Abstract
The insecticide known as neonicotinoid has negative impacts on the ecosystem, human health, and the environment; specifically, its effects on the relationship between crop yields and the death rate of natural pollinators, such as bees, affect food security. The active ingredients in neonicotinoids include imidacloprid, clothianidin, thiamethoxam, acetamiprid, sulfoxaflor, and thiacloprid, which are sold under various trade names. For many of the components of these toxic insecticides, patents have been expired; however, farmers and consumers who continue to use these chemicals are unaware of the products’ toxicity and the environmental effects they have. Thus, agricultural industries are required to consider diverse methods to minimize neonicotinoid use in farming operations and move away from the current prevailing methods. In this short review, the negative effects of neonicotinoid use; the toxic components, health effects, and environmental regulations of neonicotinoids; and sustainable methods to minimize their use are examined.
1. Introduction
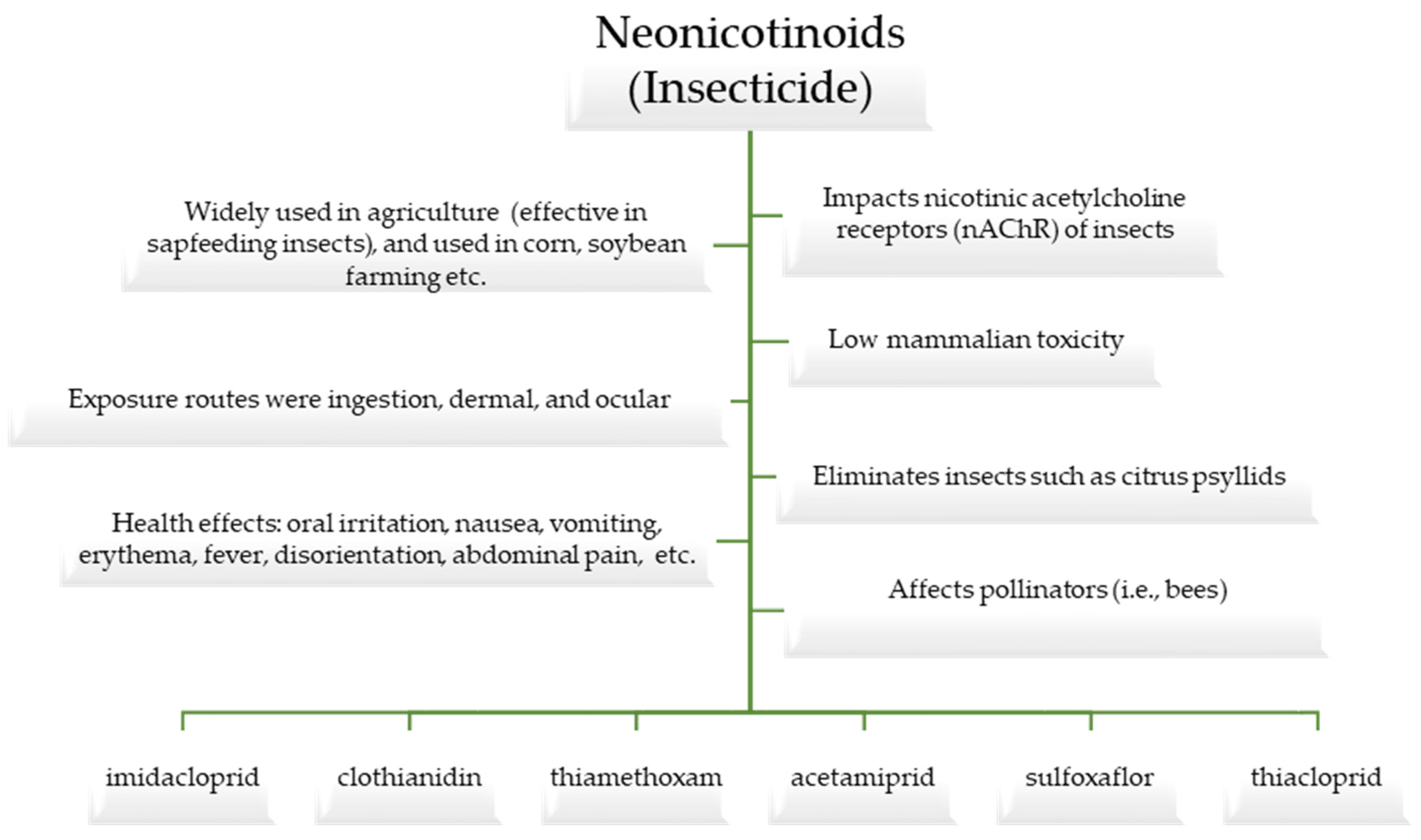
Toxic insecticides are widely used in the agricultural industry to control insects. Neonicotinoids are a class of insecticides that were introduced into the farming industry in the past few decades, and they have been in use worldwide for over 20 years [1,2]. The active ingredients in neonicotinoids include imidacloprid, clothianidin, thiamethoxam, acetamiprid, sulfoxaflor, and thiacloprid [3,4] (please refer to Figure 1).
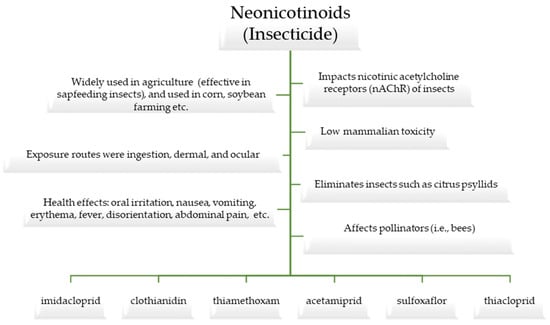
Figure 1.
An overview of neonicotinoids and their components.
