar] [CrossRef]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S. Antimicrobial assessment of polyphenolic extracts from onion (Allium cepa L.) skin of fifteen cultivars by sonication-assisted extraction method. Heliyon 2020, 6, e05478. [Google Scholar]
- Bajagai, Y.S.; Alsemgeest, J.; Moore, R.J.; Van, T.T.H.; Stanley, D. Phytogenic products, used as alternatives to antibiotic growth promoters, modify the intestinal microbiota derived from a range of production systems: An in vitro model. Appl. Microbiol. Biotechnol. 2020, 104, 10631–10640. [Google Scholar] [CrossRef]
- Crisol-Martinez, E.; Stanley, D.; Geier, M.S.; Hughes, R.J.; Moore, R.J. Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: Linking gut microbiota and growth performance in chickens. Appl. Microbiol. Biotechnol. 2017, 101, 4547–4559. [Google Scholar] [CrossRef] [PubMed]
- Qorbanpour, M.; Fahim, T.; Javandel, F.; Nosrati, M.; Paz, E.; Seidavi, A.; Ragni, M.; Laudadio, V.; Tufarelli, V. Effect of Dietary Ginger (Zingiber officinale Roscoe) and Multi-Strain Probiotic on Growth and Carcass Traits, Blood Biochemistry, Immune Responses and Intestinal Microflora in Broiler Chickens. Animal 2018, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Sirk, T.W.; Brown, E.F.; Friedman, M.; Sum, A.K. Molecular binding of catechins to biomembranes: Relationship to biological activity. J. Agric. Food Chem. 2009, 57, 6720–6728. [Google Scholar] [CrossRef]
- Duan, Y.; Jin, D.H.; Kim, H.S.; Seong, J.H.; Lee, Y.G.; Kim, D.S.; Jang, S.H. Analysis of total phenol, flavonoid content and antioxidant activity of various extraction solvents extracts from onion (Allium cepa L.) peels. J. Korean Appl. Sci. Technol. 2015, 32, 418–426. [Google Scholar] [CrossRef]
- Kim, W.; Lillehoj, H.; Min, W. Evaluation of the Immunomodulatory Activity of the Chicken NK-Lysin-Derived Peptide cNK-2. Sci. Rep. 2017, 7, 45099. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.; Yang, X. Control of brown rot on nectarines by tea polyphenol combined with tea saponin. J. Crop Prot. 2013, 45, 29–35. [Google Scholar] [CrossRef]
- Nonthakaew, A.; Matan, N.; Aewsiri, T.; Matan, N. Caffeine in foods and its antimicrobial activity. Int. Food Res. J. 2015, 22, 9–14. [Google Scholar]
- Majid, A.; Naila, C.; Rifat, K.; Shabana, N.; Sina, G. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J. Appl. Anim. Res. 2019, 47, 79–84. [Google Scholar]
- Diaz Carrasco, J.M.; Redondo, L.M.; Redondo, E.A.; Dominguez, J.E.; Chacana, A.P.; Fernandez Miyakawa, M.E. Use of Plant Extracts as an Effective Manner to Control Clostridium perfringens Induced Necrotic Enteritis in Poultry. Biomed Res. Int. 2016, 2016, 3278359. [Google Scholar] [CrossRef]
- Ibrahim, D.; Ismail, T.A.; Khalifa, E.; Abd El-Kader, S.A.; Mohamed, D.I.; Mohamed, D.T.; Shahin, S.E.; Abd El-Hamid, M.I. Supplementing Garlic Nanohydrogel Optimized Growth, Gastrointestinal Integrity and Economics and Ameliorated Necrotic Enteritis in Broiler Chickens Using a Clostridium perfringens Challenge Model. Animal 2021, 11, 2027. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, A.; Ibitoye, E.; Dabai, Y.; Garba, S. In vivo antimicrobial potentials of garlic against Clostridium perfringens and its promotant effects on performance of broiler chickens. Pak. J. Biol. Sci. 2013, 16, 1978–1984. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | Primer Sequence | |
|---|---|---|
| Necrotic enteritis toxin B (NetB) | Forward | 5′-AGTGTAATTAGTACAAGCC-3′ |
| Necrotic enteritis toxin B (NetB) | Reverse | 5′-GGCCATTTCATTTTTCCGTAA-3′ |
| Alpha toxin (α/cpa) | Forward | 5′-AGTCTACGCTTGGGATGGAA-3′ |
| Alpha toxin (α/cpa) | Reverse | 5′-TTTCCTGGGTTGTCCATTTC-3′ |
| 16SrRNA | Forward | 5′-CCTTACCTACACTTGACATCCC-3′ |
| 16SrRNA | Reverse | 5′-GGACTTAACCCAACATCTCACG-3′ |
| tpeL | Forward | 5′-GCCTGGATTTGCCTGTAGGA-3′ |
| tpeL | Reverse | 5′-GCCAGACATTATGCCACTCC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.M.; Lillehoj, H.S.; Lee, Y.; Adetunji, A.O.; Omaliko, P.C.; Kang, H.W.; Fasina, Y.O. Use of Selected Plant Extracts in Controlling and Neutralizing Toxins and Sporozoites Associated with Necrotic Enteritis and Coccidiosis. Appl. Sci. 2024, 14, 3178. https://doi.org/10.3390/app14083178
Khan MM, Lillehoj HS, Lee Y, Adetunji AO, Omaliko PC, Kang HW, Fasina YO. Use of Selected Plant Extracts in Controlling and Neutralizing Toxins and Sporozoites Associated with Necrotic Enteritis and Coccidiosis. Applied Sciences. 2024; 14(8):3178. https://doi.org/10.3390/app14083178
Chicago/Turabian StyleKhan, Md Maruf, Hyun S. Lillehoj, Youngsub Lee, Adedeji O. Adetunji, Paul C. Omaliko, Hye Won Kang, and Yewande O. Fasina. 2024. "Use of Selected Plant Extracts in Controlling and Neutralizing Toxins and Sporozoites Associated with Necrotic Enteritis and Coccidiosis" Applied Sciences 14, no. 8: 3178. https://doi.org/10.3390/app14083178






