1-Deoxynojirimycin Attenuates High-Glucose-Induced Oxidative DNA Damage via Activating NRF2/OGG1 Signaling
Abstract
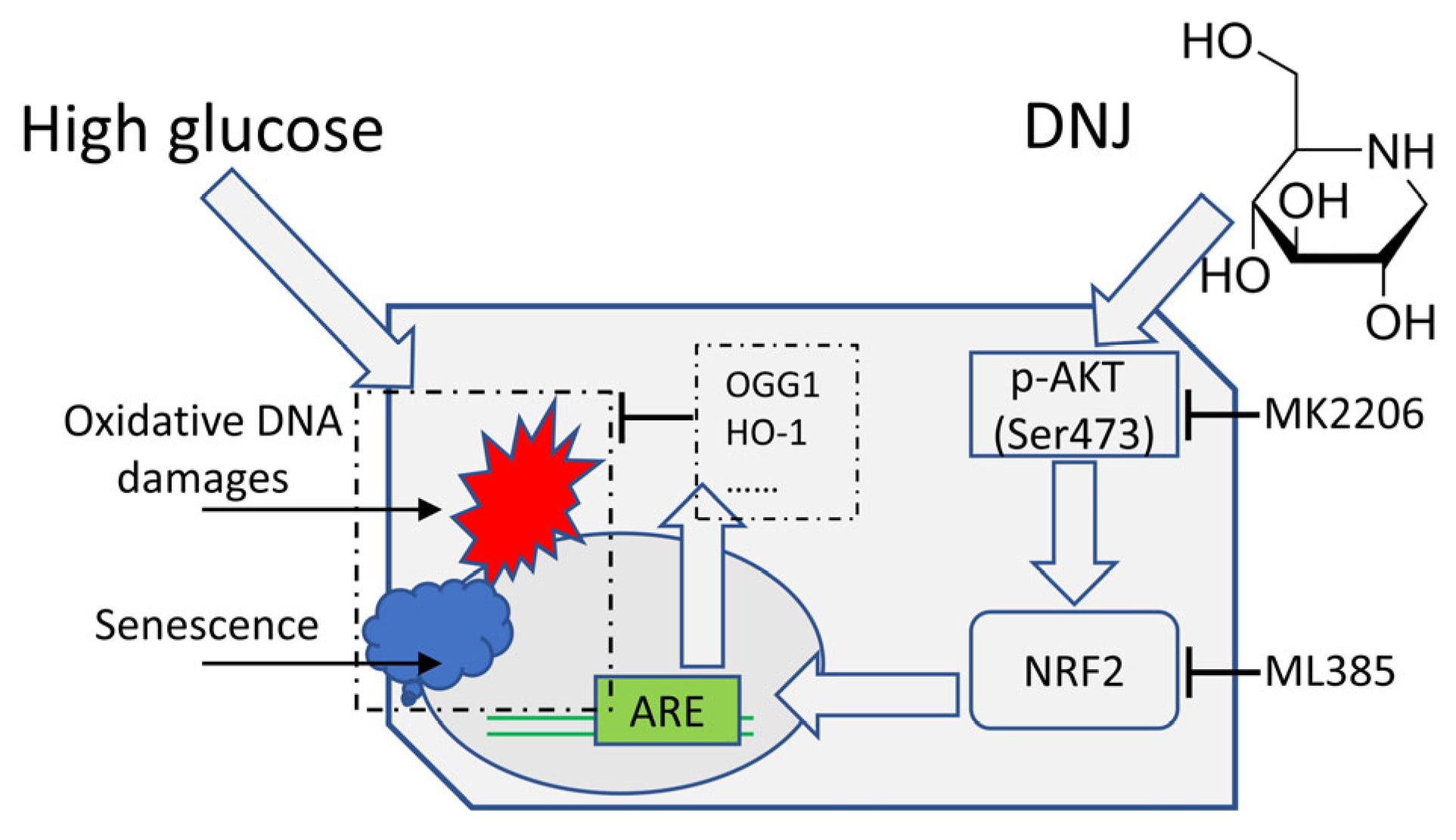
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Cell Line and Culture Condition
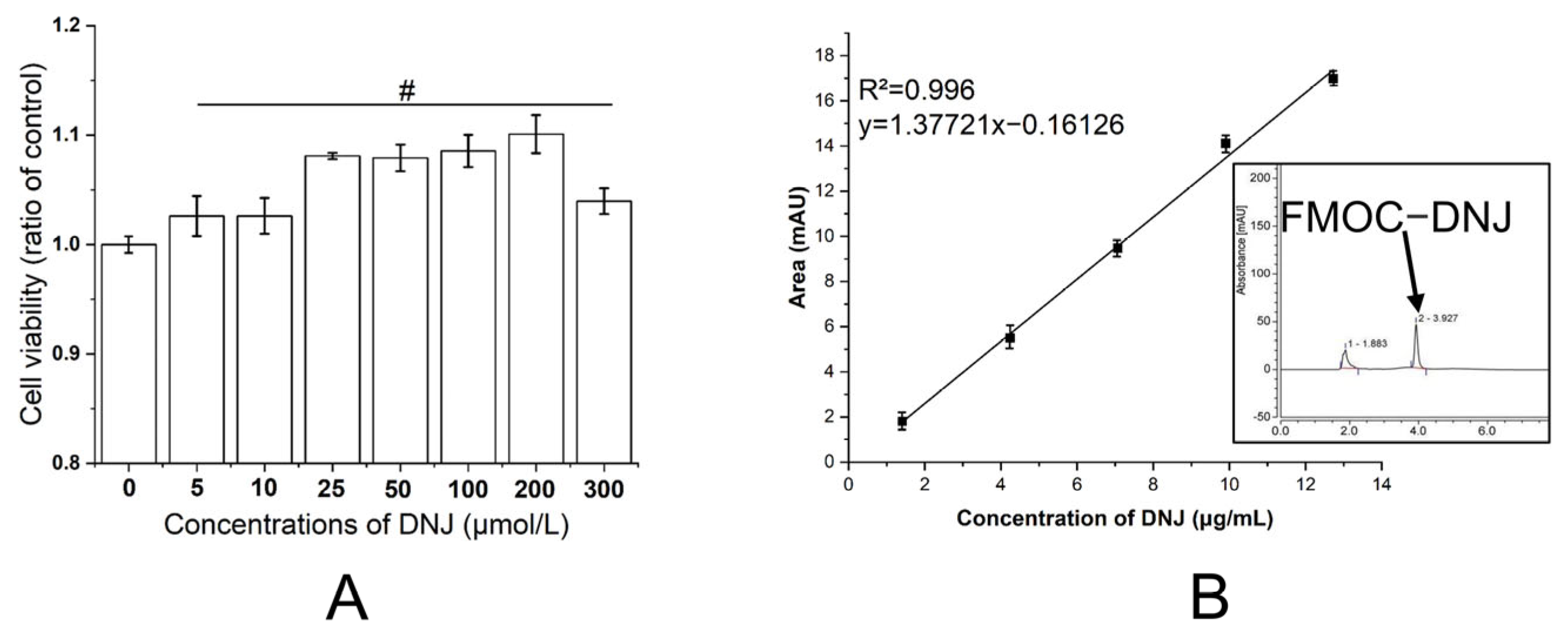
2.3. High-Performance Liquid Chromatography (HPLC) Analysis of DNJ
2.4. Cytoxicity of DNJ
2.5. Immunofluorescence Staining
2.6. Senescence-Associated β-Galactosidase (SA-β-gal) Staining
2.7. Western Blot Assay
2.8. Statistical Analysis
3. Results
3.1. Cytoxicity of DNJ
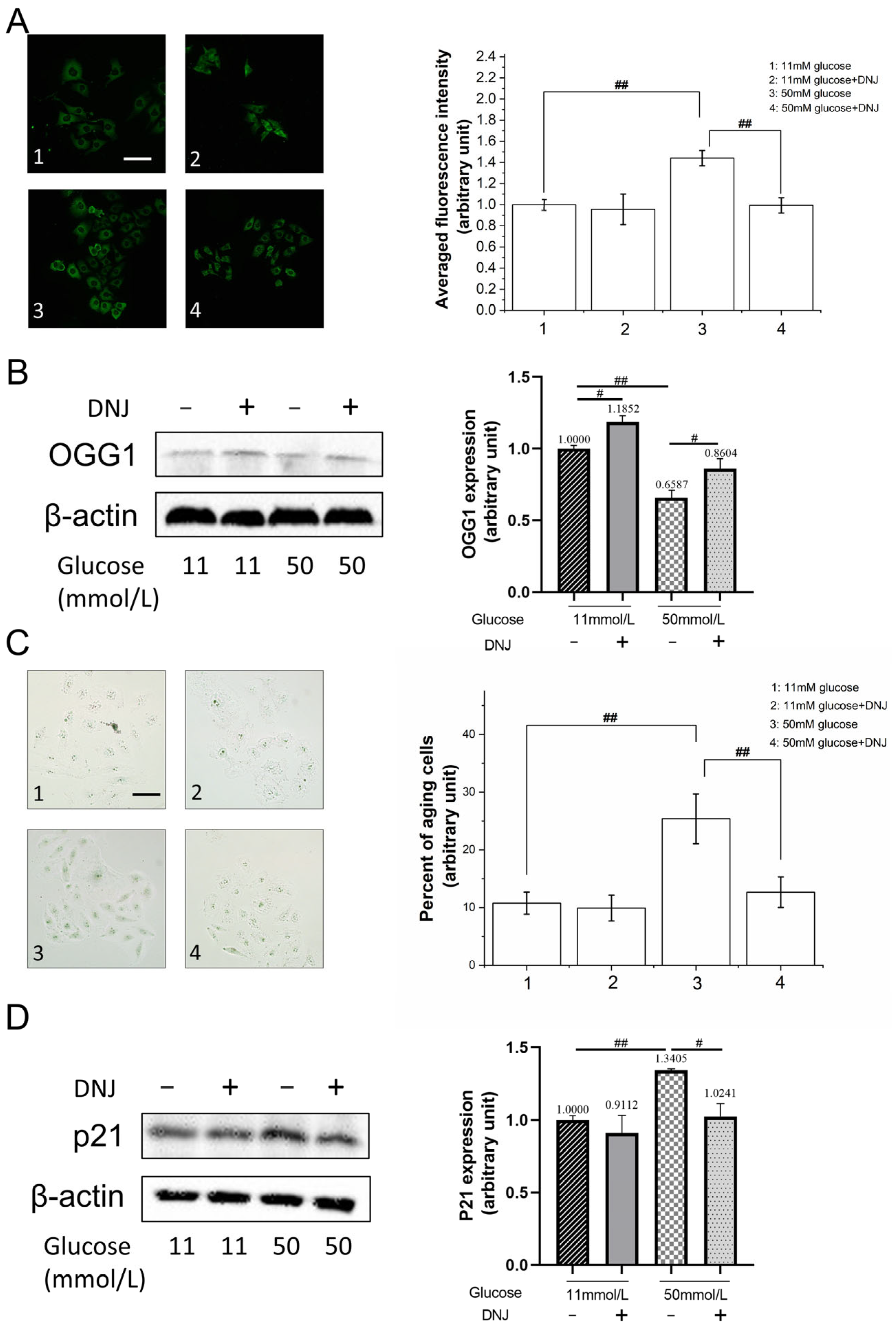
3.2. DNJ Alleviates High-Glucose-Induced Oxidative DNA Damage
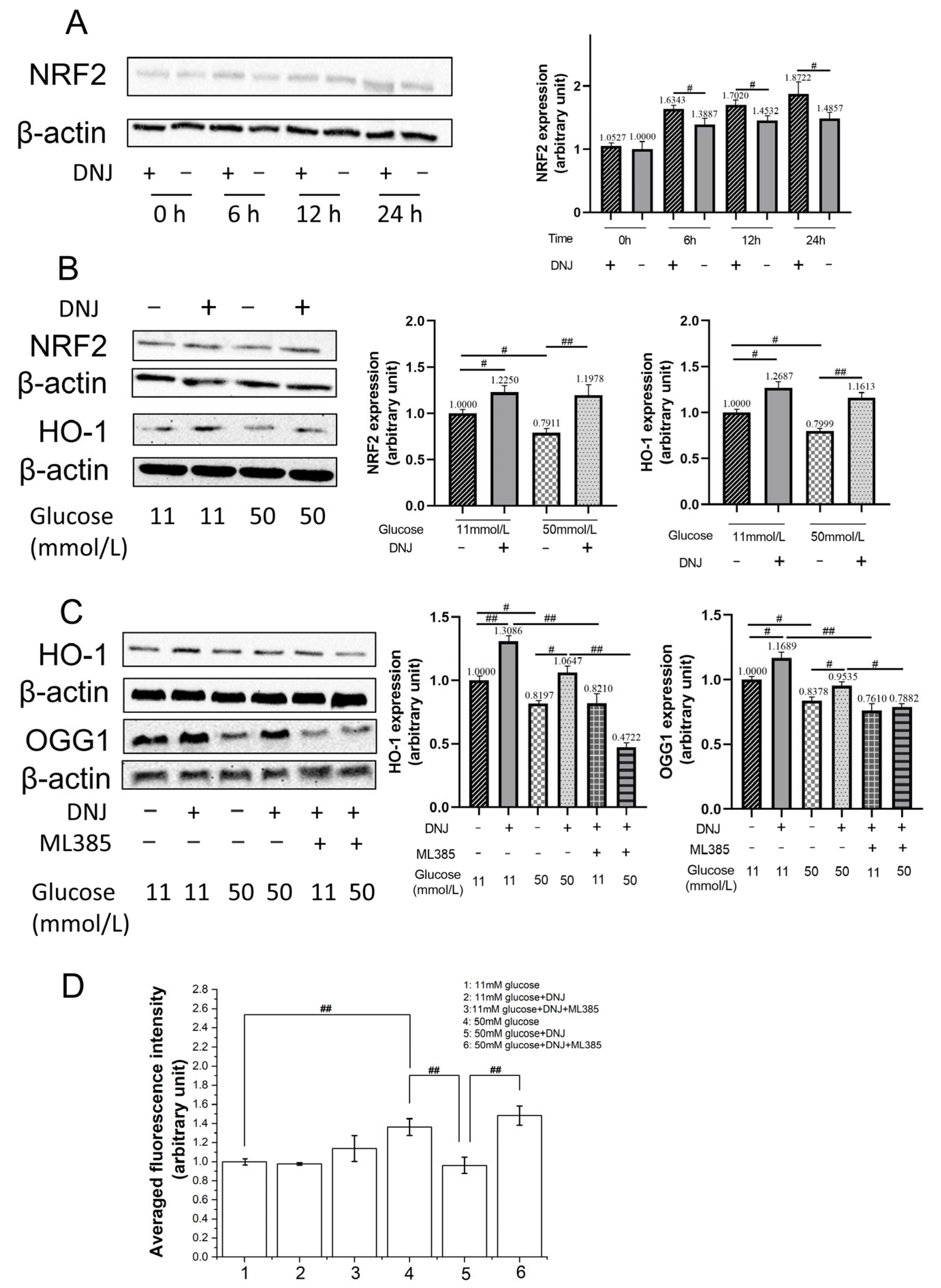
3.3. DNJ Promotes Oxidative DNA Damage Repair via Stimulating NRF2/OGG1
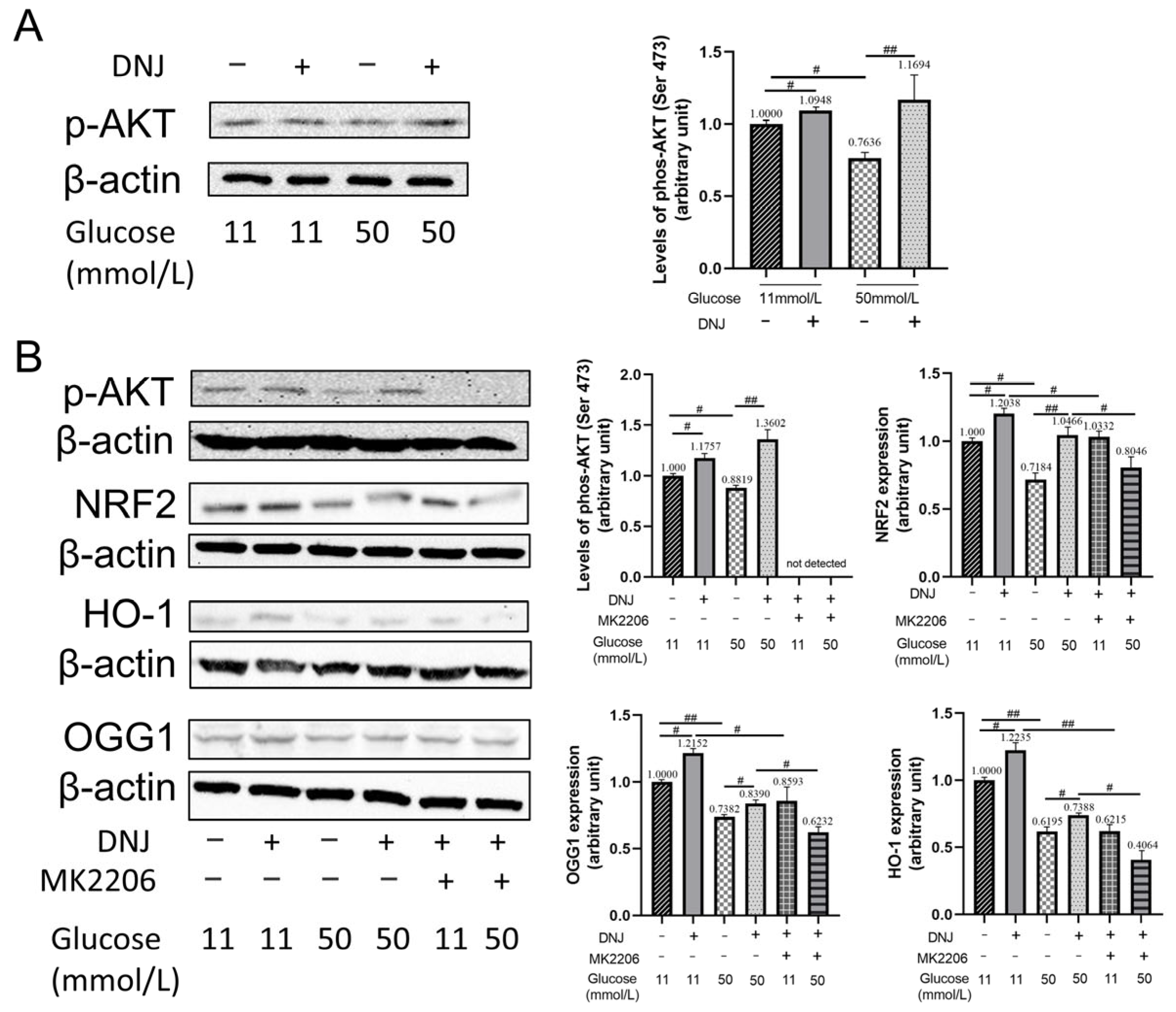
3.4. DNJ Enhances AKT Activation to Stimulate NRF2
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, C.; Mohamad, R.; Saikim, F.; Mahyudin, A.; Mohd, N. Morus alba L. Plant: Bioactive Compounds and Potential as a Functional Food Ingredient. Foods 2021, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mu, M.; Wu, H.; Liang, Z. An overview of the biological production of 1-deoxynojirimycin: Current status and future perspective. Appl. Microbiol. Biotechnol. 2019, 103, 9335–9344. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.; Saeed, R.; Sahar, A.; Khan, M. Mulberry plant as a source of functional food with therapeutic and nutritional applications: A review. J. Food Biochem. 2022, 46, e14263. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lai, J.; Tang, Q.; Zheng, X. Mulberry ethanol extract attenuates hepatic steatosis and insulin resistance in high-fat diet-fed mice. Nutr. Res. 2016, 36, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xi, M.; Gao, F.; Li, M.; Dong, T.; Geng, Z.; Liu, C.; Huang, F.; Wang, J.; Li, X.; et al. Evaluation of mulberry leaves’ hypoglycemic properties and hypoglycemic mechanisms. Front. Pharmacol. 2023, 14, 1045309. [Google Scholar] [CrossRef] [PubMed]