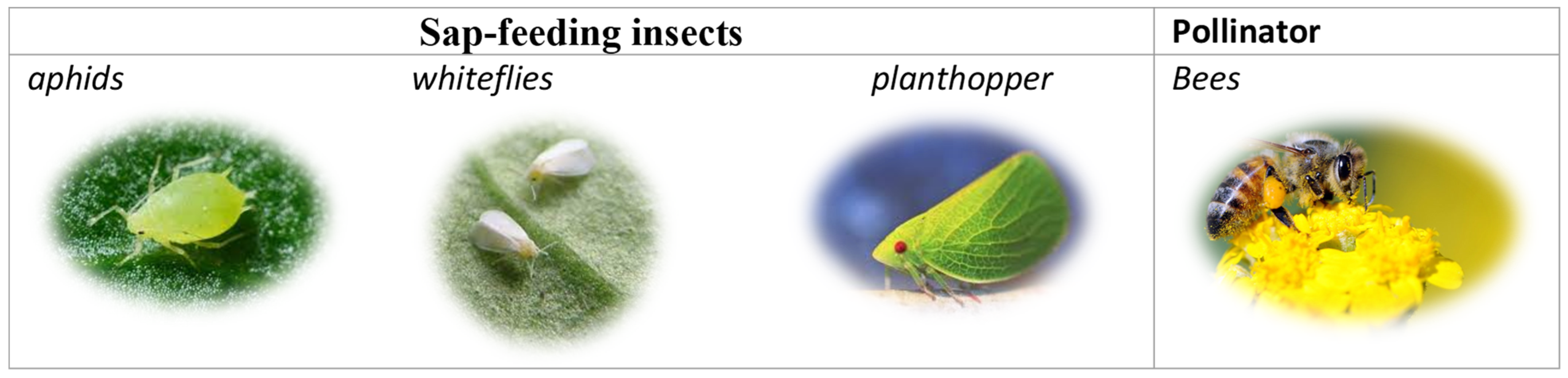
Insecticides are generally classified as substances that are toxic and used to eliminate insects. For example, disease-carrying insect citrus psyllids (Diaphorina citri and Trioza erytreae) can be controlled with neonicotinoids [5]. However, neonicotinoids are not used to control mosquitoes (i.e., vectors of human diseases) because these insecticides are slow-acting and not very effective with these nuisance insects. Neonicotinoids are a class of synthetic insecticides that eliminate insects by acting on their nicotinic receptors [6]. They are commonly used to limit pests that compromise crops or to dispatch disease-carrying insects [7]. These insecticides are widely applied in an agricultural context and are especially effective with regard to sap-feeding insects, such as aphids, whiteflies, and planthoppers, as well as insects that chew on plants [8] (Please refer to Figure 2). Imidacloprid, thiacloprid, thiamethoxam, dinotefuran, clothianidin, and acetamiprid are all potent insecticides that fall under the class of neonicotinoids. Neonicotinoids are one of the most widely used insecticides as they were specifically designed to impact the nicotinic acetylcholine receptors (nAChR) of insects, which is why they are generally believed to have relatively low mammalian toxicity [9,10].

Figure 2.
Neonicotinoids eliminate sap-feeding insects and negatively affect pollinators.
This manuscript reviews the negative impacts of neonicotinoids on human health and the ecosystem and its effects on the relationship between crop yields and the death rate of natural pollinators, as well as mitigation strategies.
2. Neonicotinoids and Its Effects after Human Exposure
While there is limited information regarding human exposure to neonicotinoids, cases of neonicotinoid poisoning are rising due to the use of these insecticides [11,12]. From 2000 to 2012, poison centers in Texas, USA received 1142 neonicotinoid exposure cases, where most products (77%) contained imidacloprid. The most common exposure routes were ingestion, dermal, and ocular. Typical adverse reactions included ocular, dermal, and oral irritation; nausea; vomiting; erythema; and red eye [11]. Mohamed et al. reported the clinical results and toxico-kinetics of the neonicotinoid insecticide imidacloprid; it causes acute self-poisoning in humans [13]. Demographic and clinical data were obtained in patients with imidacloprid exposure in three hospitals in Sri Lanka [14].
Agha et al. [14] reported that the imidacloprid poisoning rates are underestimated. They reported a case of a 62-year-old Saudi male cultivator who was admitted to the emergency department with a history of fever, disorientation, lower abdominal pain, vomiting, etc. They reported that this may be the first reported case of leukocytoclastic vasculitis due to skin contact with imidacloprid and inhalation exposure. Kim et al. [12] reported a retrospective analysis involving cases of neonicotinoid ingestion in Korean emergency departments from March 2002 to February 2010. Of the 24 patients analyzed, the most common signs of neonicotinoid toxicity were gastrointestinal symptoms, including nausea, vomiting, and abdominal pain, as well as respiratory symptoms, cardiovascular symptoms, metabolic imbalance, and renal dysfunction. Moderate toxicity was reported in 83.3% of the patients, and 16.7% sustained fatal conditions with an overall mortality rate of 4.2% [12]. Since imidacloprid, thiacloprid, thiamethoxam, dinotefuran, clothianidin, and acetamiprid fall under the class of neonicotinoids, the details of each one are presented in the below sections.
Imidacloprid: Of the many neonicotinoids, imidacloprid is the most prominent. Imidacloprid is a colorless crystal and is generally acknowledged as a useful pesticide for pest management, especially for insects with piercing and sap-feeding mouthparts [15]. Imidacloprid is used to protect crops, turf, and decorative plants from pests, such as termites and fleas, by acting on their nicotinic receptors, which are transmembrane allosteric proteins that play a critical physiological role in the body by enabling the transduction of chemo electrical signals throughout the nervous system [16]. Through calcium ion imbalance, mitochondrial dysfunction, oxidative stress, and effects on DNA, imidacloprid causes failure to the nervous system that ultimately leads to death.
Applications of imidacloprid include leaf spraying as well as soil and seed treatments, which can impact non-target organisms such as pollinators or other insects that are beneficial to farming, which can be financially unfavorable. For example, the individual immunity of bees can be affected by imidacloprid, which may diminish their defenses, making them vulnerable to parasites and pathogens [15].
