A Numerical Study of the Effect of Water Speed on the Melting Process of Phase Change Materials Inside a Vertical Cylindrical Container
Abstract
:1. Introduction
2. Phase Change Material (PCM)
3. Method
3.1. Physical Modeling
3.2. Computational Procedure
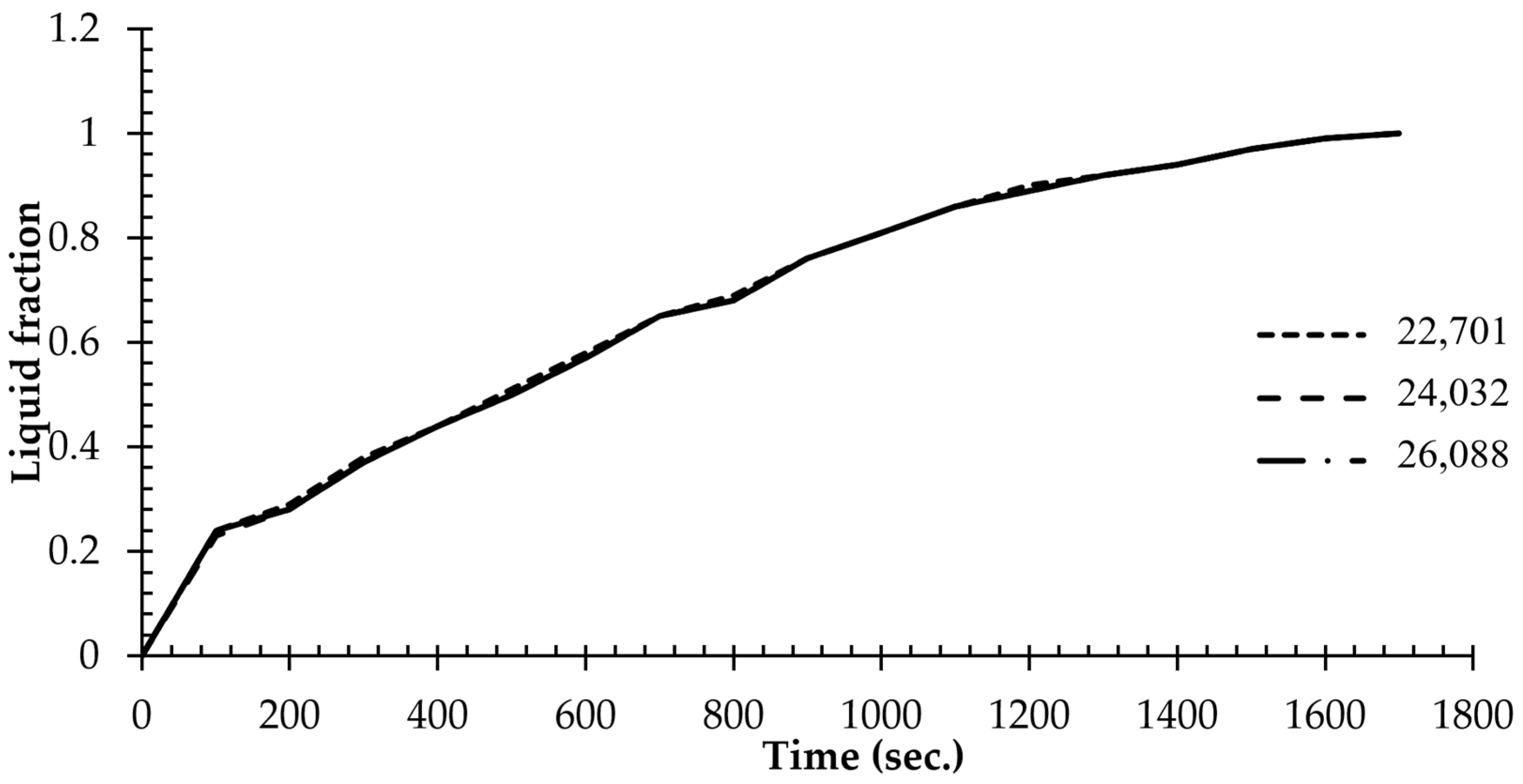
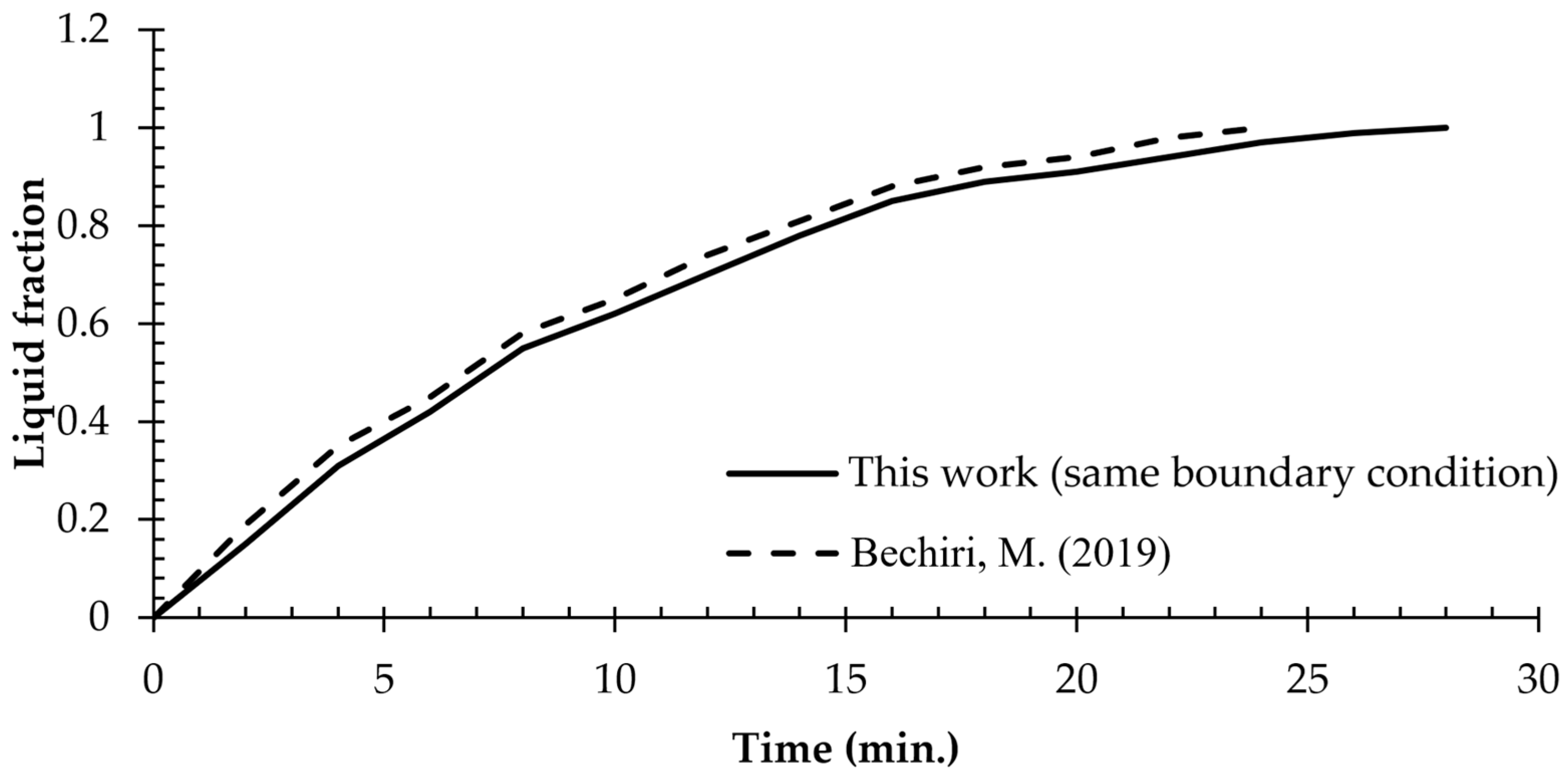
3.3. Grid Independence and the Code Validation