- Fongsodsri, K.; Thaipitakwong, T.; Rujimongkon, K.; Kanjanapruthipong, T.; Ampawong, S.; Reamtong, O.; Aramwit, P. Mulberry-Derived 1-Deoxynojirimycin Prevents Type 2 Diabetes Mellitus Progression via Modulation of Retinol-Binding Protein 4 and Haptoglobin. Nutrients 2022, 14, 4538. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Ito, M.; Kimura, T.; Ikeuchi, T.; Takita, T.; Yasukawa, K. Inhibitory effect of Morus australis leaf extract and its component iminosugars on intestinal carbohydrate-digesting enzymes. J. Biosci. Bioeng. 2021, 132, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.; Truzzi, E.; Frosi, I.; Papetti, A.; Cappellozza, S.; Saviane, A.; Pellati, F.; Bertelli, D. In vitro bioactivity evaluation of mulberry leaf extracts as nutraceuticals for the management of diabetes mellitus. Food Funct. 2022, 13, 4344–4359. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, M.; Lu, Y.; Zhou, M. An overview of therapeutic potential of N-alkylated 1-deoxynojirimycin congeners. Carbohydr. Res. 2021, 504, 108317. [Google Scholar] [CrossRef]
- Ma, Y.; Lv, W.; Gu, Y.; Yu, S. 1-Deoxynojirimycin in Mulberry (Morus indica L.) Leaves Ameliorates Stable Angina Pectoris in Patients with Coronary Heart Disease by Improving Antioxidant and Anti-inflammatory Capacities. Front. Pharmacol. 2019, 10, 569. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Z.; Jiang, J.; Li, Y.; Yu, S. Mulberry leaf attenuates atherosclerotic lesions in patients with coronary heart disease possibly via 1-Deoxynojirimycin: A placebo-controlled, double-blind clinical trial. J. Food Biochem. 2021, 45, e13573. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Chen, J.; Xu, F.; Wang, Y.; Zhao, P.; Ding, Y.; Han, Y.; Yang, J.; Tao, Y. Mixed Mulberry Fruit and Mulberry Leaf Fermented Alcoholic Beverages: Assessment of Chemical Composition, Antioxidant Capacity In Vitro and Sensory Evaluation. Foods 2022, 11, 3125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gu, L.; Xie, H.; Liu, Y.; Huang, P.; Zhang, J.; Luo, D.; Zhang, J. Glucose transport, transporters and metabolism in diabetic retinopathy. Biochim. Biophys. Acta Mol. Basis. Dis. 2023, 1870, 166995. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nor, N.; Budin, S.; Zainalabidin, S.; Jalil, J.; Sapian, S.; Jubaidi, F.; Mohamad Anuar, N. The Role of Polyphenol in Modulating Associated Genes in Diabetes-Induced Vascular Disorders. Int. J. Mol. Sci. 2022, 23, 6396. [Google Scholar] [CrossRef] [PubMed]
- Shuang, E.; Kijima, R.; Honma, T.; Yamamoto, K.; Hatakeyama, Y.; Kitano, Y.; Kimura, T.; Nakagawa, K.; Miyazawa, T.; Tsuduki, T. 1-Deoxynojirimycin attenuates high glucose-accelerated senescence in human umbilical vein endothelial cells. Exp. Gerontol. 2014, 55, 63–69. [Google Scholar]
- Zhang, Y.; Li, L.; Chai, T.; Xu, H.; Du, H.; Jiang, Y. Mulberry leaf multi-components exert hypoglycemic effects through regulation of the PI-3K/Akt insulin signaling pathway in type 2 diabetic rats. J. Ethnopharmacol. 2024, 319 (Pt 3), 117307. [Google Scholar] [CrossRef]
- He, X.; Sun, Z.; Ma, K.; Mei, Y. 1-deoxynojirimycin alleviates liver fibrosis induced by type 2 diabetes in mice. Nan Fang Yi Ke Da Xue Xue Bao 2021, 41, 1342–1349. [Google Scholar]
- Tonelli, C.; Chio, I.; Tuveson, D. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, P.; Han, X.; Ren, H.; Yu, W.; Hao, W.; Luo, B.; Khan, M.; Chen, N. Role of ionizing radiation activated NRF2 in lung cancer radioresistance. Int. J Biol. Macromol. 2023, 241, 124476. [Google Scholar] [CrossRef]
