Numerical Modeling of Venous Outflow from the Cranial Cavity in the Supine Body Position
Abstract
:1. Introduction
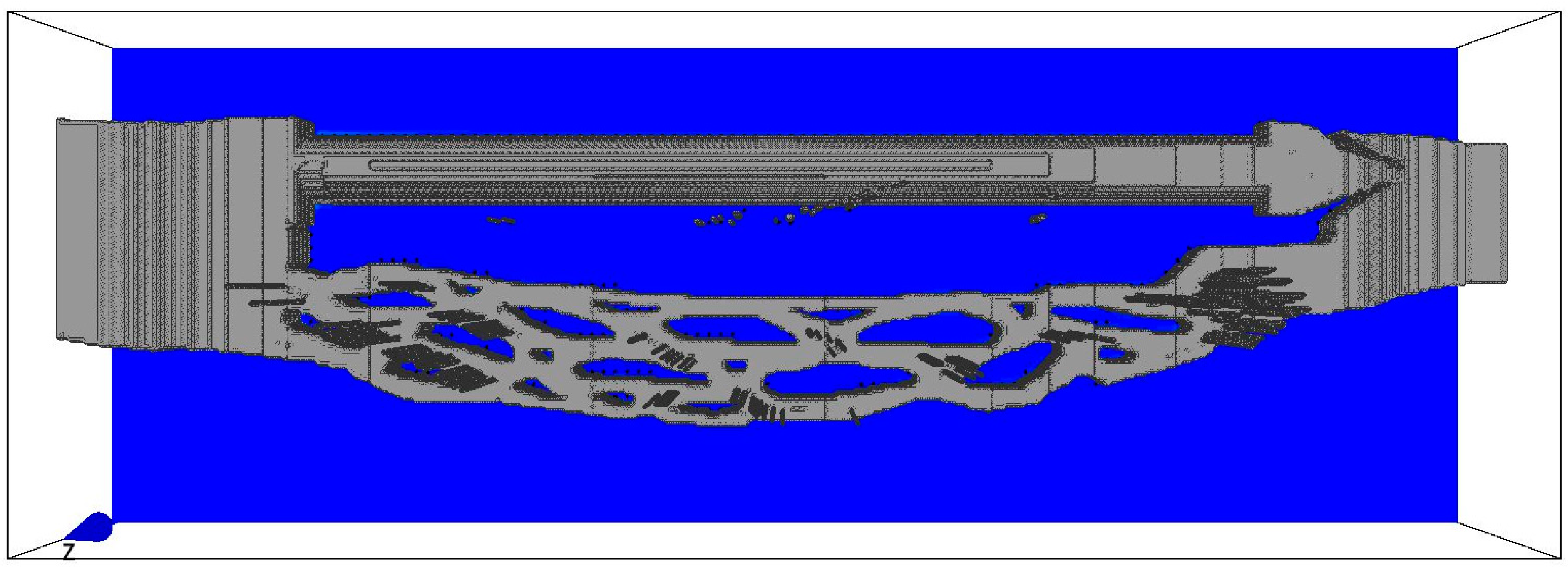
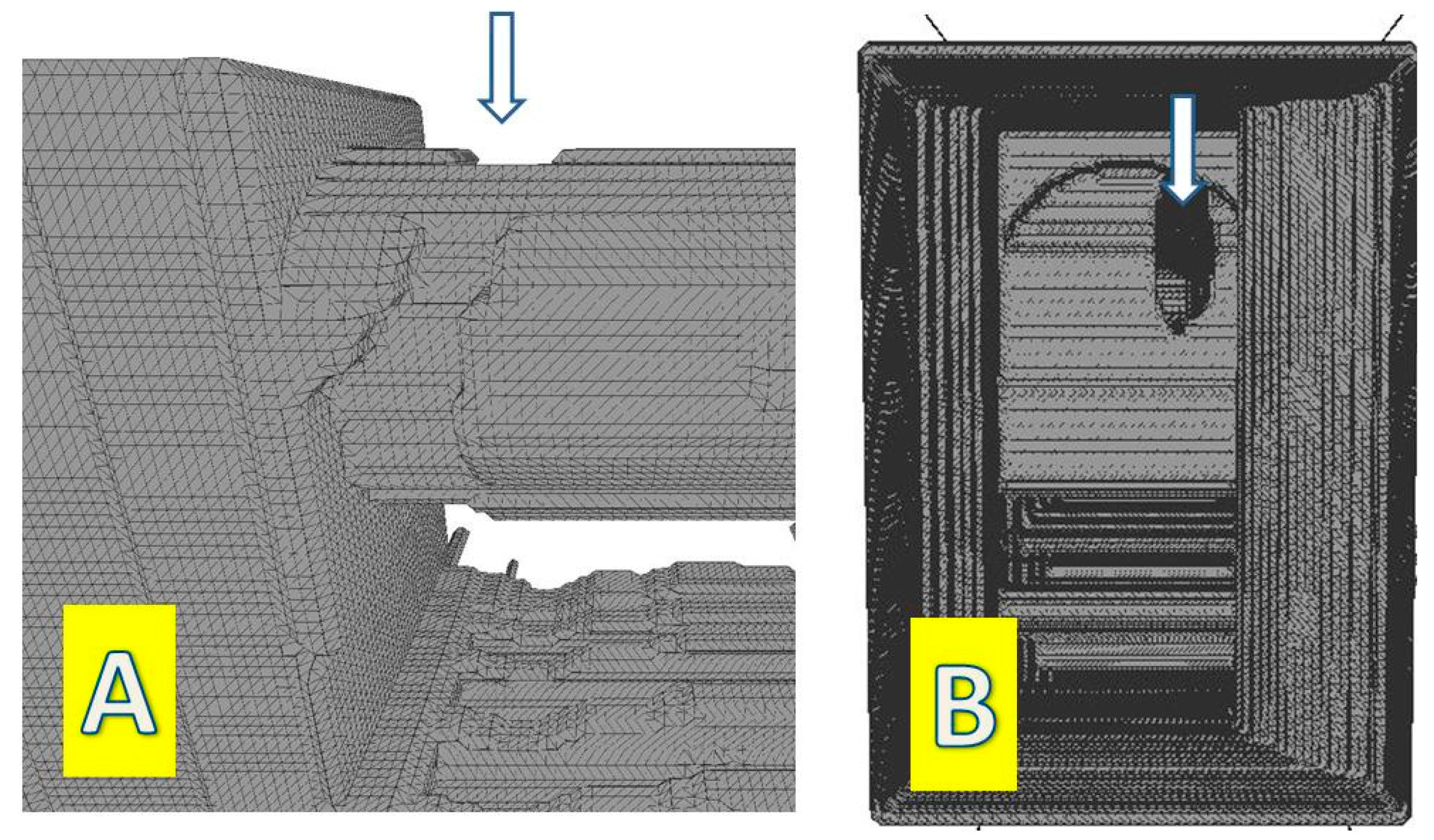
2. Materials and Methods
- No stenosis in the upper part of the IJV (cross-sectional area of 0.94 cm2);
- Minor elliptically shaped stenosis in the upper part of the IJV (cross-sectional area of 0.42 cm2);
- Minor irregularly shaped stenosis in the upper part of the IJV (cross-sectional area of 0.60 cm2);
- Major elliptically shaped stenosis in the upper part of the IJV (cross-sectional area of 0.24 cm2);
- Major elliptically shaped stenosis in the upper part of the IJV (cross-sectional area of 0.24 cm2) with opening positioned eccentrically;
- Major elliptically shaped stenosis in the upper part of the IJV (cross-sectional area of 0.24 cm2) with a longer narrowed segment (12 mm instead of 2.5 mm);
- Very narrow elliptically shaped stenosis in the upper part of the IJV (cross-sectional area of 0.07 cm2);
- Very narrow elliptically shaped stenosis in the upper part of the IJV (cross-sectional area of 0.07 cm2) with opening positioned eccentrically;
- Major stenosis in the upper part of the IJV comprising 2 eccentrically positioned openings (cross-sectional area of 0.48 cm2);
- Major stenosis in the upper part of the IJV comprising eccentrically positioned short flattening (cross-sectional area of 0.48 cm2).
