The Role of Burdock and Black Radish Powders Obtained by Low-Temperature Drying in Emulsion-Type Hair Conditioners
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Formulations
Formulation Method
2.3. Content of Active Ingredients in Plant Powders
- A—mean absorbance of the antioxidant-containing test solution;
- A0—mean absorbance of the DPPH radical solution.
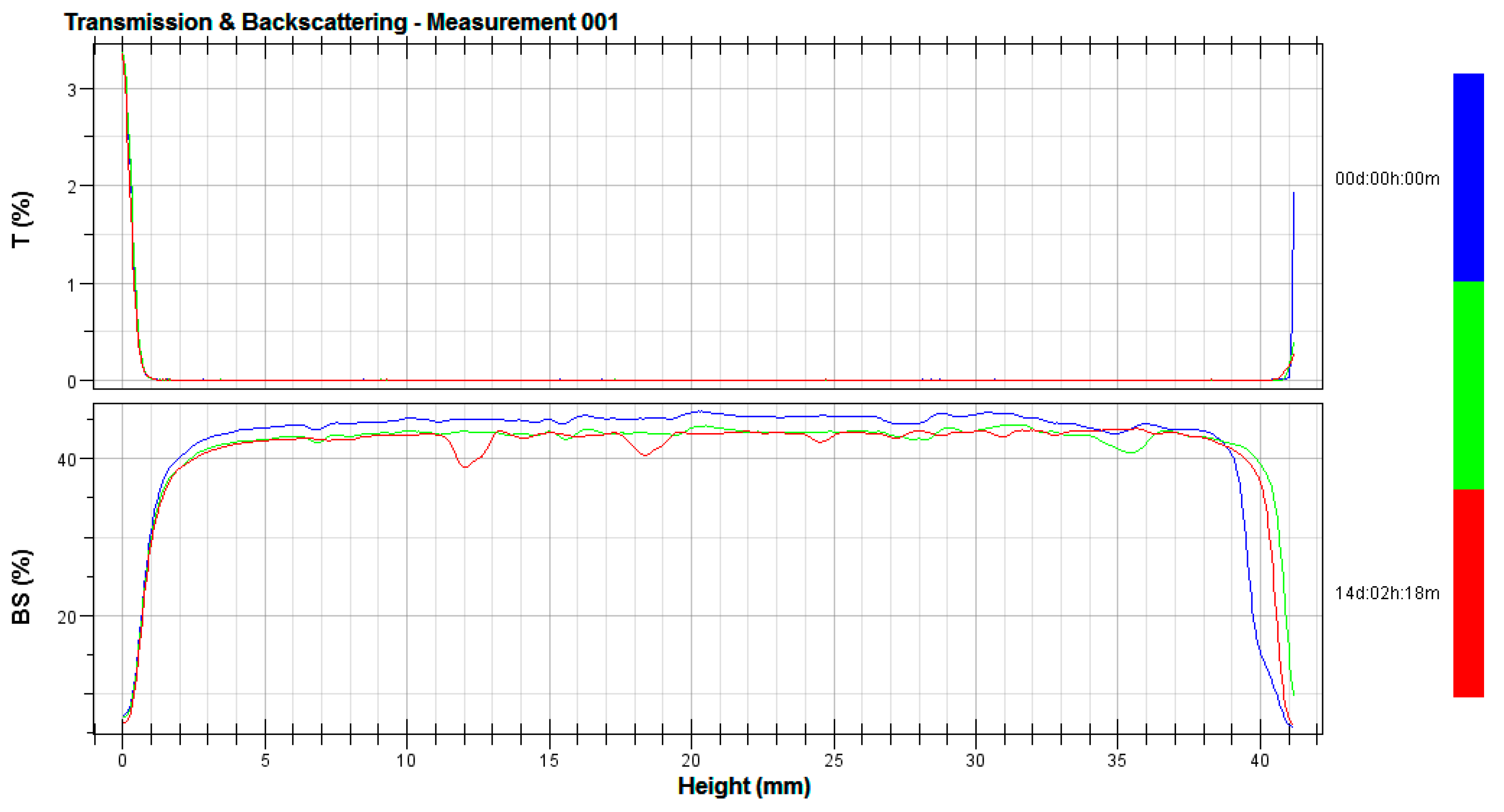
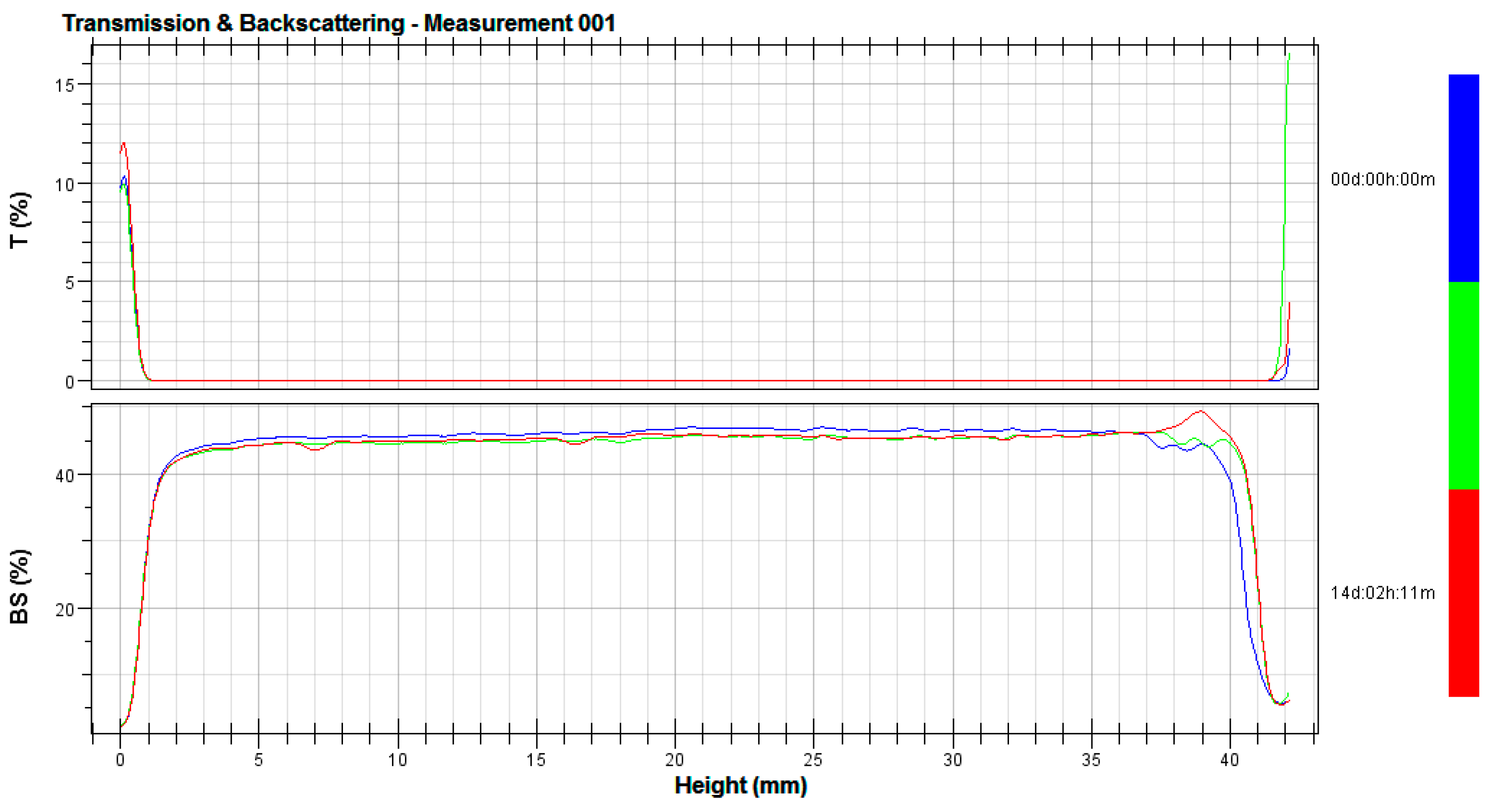
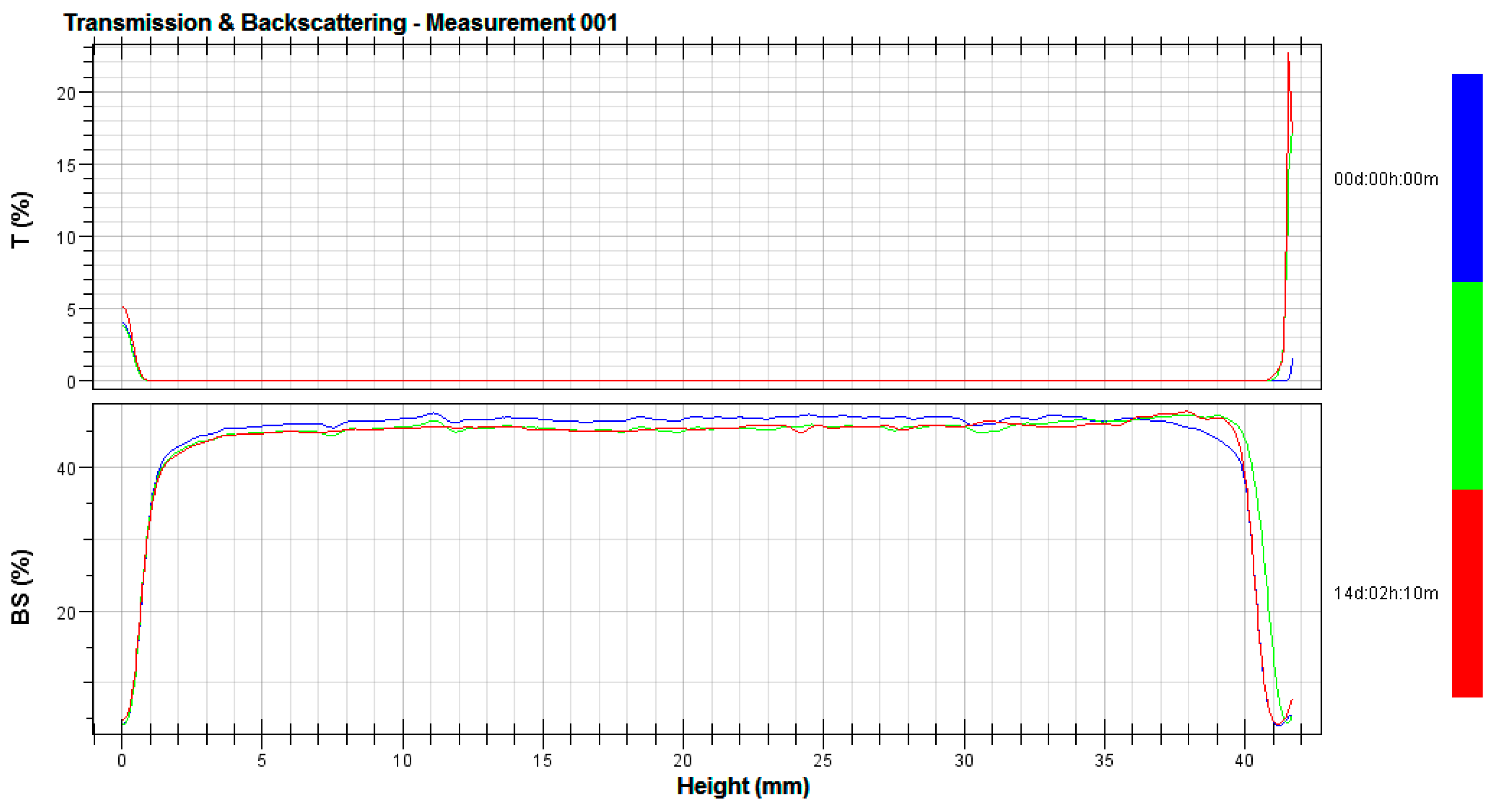
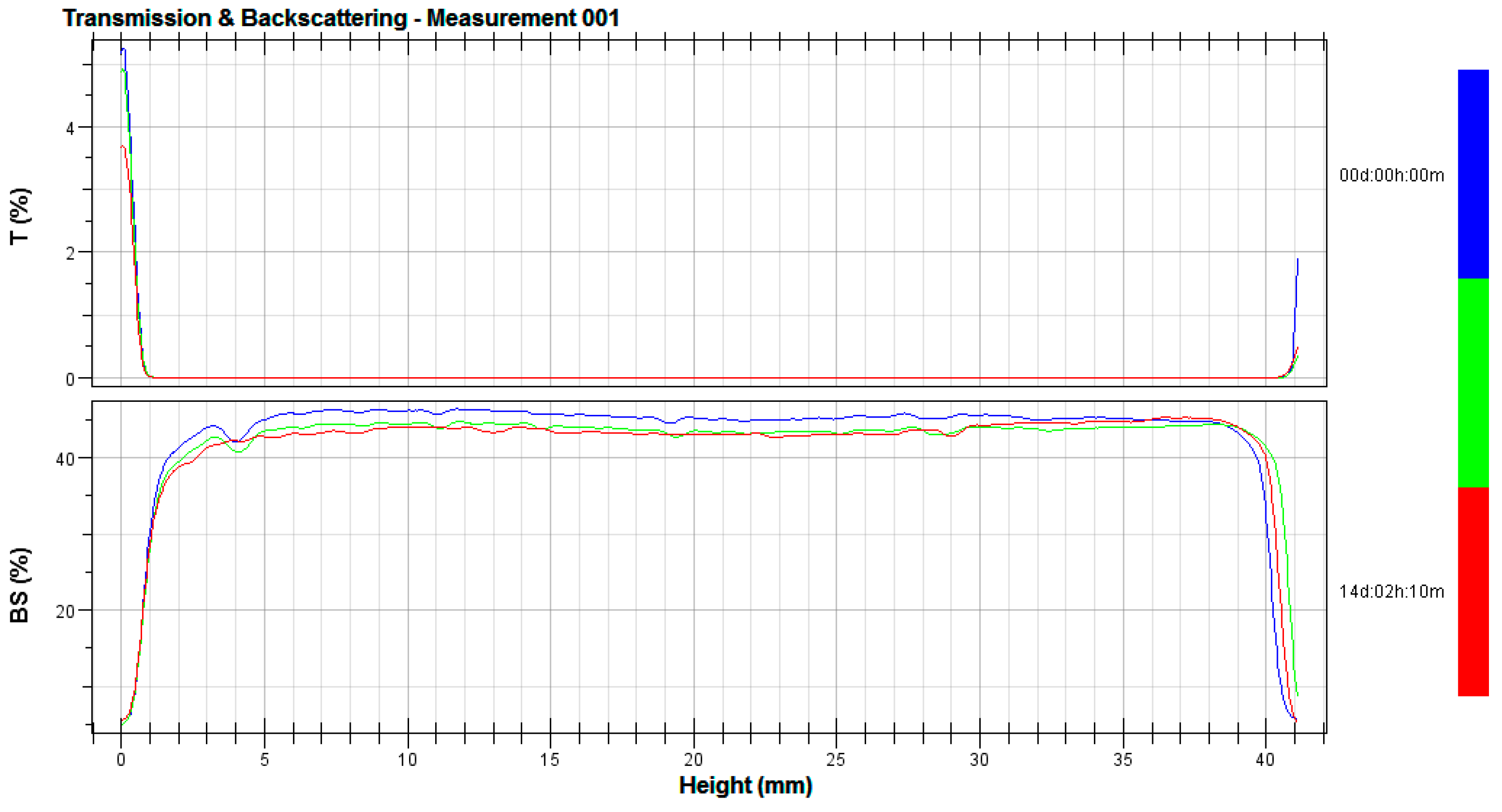
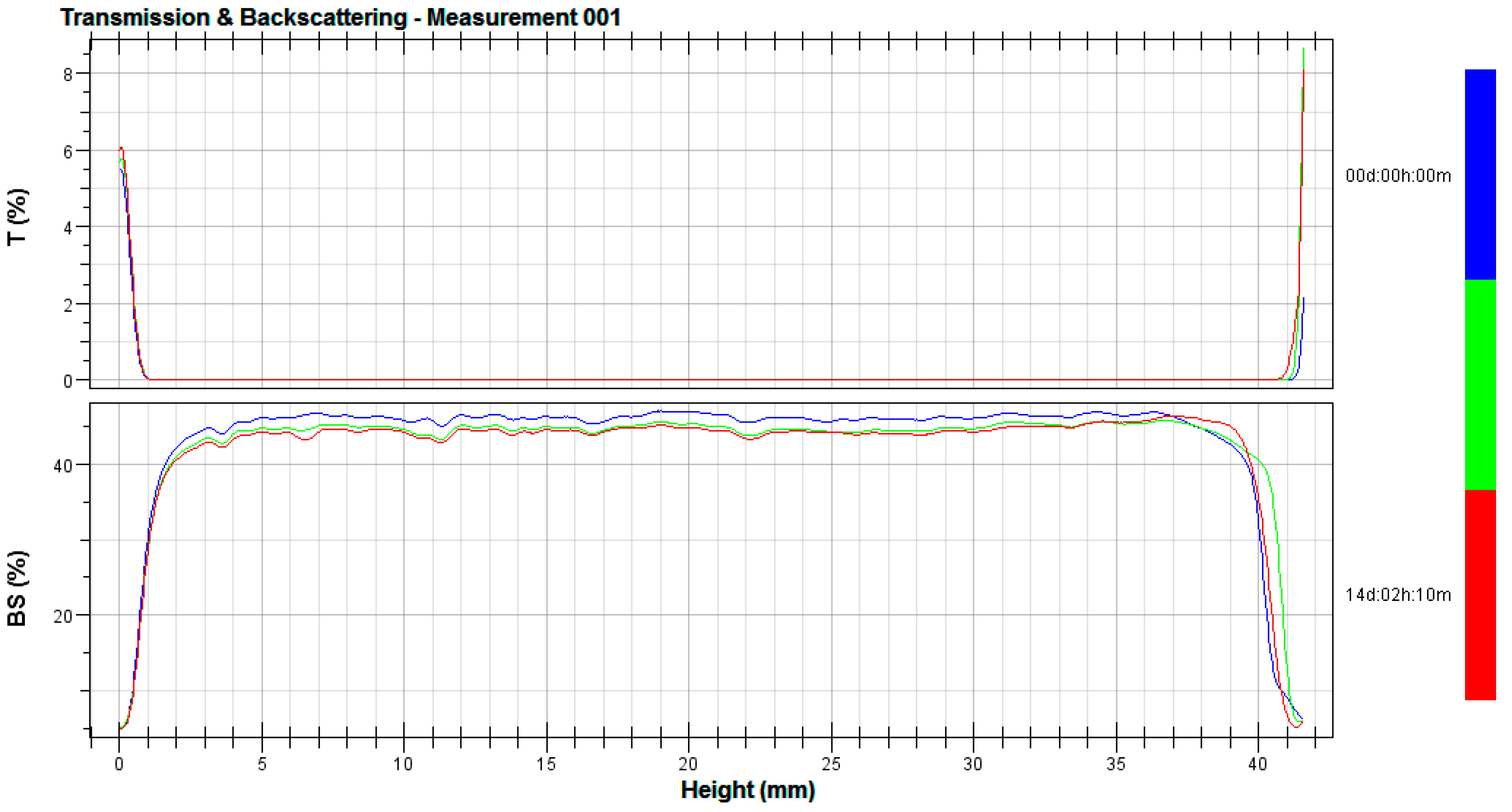
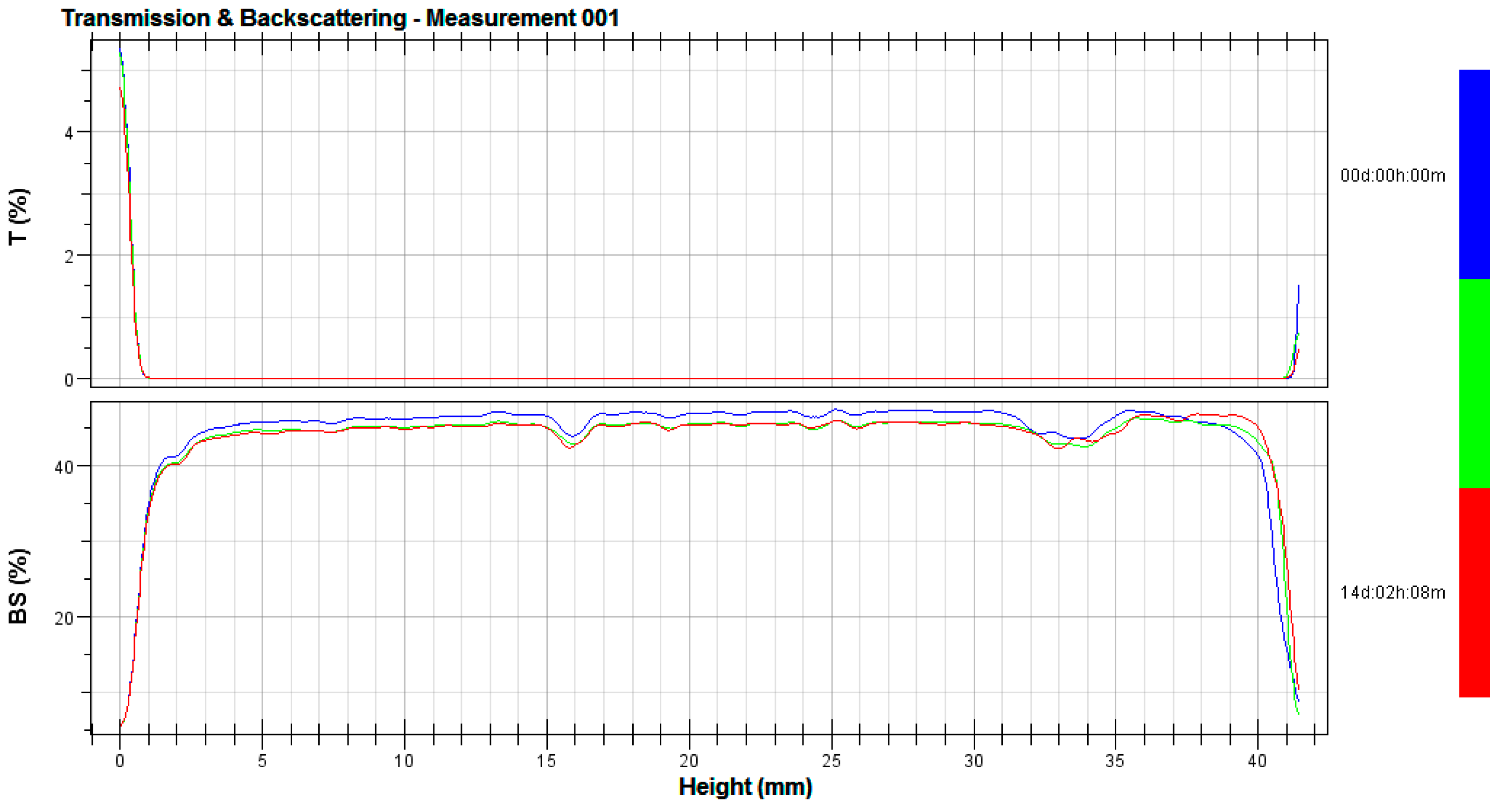
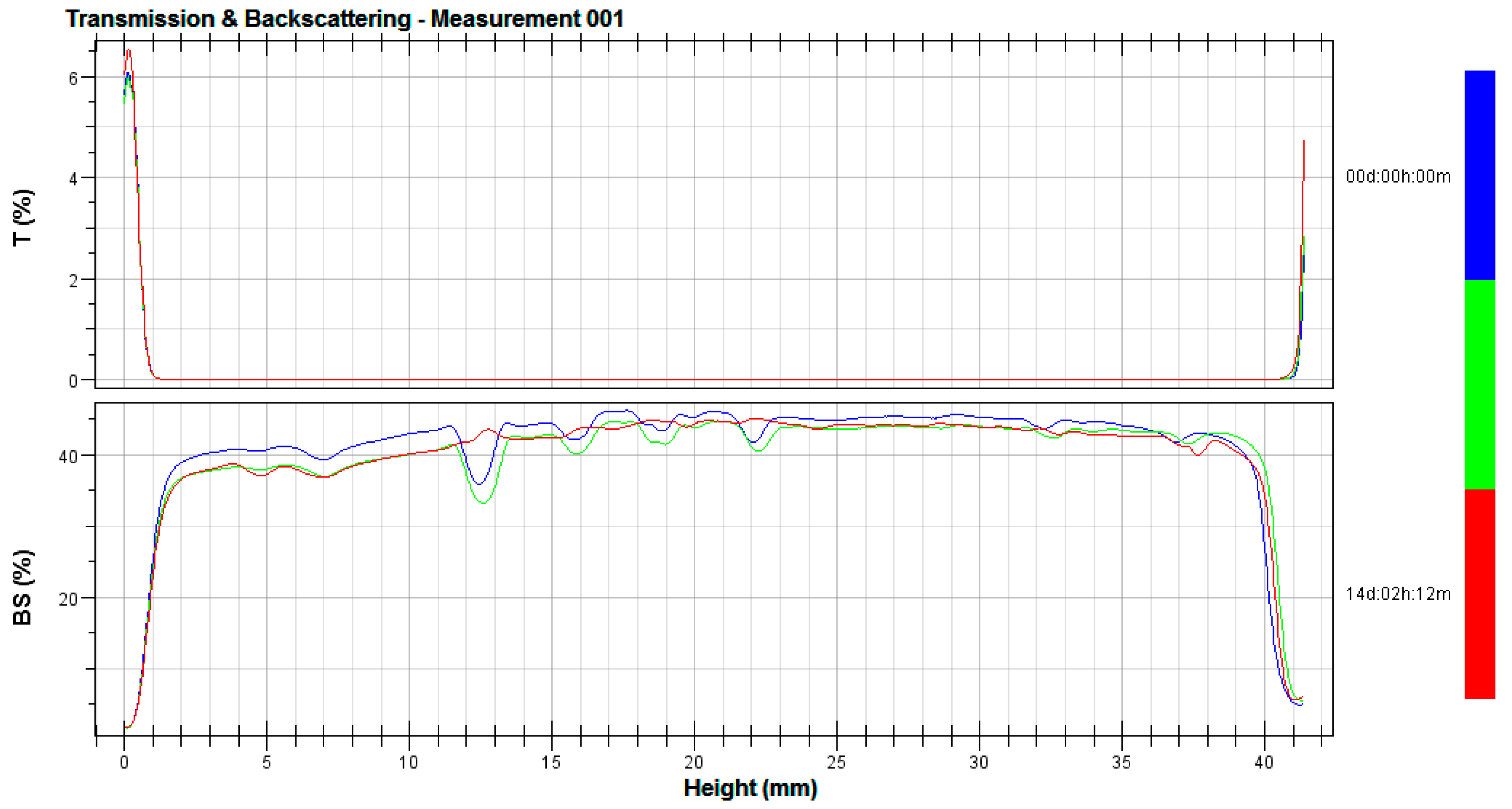
2.4. Stability Tests
