Abstract
In this study, the chemical composition of essential oils (EOs) extracted from Origanum vulgare ssp. hirtum Lamiaceae, (oregano), Salvia officinalis Lamiaceae (sage), Mentha pulegium Lamiaceae (pennyroyal), and respective hydrosols (HSs) has been investigated by Gas Chromatography–Mass Spectrometry (GC-MS). The antimicrobial activity was assessed against two oral pathogens: Gram-positive bacterium Streptococcus mutans and the fungus Candida albicans by determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal/Fungicidal concentration (MBC/MFC). Three-fold diluted solutions were dispensed into each well of a 96-well microtiter plate and, after incubation, MIC was determined by visual monitoring. The MBC/MFC was determined by transferring a small quantity of sample contained in each replicate well of the microtiter plates to appropriate culture media using a microplate replicator. The EOs of the tested herbs showed antimicrobial properties, especially the EO oil of O. vulgare, which exerted the highest antimicrobial activity. HSs of S. officinalis and M. pulegium exerted no antimicrobial activity, in contrast to oregano HS, which displayed strong antimicrobial activity. In all cases, a higher number of compounds were detected in EOs than in the corresponding HSs. The major compounds of sage EO were detected to be α-thujone (25.1%), 1,8-cineole (15.8%) and β-pinene (10.0%), while the HS was characterized by the presence of 1,8-cineole (32.6%), borneol (22.6%) and α-thujone (22.4%). Pennyroyal EO and HS consists mainly of pulegone (62.1 and 50.6%, respectively). Carvacrol was the major component present in EO (63%) and HS (97.3%) of oregano, probably contributing to the antimicrobial activity. Further research is needed in order to elucidate the antimicrobial mechanisms of specific compounds present in essential oils and hydrosols of Lamiaceae grown in Greece against oral pathogens.
Keywords:
Greek Lamiaceae; oregano; sage; pennyroyal; essential oil; hydrosol; carvacrol; antimicrobial activity; oral pathogen 1. Introduction
The oral cavity has been associated with several chronic diseases. There is emerging evidence that periodontitis is caused by oral pathogens, and it might be considered a risk factor for cardio-renal morbidity and the development of diabetes mellitus [1]. One of the most common diseases associated with significant economic impact is dental caries [2]. S. mutans is a Gram-positive, facultatively anaerobic bacterium, which is a very common cause of dental caries and other infections of organs such as the heart, skin, joints and central nervous system [2]. S. mutans is capable of synthesizing an extracellular polymer (designated as glucan) from sucrose, thus forming biofilms and allowing colonization of teeth. It is a lactic acid bacterium producing organic acid from a wide range of carbohydrates [3].
C. albicans is a yeast-like fungus causing opportunistic infections of the oral cavity (candidiasis), and is one of the most common human fungal infections [4]. C. albicans is able to metabolize glucose under both aerobic and anaerobic conditions, as well as to produce exopolysaccharides forming biofilms on dental surfaces [5,6]. Both C. albicans and S. mutans are able to interact and form dental plaque biofilm associated with early childhood caries [6]. Therefore, both microorganisms should simultaneously be a target for antimicrobial agents.
The growing bacterial resistance to antibiotics is a widespread problem nowadays [7], as well as the resistance of Candida strains to the few available antifungal agents [8]. Furthermore, bacterial resistance to the oral antiseptic chlorhexidine has been significantly increased [9]. Essential oils (EOs), as well as hydrosols (HSs), of medicinal and aromatic plants have demonstrated high antibacterial and antifungal activity against multidrug resistance strains [10,11,12,13]. These properties make EOs ideal candidates for replacing chemical antiseptics like chlorhexidine, which is commonly used in mouthwash and toothpaste against oral pathogens [9].
EOs contain numerous terpenoid and aliphatic compounds. An essential oil usually contains two or three major compounds, ranging from 20% to 70%. Despite the fact that 20 to 60 different compounds can be detected in EOs [14], the most abundant compounds exert antimicrobial, anti-inflammatory and antioxidant activities [15,16,17].
HSs are obtained during essential oil distillation from medicinal and aromatic plants. HSs consist of the condensing the water of the distillation process, and by water and non-water-soluble, polar and non-polar components. Less than 1 g/L of EOs constituent is dissolved in hydrosols during the distillation, thus contributing to their biological activity and organoleptic characteristics. HSs’ chemical composition and active compounds might be divergent from the extracted essential oil [13]. HSs have demonstrated antimicrobial activity, depending on their composition. HSs are implemented in food industry, as well as cosmetics and perfume industries [18].
Greek oregano (O. vulgare spp. hirtum), European pennyroyal (Menta pulegium), and common sage (S. officinalis) are aromatic plants belonging to Lamiaceae, and they are cultivated and widely used worldwide, due to their pharmaceutical properties [19,20,21,22]. Several studies reported the antimicrobial activity of their EOs [21,23,24,25,26,27,28,29], while the antimicrobial activity of their HSs has not been extensively studied [30]. The aim of this study was to investigate the chemical profile and the antimicrobial activity exerted by essential oils and their respective hydrosols of three important medicinal and aromatic plants grown in Thessaly against oral pathogens for the first time, thus evaluating their potential use in oral hygiene products.
2. Materials and Methods
2.1. Plant Materials
The plant material of O. vulgare ssp. hirtum, S. officinalis and M. pulegium (Figure 1) were cultivated in Thessaly, Central Greece (39°22′18.1″ N 22°58′26.2″ E). Oregano plants were aged 2 years, sage plants were aged 4 years and pennyroyal plants were aged 1 year. No fertilizer was applied during the growing season. The oregano and sage plants were not watered. Regarding the pennyroyal plant, watering was necessary twice per week during dry season. The oregano, sage and pennyroyal were harvested in July, May and September, respectively, at full blossom.

Figure 1.
Plants used in this study. (a) O. vulgare ssp. hirtum, (b) S. officinalis, (c) M. pulegium. Photo records by Alexandros Bairamis.
2.2. Isolation of Essential Oils and Hydrosols
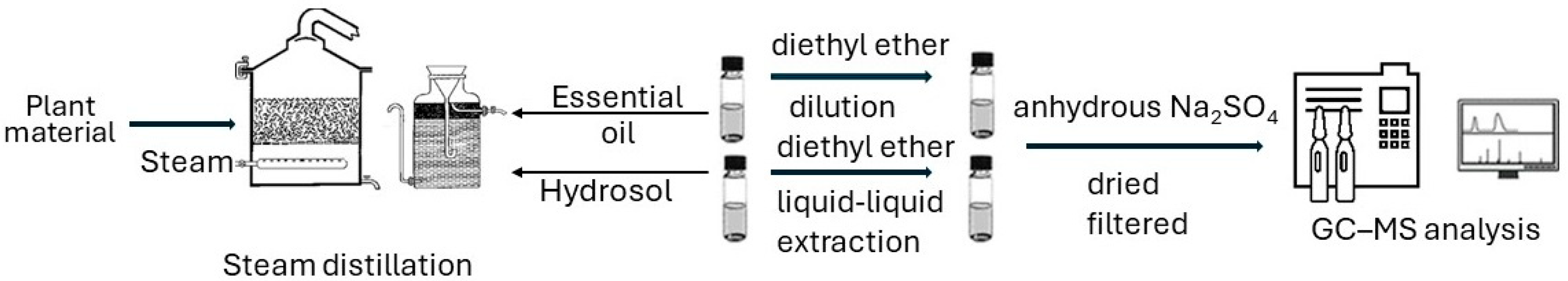
The essential oils (EOs) of herbs were isolated by steam distillation right after harvesting [31,32]. The distillation process was carried out with a steam distiller of two-hundred-liter capacity, and lasted 3 h for the O. vulgare. From 80 kg of fresh herb, 500 mL of essential oil and 22 L of hydrosol were produced [33]. S. officinalis was distilled for 2 h. From 70 kg of fresh herb, 120 mL of essential oil and 15 L of hydrosol were produced [34,35]. M. pulegium was distilled for 2 h. From 80 kg of fresh herb, 150 mL of essential oil and 15 L of hydrosol were produced [36]. The essential oils were stored in dark glass bottles at 4 °C.