Recently, there has been a growing concern about imidacloprid’s impact on the ecosystem and human health. Such concerns have been investigated, and it was uncovered that imidacloprid is toxic to humans and has acute toxic effects including severe respiratory failure and loss of consciousness [15]. Pang et al. [15] further reported that imidacloprid is toxic to mammals, including human beings, and it can kill bees, bats, earthworms, fish, and other non-target organisms; thus, the development of cost-effective and eco-friendly methods, such as biological oxidation utilizing microbial consortium with degrading enzymes, is necessary. Shadnia and Moghaddam [17] reported a fatal case of imidacloprid insecticide poisoning. They further reported that a 35-year-old male farmer ingested 350 mL of imidacloprid in a suicide attempt; thus, the patient displayed disorientation, drowsiness, dizziness, palpitations, and vomiting, which progressed to coma, tachycardia, hypertension, fever, leukocytosis, hypokalemia, etc. [17]. It is important to mention that the level of toxicity is very much dependent on the frequency and quantity or dose taken; hence, the safe field application of these chemicals may not cause such severe symptoms.
Clothianidin: Clothianidin is a neonicotinoid pesticide that is used against sucking and chewing insects [18]. As a broad-spectrum systemic insecticide, clothianidin is potent to a variety of pest species, including hemipterans, thysanopterans, orthopterans, coleopterans, lepidopterans, dipterans, hymenopterans, and isopterans [19]. It can be applied as a seed treatment or a soil or foliar spray [18]. Clothianidin products can have various agricultural or non-agricultural uses and can be formulated as granular, dust, or seed treatments; solid agars; pressurized liquids; emulsifiable or soluble concentrates; or ready-to-use solutions. Agriculturally, clothianidin can be used to protect vegetable and field crops, as well as tree fruits and nuts, from insect attacks. Clothianidin can be used non-agriculturally as well for turf and ornamental plants or for indoor and outdoor residential, commercial, and industrial sites [20].
Acetamiprid: Acetamiprid has low toxicity in mammals; however, severe toxicity can result from ingesting large amounts [21]. As it is still a relatively new insecticide, acetamiprid poisoning is fairly uncommon. Todani et al. reported that a 79-year-old man experienced consciousness disturbances, hypotension, nausea, vomiting, and hyperglycemia two hours after ingesting acetamiprid [22]. The next day, he was discharged from the hospital with improved symptoms [21,22]. Pirasath et al. reported the case of a middle-aged farmer who experienced severe lactic acidosis, myocardial ischemia, refractory hypotension, and severe hypokalemia after ingesting 150 g of acetamiprid dissolved in water [21]. Pravinson et al. reported the case of a 27-year-old man who presented vomiting, shortness of breath, and dizziness after the intake of 50 mL of acetamiprid [23]. The patient developed severe lactic acidosis, myocardial suppression, hyperglycemia, and intestinal obstruction [23].
Thiacloprid: Based on animal studies, the World Health Organization (WHO) classified thiacloprid as a class 2 pesticide that is moderately hazardous as it is likely a carcinogen [24]. Vinod et al. reported a fatal case of thiacloprid poisoning [24]. After the deliberate ingestion of 100 mL of thiacloprid suspension mixed with ethanol, a 23-year-old man experienced nausea, vomiting, tachycardia, hypertension, mydriasis with loss of light reflex, and respiratory paralysis. The patient eventually developed bradycardia, experienced cardiac arrest, and died 36 h after ingestion [24].
Thiamethoxam: First registered for use in the United States in 1999, thiamethoxam is an insecticide that is commonly used to control piercing and sucking insects, such as aphids, leafhoppers, and whitefly. Thiamethoxam is widely used for field, forage, fruit, spice, and vegetable crops, as well as for non-agricultural applications such as for residential dwellings, food handling establishments, and commercial, institutional, and industrial areas [20].
Sulfoximine: Other substitutes of neonicotinoids include sulfoximine. In a recent work, Azpiazu et al. [25] studied the acute oral toxicity of sulfoxaflor alone and in combination with a single dose of fluxapyroxad, a succinate dehydrogenase inhibitor (SDHI) fungicide, in three bee species: Apis mellifera, Bombus terrestris, and Osmia bicornis. They found that sulfoxaflor is somewhat less toxic than the recently banned neonicotinoids imidacloprid, thiamethoxam, and clothianidin, but much more toxic than other neonicotinoids (acetamiprid and thiacloprid).
Ospina et al. [26] found that 49.1% of the USA’s general population 3 years of age and older have been exposed to neonicotinoids. They report that metabolites are more suitable biomarkers to assess background exposures than parent compounds. The Centers for Disease Control and Prevention (CDC, 2019) [27] is a good source of information on human exposure to environmental chemicals, including some of the most commonly used neonicotinoids. Han et al. [28] reviewed the exposure levels, potential toxicity, and health effects of neonicotinoids on humans. They reported that methods for determining damage from neonicotinoids are still in the research stage and deserve further studies to determine whether neonicotinoids will have harmful effects on humans, especially among vulnerable populations. Table 1 lists the characteristics of the potent insecticides that fall under the class of neonicotinoids.

Table 1.
Potent insecticides that fall under the class of neonicotinoids and their characteristics.
Currently, the aforementioned active ingredients of pesticides are sold under various brand names at various concentration levels. Individual brands can either include one or more active ingredients of the compounds mentioned. From the 1990s to 2000s, neonicotinoids became widely used in corn (more than 80%) and soybean (more than 40%) farming for their proven effectiveness against pests and their ability to improve crop yields. In the USA, heavy neonicotinoid usage is found in the corn belt region, which includes South Dakota, North Dakota, Indiana, Ohio, Wisconsin, Michigan, and Kentucky. Since the 2000s, many case studies have been published, which reveal the effects of neonicotinoids on the ecosystem and crop yields [29].
3. The Reasons for Neonicotinoid Use over Other Insecticides
Neonicotinoid-based insecticides are widely used since they have a large number of advantages. Neonicotinoids are more toxic to invertebrates than mammals, meaning that the pesticide is safer for humans while being very effective for controlling pests. In fact, neonicotinoid-based pesticides are one of the safest pesticides currently on the market. Another advantage of neonicotinoids is that they are water-soluble. Their solubility allows neonicotinoids to be applied as seed treatments or directly to soil rather than as a spray to the surface of the vegetation. The vegetation can then absorb the pesticide through the vascular system from the roots, and the pesticide can migrate to the stems, leaves, flowers, pollen, and nectar [30]. Since neonicotinoid pesticides become part of the vascular systems of plants, they target aphids, sap sucking, and biting insects. Since the insecticide remains active, protection can last for an entire season [31].