- λ*—is the photon transport mean free path in the analyzed dispersion;
- ϕ—is the volume fraction of emulsion particles;
- d—is the mean diameter of particles;
- g, Qs—are the optical parameters given by the Mie theory;
- Xi—is the average backscattering for each minute of measurement;
- XBS—is the average Xi;
- n—is the number of scans [29].
2.5. Dynamic Viscosity
2.6. Yield Stress
2.7. Mechanical Properties (Texture)
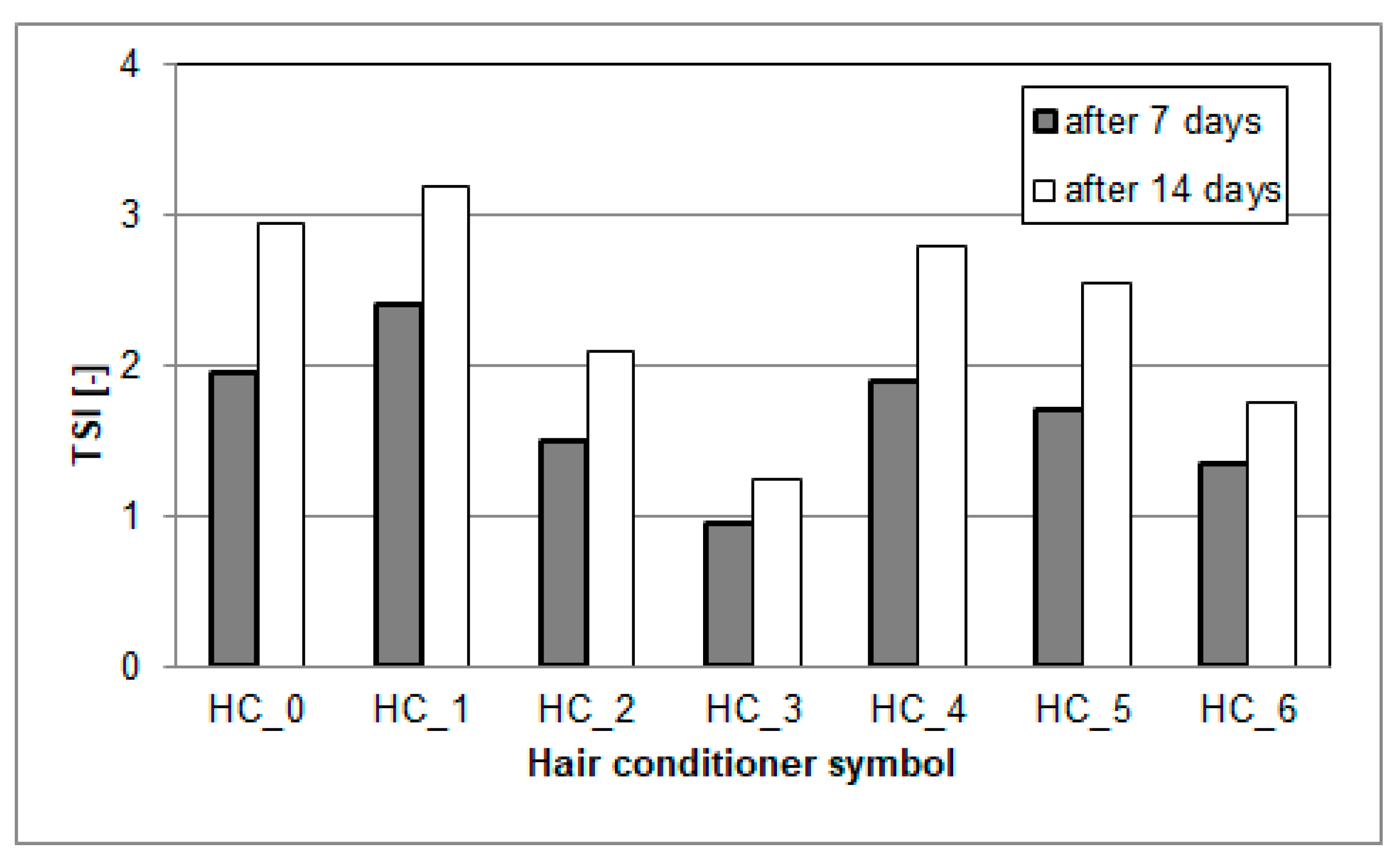
2.8. Color Assessment
- L*—lightness (intensity of color brightness; by comparing their L* values, colors can be classified as either light or dark);
- a*—value between red and green;
- b*—value between yellow and blue.