The hydrosols (HSs) recovered after the steam distillation of the herbs were extracted three times (Figure 2) using diethyl ether in a separatory funnel by a liquid–liquid extraction procedure. The organic solvent was removed via nitrogen gas flow to acquire the volatile oil. Then, HSs were dried using anhydrous Na2SO4, filtered, and stored under the same conditions as the EOs.
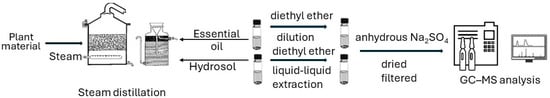
Figure 2.
Flow chart from plant material to the GC-MS analysis of the EOs and HSs extracts.
2.3. Gas Chromatography–Mass Spectrometry (GS-MS) Analysis of EOs and HDs of Herbs
The separation and identification of EOs and HSs volatile compounds was carried out using a gas chromatograph (Trace Scientific GC Ultra, Thermo Scientific, Ltd., Waltham, MA, USA), equipped with a column (30 m length, 0.25 mmID, 0.25 um film’s thickness), combined with a mass spectrometer (Thermo-5MS, Thermo Scientific, Ltd., Waltham, MA, USA). The samples were analyzed according to chromatographic conditions, as previously described, with some modifications [37]. The carrier gas was Helion (He), with a 1 m/min flow. The column temperature was initially at 60 °C, and increased gradually up to 250 °C on a 3 °C/min rate (the duration of each analysis was 63.33 min). The samples were introduced manually by the spitless mode in the impot system (GC) at 220 °C. The detector MS was set in the electron impact mode (70 ev) at a temperature of 260 °C (MS transfer line). A mixture of n-alkanes (C8-C20) was analyzed with the same method of analysis, in order to calculate the Retention Index of each compound, according to [38]. The identification of volatile compounds of EOs and HSs was based on the matching of its calculated RI and mass spectrum with data of spectral libraries (Adams07, NIST 08 and X-calibur, version 4.1), and data of bibliography. The quantification (%) was made by calculating the ratio between each chromatographic peak area and the sum of total peak area of compounds.
All data (chromatographic and spectroscopic) were recovered by X-Calibur software, version 4.1.
2.4. Streptococcus mutans and Candida albicans Strains and Growth Conditions
The S. mutans strain was isolated identified and characterized by standard laboratory methods (kindly provided by Professor Elizabeth Koulaouzidou, School of Dentistry, Aristotle University, Thessaloniki, Greece). S. mutans was routinely grown in Brain Heart Infusion (BHI) broth or agar (Conda, Madrid, Spain) according to CLSI (former NCCLS) guidelines.
C. albicans was identified and characterized by standard laboratory methods (kindly provided by Professor Timoleon-Achilleas Vyzantiadis, School of Medicine, Aristotle University, Thessaloniki, Greece). C. albicans was routinely grown in RPMI 1640 supplemented with w/25 mM Hepes w/L-Glutamine (Biosera, Cholet, France) or Sabouraud dextrose agar (Neogen, Heywood, UK) according to the CLSI (former NCCLS) guidelines.
2.5. Determination of S. mutans Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
Determination of MIC of HSs, EOs and 98% pure carvacrol (Sigma-Aldrich, Steinheim am Albuch, Germany) was carried out in sterile 96-well polystyrene microtiter plates (KiskerBiotech GmbH& Co. KG, Steinfurt, Germany). Bacterial cultures grown overnight in BHI broth were incubated under anaerobic conditions in an AnaeroJar AG25, implementing the AnaeroGen Atmosphere Generation system (Oxoid, Basingstoke, UK), at 37 °C for 24 h. Resazurin sodium salt was used as an indicator of anaerobic conditions (Alfa Aesar, ThermoFisher GmbH, Munich, Germany) (30 mg/lt). Bacterial culture was adjusted to 0.5 McFarland turbidity standard (~1.5 × CFUs/mL). HSs were diluted in BHI broth at different concentrations ranging from 100% to 10% (v/v). EOs samples and carvacrol were diluted in sterile and distilled water with Tween-20 (BDH Chemicals Ltd., Poole, UK) at concentration 0.5% v/v, then diluted in BHI broth at different concentrations ranged from 0.5% to 0.0175% (v/v). Subsequently, approximately 5 × CFUs/mL in 10 μL BHI broth was added to 190 μL of the tested diluted HSs, EOs and carvacrol and were incubated under anaerobic conditions at 37 °C for 24 h. Positive control wells containing BHI broth inoculated with bacteria tested the growth of the pathogen. Similarly, control wells containing 0.5% v/v Tween-20 and BHI broth inoculated with bacteria tested the growth of the pathogen in the presence of the detergent. Negative control wells containing hydrosols, EOs, carvacrol and Tween-20 dilutions in BHI broth without bacteria were included. Negative control wells containing only BHI broth were used to test for possible contamination. The MIC was determined as the lowest concentration which results in 100% growth inhibition [39]. MICs were determined in triplicate in at least two-independent experiments.
The lowest concentration of any antibacterial agent that could kill the tested bacteria is considered the Minimum Bactericidal Concentration (MBC). The MBC was determined by transferring a small quantity of sample contained in each replicate well of the microtiter plates to BHI agar plates by using a microplate replicator (Boekel Scientific, Feasterville-Trevose, PA, USA). The plates were incubated at 37 °C for 24 h under anaerobic conditions [40].
2.6. Determination of C. albicans Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
The susceptibility test of C. albicans was performed based on the M27-A2 method [41] with some modifications. Determination of the minimum inhibitory concentration (MIC) of hydrosols and EO and carvacrol was carried out in sterile 96-well polystyrene microtiter plates (Kisker Biotech GmbH & Co. KG, Steinfurt, Germany). Fungal cultures were grown at 35 °C for 24 h in Sabouraud dextrose agar (Neogen, Lansing, MI, USA) plus chloramphenicol at 50 mg/L (Serva, Heidelberg, Germany). The inoculum was prepared by suspending C. albicans in saline, and was adjusted to 0.5 McFarland turbidity standard (~1.5 × 108 CFUs/mL). Cell suspensions were further diluted with RPMI 1640 medium (Biosera, Cholet, France), in a 1:20 ratio. HSs were diluted in RPMI 1640 at different concentrations ranging from 100% to 10% (v/v), decreasing by 5% v/v each time. EOs samples and carvacrol were diluted in sterile and distilled water with Tween-20 (Serva, Heidelberg, Germany) at concentration 0.5% v/v, then diluted in RPMI 1640 at different concentrations ranging from 0.5% to 0.0175% (v/v). A total of 10 μL of cell suspension was added to 190 μL of the tested diluted hydrosols, EO, and carvacrol, and incubated at 35 °C for 48 h. Positive control wells containing RPMI 1640 were inoculated only with C. albicans. Similarly, control wells containing 0.5% v/v Tween-20 and RPMI 1640 broth inoculated with bacteria tested the growth of the pathogen in the presence of the detergent. Negative control wells contained hydrosols, EO, carvacrol and Tween-20 dilutions in RPMI 1640 without inoculated fungus. Furthermore, control wells containing only RPMI 1640 were used to assess possible contamination. MIC was the lowest concentration of our tested sample that inhibited visual growth of C. albicans [41]. MICs were determined in triplicate in at least two independent experiments.