4. Results and Discussion
5. Conclusions
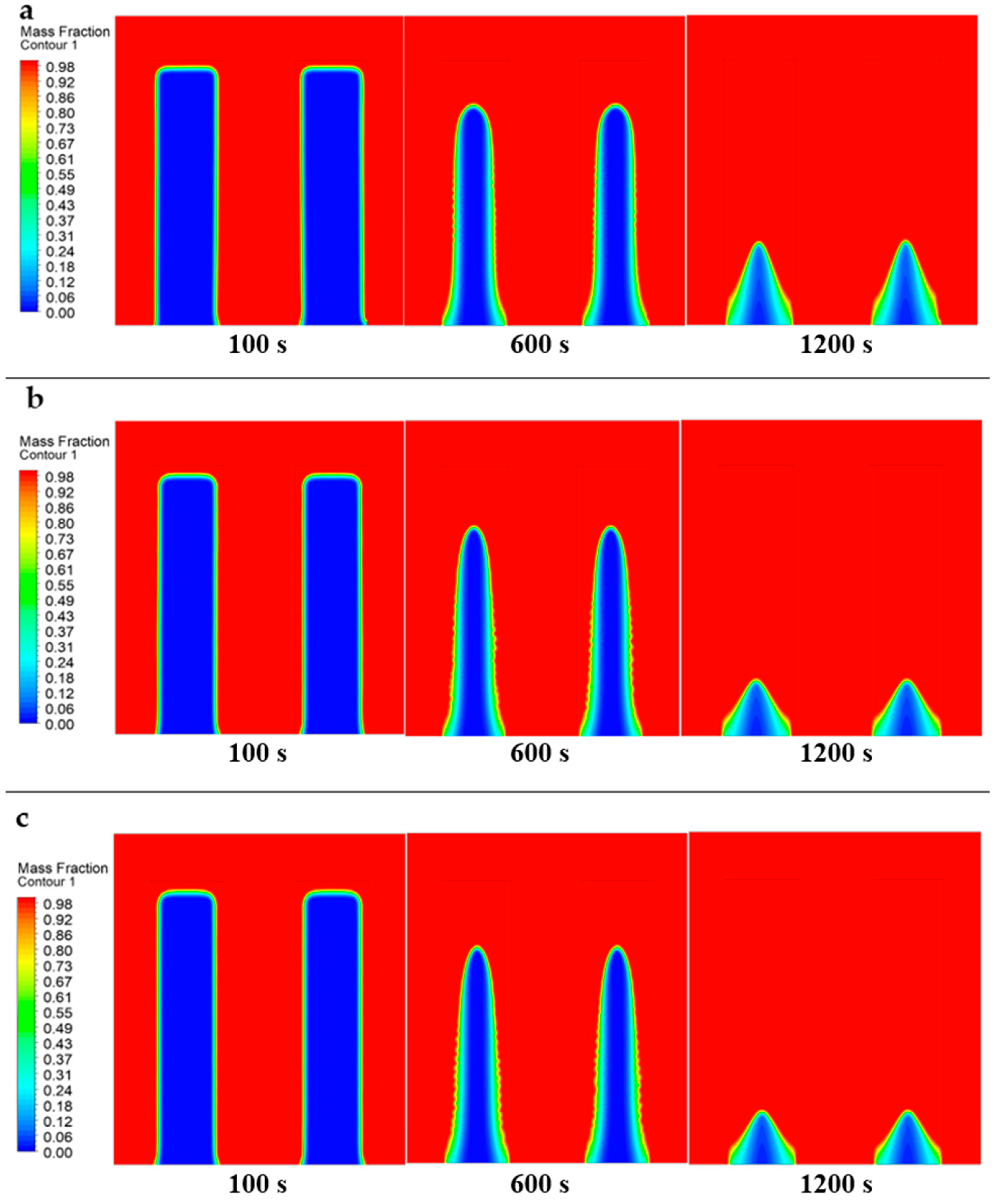
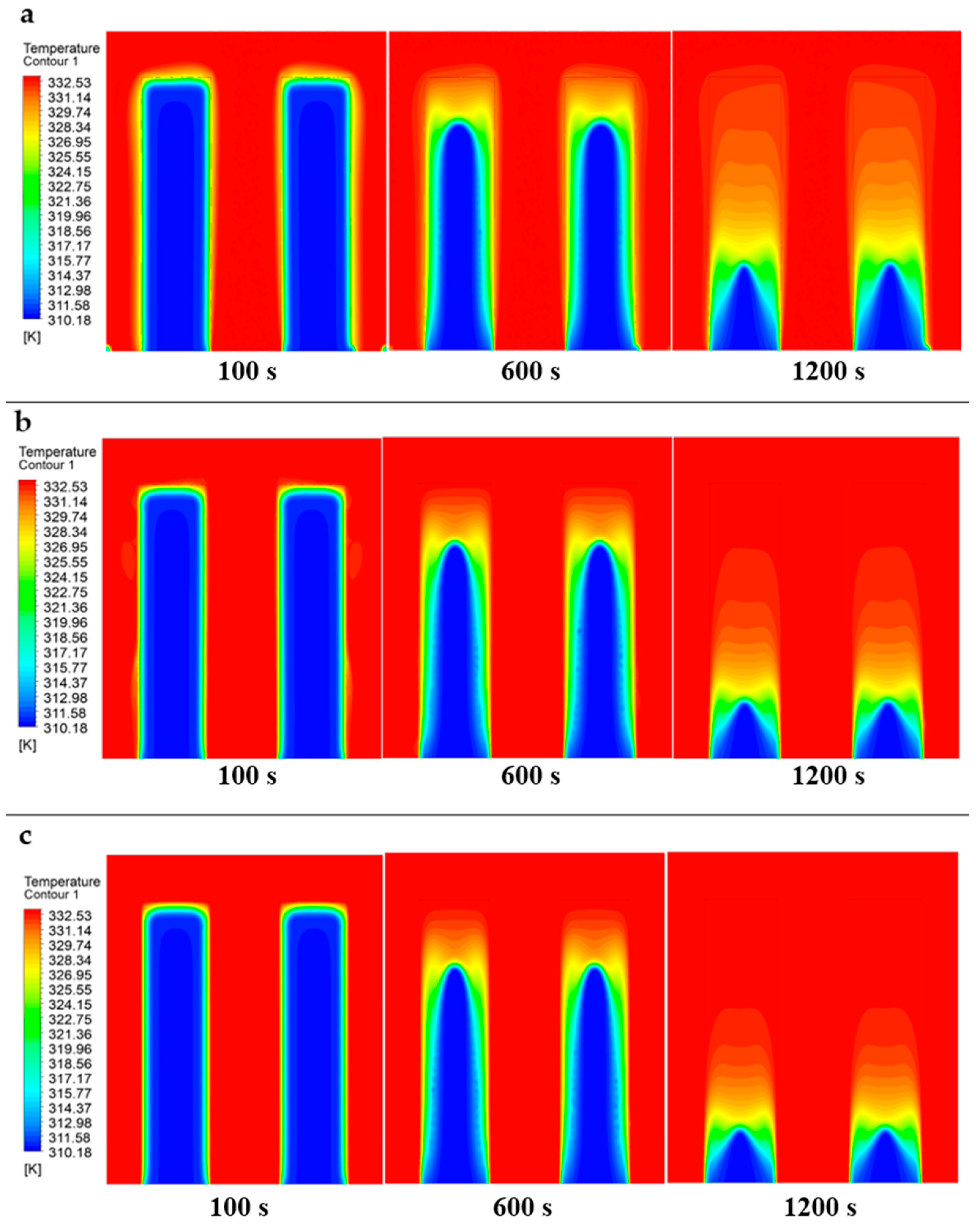
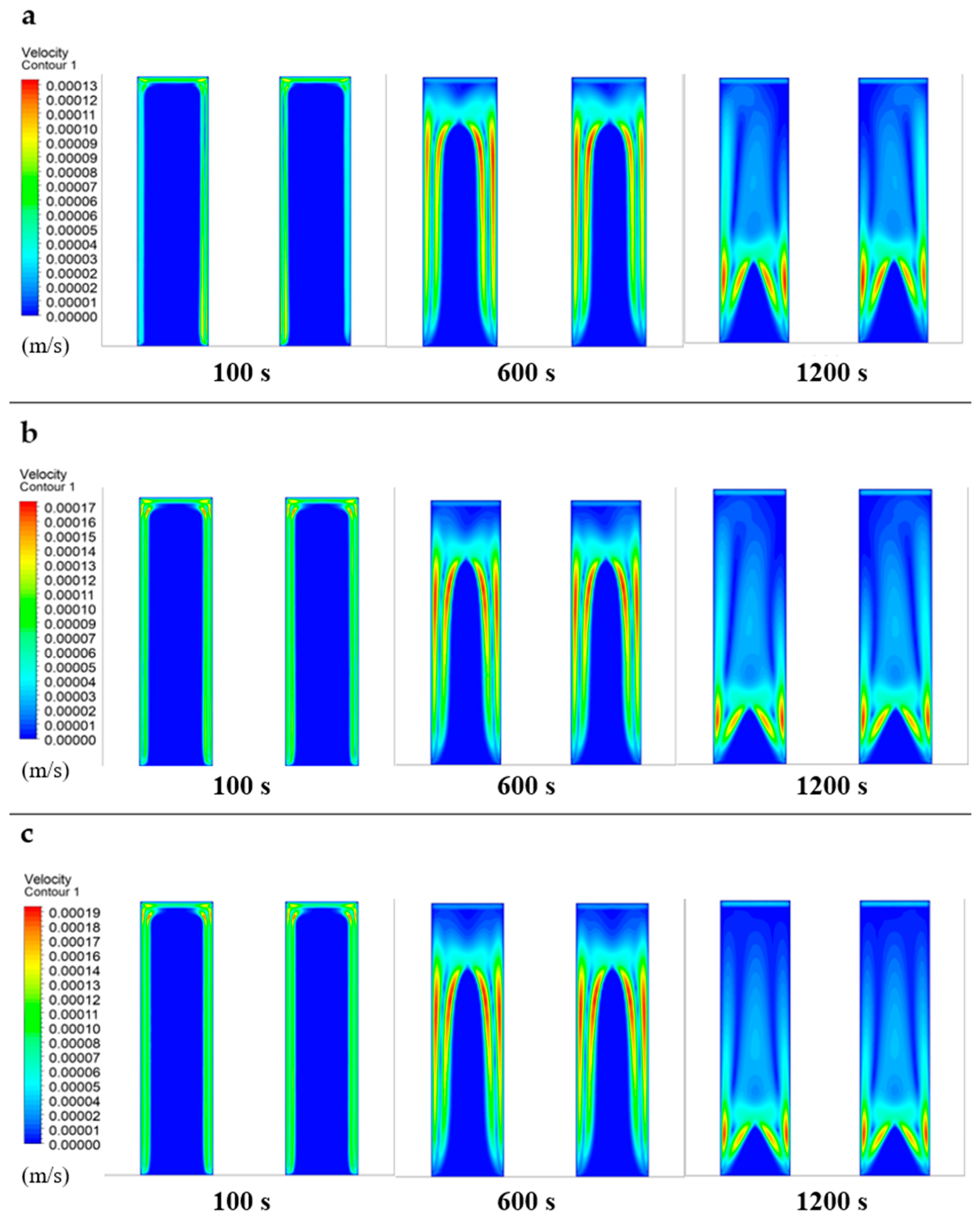
- The melting process initiates near the domain boundaries, with the solid PCM gradually transitioning to liquid state over time regardless of the heat transfer fluid (HTF) velocity.