4. The Spread of Neonicotinoids into the Ecosystem
There are two common ways that neonicotinoids enter the ecosystem. The first method is through seed treatment, and the second method involves applying the pesticide directly to soil. Seed treatment involves applying the pesticide directly to the seed prior to planting. As the plant grows and develops, the neonicotinoid pesticide becomes part of the plants’ vascular system, making it very effective against aphids and biting insects. While this is one of the most effective methods of applying the pesticide, seed treatment generates a large amount of dust containing high concentrations of neonicotinoids.
Planter dust is often generated during the planting process or shortly after the seed is planted and can migrate past field margins, reaching other vegetation. This causes exposure of the toxin to non-target insects [27]. Direct soil application involves creating trenches in the soil around the plant or crop of interest. The trenches are then filled with the neonicotinoid insecticide, which is absorbed through the roots of the plant through its vascular system. However, only about 20% of the applied pesticide is absorbed through the vascular system of the plant. The remaining 80% of the pesticide migrates through the soil beyond field margins and enters groundwater and surface water and affects other organisms.
5. Contamination of Groundwater and Surface Water
One of the main problems of neonicotinoids being in water is related to human health, as the main exposure route is through dermal contact and ingestion. Studies show that exposure to neonicotinoids results in immunosuppression, hormone disruption, reduced intelligence, reproductive distortion, and cancer [32]. Studies show that imidacloprid, clothianidin, thiamethoxam, and high levels of thiamethoxam were detected mainly in the winter and fall, as these chemicals can be immobile in dry soil [33]. To understand how neonicotinoids reach groundwater, it is important to know about the coefficients used to evaluate pesticide sorption in soil. The soil adsorption coefficient (Kd), octanol–water partition coefficient (Kow), organic content in the soil, etc., play important roles in the sorption and transport of neonicotinoids in soil and water. Neonicotinoids have a low Kow value, which means there is a low probability of them being adsorbed into the organic portion of the soil, thus contributing to the leaching of neonicotinoids into groundwater. The majority of neonicotinoids are applied as seed treatments, where only 5% of the active ingredients are absorbed by the target crop [34]; thus, the un-adsorbed amount is either degraded or enters soil and water and generally, leading to higher levels of pesticides in surface water. Zhang et al. [35] studied the human exposure potential of neonicotinoids through the consumption of safe drinking water, and the results show that drinking water had an upper middle level of neonicotinoid contamination. They reported that anthropogenic activity, the human age group, weather conditions, seasonal and regional influence, etc., play important roles.
6. Neonicotinoids’ (‘Nicotine-Like Insecticides’) Effects on Pollinators (i.e., ‘Bees’)
The word neonicotinoids means ‘new nicotine-like insecticides’ in the literal sense. Similar to nicotine-based pesticides, neonicotinoid-based pesticides are addictive to organisms and act on various receptors of the nervous system [31].
A study was conducted in which bees were given two bowls of water, one laced with sugar, and another laced with neonicotinoid. After the bees ingested a small dosage of neonicotinoids, they preferred the neonicotinoid-laced water instead of the sugar water, thus proving their addictive properties [36]. Neonicotinoids affect the nerve synapse of insects by acting on certain receptors, which leads to fatality. However, the toxicity of neonicotinoids is much higher when ingested by invertebrates (i.e., insects, worms, etc.) compared to higher organisms such as birds. The lethal dosage for honeybees is much lower than that of mammals since only less than 0.023 μg is needed to kill a honeybee, which is one of the best pollinators in the agricultural industry [29]. Although the lethal dosage is higher for other organisms, such as birds, fish, etc., the toxic effects of neonicotinoids can bio-accumulate. Bioaccumulation occurs through extended periods of pesticide exposure or through ingesting other neonicotinoid-affected organisms that are lower in the food chain [29].
When neonicotinoids were first introduced into the farming industry, they were thought to have low toxicity to beneficial insects. However, in recent years, it was recognized through studies that bees and other beneficial insects are negatively affected by contact with nectar and pollen that are laced with neonicotinoid-based pesticides. Low-level exposure to neonicotinoids may not result in immediate death for beneficial insects, but the toxins reduce the insects’ ability to function correctly. For example, when natural pollinators such as bees are exposed to neonicotinoids, their ability to forage nectar may be reduced. Also, bees may cease to remember the locations of flowers or the way back to their hives [31].
Ihara and Matsuda [37] reported that neonicotinoids currently make up of about 30% of insecticide sales worldwide, and due to their adverse impacts on pollinators such as honeybees and bumble bees, neonicotinoids are being banned in the EU. Since it is crucial to understand the mechanism underlying neonicotinoid actions on pollinators as well as on other pests, they researched the molecular mechanisms of neonicotinoid actions at an atomic level and proposed relevant research topics for further studies for pest management. In a recent study, Dirilgen et al. [38] presented the existing research on other herbicides, fungicides, and insecticides which come with a range of environmental concerns, such as the effects of insecticides on non-target organisms such as bees. Their study also focused on non-neonicotinoid insecticides and non-honeybees.
Furthermore, neonicotinoids caused alterations in the bees’ immune systems, causing the bees to be susceptible to viral infections that healthy bees normally have immunity to. Aside from bees, neonicotinoids also have a negative impact on other wildlife, such as birds and aquatic invertebrates. Neonicotinoids cannot be completely broken down into harmless chemicals by the natural ecosystem or even with chemical treatment methods. Residues of neonicotinoid pesticides can come from soil and seeds, which lead to residues in plants. Neonicotinoids can also affect pollen, nectar, surface water, etc. [2].
In addition to environmental effects, neonicotinoids, when applied as seed treatments, become more and more dilute in plants as they grow. The pesticides become ineffective after a specific duration depending on the type of vegetation, and the effects of the pesticides often do not last until harvest. This means that vegetation is potentially susceptible to pests before it is harvested. A study revealed that when soybean seeds have been treated with neonicotinoids, the concentration begins to decline during the growing cycle and becomes very low right before the flowering stage. As a result, the plants are no longer protected against aphids (i.e., soybean aphids) [39].