The lowest concentration of any antifungal agent that might kill tested fungus is considered the Minimum Fungicidal Concentration (MFC). MFC was determined by transferring a small quantity of sample contained in each replicate well of the microtiter plates to Sabouraud dextrose agar plus chloramphenicol plates by using a microplate replicator. The plates were incubated at 35 °C for 48 h. The MFC was determined as the lowest concentration of tested sample at which no fungal growth was observed.
3. Results and Discussion
3.1. Essential Oil and Hydrosol Extract Chemical Profile Implementing GS-MS Analysis
The chemical profile of O. vulgare ssp. hirtum, S. officinalis and M. pulegium essential oils (EOs) and hydrosols (HSs) were determined by GC-MS analysis. The chemotype of EOs described in the literature and all identified compounds in this study, as well as their relative percentage (%), are presented in Table 1 and Table 2.
3.1.1. O. vulgare Essential Oil and Hydrosol Extract Chemical Profile
The essential oil of O. vulgare contained eight major compounds, comprising 92.2% of the total EO. The major compound was carvacrol (63.0%), followed by its precursors p-cymene (11.8%) and γ-terpinene (8.4%). Other compounds were detected at relatively low levels: β-myrcene (2.0%), α-pinene and E-caryophyllene (1.9%), thymol (1.8%), and α-terpinene (1.6%). Similar findings have been reported by other studies, demonstrating that the essential oil of O. vulgare, cultivated in Greece, consists of mainly carvacrol, p-cymene, γ-terpinene, while α-terpinene, thymol, E-caryophyllene, α-pinene were reported as minor constituents [19,20,42,43]. Baranauskiene et al. [23], reported that this oregano subspecies, which is characterized by carvacrol chemotype (72.4–88.2%), contains its isomer thymol in low percentages or traces, which is typical for Greek oregano (O. vulgare spp. hirtum) [44,45]. In contrast, other studies that reported the chemical profile of oregano derived from other Mediterranean areas showed differences in chemotype of O. vulgare (Table 1). The main compound of Italian O. vulgare EO was trans-sabinene hydrate [24], and was thymol and γ-terpinene for Sicilian [46]. These data demonstrate that the chemotype of carvacrol Origanum vulgare spp. hirtum might be detected mainly in Greece.
As shown in Table 2, the O. vulgare HS was consisted almost entirely of carvacrol at (97.3%). Of note, carvacrol was detected in O. vulgare HS, apparently in a much higher percentage compared to EO. This profound difference could be attributed to the fact that some non-polar molecules, especially terpene hydrocarbons (α-pinene, α-myrcene, o-cymene, α- and β-terpinene), are detected only in EOs [47].

Table 1.
Chemotype of O. vulgare ssp. hirtum, S. officinalis and M. pulegium essential oil from according to country origin, as described in the literature.
Table 1.
Chemotype of O. vulgare ssp. hirtum, S. officinalis and M. pulegium essential oil from according to country origin, as described in the literature.
| Chemotype | Main Compounds | Country Origin | References |
|---|---|---|---|
| Origanum vulgare ssp. hirtum | |||
| Carvacrol | Carvacrol (80%), γ-terpinene (6.5%), p-cymene (2.5%), caryophyllene (2.5%) | Lithuania | [23] |
| Carvacrol/γ-terpinene | Carvacrol (30%), γ-terpinene (23%), p-cymene (10%) | Poland | [45] |
| trans-Sabinene hydrate/carvacrol | trans-Sabinene hydrate (31%), carvacrol (23%), 4-terpineol (10%), linalyl acetate (5%) | Italy | [24] |
| Thymol/γ-terpinene | Thymol (54%), γ-terpinene (13%), p-cymene (6.5%), α-terpinene (2%), β-myrcene (1.5%) | Italy | [46] |
| Salvia officinalis | |||
| α-Thujone/1,8-cineole/β-pinene | α-Thujone, 1,8-cineole, β-pinene (16%), β-caryophyllene (9%), α-humulene (8.5%), borneol (6%), β-thujone (4%), camphene (2%) | Germany | [48] |
| Inflorescence: β-thujone/1,8-cineole/camphor/borneol, Leaves: α-thujone/β-pinene/1,8-cineole | Inflorescence: β-thujone (15%), 1,8-cineole (15%), camphor (13%), borneol (10%), α-thujone (6%), ledol (6%), β-pinene (3%), Leaves: α-thujone (20%), β-pinene (15%), 1,8-cineole (15%), ledol (8%), borneol (7%), β-thujone (6%) | China | [49] |
| Inflorescence: α-thujone/E-caryophyllene/manool, Leaves: α-thujone/1,8-cineole/camphor, Stems: α-thujone/manool | α-Thujone (20–42%), (E)-caryophyllene (1–16%), manool (4–15%), viridiflorol (3–13%), 1,8-cineole (3–14%), camphor (1–22%), borneol (1–5%), α-humulene (1.5–4.5%), β-pinene (1–4%), β-thujone (1–4%) | India | [50] |
| Camphor/α-thujone | Camphor (24%), α-thujone (23%), sclareol (10%), camphene (9%), β-thujone (8%) | Egypt | [51] |
| α-Thujone/camphor/viridiflorol | α-Thujone (22%), camphor (12%), viridiflorol (12%), manool (9%), 1-octen-3-ol (8%), 1,8-cineol (7%), β-thujone (5.5%) | Romania | [52] |
| α-Thujone/1,8-cineole/borneol | α-Thujone (25%), 1,8-cineole (15%), borneol (11%), camphor (11%), β-pinene (10%), δ-gurjunene (8%) | South Brazil | [27] |
| Camphor/1,8-cineole | Camphor (34%), 1,8-cineole (22%), α-thujone (21%), camphene (5%), β-thujone (4%), borneol (3%), α-pinene (2%), p-cymene (1%), β-pinene (1%) | Tunisia | [53] |
| α-Thujone/camphor/caryophyllene | α-Τhujone (8–20%), camphor (8–20%), borneol (3–17%), γ-muurolene (3–4%), sclareol (6–23%). | Italy | [22] |
| Mentha pulegium | |||
| Pulegone | Pulegone (71%), neo-menthol (11%), iso-pulegol (2%), piperitenone (1.5%) | Algeria | [21] |
| Menthone/pulegone | Menthone (36%), pulegone (23%), neo-menthol (9%), 8-hydroxy-δ-4(5)-p-menthen-3-one (2%) | Portugal | [54] |
| Pulegone | Pulegone (75%), D-limonene (9%), 2-(2,2,4-trimethyl-3-cyclopenten-1-yl) ethanol (5%), verbenone (3%) | Morocco | [55] |
| Pulegone/menthone | Pulegone (41%), menthone (21%), α-terpineol (8%), humulene (5%) | Morocco | [25] |
| Pulegone/α-terpinyl acetate | Pulegone (34%), α-terpinyl acetate (24%),bicyclo [3.1.0] hexane, 6-isopropylidene-1-methyl (13%), 1,8-cineole (10%), α-humulene (5%), α-pinene (5%) | Morocco | [26] |
| Pulegone, pulegone/piperitenone oxide, piperitenone oxide/trans-piperitone epoxide, pylegone/1,8-cineole, pulegone/limonene, pulegone/menthone, pulegone/piperitenone/ment-hone, 1,8-cineole/trans-piperitone epoxide | Pulegone (2.5–52%), piperitenone oxide (0.2–45%), trans-piperitone epoxide (0–29%), 1,8-cineole (0–33%), limonene (0–34%), menthone (0.2–30%), piperitenone (0.2–13%), caryophyllene oxide (0.2–8%), neo-iso-menthol (0.4–8%), menthol (0.2–5%) | Iran | [56] |

Table 2.