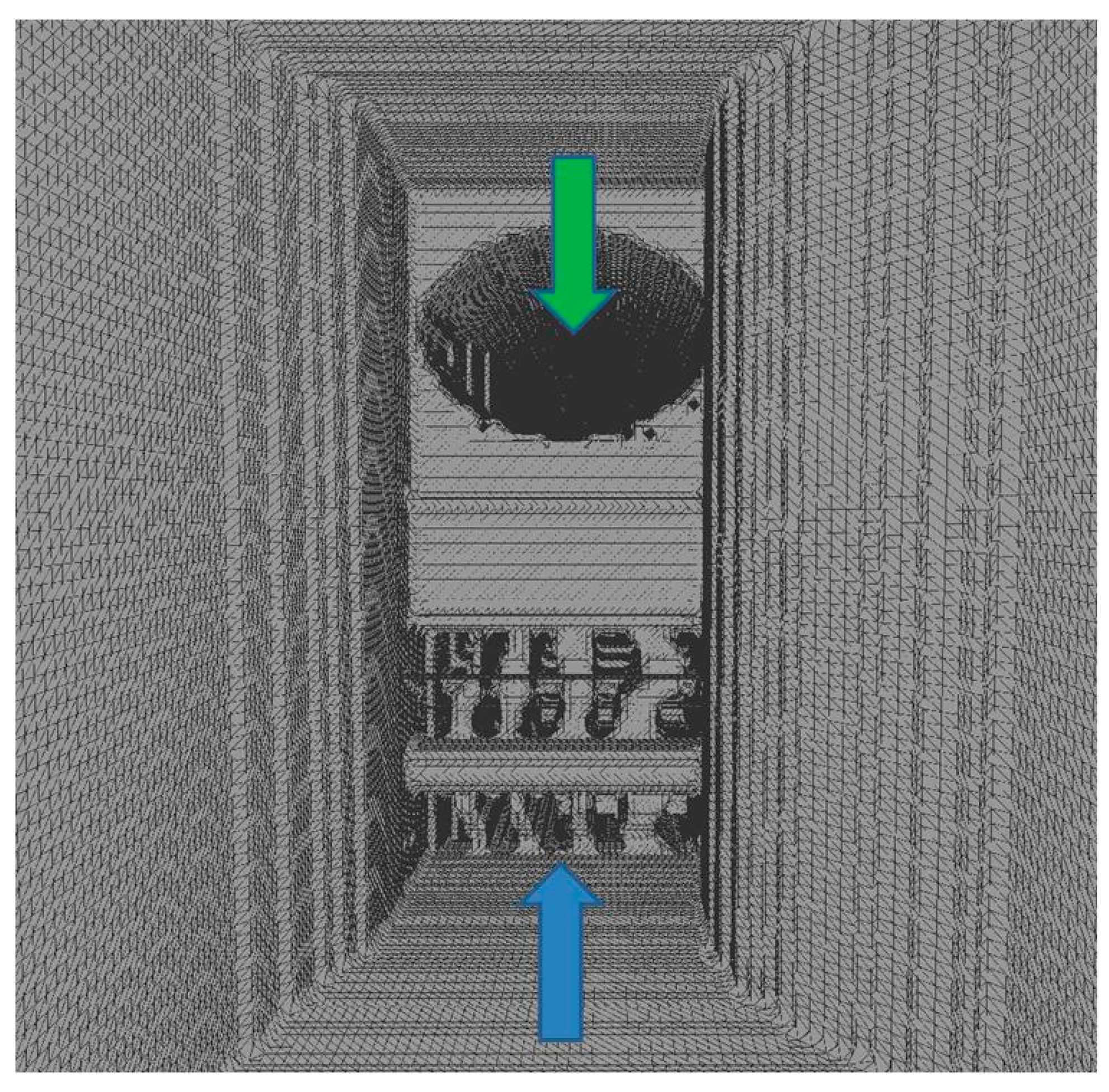
- No valve;
- Bicuspid valve, distance between leaflets set to 4.3 mm (opening located centrally, 4.3 mm × 8.5 mm);
- Bicuspid valve, distance between leaflets set to 1.5 mm (opening located centrally, 1.5 mm × 8.5 mm);
- Septum instead of a valve, almost completely obstructing the IJV (opening located next to the wall, 1.5 mm × 8.5 mm).