7. Environmental Regulations on the Use of Neonicotinoids
Since the early 1990s, neonicotinoids have been used in Ontario, Canada. For two decades, after neonicotinoids became available to the public, their use drastically escalated. Many patents for neonicotinoid products will be expiring in the future, and this will lead to a flood of neonicotinoid-related products that are generic copies of pre-existing products in the market. Farmers will have to pay more attention to the contents of active ingredients shown on the labels. In Ontario, Canada, labels are available for all neonicotinoids that are registered for use on the Pest Management Regulatory Agency (PMRA) label site [4]. The labels clearly indicate that neonicotinoid-related active ingredients are toxic to bees, aquatic invertebrates, fish, birds, and small mammals. On the PMRA label site, the labels for all registered products can be easily identified based on active ingredients. However, Ontario farmers have an overall lack of awareness of products that contain neonicotinoids since they tend to purchase products based on brand names rather than by their designated pesticide classes. In many cases, farmers can be unaware of the properties of neonicotinoids.
Within Ontario, Canada, hundreds of crops have been registered for neonicotinoid use. The types of crops registered include both ‘major’ crops as well as ‘minor’ crops. Examples of ‘major’ crops include soybeans, wheat, corn, and canola. Examples of ‘minor’ crops include field crops, greenhouse vegetable crops, and orchard crops. Neonicotinoids are also used for specialty crops, such as ginseng, turf grass, sweet potatoes, herbs, and non-food crops. They are used in golf courses, but the allowable rate is much higher compared to that for food crops. In 2008, the Ontario Ministry of Agriculture conducted a pesticide census for Ontario [4]. This census does not include information on using neonicotinoids as seed treatments. Aside from the 2008 census, another study was conducted in 2014, which is more comprehensive in terms of information on neonicotinoids. Treated seeds are produced by applying neonicotinoids to seeds before they are purchased by farmers. The use of neonicotinoid products is difficult to regulate since the treated seeds are more likely to be produced outside of Ontario or even outside of Canada. In Canada, each provincial government is responsible for classifying and regulating pesticides for their sale, use, storage, transportation, and disposal. Class 12 pesticides are a class of pesticides that refer to corn and soybean seeds treated with neonicotinoids that include one of the three active ingredients discussed above: imidacloprid, thiamethoxam, or clothianidin. Based on the Ontario regulations for neonicotinoid-related products, growers are required to follow the Pest Assessment Guideline. When growers order neonicotinoid-treated seeds, they will be required to provide evidence that mitigation strategies have been considered for the growing season. Furthermore, growers must carefully review the instructions provided on the neonicotinoid-treated seed bag, and they are required to keep record of all purchased and planted seeds that were treated with neonicotinoids. Neonicotinoid-treated seeds can only be planted in farm areas that have been clearly identified in a pest assessment report or seed amount declaration. In order to purchase products from vendors, farmers must provide the declaration, which must include information on the amount of land on which they intend to plant neonicotinoid-treated corn or soybean seeds as well as the amount of land on which they intend to plant non-treated corn and soybean seeds [40]. Integrated pest management (IPM) focuses on using environmentally and economically sustainable ways to control and manage pests. Based on IPM concepts, pesticides should be considered as the last resort after all other possible available options have been considered for pest control.
In Europe, regulations regarding the active ingredients of neonicotinoids are stated in Annex I of the EU Commission Implementing Regulation (EU) 540/2011 [41]. The active ingredients include acetamiprid, clothianidin, thiamethoxam, thiacloprid, and imidacloprid. There are several provisions that have been set out for these substances. For example, one of the provisions states that to protect non-target organisms, particularly honeybees, seed coating should be carried out in professional seed treatment facilities.
8. Ways to Minimize Neonicotinoid Use
Endorsing immediate bans on the use of neonicotinoids may cause serious economic instability among farmers and communities. Along with regulations that restrict the use of neonicotinoids, different farming operations or prevention methods need to be investigated. The benefits of each method need to be looked into based on their economic, environmental, and social values. The general public mostly obtains its food supply from farms that involve large-scale outdoor operations.
According to the U.S. Department of Agriculture (USDA), the average size of a farm in America is 434 acres. Operations of this size require the use of large machinery [42]. All of the essential components of growing crops, such as seeding, harvesting, watering, eliminating pests, etc., must be carried out mechanically. Such activities use an incredible number of resources, and the infrastructure does not support sustainable expansion.
Arguably, the most labor-intensive part of the farming process is pest control. Naksata et al. [43] reported that wearing appropriate personal protective equipment during the application of pesticides is important in reducing dermal exposure to pesticides. They developed a type of personal protective clothing (PPC) coated with gum rosin and investigated the efficiency of its level of protection. A comparison of its protection efficiency against that of cotton fabrics was made, and they reported that the gum-rosin-coated clothing provided satisfactory levels of protection against insecticides and could be considered suitable protective clothing for pesticide applicators.
Matthews et al.’s study [44] is a good reference for proper pesticide application methods that cover integrated pest management, the formulation of pesticides, different types of sprayers, controlled droplet application, aerial application, seed treatment, dust and granule application, space treatment by fogging, specialist application techniques (injection, fumigation, and other techniques), and safety precautions.
8.1. Organic Farming
Organic farming follows the idea that every part of the farming process should be handled in the most natural way possible. This means that organic farmers cover their plots for months prior to growing in order to kill weeds, utilize some type of composting system to make their own fertilizer, and avoid the use of commercial pesticides or fertilizers.
There are many varying beliefs within the organic farming community on what exactly ‘organic’ is supposed to mean. In areas where regulations specify what is classified as organic or not organic, there are inevitable shortfalls that appear. In certain jurisdictions, the bar is set so low for what constitutes an organic item that it is a seemingly arbitrary concept. Sometimes, it has been reduced to nothing more than a marketing buzzword that is used with the goal of selling more produce. Even among the community, there is a multitude of different practices that are debated over. An operation that is run in a truly organic way will not cause anywhere near the environmental impact that large-scale farms will. Without the use of pesticides and fertilizers, there are no pollutants from organic farming that will harm the environment, and without the use of heavy machinery, there is no release of greenhouse gases.