Chemical composition of Origanum vulgare ssp. hirtum, Salvia officinalis and Mentha pulegium essential oils and hydrosols.
Table 2.
Chemical composition of Origanum vulgare ssp. hirtum, Salvia officinalis and Mentha pulegium essential oils and hydrosols.
| No. | Compounds * | RI | LRI | Relative Percentage Area (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| EOs | HSs | ||||||||
| OVH | SO | MP | OVH | SO | MP | ||||
| 1 | α-Pinene | 939 | 932 | 1.9 | 2.9 | - | - | - | - |
| 2 | Camphene | 957 | 946 | - | 2.0 | - | - | - | - |
| 3 | 3-Octanol | 970 | 988 | - | - | 2.5 | - | - | - |
| 4 | β-Pinene | 984 | 974 | - | 10.0 | - | - | - | - |
| 5 | β-Myrcene | 990 | 988 | 2.0 | - | - | - | - | - |
| 6 | α-Terpinene | 1020 | 1014 | 1.6 | - | - | - | - | - |
| 7 | o-Cymene | 1032 | 1022 | 11.6 | - | - | - | - | - |
| 8 | 1,8-Cineole | 1040 | 1026 | - | 15.8 | - | - | 32.6 | - |
| 9 | γ-Terpinene | 1063 | 1054 | 8.4 | - | - | - | - | - |
| 10 | α-Thujone | 1113 | 1101 | - | 25.1 | - | - | 22.4 | - |
| 11 | β-Thujone | 1126 | 1112 | - | 5.0 | - | - | 3.4 | - |
| 12 | Menthone | 1132 | 1148 | - | - | 7.8 | - | - | 2.6 |
| 13 | Isomenthone | 1134 | 1158 | - | - | 7.7 | - | - | 1.0 |
| 14 | Camphor | 1160 | 1141 | - | 2.9 | - | - | 11.3 | - |
| 15 | Borneol | 1181 | 1165 | - | 5.1 | - | - | 22.6 | - |
| 16 | Terpinen-4-ol | 1187 | 1174 | - | - | - | - | 3.0 | - |
| 17 | Pulegone | 1213 | 1133 | - | - | 62.1 | - | - | 50.6 |
| 18 | Piperitone | 1230 | 1249 | - | - | 11.3 | - | - | 32.4 |
| 19 | Thymol | 1296 | 1289 | 1.8 | - | - | - | - | - |
| 20 | Carvacrol | 1269 | 1298 | 63.0 | - | - | 97.3 | - | - |
| 21 | E-Caryophyllene | 1419 | 1417 | 1.9 | 7.8 | - | - | - | - |
| 22 | α-Humulene | 1475 | 1452 | - | 7.8 | - | - | - | - |
* Compounds are presented according to elution order from Thermo-5MS column. RI: Retention Index from calculation, LRI: Retention Index from literature. EOs: essential oils, HSs: hydrosols, OVH: O. vulgare ssp. hirtum, SO: S. officinalis, MP: M. pulegium.
3.1.2. S. officinalis Essential Oil and Hydrosol Extract Chemical Profile
In total, ten major compounds were detected in S. officinalis EO, accounting for 84.4% of the total essential oil. The most abundant compounds, representing 50% of essential oil, were α-thujone (25.1%), 1,8-cineole (15.8%) and β-pinene (10.0%). Moreover, E-caryophyllene and α-humulene were found at equal and lower percentage (7.8%), as well as β-thujone and borneol (5.0%). The lowest percentages corresponded to α-pinene and camphor (2.9%), as well as camphene (2.0%). These compounds were commonly found in the essential oil of S. officinalis, though with different percentages depending on the season, geographic origin, environmental factors, extraction methods, plant organs, sampling techniques, and genetic differences. In agreement with this study, Tibaldi et al. [48], reported that the EO of S. officinalis inflorescence cultivated in Germany (Table 1) was mainly composed of α-thujone, 1,8-cineole, β-pinene and E-caryophyllene. In addition, Li et al. [49] attributed the difference regarding the chemical composition of S. officinalis EO to the part of the plant. The compounds β-thujone, 1,8-cineole and camphor were most abundant in the EO of S. officinalis leaves, while α-thujone, β-pinene and 1,8-cineole were most abundant in the EO of S. officinalis flowers. Furthermore, in another study of whole aerial parts of S. officinalis from India, the percentages of α-thujone, 1,8-cineole, E-caryophyllene, viridiflorol, β-pinene and borneol were found to be higher in the essential oil. Similarly, it was reported that the total content of thujone was lower in inflorescence in comparison to stems, contrary to the observed trend of β-pinene accumulation [50]. On the other hand, data presented in previous studies on S. officinalis EO indicated that the most abundant constituents were α-thujone and camphor [51,52]. Moreover, recent studies of S. officinalis cultivated in South Brazil [27] and Tunisia [29,53], reported that the EOs mainly contained α-thujone, 1,8-cineole and camphor. Russo et al. [22] reported that the most abundant compounds in EO samples of S. officinalis cultivated in Italy were α-thujone, camphor, borneol, γ-murolene and sclareol. Besides well-explained differences among Salvia species, intra-species variability is known to affect chemical composition. Chemical composition is also affected by geographical origin, plant part, phenological stage and culture conditions [57]. It has been previously reported that clary sage under water stress exhibited reduced growth and altered chemical composition than clary sage under regular irrigation [58]. Moreover, different chemotypes of S. officinalis might be attributed to the plant age and drying process [48]. Usano-Alemany et al. conducted an analysis of essential oils extracted from eleven mother plants of Spanish sage, some of them collected in the wild and the rest cultivated, over four years at three different phenological stages, and they concluded that the chemical composition was strongly affected by the genotype. It was also clear that the year of harvest significantly affects EO chemical profile, while the phenological stage of the plant was not that important [59].
The chemical profile of S. officinalis HS showed six identified compounds accounting for roughly 95.3% of the overall extract. The most abundant compounds were 1,8-cineole (32.6%), borneol (22.6%), α-thujone (22.4%) and camphor, alongside β-thujone (3.4%) and terpinene-4-ol (3.0%). In the case of S. officinalis, in HSs, fewer compounds were identified, and in higher percentages relative to the EO. Of note, only in S. officinalis HS was terpinene-4-ol found, while α-pinene, camphene, E-caryophyllene and α-humulene were not detected at all. This compound variability could be explained, besides the low water-solubility of terpene hydrocarbons (camphene, α-and β-pinene), because the rest of compounds are oxygenated terpenes exhibiting greater tendency to form hydrogen bonds, thus leading to higher solubility in hydrosol [60].