- —difference in color saturation between the base hair conditioner formulation and the hair conditioner containing plant powder;
- HC_0—base hair conditioner formulation;
- HC_X—hair conditioner with plant powder at a concentration of X;
- X = 0.25; 0.5, 1.0.
2.9. Statistical Analysis
3. Results and Discussion
3.1. Content of Active Ingredients in Plant Powders
3.2. Stability Tests
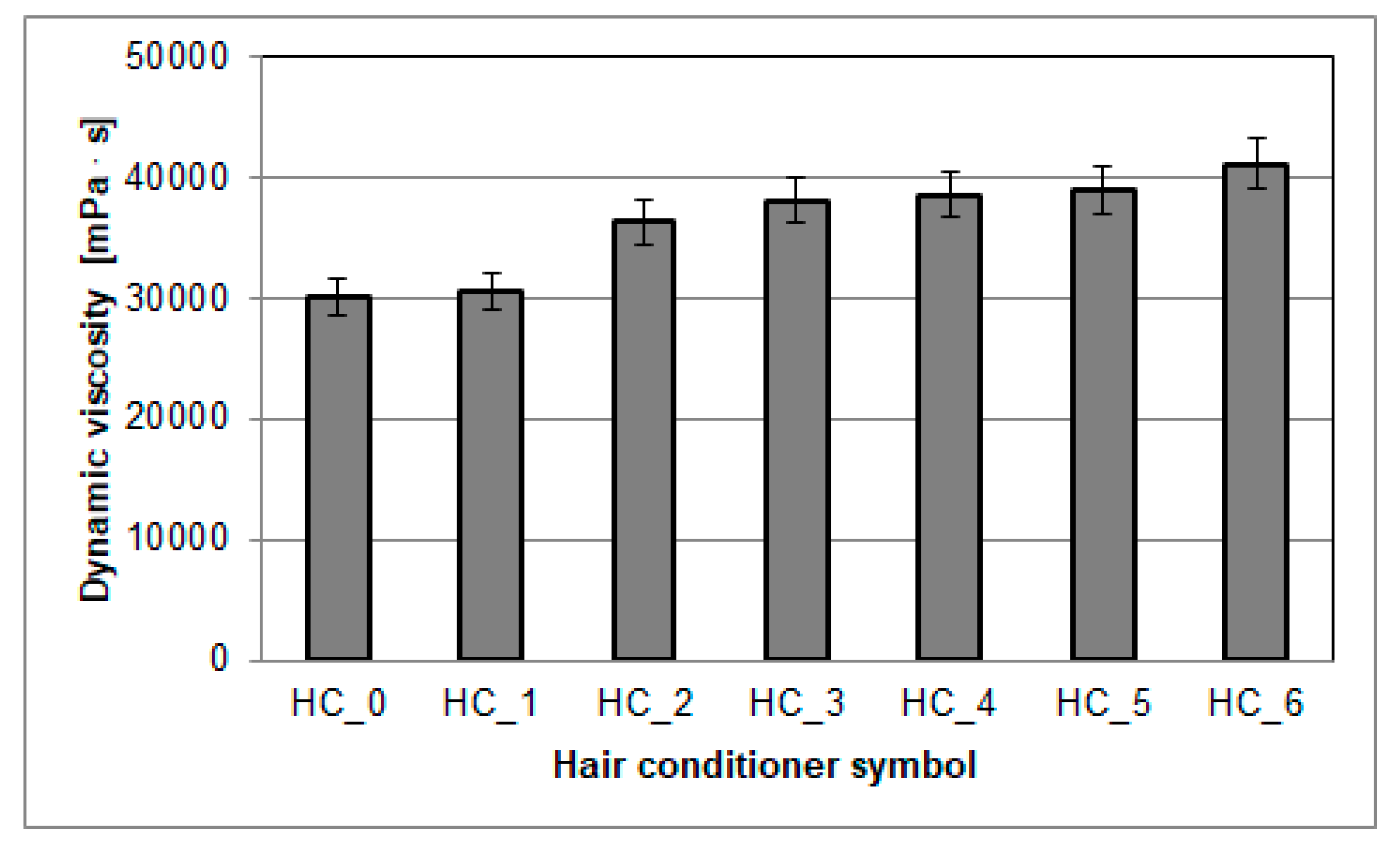
3.3. Dynamic Viscosity
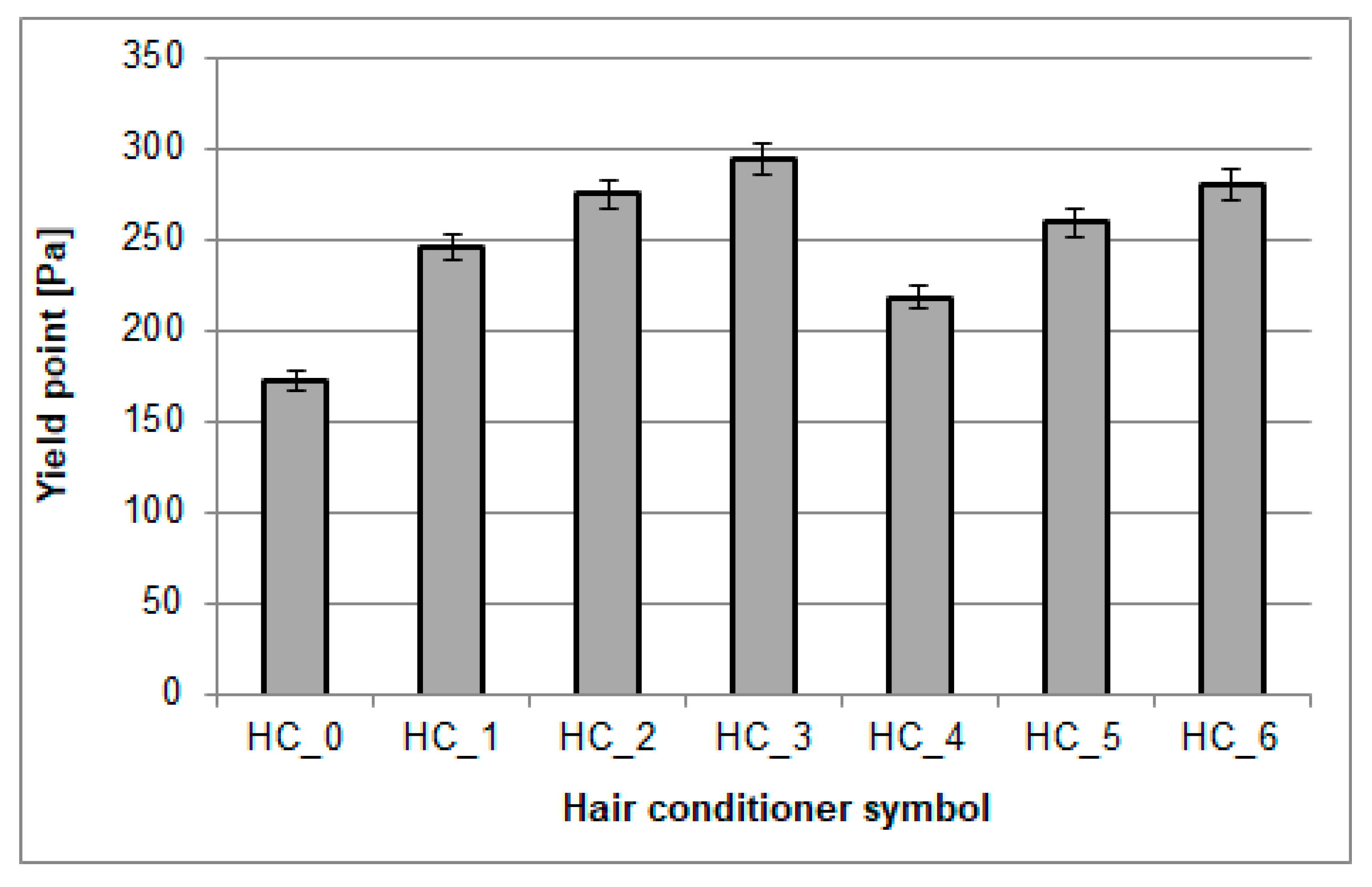
3.4. Yield Stress
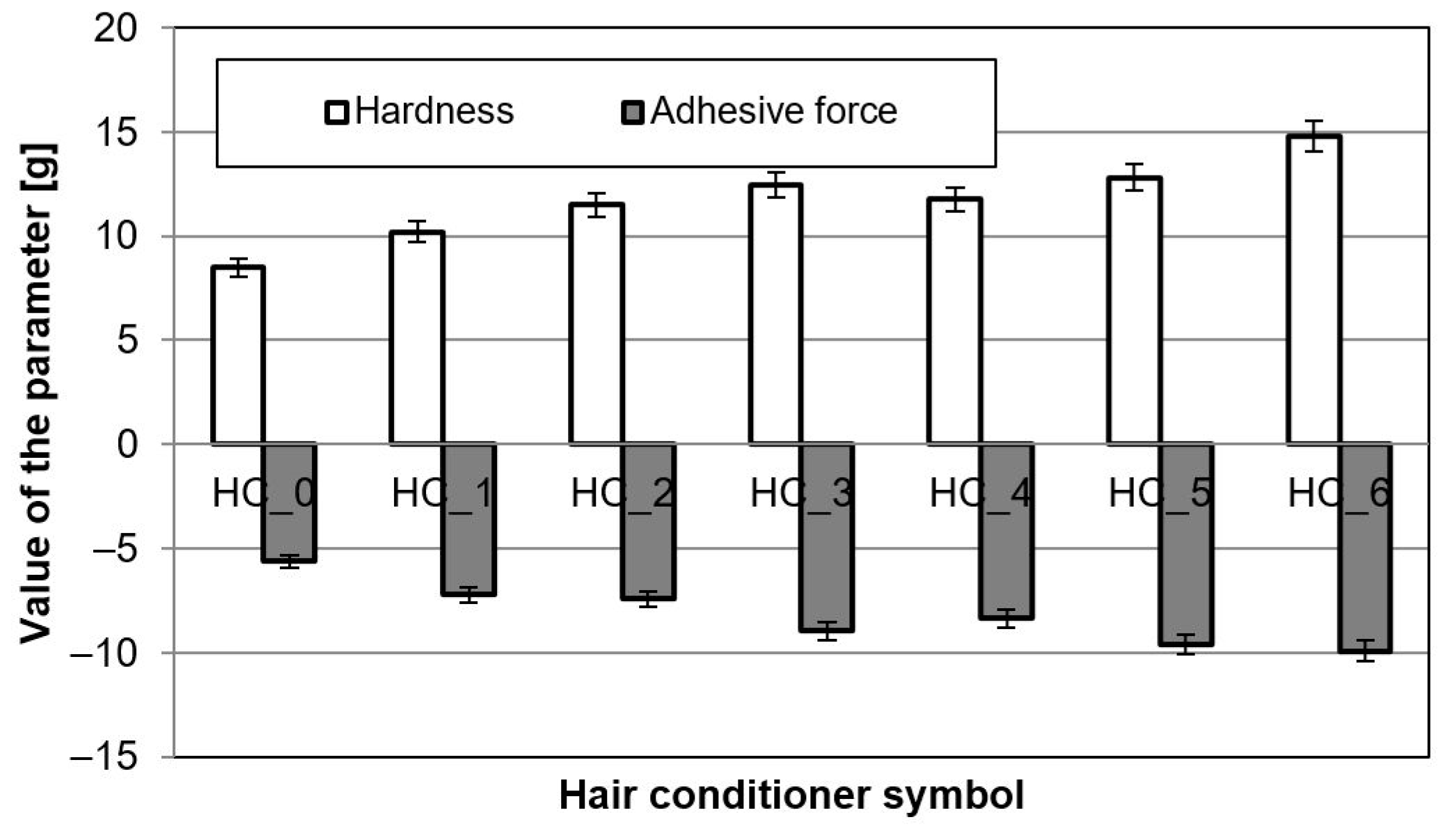
3.5. Mechanical Properties of Emulsions (Texture)
3.6. Color Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Fernandes, C.; Medronho, B.; Alves, L.; Rasteiro, M.G. On Hair Care Physicochemistry: From Structure and Degradation to Novel Biobased Conditioning Agents. Polymers 2023, 15, 608. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.R. Interactions of Shampoo and Conditioner Ingredients with Hair. In Chemical and Physical Behavior of Human Hair; Springer: Berlin/Heidelberg, Germany, 2012; pp. 329–443. [Google Scholar]
- Marsh, J.; Gray, J.; Tosti, A. Understanding Hair Damage. In Healthy Hair; Springer International Publishing: Cham, Switzerland, 2015; pp. 45–70. [Google Scholar]
- Goyal, A.; Sharma, A.; Kaur, J.; Kumari, S.; Garg, M.; Sindhu, R.K.; Rahman, M.H.; Akhtar, M.F.; Tagde, P.; Najda, A.; et al. Bioactive-Based Cosmeceuticals: An Update on Emerging Trends. Molecules 2022, 27, 828. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Peña, L.; Guzmán, E. Physicochemical aspects of the performance of hair-conditioning formulations. Cosmetics 2020, 7, 26. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, L.A.; Ali, T.; Kareem, O.; Rashid, R.; Khan, N.A.; Chashoo, I.A.; Bader, G.N. Arctium lappa: A Review on Its Phytochemistry and Pharmacology. In Edible Plants in Health and Diseases; Masoodi, M.H., Rehman, M.U., Eds.; Springer: Singapore, China, 2022; Volume 2, pp. 327–348. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Zhang, M.; Gao, C.; Ma, C.; Wang, Z.L. Improved extraction andidentification by ultra-performance liquid chromatography tandem mass spectrometry of phenolic compounds in burdock leaves. J. Chromatogr. A 2010, 1217, 2441–2446. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, R.; Graziani, G.; Gallo, M.; Fogliano, V.; Ritieni, A. Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J. Pharm. Biomed. Anal. 2010, 51, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Yamabe, S.; Shimada, M. Arctium lappa Lam. And Its Related Lignans Improve Hyperglycemia and Dyslipidemia in Diabetic Rodent Models: A Systematic Review and Meta-Analysis. Nutraceuticals 2022, 2, 335–349. [Google Scholar] [CrossRef]
- Zhang, X.; Herrera-Balandrano, D.D.; Huang, W.; Chai, Z.; Beta, T.; Wang, J.; Feng, J.; Li, Y. Comparison of Nutritional and Nutraceutical Properties of Burdock Roots Cultivated in Fengxian and Peixian of China. Foods 2021, 10, 2095. [Google Scholar] [CrossRef]
- Lucia Pirvu, L.; Nicorescu, I.; Hlevca, C.; Albu, B.; Nicorescu, V. Burdock (Arctium lappa) Leaf Extracts Increase the In Vitro Antimicrobial Efficacy of Common Antibiotics on Gram-positive and Gram-negative Bacteria. Open Chem. 2017, 15, 92–102. [Google Scholar] [CrossRef]
- Chan, Y.-S.; Cheng, L.-N.; Wu, J.-H.; Chan, E.; Kwan, Y.-W.; Lee, S.M.-Y.; Leung, G.P.-H.; Yu, P.H.-F.; Chan, S.-W. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology 2011, 19, 245–254. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Georgiev, M.I.; Fierascu, I.; Ungureanu, C.; Avramescu, S.M.; Ortan, A.; Georgescu, M.I.; Sutan, A.N.; Zanfirescu, A.; Dinu-Pirvu, C.E.; et al. Mitodepressive, antioxidant, antifungal and anti-inflammatory effects of wild-growing Romanian native Arctium lappa L. (Asteraceae) and Veronica persica Poiret (Plantaginaceae). Food Chem. Toxicol. 2018, 111, 44–52. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Heidari, H.; Oroojan, A.A.; Mirzavandi, F.; Nasr Esfehani, K.; Dehghan Mohammadi, Z. Antidiabetic, hypolipidemic and hepatoprotective effects of Arctium lappa root’s hydro-alcoholic extract on nicotinamide-streptozotocin induced type 2 model of diabetes in male mice. Avicenna J. Phytomed. 2017, 7, 169–179. [Google Scholar] [PubMed]