Souto et al. [45] reported that the indiscriminate use of synthetic pesticides over time creates several issues, including pesticide resistance and the contamination of water, air, and soil. They suggest that in order to improve the efficiency of crop production and reduce the food crisis, plant-derived pesticides can be used as a green alternative to synthetic ones. Plant-derived pesticides are inexpensive, biodegradable, eco-friendly, and less of a hazard to humans and the environment. They further reported that plant products with bioactivity toward insects include several classes of molecules, such as terpenes, flavonoids, alkaloids, polyphenols, cyanogenic glucosides, quinones, amides, aldehydes, thiophenes, amino acids, saccharides, and polyketides. They further reported on the factors that contribute to the commercialization of plant-derived pesticides.
8.2. Effective Use of NPK Fertilizer
All plants require a healthy and specific mix of nitrogen (N), phosphorus (P), and potassium (K). Most fertilizers sold commercially are called NPK fertilizers. Nitrogen is generally thought to be a contributor to a plant’s leaf count and overall greenness, while phosphorus promotes stem health, and potassium is used by plants to strengthen their roots [46]. The use of these fertilizers as a wide-spread solution results in several consequences to the environment.
Approximately 40–60% (depending on crop) of any given component in NPK fertilizers is taken up by the plant. This leaves the rest to accumulate in the soil, leach into surrounding groundwater reservoirs, travel via runoff, and harm ecosystems. The excess fertilizer has many negative effects on the wildlife of small bodies of water. These added nutrients will cause an explosion of growth in primary species, like algae. The layer of algae that forms on top of a pond will block any potential sunlight from reaching the plants or organisms living below the surface of the water. Furthermore, the oxygen concentration in the water will reduce significantly and start to suffocate the species living within. This effect is compounded by the thick layer of algae, which will start to decompose and siphon even more oxygen from the water. Thus, the oversupply of nutrients leads to the suffocation of all life within a pond. This means that any type of fertilizer use will require close monitoring of the amounts so as not to destroy the surrounding ecosystems.
Monitoring is needed in areas where crops are planted because they all require a tailored combination of the three primary nutrients. For example, a section of land is used to grow lettuce. Lettuce is mostly composed of leaves, and it requires a larger amount of nitrogen than either phosphorus or potassium. After repeatedly growing lettuce in an area, the soil will become drained of its nitrogen content while simultaneously accumulating an abundance of both phosphorus and potassium. This plot of land will soon become unavailable for growing lettuce because it needs nitrogen. The plot of land will also become completely unavailable for growing anything else once the phosphorus and potassium levels become too high to support other species. It is therefore in a grower’s best interest to rotate crops in a way that will balance these three primary nutrients.
8.3. Soil-Free Hydroponic Farm Operations
Operations that involve growing crops in a medium that is not soil are referred to as hydroponic farm operations. In these methods, the roots of the crop sit in a nutrient solution that contains the perfect amount for the application. If a certain crop requires certain nutrients, the concentrations of said nutrients can be easily controlled by a variety of methods. A grow tray holds the plants via some type of pot or permeable container. This grow tray is kept at a desirable level and is connected to the reservoir by a circulation pump. This pump periodically floods the grow tray with the nutrient solution in order to maintain ideal nutrient concentrations.
The benefits of growing in water versus soil alone should be enough to change the perspective of the general public. Hydroponic operations can more easily be implemented in indoor settings. This means that they can operate without ever inadvertently polluting nearby ecosystems. The hydroponic system can be run in a near-closed manner. The water in a hydroponic setup is recycled until it is not suitable for use, thus maximizing water efficiency, preventing pollution, and eliminating neonicotinoid use.
Aquaponics are a subset of hydroponics, and they involve the use of fish in the nutrient cycle. In the nutrient cycle, fish food is supplied to grow fish; subsequently, the ammonia produced by fish is metabolized, with the help of microbes, into nitrites and nitrates, which are then absorbed by the plants. The incorporation of multiple types of farm operations, as discussed above, is needed to meet food demands while maintaining environmental sustainability.
Rajaseger et al. [47] reported an interesting technology that incorporates hydroponics with smart technology in farming operations. This technology is novel and promising for effective and environmentally friendly crop production, and it eliminates the need for soil and reduces water usage. The IoT (Internet of Things) and automation allow for the constant monitoring of soil conditions, nutrient levels, and plant vitality. Smart farming lessens the need for organic chemical inputs, and it promotes safe methods of pest management.
A life cycle analysis (LCA) helps to assess the environmental impacts and aids in decision making by prioritizing values and resources to determine the best and most innovative countermeasures [48]. One can compare hydroponic versus conventional farm operations using an LCA. The indicators for environmental sustainability can include land use, water footprint, and the level of pollution. The indicators used for economic sustainability can include the production rates and efficiency of production. Social sustainability can be based on speculation from both environmental and economic indicators.
It is reported that hydroponically grown tomato plants have, on average, a higher leaf count and higher quality than those grown in soil [49]. A hydroponic producer is able to control the growing environment better; on the other hand, a soil growing environment is primarily dependent on weather conditions. Hydroponic operations require much less water usage and typically cost less to initiate and operate than conventional farms. The price of land makes it much more expensive if multiple acres of land are needed for soil farming. If the price of electricity is very high (i.e., in extreme hot/cold climate regions), there is a necessity for constant heating/cooling; thus, it may become prohibitively expensive to operate a hydroponic farm. The water demand for conventional farming will always be much higher than that of a hydroponic farm. These factors suggest that, from environmental and social perspectives, future farming methods will include a balanced combination of outdoor soil farming and hydroponic methods. Since indoor operations do not have a requirement for pesticides, such as neonicotinoids, hybrid operations will minimize the release of pesticides into the environment.