3.1.3. M. pulegium Essential Oil and Hydrosol Extract Chemical Profile
Five compounds were detected in EO of M. pulegium, accounting for 91.4% of the total oil. The most abundant compound was the monoterpene ketone, pulegone (62.1%). Piperitone was found to be the second most abundant compound (11.3%). The two isomers, methone and isomethone, were present at a lower percentage (7.8%), while 3-octanol was found at the lowest percentage (2.5%). According to previous studies, the chemical composition of M. pulegium EO, is qualitatively and quantitatively variable regarding the main compounds, depending on the region of cultivation. The GC-MS analysis of M. pulegium EO, from Algeria, produced by steam distillation showed that pulegone and neo-menthol were the major compounds, followed by menthone, cis-isopulegone and piperitenone [21]. Another study of M. pulegium EO, from Portugal, revealed that contained a complex mixture consisting mainly of menthone and pulegone, and to less extend by neo-menthol and 8-hydroxy-δ-4(5)-p-menthen-3-one [54]. In similar studies on the chemical composition of Moroccan M. pulegium EO, significant variability has been reported. One study reported that pulegone was the most abundant compound, followed by D-limonene, 2-(2,2,4-trimethyl-3-cyclopenten-1-yl) ethanol and verbenone [55], whereas in another study, pulegone and menthone were the major constituents, and 1,8-cineole, α-terpineol, verbenone and humulene were minor [25]. Mollaei et al. [56] studied EOs produced from twelve M. pulegium populations from Iran, and they reported that the most abundant compounds were pulegone, menthone, limonene, 1,8-cineol, piperitenone oxide and trans-piperitone epoxide. The variability of M. pulegium, chemotypes described in the literature, could be attributed to the growing conditions, drying process, harvest year, vegetation phase, and essential oil quality, as well as environmental conditions and plant parts that undergo distillation [48]. It is known that morphology, growth, yield, and secondary metabolite production in Mentha species are affected by (micro)climate [61]. Furthermore, the diversity and concentration of secondary metabolites of M. pulegium could be affected by plant growth factors such as salinity, irrigation, and genetic background [62].
Four compounds were detected in M. pulegium HS, accounting for 86.6% of the total extract. The major compounds were pulegone (50.6%) and piperitone (32.4%), while the isomers menthone (2.6%) and isomenthone (1.0%) were found to be minor compounds. Comparison of the chemical composition of EOs and HSs of herbs showed that EOs contained more compounds, and several studies are in agreement with that [18,47,63]. The chemical profile of M. pulegium HS was the same with the respective EO, but in lower proportions, with the notable exception the absence of 3-octanol. Pulegone, piperitone, menthone and isomenthone are monoterpene ketones with high polarity, thus hydrophilic tendency [18].
3.2. Antimicrobial Activity
Sage and pennyroyal HSs did not exert high enough antimicrobial activity against the tested oral pathogens so neither MIC nor MBC/MFC could be determined. However, oregano HS exerted antibacterial activity against both tested pathogens. MIC was determined at 25% v/v and 35% v/v for S. mutans and C. albicans, respectively. MFC value was equal to MIC for C. albicans, whereas MBC was determined at slightly higher value of 30% v/v for S. mutans (Table 3). On the contrary, all tested EOs demonstrated antimicrobial activity against both pathogens, ranging from 0.05% v/v to 0.45% v/v regarding MIC for S. mutans, and 0.05% v/v to 0.4% v/v regarding MIC for C. albicans. Similarly, MBC/MFC values were determined, ranging from 0.05% v/v to 0.45% v/v for S. mutans, and from 0.05% v/v to >0.5% v/v for C. albicans (Table 3). Apparently, oregano essential oil exerted the strongest antimicrobial activity against both tested pathogens. Sage EO demonstrated the second highest antibacterial activity against S. mutans (MIC 0.25% v/v and MBC at 0.35% v/v), whereas pennyroyal EO was the second most active against C. albicans (both MIC and MFC determined at 0.25% v/v).

Table 3.
MIC, MBC and MFC results. ND: Not determined.
The most abundant active substance carvacrol, present in oregano EO was tested as control for comparison. Both MIC and MBC were determined at 0.0315% v/v, corresponding to roughly 63% of carvacrol measured by GC-MS in oregano EO. Similarly, carvacrol MIC and MFC against C. albicans was determined at 0.0175% v/v and 0.0315% v/v, respectively.
The quest to develop novel antimicrobial agents against resistant microorganisms is continuous nowadays [64,65]. The EOs of aromatic and medicinal plants is a great source of antimicrobial and food preservative compounds [66]. EOs have been proposed for many applications, such as postharvest antifungal agents, against phytopathogenic bacteria, food preservatives, or even as compounds in active packaging [66,67,68,69]. Antimicrobial activity might be attributed to EO synthesis rich in terpenoids, alcoholic compounds, ketogenic bodies, phenols, aromatic phenols, and other secondary plant metabolites [66].The main issue regarding the application of EOs in the health, agriculture, cosmetics, and food industries is the compound volatility EOs extracted from the same herbal species, which might have a different chemical synthesis depending on the extraction method, climate diversity, or even seasonal variation [14,70].
Oregano EO exerted the highest antimicrobial activity. The very high antifungal activity of oregano EO against C. albicans compared to other EOs like cinnamon, thyme, and ginger, has been demonstrated in a previous study, whereas MIC and MFC values were ranging from 200 to 800 μg/mL regarding 60 C. albicans isolates (30 resistant and 30 susceptible to fluconazole), which are comparable to our results [71]. Our findings demonstrated the high antimicrobial activity exerted by carvacrol, present in oregano essential oil. MBC and MFC values at 0.05% v/v for the oregano essential oil which, according to GS-MS analysis, contained 63% carvacrol, was directly comparable to the MBC and MFC value at 0.03 % v/v of pure (98% v/v) carvacrol used as a control. Identification of carvacrol as the major antimicrobial compound in oregano EO might be used to form a classification system of antimicrobial activity exerted by oregano EOs of even different chemical compositions. However, other compounds present in oregano EO might negatively affect antimicrobial activity. A recent study on the antibacterial activity against Staphylococcus aureus demonstrated that thymol and carvacrol present in oregano EO exhibit antagonism [72]. Thymol and carvacrol ratio might vary in oregano essential oils [73], so it is imperative to conduct further antimicrobial studies on oregano EOs of different chemical composition.
Despite the fact that sage EO in this study has exerted lower antibacterial activity (MBC 0.35% v/v) compared to oregano EO, a recent study has demonstrated increased activity against Streptococcus pyogens biofilm formation [74]. Furthermore, a clinical study showed that an implemented sage extract reduced the colony number of S. mutans in dental plaque in 11–14 year old children [75]. Bacterial species like S. mutans adhere to the primary colonizers, mainly Streptococcus sanquis and Actinomyces viscosus, by cell-to-cell interactions, leading to biofilm formation on teeth [2]. Therefore, sage EO might be an essential compound in mouthwash solutions.
Pennyroyal essential oil in this study exerted lower antibacterial activity at 0.45% v/v than antifungal activity against C. albicans (0.25% v/v). Pennyroyal is an herb belonging to the Mentha genus. These herbs are widely used in EO production of high commercial value, exceeding USD 400 million. Moreover, Mentha species are used as additives in many spice mixtures used in foods because of their aroma and flavor [76]. The antimicrobial activity exerted by Mentha Eos, in combination with their intense aroma and flavor, make them attractive constituents in oral hygiene products. Nevertheless, according to European Medicines Agency (EMA), there is a maximum daily intake for pulegone, which is the major substance of pennyroyal essential oil (62.1%) [77]. Therefore, the implementation of pennyroyal EO should be limited, and the maximum daily intake should be taken into account in commercial product development.