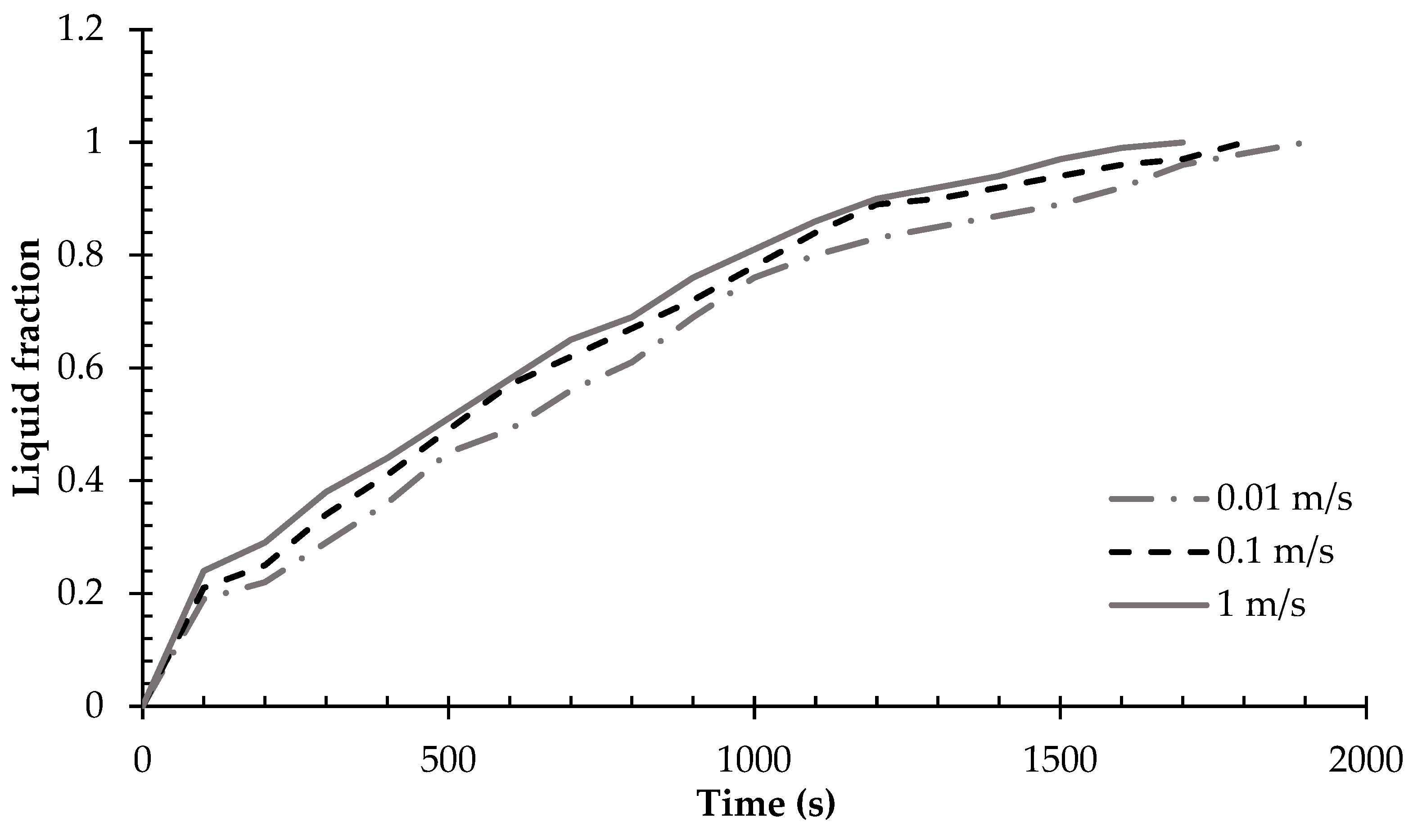
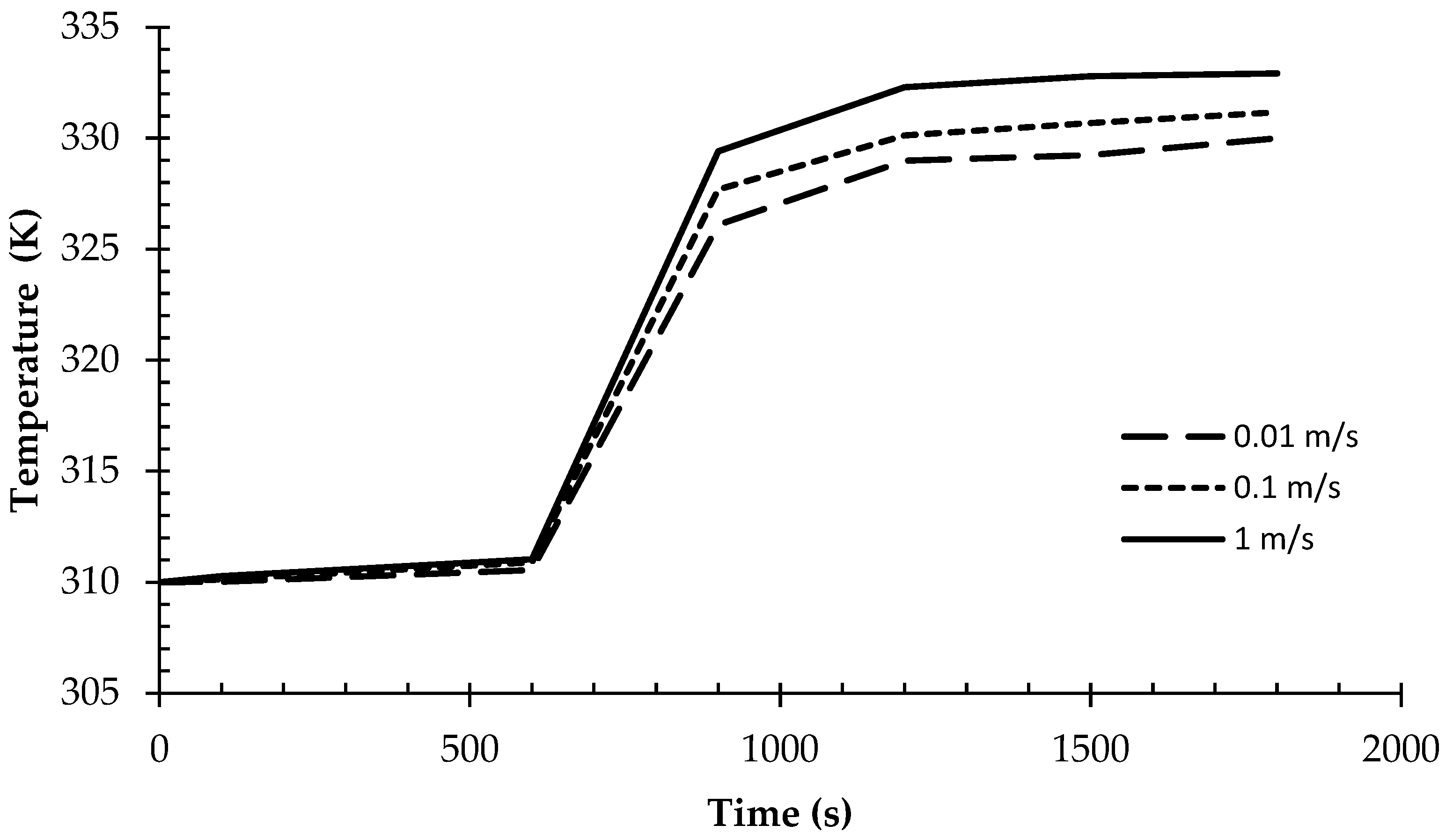
- Higher HTF velocities result in a more rapid increase in the liquid phase size and a corresponding decrease in the solid phase, indicating a faster melting process.