- Niture, S.; Khatri, R.; Jaiswal, A. Regulation of Nrf2-an update. Free. Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, J.; Park, S.; Yang, S. An Update on the Role of Nrf2 in Respiratory Disease: Molecular Mechanisms and Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 8406. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Pandita, R.; Eskiocak, U.; Ly, P.; Kaisani, A.; Kumar, R.; Cornelius, C.; Wright, W.; Pandita, T.; Shay, J. Targeting of Nrf2 induces DNA damage signaling and protects colonic epithelial cells from ionizing radiation. Proc. Natl. Acad. Sci. USA 2012, 109, E2949–E2955. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Chatterjee, A.; Ronghe, A.; Bhat, N.; Bhat, H. Antioxidant-mediated up-regulation of OGG1 via NRF2 induction is associated with inhibition of oxidative DNA damage in estrogen-induced breast cancer. BMC Cancer 2013, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Feng, Y.; Li, T.; Zhao, C.; Barcenas, A.; Serrano, B.; Qu, L.; Shen, M.; Zhao, W. The Effects of 1-Deoxynojirimycin from Mulberry on Oxidative Stress and Inflammation in Laying Hens and the Direct Effects on Intestine Epithelium Cells In Vitro. Animals 2023, 13, 2830. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, Y.; Shi, X.; Ye, C.; Wang, J.; Huang, J.; Zhang, B.; Deng, Z. Hepatoprotective effects of different mulberry leaf extracts against acute liver injury in rats by alleviating oxidative stress and inflammatory response. Food Funct. 2022, 13, 8593–8604. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, F.; Li, Y.; Wang, Z.; Kong, D. Cellular signaling perturbation by natural products. Cell Signal. 2009, 21, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- King, G.; Loeken, M. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem. Cell Biol. 2004, 122, 333–338. [Google Scholar] [CrossRef]