Although hydrosols are considered distillation byproducts [18,78], they exert some biological properties. Hydrosols are chemically different regarding the volatile compounds compared to EOs [18]. In this study, GS-MS analysis demonstrated that the main oregano HS volatile component is carvacrol, which exerts strong antimicrobial activity [60]. This finding implies that oregano hydrosol might be an ingredient of oral hygiene products exerting antimicrobial activity.
Hydrosols from sage and pennyroyals have not demonstrated any antibacterial or antifungal activity. However, they contain active volatile components, as depicted in the GS-MS analysis. A common usage of HSs in cosmetics is to replace water by adding active ingredients [18]. In the case of a mouthwash solution produced by natural ingredients, hydrosols might have the same application. HSs could make more palatable the flavor of the product, and might increase its antioxidant activity. In that respect, the application of hydrosols from sage, thyme, and peppermint as mouthwash ingredients clearly reduced mucositis in patients undergoing chemotherapy in a randomized controlled pilot study [79]. Nevertheless, further studies (including in vivo studies and clinical trials) must be carried out in order to fully elucidate the spectra and the mechanisms of antimicrobial activity exerted by EOs and their respective hydrosols distilled from a plethora of herbs grown in regions characterized by high plant diversity and certain (micro)climates (especially xerothermic) in order to develop commercial products.
4. Conclusions
This study reported, for the first time, the detailed chemical profiles of EOs and HSs produced by three important Lamiaceae species cultivated in Thessaly (central Greece). Furthermore, this study demonstrated the high antimicrobial activity exerted by O. vulgare ssp. hirtum EO against oral pathogens, which is attributed mainly to carvacrol. Similarly, significant antimicrobial activity has been exerted by S. officinalis and M. pulegium EOs. Oregano HS was the only one that exerted antimicrobial activity. Nevertheless, GC-MS analysis of S. officinalis and M. pulegium HSs detected certain terpenes, which add flavor and aroma. Therefore, it is feasible that the EOs, as well as HSs, of the tested Lamiaceae species could be implemented to food and cosmetics industries as antimicrobial ingredients or preservatives. Our finding regarding the chemical composition and the in vitro antimicrobial activity of EOs and HSs against oral pathogens are promising, and could lead to oral hygiene product development. However, further in vivo studies, as well as clinical trials, should be conducted in order to assess the efficacy and safety of Lamiaceae EOs and HSs.
Author Contributions
Conceptualization, D.M. and P.T.; methodology, D.M.; investigation, A.B. and N.-S.D.S.; resources, P.T. and D.M.; formal analysis, C.T., P.T. and D.M.; writing—original draft preparation, A.B. and D.M.; writing—review and editing, A.B., N.-S.D.S., P.T. and D.M.; supervision, D.M. and P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kudiyirickal, M.G.; Pappachan, J.M. Diabetes mellitus and oral health. Endocrine 2015, 49, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus mutans, caries and simulation models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Pradebon Brondani, L.; Alves da Silva Neto, T.; Antonio Freitag, R.; Guerra Lund, R. Evaluation of anti-enzyme properties of Origanum vulgare essential oil against oral Candida albicans. J. Mycol. Med. 2018, 28, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, S.; Xiao, J.; Silva, B.B.; Gonzalez, I.; Agidi, P.S.; Klein, M.I.; Ambatipudi, K.S.; Rosalen, P.L.; Bauserman, R.; Waugh, R.E.; et al. Role of glucosyltransferase B in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Appl. Environ. Microbiol. 2011, 77, 6357–6367. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential Oils, A New Horizon in Combating Bacterial Antibiotic Resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, A.; Shokri, H.; Abbaszadeh, S. Interaction of carvacroland voriconazole against drug-resistant Candida strains isolated from patients with candidiasis. J. Mycol. Med. 2019, 29, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance toward chlorhexidine in oral bacteria-is there cause for concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef]
- Abdi-Moghadam, Z.; Mazaheri, Y.; Rezagholizade-shirvan, A.; Mahmoudzadeh, M.; Sarafraz, M.; Mohtashami, M.; Shokri, S.; Ghasemi, A.; Nickfar, F.; Darroudi, M.; et al. The significance of essential oils and their antifungal properties in the food industry: A systematic review. Heliyon 2023, 9, e21386. [Google Scholar] [CrossRef]
- Hou, T.; Sana, S.S.; Li, H.; Xing, Y.; Nanda, A.; Netala, V.R.; Zhang, Z. Essential oils and its antibacterial, antifungal and anti-oxidant activity applications: A review. Food Biosci. 2022, 47, 101716. [Google Scholar] [CrossRef]
- Solórzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef]
- D’Amato, S.; Serio, A.; López, C.C.; Paparella, A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control 2018, 86, 126–137. [Google Scholar] [CrossRef]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the antimicrobial activity and cytotoxicity of different components of natural origin present in essential oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.; Thangavelu, L. Salvia officinalis in dentistry. Dent. Hypotheses 2015, 6, 27–30. [Google Scholar] [CrossRef]
- Aćimović, M.; Tešević, V.; Smiljanić, K.; Cvetković, M.; Stanković, J.; Kiprovski, B.; Sikora, V. Hydrolates: By-products of essential oil distillation: Chemical composition, biological activity and potential uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Economoy, G.; Panagopoulos, G.; Tarantilis, P.; Kalivas, D.; Kotoulas, V.; Travlos, I.S.; Polysiou, M.; Karamanos, A. Variability in essential oil content and composition of Origanum hirtum L., Origanum onites L., Coridothymus capitatus (L.) and Satureja thymbra L. populations from the Greek island Ikaria. Ind. Crop. Prod. 2011, 33, 236–241. [Google Scholar] [CrossRef]
- Giannoulis, K.D.; Kamvoukou, C.A.; Gougoulias, N.; Wogiatzi, E. Irrigation and nitrogen application affect Greek oregano (Origanum vulgare ssp. hirtum) dry biomass, essential oil yield and composition. Ind. Crops Prod. 2020, 150, 112392. [Google Scholar] [CrossRef]
- Addelli, M.; Moghrani, H.; Aboun, A.; Maachi, R. Algerian Mentha pulegium L. leaves essential oil: Chemical composition, antimicrobial, insecticidal and antioxidant activities. Ind. Crop. Prod. 2016, 94, 197–205. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Baranauskiene, R.; Venskutonis, P.R.; Dambrauskiene, E.; Viškelis, P. Harvesting time influences the yield and oil composition of Origanum vulgare L. ssp. vulgare and ssp. hirtum. Ind. Crops Prod. 2013, 49, 43–51. [Google Scholar] [CrossRef]