- Enkhtuya, E.; Tsend, M. The effect of peeling on antioxidant capacity of black radish root. Ital. J. Food Sci. 2020, 32, 701–711. [Google Scholar] [CrossRef]
- Janjua, S.; Shahid, M.; Fakhir, A. Phytochemical analysis and in vitro antibacterial activity of root peel extract of Raphanus sativus L. var niger. Adv. Med. Plant Res. 2013, 1, 1–7. [Google Scholar]
- Kumar, R.; Patwa, R. Antioxidant activity of Raphanus sativus L. of Jhansi district, Uttar Pradesh, India. Int. Res. J. Pharm. 2018, 9, 98–102. [Google Scholar] [CrossRef]
- Lugasi, A.; Dworschák, A.; Blázovics, A.; Kéry, Á. Antioxidant and Free Radical Scavenging Properties of Squeezed Juice from Black Radish (Raphanus sativus L. var niger) Root. Phytother. Res. 1998, 12, 502–506. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, X.; Tan, Q.; Xiao, Q.; Mei, S. A Comparative Metabolomics Study of Flavonoids in Radish with Different Skin and Flesh Colors (Raphanus sativus L.). J. Agric. Food Chem. 2020, 68, 14463–14470. [Google Scholar] [CrossRef] [PubMed]
- Łuczak, A.; Fryźlewicz-Kozak, B. Methods of research into hair conditioners stability. Tech. Trans. Chem. 2013, 1, 29–38. [Google Scholar]
- Liu, Z.; Graf, K.; Hub, J.; Kellermeier, M. Effects of Cosmetic Emulsions on the Surface Properties of Mongolian Hair. ACS Omega 2022, 7, 10910–10920. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska, E.; Zieba, M.; Gregorczyk, K.; Markuszewski, L. Application of Blue Honeysuckle Powder Obtained by an Innovative Method of Low-Temperature Drying in Skin care Face Masks. Molecules 2021, 26, 7184. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Peña, L.; Guzmán, E.; Leonforte, F.; Serrano-Pueyo, A.; Regulski, K.; Tournier-Couturier, L.; Ortega, F.; Rubio, R.G.; Luengo, G.S. Effect of molecular structure of eco-friendly glycolipid biosurfactants on the adsorption of hair-care conditioning polymers. Colloids Surf. B Biointerfaces 2020, 185, 110578. [Google Scholar] [CrossRef]
- Treford, E. Product claims. In Practical Modern Hair Science; Evans, T., Wickett, R.R., Eds.; Allured Business Media: Carol Stream, IL, USA, 2012; pp. 498–531. [Google Scholar]
- Gonçalves, R.A.; Lam, Y.-M.; Lindman, B. Double-Chain Cationic Surfactants: Swelling, Structure, Phase Transitions and Additive Effects. Molecules 2021, 26, 3946. [Google Scholar] [CrossRef] [PubMed]
- Keis, K.; Persaud, D.; Kamath, Y.K.; Rele, A.S. Investigation of penetration abilities of various oils into human hair fibers. J. Cosmet. Sci. 2005, 56, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Rele, A.S.; Mohile, R.B. Effect of mineral oil, sunflower oil, and coconut oil on prevention of hair damage. J. Cosmet. Sci. 2003, 54, 175–192. [Google Scholar] [PubMed]
- Bontozoglou, C.; Xiao, P. Applications of capacitive imaging in human skin texture and hair analysis. Appl. Sci. 2020, 10, 256. [Google Scholar] [CrossRef]
- Tinoco, A.; Costa, A.F.; Luís, S.; Martins, M.; Cavaco-Paulo, A.; Ribeiro, A. Proteins as hair styling agents. Appl. Sci. 2021, 11, 4245. [Google Scholar] [CrossRef]
- Shin, S.-Y.; Kwon, J.-E.; Kim, S.; Lee, Y.-G.; Park, S.; Kang, S.-C. Hair regeneration effects of lespedeza bicolor extract in vivo and in vitro. Appl. Sci. 2022, 12, 2863. [Google Scholar] [CrossRef]
- Mitsui, T. New Cosmetic Science; Elsevier Science: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Zięba, M.; Klimaszewska, E.; Ruszkowska, M. Evaluation of physicochemical properties of plant raw materials in powder form and dry hair shampoos obtained with their addition. Przem. Chem. 2022, 101, 404–411. [Google Scholar] [CrossRef]
- PN-A-04019:1998; Food Products: Determination of Vitamin C Content. Polish Committee for Standarisation: Warsaw, Poland, 1998.
- Peri, C.; Pompei, C. An assay of different phenolic fractions in wines. Am. J. Enol. Vitic. 1971, 22, 55–58. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, F.; Saito, M.; Tatsumi, E.; Yin, L. Effect of magnesium salt concentration in water-in-oil emulsions on the physical properties and microstructure of tofu. Food Chem. 2016, 201, 197–204. [Google Scholar] [CrossRef]