- The upward movement of liquid PCM within the domain is primarily influenced by convective currents, buoyancy forces, and thermal gradients, rather than solely by density differences.
- The density variations caused by melting solid PCM lead to natural convection, where less dense liquid rises due to increased temperature and denser, cooler liquid sinks.
- Enhanced HTF velocities lead to faster melting rates and a decrease in the remaining solid phase, underscoring the importance of fluid dynamics in affecting phase change kinetics.
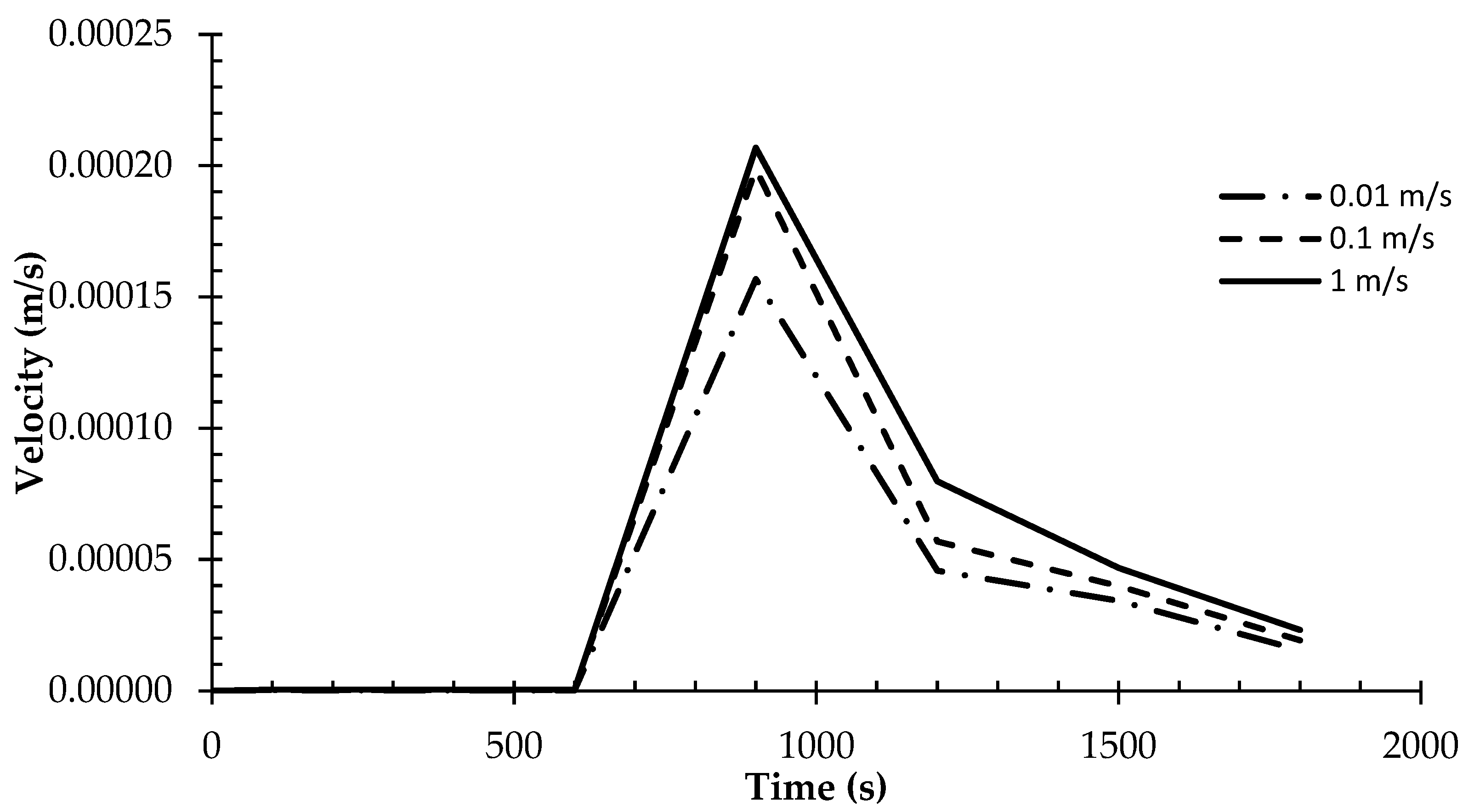
- The velocity of molten PCM within the domain directly correlates with HTF speed, highlighting the significant impact of HTF velocity on PCM movement and the overall efficiency of phase change processes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Cp | specific heat capacity J/(kg·K) |
| k | thermal conductivity W/(m·K) |
| L | latent heat J/kg |
| T | temperature K |
| β | melting fraction |
| dynamic viscosity kg/(m·s) | |
| density kg/m3 |
References
- Mousavi Ajarostaghi, S.S.; Hosseinian-Sorkhi, A.; Arıcı, M. Influence of immiscible intermediate fluid on melting process in a horizontal shell-and-tube phase change material storage. J. Therm. Anal. Calorim. 2023, 148, 11013–11027. [Google Scholar] [CrossRef]
- Bull, S.R. Renewable Energy Today and Tomorrow. Proc. IEEE 2001, 89, 1216–1226. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on Thermal Energy Storage with Phase Change Materials and Applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Younsi, Z.; Joulin, A.; Zalewski, L.; Lassue, S.; Rousse, D.R. Phase Change Materials: A Numerical Method for the Behavior Predictions. In Proceedings of the Fourth International Conference on Thermal Engineering: Theory and Applications, Abu Dhabi, United Arab Emirates, 12–14 January 2009; pp. 12–14. [Google Scholar]
- Shokouhmand, H.; Kamkari, B. Experimental Investigation on Melting Heat Transfer Characteristics of Lauric Acid in a Rectangular Thermal Storage Unit. Exp. Therm. Fluid. Sci. 2013, 50, 201–212. [Google Scholar] [CrossRef]
- Sun, X.; Chu, Y.; Mo, Y.; Fan, S.; Liao, S. Experimental Investigations on the Heat Transfer of Melting Phase Change Material (PCM). Energy Procedia 2018, 152, 186–191. [Google Scholar] [CrossRef]
- Yadav, A.; Samir, S. Experimental and Numerical Investigation of Spatiotemporal Characteristics of Thermal Energy Storage System in a Rectangular Enclosure. J. Energy Storage 2019, 21, 405–417. [Google Scholar] [CrossRef]
- Abderrahmane, A.; Qasem, N.A.A.; Mourad, A.; Al-Khaleel, M.; Said, Z.; Guedri, K.; Younis, O.; Marzouki, R. Enhancing the Melting Process of Shell-and-Tube PCM Thermal Energy Storage Unit Using Modified Tube Design. Nanomaterials 2022, 12, 3078. [Google Scholar] [CrossRef]
- Alshara, A.K.; Kadhim, M.K. Numerical Investigation of Energy Storage in Packed Bed of Cylindrical Capsules of PCM. Eng. Technol. J. 2014, 32, 494–510. [Google Scholar] [CrossRef]