- Semenzato, G.; Del Duca, S.; Vassallo, A.; Zaccaroni, M.; Mucci, N.; Greco, C.; Padula, A.; Castronovo, L.M.; Chioccioli, S.; Pistelli, L.; et al. Exploring the nexus between the composition of essential oil and the bacterial phytobiome associated with different compartments of the medicinal plants Origanum vulgare ssp. vulgare, O. vulgare ssp. hirtum, and O. heracleoticum. Ind. Crop. Prod. 2023, 191, 115997. [Google Scholar] [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Bakri, Y.; Talbaui, A.; Fellah, H.; Abrini, J.; Dakka, N. Chemical composition of Mentha pulegium and Rosmarinus officinalis essential oils and their antileishmanial, antibacterial and antioxidant activities. Microb. Pathog. 2017, 111, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Cherrat, L.; Espina, L.; Bakkali, M.; Pagan, R.; Laglaoui, A. Chemical composition, antioxidant and antimicrobial properties of Mentha pulegium, Lavandula stoechas and Satureja calamintha Scheele essential oils and an evaluation of their bactericidal effect in combined processes. Innov. Food Sci. Emerg. Technol. 2014, 22, 221–229. [Google Scholar] [CrossRef]
- Delamare, A.P.L.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Durović, S.; Micić, D.; Pezo, L.; Radić, D.; Bazarnova, J.G.; Smyatskaya, Y.; Blagijević, S. The effect of various extraction techniques on the quality of sage (Salvia officinalis L.) essential oil, expressed by chemical composition, thermal properties and biological activity. Food Chem. 2022, 13, 100213. [Google Scholar] [CrossRef]
- Hayouni, E.A.; Chraief, I.; Abedrabba, M.; Bouix, M.; Leveau, J.-Y.; Mohammed, H.; Hamdi, M. Tunisian Salvia officinalis L. and Schinus molle L. essential oils: Their chemical compositions and their preservative effects against Salmonella inoculated in minced beef meat. Int. J. Food Microbiol. 2008, 125, 242–251. [Google Scholar] [CrossRef]
- Saǧdiç, O.; Özcan, M. Antibacterial activity of Turkish spice hydrosols. Food Control 2002, 14, 141–143. [Google Scholar] [CrossRef]
- Valderrama, F.; Ruiz, F. An optimal control approach to steam distillation of essential oils from aromatic plants. Comput. Chem. Eng. 2018, 117, 25–31. [Google Scholar] [CrossRef]
- Masango, P. Cleaner production of essential oils by steam distillation. J. Clean. Prod. 2005, 13, 833–839. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Schlegel, V. Distillation time changes oregano essential oil yields and composition but not the antioxidant or antimicrobial activities. HortScience 2012, 47, 777–784. [Google Scholar] [CrossRef]
- Miguel, G.; Cruz, C.; Faleiro, M.L.; Simões, M.T.F.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Salvia officinalis L. essential oils: Effect of hydrodistillation time on the chemical composition, antioxidant and antimicrobial activities. Nat. Prod. Res. 2011, 25, 526–541. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Shiwakoti, S.; Poudyal, S.; Horgan, T.; Kovatcheva, N.; Dobreva, A. Essential oil yield and composition of garden sage as a function of different steam distillation times. HortScience 2014, 49, 785–790. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T. Effect of distillation time on mentha canadensis essential oil yield and composition. HortScience 2012, 47, 643–647. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Daferera, D.J.; Mitsi, C.; Trigas, P.; Polissiou, M.; Tarantilis, P.A. Comparative chemotype determination of Lamiaceae plants by means of GC–MS, FT-IR, and dispersive-Raman spectroscopic techniques and GC-FID quantification. Ind. Crops Prod. 2014, 62, 22–33. [Google Scholar] [CrossRef]
- Lucero, M.; Estell, R.; Tellez, M.; Fredrickson, E. A Retention Index Calculator Simplifi es Identifi cation of Plant Volatile Organic Compounds. Phytochem. Anal. 2009, 20, 378–384. [Google Scholar] [CrossRef]
- Tsavea, E.; Mossialos, D. Antibacterial activity of honeys produced in Mount Olympus area against nosocomial and foodborne pathogens is mainly attributed to hydrogen peroxide and proteinaceous compounds. J. Apic. Res. 2019, 58, 756–763. [Google Scholar] [CrossRef]
- Didaras, N.A.; Kafantaris, I.; Dimitriou, T.G.; Mitsagga, C.; Karatasou, K.; Giavasis, I.; Stagos, D.; Amoutzias, G.D.; Hatjina, F.; Mossialos, D. Biological properties of bee bread collected from apiaries located across Greece. Antibiotics 2021, 10, 555. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Haturvedi, V.; Espinel-Ingroff, A.; Ghannoum, M.A.; Gosey, L.L.; Odds, F.C.; Rex, J.H.; Rinaldi, M.G.; Sheehan, D.J.; Walsh, T.J.; et al. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. In Approved Standard—Second Edition Serving the World’ s Medical Science Community Through Voluntary Consensus; NCCLS: Wayne, PA, USA, 2002; Volume 22, ISBN 1562384694. [Google Scholar]
- Karamanos, A.J.; Sotiropoulou, D.E.K. Field studies of nitrogen application on Greek oregano (Origanum vulgare ssp. hirtum (Link) Ietswaart) essential oil during two cultivation seasons. Ind. Crop. Prod. 2013, 46, 246–252. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Oreopoulou, V. A kinetic study of essential oil components distillation for the recovery of carvacrol rich fractions. J. Appl. Res. Med. Aromat. Plants 2018, 9, 117–123. [Google Scholar] [CrossRef]
- Król, B.; Kołodziej, B.; Kędzia, B.; Hołderna-Kędzia, E.; Sugier, D.; Luchowska, K. Date of harvesting affects yields and quality of Origanum vulgare ssp. hirtum (Link) Ietswaart. J. Sci. Food Agric. 2019, 99, 5432–5443. [Google Scholar] [CrossRef] [PubMed]
- Węglarz, Z.; Kosakowska, O.; Przybył, J.L.; Pióro-Jabrucka, E.; Baczek, K. The Quality of Greek Oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. vulgare) Cultivated in the Temperate Climate of Central Europe. Foods 2020, 9, 1671. [Google Scholar] [CrossRef] [PubMed]
- Napoli, E.; Mazzaglia, A.; Restuccia, C.; Ragni, P.; Lanza, C.M.; Ruberto, G. The effect of γ-irradiation on chemical composition, microbial load and sensory properties of Sicilian oregano. Lwt 2016, 72, 566–572. [Google Scholar] [CrossRef]
- Moukhles, A.; Ellaghdach, A.; Driss, A.B.; El Amrani, M.A.; Aghmiz, A.; Mansour, A.I. Chemical profile and in vitro antibacterial potential of essential oils and hydrolat extracts from aerial parts of three wild species of Moroccan Thymus. Sci. African 2022, 18, e01434. [Google Scholar] [CrossRef]
- Tibaldi, G.; Hazrati, S.; Hosseini, S.J.; Ertani, A.; Bulgari, R.; Nicola, S. Cultivation techniques and drying process can affect the inflorescence essential oil composition of three selections of Salvia officinalis. Ind. Crops Prod. 2022, 183, 114923. [Google Scholar] [CrossRef]
- Li, B.; Zhang, C.; Peng, L.; Liang, Z.; Yan, X.; Zhu, Y.; Liu, Y. Comparison of essential oil composition and phenolic acid content of selected Salvia species measured by GC-MS and HPLC methods. Ind. Crops Prod. 2015, 69, 329–334. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A. Harvesting season and plant part dependent variations in the essential oil composition of Salvia officinalis L. grown in northern India. J. Herb. Med. 2015, 5, 165–171. [Google Scholar] [CrossRef]