- Klimaszewska, E.; Seweryn, A.; Małysa, A.; Zięba, M.; Lipińska, J. The effect of chamomile extract obtained in supercritical carbon dioxide conditions on physicochemical and usable properties of pharmaceutical ointments. Pharm. Dev. Technol. 2018, 23, 780–786. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; de Fariña, L.O.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Bharadvaja, N.; Gautam, S.; Singh, H. Natural polyphenols: A promising bioactive compounds for skin care and cosmetics. Mol. Biol. Rep. 2023, 50, 1817–1828. [Google Scholar] [CrossRef]
- Liston, L.S.; Rivas, P.L.; Sakdiset, P.; See, G.L.; Arce, F. Chemical Permeation Enhancers for Topically-Applied Vitamin C and Its Derivatives: A Systematic Review. Cosmetics 2022, 9, 85. [Google Scholar] [CrossRef]
- Wargala, E.; Sławska, M.; Zalewska, A.; Toporowska, M. Health Effects of Dyes, Minerals, and Vitamins Used in Cosmetics. Women 2021, 1, 223–237. [Google Scholar] [CrossRef]
- Enkhtuya, E.; Lhamsuren, E.; Tsend, M. Effect of Heat on Antioxidant Capacity of Black Radish (Raphanus sativus L. var niger) Root. J. Food Nutr. Res. 2022, 10, 221–227. [Google Scholar] [CrossRef]
- Hanlon, P.R.; Robbins, M.G.; Hammon, L.D.; Barnes, D.M. Aqueous extract from the vegetative portion of Spanish black radish (Raphanus sativus L. var. niger) induces detoxification enzyme expression in HepG2 cells. J. Agric. Food Chem. 2007, 55, 6439–6446. [Google Scholar] [CrossRef]
- de Souza, A.R.C.; Guedes, A.R.; Rodriguez, J.M.F.; Bombardelli, M.C.M.; Corazza, M.L. Extraction of Arctium Lappa leaves using supercritical CO2+ethanol: Kinetics, chemical composition, and bioactivity assessments. J. Supercrit. Fluids 2018, 140, 137–146. [Google Scholar] [CrossRef]
- Tafuro, G.; Di Domenico, E.; Costantini, A.; Francescato, S.; Busata, L.; Baratto, G.; Semenzato, A. Stability and Application Properties of Surfactant-Free Cosmetic Emulsions: An Instrumental Approach to Evaluate Their Potential. Cosmetics 2022, 9, 123. [Google Scholar] [CrossRef]
- Wasilewski, T.; Nizioł-Łukaszewska, Z.; Bujak, T.; Szmuc, E.; Czerwonka, D.; Mucha, M.; Sarna, K. The Role of Solid Particles Obtained from Plant Materials in Improvement the Quality of Cosmetic Care Balms. Tenside Surf. Det. 2021, 58, 33–43. [Google Scholar] [CrossRef]
- Jeon, T.Y.; Hong, J.S. Stabilization of O/W emulsion with hydrophilic/hydrophobic clay particles. Colloid Polym. Sci. 2014, 292, 2939–2947. [Google Scholar] [CrossRef]
- Kowalska, M.; Żbikowska, A.; Woźniak, M.; Amanowicz, A. Quality of Emulsions Based on Modified Watermelon Seed Oil, Stabilized with Orange Fibres. Molecules 2022, 27, 513. [Google Scholar] [CrossRef] [PubMed]
- Nizioł-Łukaszewska, Z.; Wasilewski, T.; Bujak, T.; Gaweł-Bęben, K.; Osika, P.; Czerwonka, D. Cornus mas L. extract as a multifunctional material for manufacturing cosmetic emulsions. Chin. J. Nat. Med. 2018, 16, 284–292. [Google Scholar] [CrossRef]
- Lukic, M.; Jaksic, I.; Krstonosic, V.; Dokic, L.; Savic, S. Effect of small change in oil phase composition on rheological and textural properties of w/o emulsion. J. Texture Stud. 2013, 44, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska, E.; Małysa, A.; Zięba, M.; Rój, E.; Wasilewski, T. Use of the hydrophobic blackberry extract obtained by ex-traction with supercritical carbon dioxide for the preparation in cosmetic masks. Przem. Chem. 2016, 95, 1000–1005. [Google Scholar] [CrossRef]
- Brummer, R.; Godersky, S. Rheological studies to objectify sensations occurring when cosmetic emulsions are applied to the skin. Colloids Surf. A Physicochem. Eng. 1999, 152, 89–94. [Google Scholar] [CrossRef]
- Ogorzałek, M.; Klimaszewska, E. Applications of Hydroxyethyl Urae in Skin Care Cosmetics. In The Role of Commodity Science in Quality Management in a Knowledge-Based Economy. Innovation in Quality Development of Products and Services; Dmowski, P., Ed.; Wydawnictwo Uniwersytetu Morskiego w Gdyni: Gdynia, Poland, 2022; Volume 1, pp. 81–103. [Google Scholar]