9. Conclusions
In summary, neonicotinoid insecticides are toxic to human health, have adverse effects on our ecosystem and ground and surface water bodies, and pose a threat to pollinating species that contribute to the food industry through healthy farming operations. This review provides a summary on the class of neonicotinoids that includes imidacloprid, thiacloprid, thiamethoxam, dinotefuran, clothianidin, acetamiprid, and sulfoximine, which have varying degrees of human toxicity and effects on soil, drinking water, and pollinating species such as bees. The application of neonicotinoids and its components are strictly regulated by governments. Thus, taking precautions during insecticide application and wearing appropriate personal protective clothing, such as clothing coated with gum rosin, can provide protection against insecticide poisoning. Furthermore, mitigation methods to reduce neonicotinoid insecticide use include soil-free hydroponic farm operations, the use of hydroponics with smart technology (IoT) applications, the use of plant-derived pesticides, and the effective use of NPK fertilizers. Sustainable farming operations are possible with applications of innovative technology and best hazardous waste management practices, which will keep humans, our ecosystems, farming operations, and beneficial pollinators safe.
Author Contributions
Both Z.S. and A.E. contributed to the conceptualization, methodology, and initial draft preparation. The reviews, preparation of figures and tables, and editing was carried out by Z.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The work in this paper was supported, in part, by the Open Access Program at the American University of Sharjah. This paper represents the opinions of the author(s) and does not represent the positions or opinions of the American University of Sharjah.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jeschke, P.; Nauen, R. Neonicotinoids-from zero to hero in insecticide chemistry. Pest Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Myths and Truths about Neonicotinoids, Chemicals and the Pesticides Industry. Available online: https://cdn.buglife.org.uk/downloads/The-bee-coalition-2014-Myths-and-truths-about-neonicotinoids.pdf (accessed on 16 October 2022).
- Simon-Delso, N.; Amaralrogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Chan, S. 10 Facts about Neonicotinoids in Ontario. Available online: https://www.ontariobee.com/sites/ontariobee.com/files/10%20factsneonicsChan.pdf (accessed on 16 October 2022).
- Asian Citrus Psyllid. Available online: https://cisr.ucr.edu/invasive-species/asian-citrus-psyllid (accessed on 16 October 2022).
- Ensley, S.M. Neonicotinoids. In Veterinary Toxicology; Academic Press: Cambridge, MA, USA, 2012; pp. 596–598. [Google Scholar] [CrossRef]
- Bolognesi, C.; Merlo, F.D. Pesticides: Human health effects. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 118–132. [Google Scholar] [CrossRef]
- Bass, C.; Field, L.M. Neonicotinoids. Curr. Biol. 2018, 28, R772–R773. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Buckingham, S.D.; Kleier, D.; Rauh, J.J.; Grauso, M.; Sattelle, D.B. Neonicotinoids: Insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2001, 22, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Casida, J.E. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu. Rev. Entomol. 2003, 48, 339–364. [Google Scholar] [CrossRef] [PubMed]
- Forrester, M.B. Neonicotinoid insecticide exposures reported to six poison centers in Texas. Hum. Exp. Toxicol. 2014, 33, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-C.; So, B.-H.; Kim, H.-J.; Kim, H.-M.; Park, J.-H.; Choi, S.-M.; Park, K.-N.; Choi, K.-H. Clinical Characteristics of Patients with Neonicotinoid Insecticide Poisoning. J. Korean Soc. Clin. Toxicol. 2010, 8, 24–29. [Google Scholar]
- Mohamed, F.; Gawarammana, I.; Robertson, T.A.; Roberts, M.S.; Palangasinghe, C.; Zawahir, S.; Jayamanne, S.; Kandasamy, J.; Eddleston, M.; Buckley, N.A.; et al. Acute Human Self-Poisoning with Imidacloprid Compound: A Neonicotinoid Insecticide. PLoS ONE 2009, 4, e5127. [Google Scholar] [CrossRef] [PubMed]
- Agha, A.; Bella, A.; Aldosary, B.; Kazzi, Z.N.; AlHumaidi, M.A. Imidacloprid poisoning presenting as leukoclastic vasculitis with renal and hepatic dysfunction. Saudi J. Kidney Dis. Transplant. 2012, 23, 1300–1303. [Google Scholar]
- Pang, S.; Lin, Z.; Zhang, Y.; Zhang, W.; Alansary, N.; Mishra, S.; Bhatt, P.; Chen, S. Insights into the toxicity and degradation mechanisms of imidacloprid via physicochemical and microbial approaches. Toxics 2020, 8, 65. [Google Scholar] [CrossRef]
- Changeux, J.-P.; Paas, Y. Nicotinic acetylcholine receptors. In Encyclopedia of Neuroscience; Academic Press: Cambridge, MA, USA, 2009; pp. 1129–1133. [Google Scholar] [CrossRef]
- Shadnia, S.; Moghaddam, H.H. Fatal intoxication with imidacloprid insecticide. Am. J. Emerg. Med. 2008, 26, 624. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Dyer, D.G.; McConnell, L.L.; Bondarenko, S.; Allen, R.; Heinemann, O. Clothianidin in agricultural soils and uptake into corn pollen and canola nectar after multiyear seed treatment applications. Environ. Toxicol. Chem. 2016, 35, 311–321. [Google Scholar] [CrossRef]
- Tanaka, T. Re-evaluation of neurobehavioral toxicity of clothianidin using statistical methods for ordered alternatives assuming dose-response effect. Toxicol. Ind. Health 2020, 37, 90–97. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Clothianidin and Thiamethoxam Proposed Interim Registration Review Decision Case Numbers 7620 and 7614; January 2020. Available online: https://19january2021snapshot.epa.gov/sites/static/files/2020-01/documents/clothianidin_and_thiamethoxam_pid_final_1.pdf (accessed on 27 December 2021).