- Hlimi, M.; Hamdaoui, S.; Mahdaoui, M.; Kousksou, T.; Ait Msaad, A.; Jamil, A.; El Bouardi, A. Melting inside a Horizontal Cylindrical Capsule. Case Stud. Therm. Eng. 2016, 8, 359–369. [Google Scholar] [CrossRef]
- Bechiri, M.; Mansouri, K. Study of Heat and Fluid Flow during Melting of PCM inside Vertical Cylindrical Tube. Int. J. Therm. Sci. 2019, 135, 235–246. [Google Scholar] [CrossRef]
- Al-Mudhafar, A.H.N.; Tarish, A.L. Enhancing PCM melting in a triple tube heat exchanger by utilizing innovative fins configurations. J. Braz. Soc. Mech. Sci. Eng. 2024, 46, 262. [Google Scholar] [CrossRef]
- Soodmand, A.M.; Azimi, B.; Nejatbakhsh, S.; Pourpasha, H.; Farshchi, M.E.; Aghdasinia, H.; Mohammadpourfard, M.; Heris, S.Z. A comprehensive review of computational fluid dynamics simulation studies in phase change materials: Applications, materials, and geometries. J. Therm. Anal. Calorim. 2023, 148, 10595–10644. [Google Scholar] [CrossRef]
- Rizan, M.Z.M.; Tan, F.L.; Tso, C.P. An Experimental Study of N-Octadecane Melting inside a Sphere Subjected to Constant Heat Rate at Surface. Int. Commun. Heat. Mass. Transf. 2012, 39, 1624–1630. [Google Scholar] [CrossRef]
- Ismail, K.A.R.; Moura, L.F.; Lago, T.; Lino, F.A.M.; Nobrega, C. Experimental Study of Fusion and Solidification of Phase Change Material (PCM) in Spherical Geometry. In Proceedings of the 12th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics, Costa de Sol, Spain, 11–13 July 2016; pp. 1243–1248. [Google Scholar]
- Sattari, H.; Mohebbi, A.; Afsahi, M.M.; Azimi Yancheshme, A. CFD Simulation of Melting Process of Phase Change Materials (PCMs) in a Spherical Capsule. Int. J. Refrig. 2017, 73, 209–218. [Google Scholar] [CrossRef]
- Agarwal, A.; Sarviya, R.M. An Experimental Investigation of Shell and Tube Latent Heat Storage for Solar Dryer Using Paraffin Wax as Heat Storage Material. Eng. Sci. Technol. Int. J. 2016, 19, 619–631. [Google Scholar] [CrossRef]
- Fahmy, M.; Morsy, M.; Abd Elshakour, H.; Belal, A.M. Effect of Thermal Insulation on Building Thermal Comfort and Energy Consumption in Egypt. J. Adv. Res. Appl. Mech. 2018, 43, 8–19. [Google Scholar]
- Izadi, M.; Saleem, A.; Alshehri, H.M.; Ambreen, T.; Yasuri, A.K. Influence of Geometric Parameters on the Charging process of PCM in Semi-circular thermal storages for energy management. Environ. Sci. Pollut. Res. 2023, 30, 59765–59780. [Google Scholar] [CrossRef] [PubMed]
- Esapour, M.; Hosseini, M.J.; Ranjbar, A.A.; Pahamli, Y.; Bahrampoury, R. Phase Change in Multi-Tube Heat Exchangers. Renew. Energy 2016, 85, 1017–1025. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Hosseini, M.J.; Ranjbar, A.A.; Rahimi, M.; Bahrampoury, R. Melting Process Investigation of Phase Change Materials in a Shell and Tube Heat Exchanger Enhanced with Heat Pipe. Renew. Energy 2019, 138, 378–394. [Google Scholar] [CrossRef]