- Savić, S.M.; Cekić, N.D.; Savić, S.R. D-optimal design of experiments and comprehensive rheological analysis in the development of natural anti-aging creams. Adv. Technol. 2020, 9, 29–40. [Google Scholar] [CrossRef]
- Tai, A.; Bianchini, R.; Jachowicz, J. Texture analysis of cosmetic/pharmaceutical raw materials and formulations. Int. J. Cosmet. Sci. 2014, 36, 291–304. [Google Scholar] [CrossRef]
- Gilbert, L.; Picard, C.; Savary, G.; Grisel, M. Rheological and textural characterization of cosmetic emulsions containing, natural and synthetic polymers: Relationships between both data. Colloids Surf. A Physicochem. Eng. 2013, 421, 150–163. [Google Scholar] [CrossRef]
- Brudzynska, P.; Kurzawa, M.; Sionkowska, A.; Grisel, M. Antioxidant activity of plant-derived colorants for potential cosmetic application. Cosmetics 2022, 9, 81. [Google Scholar] [CrossRef]
- Brudzynska, P.; Sionkowska, A.; Grisel, M. Plant-derived colorants for food, cosmetic and textile industries: A review. Materials 2021, 14, 3484. [Google Scholar] [CrossRef] [PubMed]
- Bujak, T.; Zagórska-Dziok, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Lal, K.; Wasilewski, T.; Hordyjewicz-Baran, Z. Flower Extracts as Multifunctional Dyes in the Cosmetics Industry. Molecules 2022, 27, 922. [Google Scholar] [CrossRef] [PubMed]
- Bujak, T.; Zagórska-Dziok, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Wasilewski, T.; Hordyjewicz-Baran, Z. Antioxidant and cytoprotective properties of plant extract from dry flowers as functional dyes for cosmetic products. Molecules 2021, 26, 2809. [Google Scholar] [CrossRef] [PubMed]

| Phase | Ingredients [According to INCI] | Symbol of Hair Conditioner | ||||||
|---|---|---|---|---|---|---|---|---|
| HC_0 | HC_1 | HC_2 | HC_3 | HC_4 | HC_5 | HC_6 | ||
| Concentration [% w/w] | ||||||||
| I | Cetrimonium Chloride | 2.0 | ||||||
| Ceteareth-20 | 2.5 | |||||||
| Cetearyl Alcohol | 4.0 | |||||||
| Glyceryl Stearate | 1.5 | |||||||
| Paraffinum Liquidum | 2.0 | |||||||
| Ethylhexyl Hydroxystearate | 1.0 | |||||||
| II | Aqua | at 100 | ||||||
| Glycerin | 1.0 | |||||||
| III | Sodium Benzoate and Potassium Sorbate | 1.0 | ||||||
| Citric Acid | to pH = 5.5 | |||||||
| Arctium lappa Powder | - | 0.25 | 0.5 | 1.0 | - | - | - | |
| Raphanus sativus var. niger Powder | - | - | - | - | 0.25 | 0.5 | 1.0 | |
| Plant Powder | Total Polyphenolic Content [mg GAE/100 g DM] | Oxidative Activity [% Inhibition] | Vitamin C Content [mg/100 g DM] | |||
|---|---|---|---|---|---|---|
| Mean Value | SD | Mean Value | SD | Mean Value | SD | |
| Black radish | 15.46 | 1.23 | 13.83 | 0.57 | 266.34 | 0.000 |
| Burdock | 41.52 | 1.25 | 68.75 | 2.91 | 4.14 | 0.447 |
| Hair Conditioner Symbol | Parameter | |||
|---|---|---|---|---|
| L* | a* | b* | ΔC*HC | |
| HC_0 | 71.54 | −1.54 | −1.62 | - |
| HC_1 | 70.51 | −1.52 | 2.26 | 0.63 |
| HC_2 | 67.16 | −1.47 | 3.38 | 1.49 |
| HC_3 | 66.71 | −1.39 | 5.37 | 3.31 |
| HC_4 | 66.63 | −0.48 | 4.01 | 1.80 |
| HC_5 | 63.12 | 0.07 | 4.79 | 2.55 |
| HC_6 | 56.84 | 0.57 | 8.67 | 6.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zięba, M.; Klimaszewska, E.; Ogorzałek, M.; Ruszkowska, M. The Role of Burdock and Black Radish Powders Obtained by Low-Temperature Drying in Emulsion-Type Hair Conditioners. Appl. Sci. 2024, 14, 3390. https://doi.org/10.3390/app14083390
Zięba M, Klimaszewska E, Ogorzałek M, Ruszkowska M. The Role of Burdock and Black Radish Powders Obtained by Low-Temperature Drying in Emulsion-Type Hair Conditioners. Applied Sciences. 2024; 14(8):3390. https://doi.org/10.3390/app14083390
Chicago/Turabian StyleZięba, Małgorzata, Emilia Klimaszewska, Marta Ogorzałek, and Millena Ruszkowska. 2024. "The Role of Burdock and Black Radish Powders Obtained by Low-Temperature Drying in Emulsion-Type Hair Conditioners" Applied Sciences 14, no. 8: 3390. https://doi.org/10.3390/app14083390
APA StyleZięba, M., Klimaszewska, E., Ogorzałek, M., & Ruszkowska, M. (2024). The Role of Burdock and Black Radish Powders Obtained by Low-Temperature Drying in Emulsion-Type Hair Conditioners. Applied Sciences, 14(8), 3390. https://doi.org/10.3390/app14083390