- Pirasath, S.; Senthuran, R.; Athirayan, C.; Gevakaran, M.; Guruparan, M.; Gnanathasan, A. Acute poisoning with acetamiprid: A case report. J. Med. Case Rep. 2021, 15, 419. [Google Scholar] [CrossRef] [PubMed]
- Todani, M.; Kaneko, T.; Hayashida, H.; Kaneda, K.; Tsuruta, R.; Kasaoka, S.; Maekawa, T. [Acute poisoning with neonicotinoid insecticide acetamiprid]. Chudoku Kenkyu 2008, 21, 387–390. [Google Scholar] [PubMed]
- Pravinson, M.; Pirasath, S.; Ghetheeswaran, S.; Uthayakumaran, S. Acute poisoning with acetamiprid, a type of neonicotinoid insecticide causing severe lactic acidosis: A case report. SAGE Open Med. Case Rep. 2021, 9, 2050313X211059296. [Google Scholar] [CrossRef] [PubMed]
- Vinod, K.V.; Srikant, S.; Thiruvikramaprakash, G.; Dutta, T.K. A fatal case of thiacloprid poisoning. Am. J. Emerg. Med. 2015, 33, 310.e5–310.e6. [Google Scholar] [CrossRef] [PubMed]
- Azpiazu, C.; Bosch, J.; Bortolotti, L.; Medrzycki, P.; Teper, D.; Molowny-Horas, R.; Sgolastra, F. Toxicity of the insecticide sulfoxaflor alone and in combination with the fungicide fluxapyroxad in three bee species. Sci. Rep. 2021, 11, 6821. [Google Scholar] [CrossRef]
- Ospina, M.; Wong, L.-Y.; Baker, S.E.; Serafim, A.B.; Morales-Agudelo, P.; Calafat, A.M. Exposure to neonicotinoid insecticides in the U.S. general population: Data from the 2015–2016 national health and nutrition examination survey. Environ. Res. 2019, 176, 108555. [Google Scholar] [CrossRef]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019, Centers for Disease Control and Prevention. National Center for Environmental Health; Division of Laboratory Sciences. 2019. Available online: https://stacks.cdc.gov/view/cdc/75822 (accessed on 9 April 2024).
- Han, W.; Tian, Y.; Shen, X. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere 2018, 192, 59–65. [Google Scholar] [CrossRef]
- Richmond, D.; Patton, A. Neonicotinoid Insecticides and Pollinators: What’s the Buzz All about? Available online: https://extension.entm.purdue.edu/neonicotinoids/PDF/Neonicotinoidinsecticidesandbeeswhatsallthebuzzabout.pdf (accessed on 16 September 2021).
- What Is a Neonicotinoid? Available online: https://citybugs.tamu.edu/factsheets/ipm/what-is-a-neonicotinoid/ (accessed on 16 September 2021).
- Neonicotinoids: Trying to Make Sense of the Science. September 2012. Available online: http://scientificbeekeeping.com/neonicotinoids-trying-to-make-sense-of-the-science-part-2/ (accessed on 16 September 2021).
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-Onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in Drinking Water—A Review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef] [PubMed]
- Browne, D.; Levison, J.; Limay-Rios, V.; Novakowski, K.; Schaafsma, A. Neonicotinoids in groundwater: Presence and fate in two distinct hydrogeologic settings in Ontario, Canada. Hydrogeol. J. 2021, 29, 651–666. [Google Scholar] [CrossRef]
- Goulson, D. Pesticides linked to bird declines. Nature 2014, 511, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yi, X.; Xie, L.; Liu, H.; Tian, D.; Yan, B.; Li, D.; Li, H.; Huang, M.; Ying, G.-G. Contamination of drinking water by neonicotinoid insecticides in China: Human exposure potential through drinking water consumption and percutaneous penetration. Environ. Int. 2021, 156, 106650. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J. Bees Could Be Getting Hooked on Nicotine in Pesticides—Is This the Cause of Colony Collapse Disorder? 22 April 2015. Available online: https://www.techtimes.com/articles/48085/20150422/bees-getting-hooked-nicotine-pesticides-cause-colony-collapse-disorder.htm (accessed on 16 September 2021).
- Ihara, M.; Matsuda, K. Neonicotinoids: Molecular mechanisms of action, insights into resistance and impact on pollinators. Curr. Opin. Insect Sci. 2018, 30, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Dirilgen, T.; Herbertsson, L.; O’Reilly, A.; Mahon, N.; Stanley, D. Moving past neonicotinoids and honeybees: A systematic review of existing research on other insecticides and bees. Environ. Res. 2023, 235, 116612. [Google Scholar] [CrossRef]
- Pack, D. Publication Recaps Academic Research on Neonicotinoids. 15 March 2016. Available online: https://www.purdue.edu/newsroom/releases/2016/Q1/publication-recaps-academic-research-on-neonicotinoids.html (accessed on 16 September 2021).
- Neonicotinoid Regulations for Growers. Available online: https://www.ontario.ca/page/neonicotinoid-rules-growers (accessed on 16 September 2021).
- Commission Implementing Regulation (EU) No 540/2011. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:153:0001:0186:EN:PDF (accessed on 16 September 2021).
- Farms and Farmland: Numbers, Acreage, Ownership, and Use. Census of Agriculture. Available online: https://www.nass.usda.gov/Publications/Highlights/2014/Highlights_Farms_and_Farmland.pdf (accessed on 16 September 2021).
- Naksata, M.; Watcharapasorn, A.; Hongsibsong, S.; Sapbamrer, R. Development of Personal Protective Clothing for Reducing Exposure to Insecticides in Pesticide Applicators. Int. J. Environ. Res. Public Health 2020, 17, 3303. [Google Scholar] [CrossRef]
- Matthews, G.; Bateman, R.; Miller, P. Pesticide Application Methods, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, March 2014; Available online: https://www.wiley.com/en-ae/Pesticide+Application+Methods,+4th+Edition-p-9781118351307 (accessed on 9 April 2024).
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-Derived Pesticides as an Alternative to Pest Management and Sustainable Agricultural Production: Prospects, Applications and Challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef]
- Why Is Eutrophication Such a Serious Pollution Problem. Retrieved November 2016. Available online: http://www.unep.or.jp/ietc/publications/short_series/lakereservoirs-3/1.asp (accessed on 9 April 2024).
- Rajaseger, G.; Chan, K.L.; Tan, K.Y.; Ramasamy, S.; Khin, M.C.; Amaladoss, A.; Haribhai, P.K. Hydroponics: Current trends in sustainable crop production. Bioinformation 2023, 19, 925–938. [Google Scholar] [CrossRef]
- Benefits of Life Cycle Approaches. Available online: https://www.lifecycleinitiative.org/starting-life-cycle-thinking/benefits/ (accessed on 16 September 2021).
- Hydroponic and Soil Tomato Growth. Available online: https://www.flickr.com/photos/aquamech-utah/24443777644 (accessed on 20 December 2021).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).