- Xue, H.; Zhang, P.; Zhang, C.; Gao, Y.; Tan, J. Research progress in the preparation, structural characterization, and biological activities of polysaccharides from traditional Chinese medicine. Int. J. Biol. Macromol. 2024, 262 (Pt 1), 129923. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Hernández-Hernández, O.; Ruiz-Matute, A.; Sanz, M. A derivatization procedure for the simultaneous analysis of iminosugars and other low molecular weight carbohydrates by GC–MS in mulberry (Morus sp.). Food Chem. 2011, 126, 353–359. [Google Scholar] [CrossRef]
- Shreelakshmi, S.; Nazareth, M.; Kumar, S.; Giridhar, P.; Prashanth, K.; Shetty, N. Physicochemical Composition and Characterization of Bioactive Compounds of Mulberry (Morus indica L.) Fruit during Ontogeny. Plant Foods Hum. Nutr. 2021, 76, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Guo, Y.; Chen, L.; Chen, G.; Liang, Z. A Novel Strategy to Regulate 1-Deoxynojirimycin Production Based on Its Biosynthetic Pathway in Streptomyces lavendulae. Front. Microbiol. 2019, 10, 1968. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, M.; Lu, Y.; Wu, N.; Chen, J.; Ji, Z.; Zhan, Y.; Ma, X.; Chen, J.; Cai, D.; et al. Metabolic engineering of Bacillus amyloliquefaciens for efficient production of α-glucosidase inhibitor1-deoxynojirimycin. Synth. Syst. Biotechnol. 2023, 8, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, C.; Hu, M. 1-Deoxynojirimycin inhibits metastasis of B16F10 melanoma cells by attenuating the activity and expression of matrix metalloproteinases-2 and -9 and altering cell surface glycosylation. J. Agric. Food Chem. 2010, 58, 8988–8993. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.; Li, S.; Sui, X.; Guo, L.; Liu, X.; Li, H.; Gao, L.; Cai, S.; Li, Y.; Wang, T.; et al. 1-Deoxynojirimycin (DNJ) Ameliorates Indomethacin-Induced Gastric Ulcer in Mice by Affecting NF-kappaB Signaling Pathway. Front. Pharmacol. 2018, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Du, A.; Zhou, L.; Zhang, T.; Lu, B.; Wang, Z.; Ji, L. Chlorogenic acid improves diabetic retinopathy by alleviating blood-retinal-barrier dysfunction via inducing Nrf2 activation. Phytother. Res. 2022, 36, 1386–1401. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Jia, H.; Zhang, Y.; Zhou, J.; Chen, X.; Wu, L.; Wang, J. Geniposidic Acid from Eucommia ulmoides Oliver Staminate Flower Tea Mitigates Cellular Oxidative Stress via Activating AKT/NRF2 Signaling. Molecules 2022, 27, 8568. [Google Scholar] [CrossRef] [PubMed]
- McWalter, G.; Higgins, L.; McLellan, L.; Henderson, C.; Song, L.; Thornalley, P.; Itoh, K.; Yamamoto, M.; Hayes, J. Transcription factor Nrf2 is essential for induction of NAD(P)H: Quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J. Nutr. 2004, 134 (Suppl. 12), 3499S–3506S. [Google Scholar] [CrossRef]
- You, A.; Nam, C.; Wakabayashi, N.; Yamamoto, M.; Kensler, T.; Kwak, M. Transcription factor Nrf2 maintains the basal expression of Mdm2: An implication of the regulation of p53 signaling by Nrf2. Arch. Biochem. Biophys. 2011, 507, 356–364. [Google Scholar] [CrossRef]
- Yang, C.; Cui, X.; Ding, Z.; Jiang, T.; Feng, X.; Pan, Y.; Lin, Y.; Shang, T.; Wang, Q.; Pan, J.; et al. Gankyrin and TIGAR cooperatively accelerate glucose metabolism toward the PPP and TCA cycle in hepatocellular carcinoma. Cancer Sci. 2022, 113, 4151–4164. [Google Scholar] [CrossRef]
- Singh, B.; Shoulson, R.; Chatterjee, A.; Ronghe, A.; Bhat, N.; Dim, D.; Bhat, H. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis 2014, 35, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, I.; Kang, K.; Piao, M.; Ryu, M.; Kim, J.; Lee, N.; Hyun, J. Triphlorethol-A from Ecklonia cava up-regulates the oxidant sensitive 8-oxoguanine DNA glycosylase 1. Mar. Drugs 2014, 12, 5357–5371. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Pan, C.; Zhang, X.; Yang, T.; Hu, T.; Zheng, L.; Cao, S.; Feng, C.; Hu, X.; Chai, X.; et al. Nuclear factor Nrf2 promotes glycosidase OGG1 expression by activating the AKT pathway to enhance leukemia cell resistance to cytarabine. J. Biol. Chem. 2023, 299, 102798. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Wu, Y.; Liu, S.; Luo, C.; Lu, Y.; Liu, M.; Wang, L.; Zhang, Y.; Liu, X. Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J. Ethnopharmacol. 2022, 289, 115021. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, Y.; Zhong, Y.; Che, T.; Hu, Y.; Bao, J.; Meng, N. Protective Effect of Flavonoids from a Deep-Sea-Derived Arthrinium sp. against ox-LDL-Induced Oxidative Injury through Activating the AKT/Nrf2/HO-1 Pathway in Vascular Endothelial Cells. Mar. Drugs 2021, 19, 712. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Li, C.; Zheng, Y.; Peng, G. 1-Deoxynojirimycin Alleviates Insulin Resistance via Activation of Insulin Signaling PI3K/AKT Pathway in Skeletal Muscle of db/db Mice. Molecules 2015, 20, 21700–21714. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Park, M.; Lee, H. Mulberry (Morus alba L.) Leaf Extract and 1-Deoxynojirimycin Improve Skeletal Muscle Insulin Resistance via the Activation of IRS-1/PI3K/Akt Pathway in db/db Mice. Life 2022, 12, 1630. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Lin, M.; Huang, C.; Chang, W.; Wang, C. Mulberry 1-deoxynojirimycin pleiotropically inhibits glucose-stimulated vascular smooth muscle cell migration by activation of AMPK/RhoB and down-regulation of FAK. J. Agric. Food Chem. 2013, 61, 9867–9875. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhu, L.; Ma, M.; Chen, X.; Gao, Y.; Yu, T.; Yang, G.; Pang, W. Mulberry 1-Deoxynojirimycin Inhibits Adipogenesis by Repression of the ERK/PPARγ Signaling Pathway in Porcine Intramuscular Adipocytes. J. Agric. Food Chem. 2015, 63, 6212–6220. [Google Scholar] [CrossRef]
- Ren, X.; Guo, Q.; Jiang, H.; Han, X.; He, X.; Liu, H.; Xiu, Z.; Dong, Y. Combinational application of the natural products 1-deoxynojirimycin and morin ameliorates insulin resistance and lipid accumulation in prediabetic mice. Phytomedicine 2023, 121, 155106. [Google Scholar] [CrossRef]