- Abou Baker, D.H.; Amarowicz, R.; Kandeil, A.; Ali, M.A.; Ibrahim, E.A. Antiviral activity of Lavandula angustifolia L. and Salvia officinalis L. essential oils against avian influenza H5N1 virus. J. Agric. Food Res. 2021, 4, 100135. [Google Scholar] [CrossRef]
- Radulescu, V.; Chiliment, S.; Oprea, E. Capillary gas chromatography-mass spectrometry of volatile and semi-volatile compounds of Salvia officinalis. J. Chromatogr. A 2004, 1027, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Kammoun El Euch, S.; Hassine, D.B.; Cazaux, S.; Bouzouita, N.; Bouajila, J. Salvia officinalis essential oil: Chemical analysis and evaluation of anti- enzymatic and antioxidant bioactivities. S. Afr. J. Bot. 2019, 120, 253–260. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Batistam, I.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. European pennyroyal (Mentha pulegium) from Portugal: Chemical composition of essential oil and antioxidant and antimicrobial properties of extracts and essential oil. Ind. Crop. Prod. 2012, 36, 81–87. [Google Scholar] [CrossRef]
- Oualdi, I.; Elfazazi, K.; Azzouzo, H.; Oussaid, A.; Touzani, R. Chemical composition and antimicrobial properties of Moroccan Mentha pulegium L. essential oil. Mater. Today Proccedings 2023, 72, 3768–3774. [Google Scholar] [CrossRef]
- Mollaei, S.; Ebadi, M.; Hazrati, S.; Habibi, B.; Gholami, F.; Sourestani, M.M. Essential oil variation and antioxidant capacity of Mentha pulegium populations and their relation to ecological factors. Biochem. Syst. Ecol. 2020, 91, 104084. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phyther. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Laoutari, S.; Litskas, V.D.; Stavrinides, M.C.; Tzortzakis, N. Effects of water stress on lavender and sage biomass production, essential oil composition and biocidal properties against Tetranychus urticae (Koch). Sci. Hortic. 2016, 213, 96–103. [Google Scholar] [CrossRef]
- Usano-Alemany, J.; Palá-Paúl, J.; Herráiz-Peñalver, D. Essential oil yields and qualities of different clonal lines of Salvia lavandulifolia monitored in Spain over four years of cultivation. Ind. Crops Prod. 2016, 80, 251–261. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, N.A.; Mahmood, A.; Al-Kedhairy, A.A.; Alkhathlan, H.Z. The composition of the essential oil and aqueous distillate of Origanum vulgare L. growing in Saudi Arabia and evaluation of their antibacterial activity. Arab. J. Chem. 2018, 11, 1189–1200. [Google Scholar] [CrossRef]
- Thakur, M.; Kumar, R. Microclimatic buffering on medicinal and aromatic plants: A review. Ind. Crop. Prod. 2021, 160, 113144. [Google Scholar] [CrossRef]
- Assaf, M.; Korkmaz, A.; Karaman, Ş.; Kulak, M. Effect of plant growth regulators and salt stress on secondary metabolite composition in Lamiaceae species. S. Afr. J. Bot. 2022, 144, 480–493. [Google Scholar] [CrossRef]
- Politi, M.; Ferrante, C.; Menghini, L.; Angelini, P.; Flores, G.A.; Muscatello, B.; Braca, A.; Leo, M. De Activity Evaluation. JCR J. Clin. Rheumatol. 2004, 10, S41. [Google Scholar] [CrossRef]
- Cunha, B.A. Antibiotic side effects. Med. Clin. N. Am. 2001, 85, 149–185. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Tragiannidis, A.; München, S.; Andreas, H. Clinical Hepatotoxicity Associated with Antifungal Agents; Taylor and Francis: Abingdon, UK, 2017; ISBN 4925183478. [Google Scholar]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [PubMed]
- Božik, M.; Císarová, M.; Tančinová, D.; Kouřimská, L.; Hleba, L.; Klouček, P. Selected essential oil vapours inhibit growth of Aspergillus spp. in oats with improved consumer acceptability. Ind. Crops Prod. 2017, 98, 146–152. [Google Scholar] [CrossRef]
- Frankova, A.; Smid, J.; Bernardos, A.; Finkousova, A.; Marsik, P.; Novotny, D.; Legarová, V.; Pulkrabek, J.; Kloucek, P. The antifungal activity of essential oils in combination with warm air flow against postharvest phytopathogenic fungi in apples. Food Control 2016, 68, 62–68. [Google Scholar] [CrossRef]
- Pola, C.C.; Medeiros, E.A.A.; Pereira, O.L.; Souza, V.G.L.; Otoni, C.G.; Camilloto, G.P.; Soares, N.F.F. Cellulose acetate active films incorporated with oregano (Origanum vulgare) essential oil and organophilic montmorillonite clay control the growth of phytopathogenic fungi. Food Packag. Shelf Life 2016, 9, 69–78. [Google Scholar] [CrossRef]
- Napoli, E.; Giovino, A.; Carrubba, A.; Siong, V.H.Y.; Rinoldo, C.; Nina, O.; Ruberto, G. Variations of essential oil constituents in oregano (Origanum vulgare subsp. viridulum (= o. heracleoticum) over cultivation cycles. Plants 2020, 9, 1174. [Google Scholar] [CrossRef] [PubMed]
- Pozzatti, P.; Scheid, L.A.; Spader, T.B.; Atayde, M.L.; Santurio, J.M.; Alves, S.H. In vitro activity of essential oils extracted from plants used as spices against fluconazole-resistant and fluconazole-susceptible Candida spp. Can. J. Microbiol. 2008, 54, 950–956. [Google Scholar] [CrossRef]
- Rúa, J.; Del Valle, P.; De Arriaga, D.; Fernández-Álvarez, L.; García-Armesto, M.R. Combination of Carvacrol and Thymol: Antimicrobial Activity Against Staphylococcus aureus and Antioxidant Activity. Foodborne Pathog. Dis. 2019, 16, 622–629. [Google Scholar] [CrossRef]
- Leyva-l, N.; Guti, E.P.; Vazquez-olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Wijesundara, N.M.; Rupasinghe, H.P.V. Essential oils from Origanum vulgare and Salvia officinalis exhibit antibacterial and anti-biofilm activities against Streptococcus pyogenes. Microb. Pathog. 2018, 117, 118–127. [Google Scholar] [CrossRef]
- Beheshti-Rouy, M.; Azarsina, M.; Rezaie-Soufi, L.; Alikhani, M.Y.; Roshanaie, G.; Komaki, S. The antibacterial effect of sage extract (Salvia officinalis) mouthwash against Streptococcus mutans in dental plaque: A randomized clinical trial. Iran. J. Microbiol. 2015, 7, 173–177. [Google Scholar] [PubMed]
- Ouakouak, H.; Chohra, M.; Denane, M. Chemical Composition, Antioxidant Activities of the Essential Oil of Mentha pulegium L., South East of Algeria. Int. Lett. Nat. Sci. 2015, 39, 49–55. [Google Scholar] [CrossRef]
- European Medicines Agency. Public Statement on the Use of Herbal Medicinal Products Containing Pulegone and Menthofuran; European Medicines Agency: Amsterdam, The Netherlands, 2016; Volume 44, pp. 1–24. [Google Scholar]
- Politi, M.; Menghini, L.; Conti, B.; Bedini, S.; Farina, P.; Cioni, P.L.; Braca, A.; De Leo, M. Reconsidering Hydrosols as Main Products of Aromatic Plants Manufactory: The Lavandin (Lavandula × intermedia) Case Study in Tuscany. Molecules 2020, 25, 2225. [Google Scholar] [CrossRef]
- Yayla, E.M.; Izgu, N.; Ozdemir, L.; Erdem, S.A.; Kartal, M. Sage tea–thyme–peppermint hydrosol oral rinse reduces chemotherapy-induced oral mucositis: A randomized controlled pilot study. Complement. Ther. Med. 2015, 27, 58–64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).