- Khalaf, A.F.; Rashid, F.L.; Basem, A.; Abbas, M.H. Numerical analysis in a lid-driven square cavity with hemispherical obstacle in the bottom. Math. Model. Eng. Probl. 2022, 9, 1639–1647. [Google Scholar] [CrossRef]
- Jesumathy, S.; Udayakumar, M.; Suresh, S. Experimental Study of Enhanced Heat Transfer by Addition of CuO Nanoparticle. Heat. Mass. Transf. 2012, 48, 965–978. [Google Scholar] [CrossRef]
- Harikrishnan, S.; Kalaiselvam, S. Preparation and Thermal Characteristics of CuO–Oleic Acid Nanofluids as a Phase Change Material. Thermochim. Acta 2012, 533, 46–55. [Google Scholar] [CrossRef]
- Rashid, F.L.; Basem, A.; Khalaf, F.A.A.; Abbas, M.H.; Hashim, A. Recent Breakthroughs and Improvements in Phase Change Material Melting in a Triple-Tube Thermal Storage Unit. Rev. Compos. Mater. Av. 2022, 32, 295. [Google Scholar] [CrossRef]
- Tiji, M.E.; Al-Azzawi, W.K.; Mohammed, H.I.; Dulaimi, A.; Rashid, F.L.; Mahdi, J.M.; Majdi, H.S.; Talebizadehsardari, P.; Ali, H.M. Thermal Management of the Melting Process in a Latent Heat Triplex Tube Storage System Using Different Configurations of Frustum Tubes. J. Nanomater. 2022, 2022, 7398110. [Google Scholar] [CrossRef]
- Uniyal, A.; Prajapati, Y.K. Impact of Fin Arrangement on Heat Transfer and Melting Characteristics of Phase Change Material. J. Therm. Sci. 2024, 33, 435. [Google Scholar] [CrossRef]
- Kong, D.; Zhang, Y.; Liu, S. Enhanced Natural Convection for Accelerating Melting of Phase Change Material in Cellular Structure through Inserting Fin. J. Therm. Sci. 2024, 33, 548. [Google Scholar] [CrossRef]
- Mao, Q.; Zhu, Y.; Li, T. Heat Storage Performance of PCM in a Novel Vertical Pointer-Shaped Finned Latent Heat Tank. J. Therm. Sci. 2024, 33, 422. [Google Scholar] [CrossRef]

| Property | Value | Units |
|---|---|---|
| Density (liquid, solid), | 760 | kg/m3 |
| Specific heat capacity, Cp | 2000 | J/(kg·K) |
| Thermal conductivity, k | 0.2 | W/(m·K) |
| Latent heat L | 165,000 | J/kg |
| Dynamic viscosity, | 0.02351 | kg/(m·s) |
| Thermal expansion coefficient | 0.0005 | 1/K |
| Solid temperature | 311 | K |
| Liquid temperature | 315 | K |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalaf, A.F.; Rashid, F.L.; Letif, S.A.; Ameen, A.; Mohammed, H.I. A Numerical Study of the Effect of Water Speed on the Melting Process of Phase Change Materials Inside a Vertical Cylindrical Container. Appl. Sci. 2024, 14, 3212. https://doi.org/10.3390/app14083212
Khalaf AF, Rashid FL, Letif SA, Ameen A, Mohammed HI. A Numerical Study of the Effect of Water Speed on the Melting Process of Phase Change Materials Inside a Vertical Cylindrical Container. Applied Sciences. 2024; 14(8):3212. https://doi.org/10.3390/app14083212
Chicago/Turabian StyleKhalaf, Abbas Fadhil, Farhan Lafta Rashid, Shaimaa Abdel Letif, Arman Ameen, and Hayder I. Mohammed. 2024. "A Numerical Study of the Effect of Water Speed on the Melting Process of Phase Change Materials Inside a Vertical Cylindrical Container" Applied Sciences 14, no. 8: 3212. https://doi.org/10.3390/app14083212