- Kimura, T.; Nakagawa, K.; Kubota, H.; Kojima, Y.; Goto, Y.; Yamagishi, K.; Oita, S.; Oikawa, S.; Miyazawa, T. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of postprandial blood glucose in humans. J. Agric. Food Chem. 2007, 55, 5869–5874. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Supasyndh, O.; Siritienthong, T.; Bang, N. Mulberry leaf reduces oxidation and C-reactive protein level in patients with mild dyslipidemia. Biomed. Res. Int. 2013, 2013, 787981. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Sakamoto, Y.; Mizowaki, Y.; Iwagaki, Y.; Kimura, T.; Nakagawa, K.; Miyazawa, T.; Tsuduki, T. Intake of mulberry 1-deoxynojirimycin prevents colorectal cancer in mice. J. Clin. Biochem. Nutr. 2017, 61, 47–52. [Google Scholar]
- Thaipitakwong, T.; Numhom, S.; Aramwit, P. Mulberry leaves and their potential effects against cardiometabolic risks: A review of chemical compositions, biological properties and clinical efficacy. Pharm. Biol. 2018, 56, 109–118. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, J. 1-Deoxynojirimycin Attenuates High-Glucose-Induced Oxidative DNA Damage via Activating NRF2/OGG1 Signaling. Appl. Sci. 2024, 14, 3186. https://doi.org/10.3390/app14083186
Chen Y, Wang J. 1-Deoxynojirimycin Attenuates High-Glucose-Induced Oxidative DNA Damage via Activating NRF2/OGG1 Signaling. Applied Sciences. 2024; 14(8):3186. https://doi.org/10.3390/app14083186
Chicago/Turabian StyleChen, Yuwei, and Jun Wang. 2024. "1-Deoxynojirimycin Attenuates High-Glucose-Induced Oxidative DNA Damage via Activating NRF2/OGG1 Signaling" Applied Sciences 14, no. 8: 3186. https://doi.org/10.3390/app14083186
APA StyleChen, Y., & Wang, J. (2024). 1-Deoxynojirimycin Attenuates High-Glucose-Induced Oxidative DNA Damage via Activating NRF2/OGG1 Signaling. Applied Sciences, 14(8), 3186. https://doi.org/10.3390/app14083186