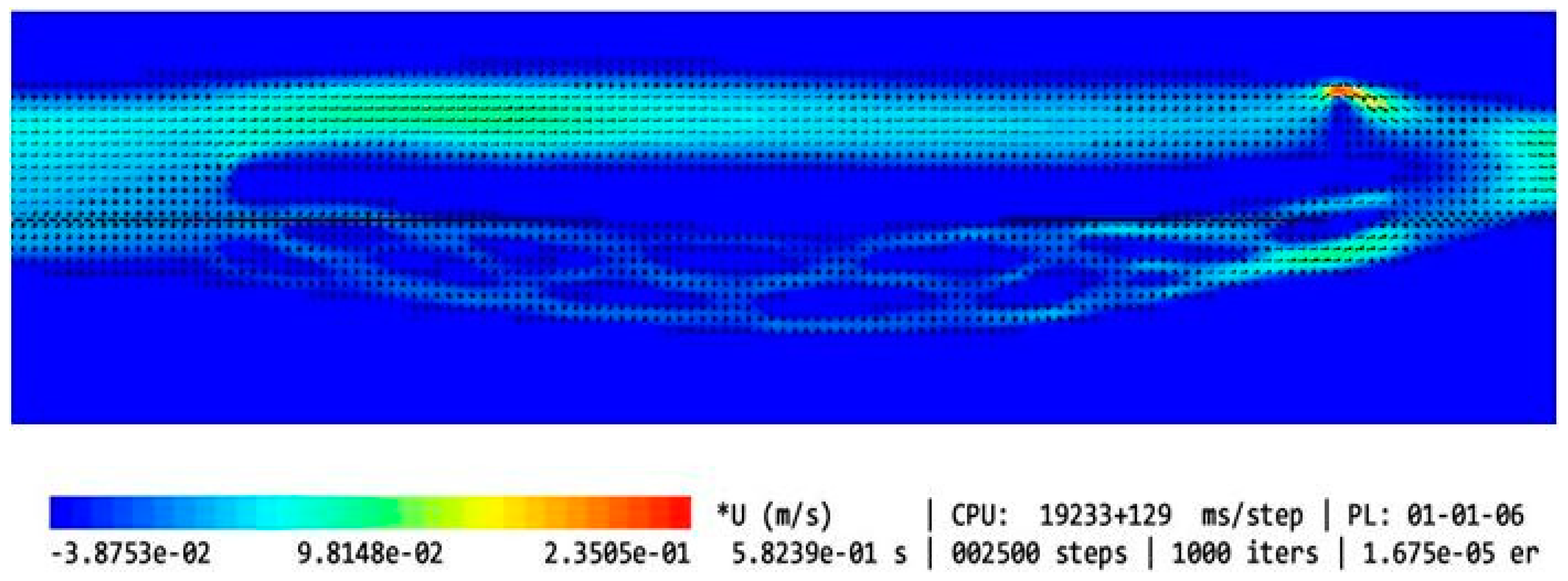
3. Results
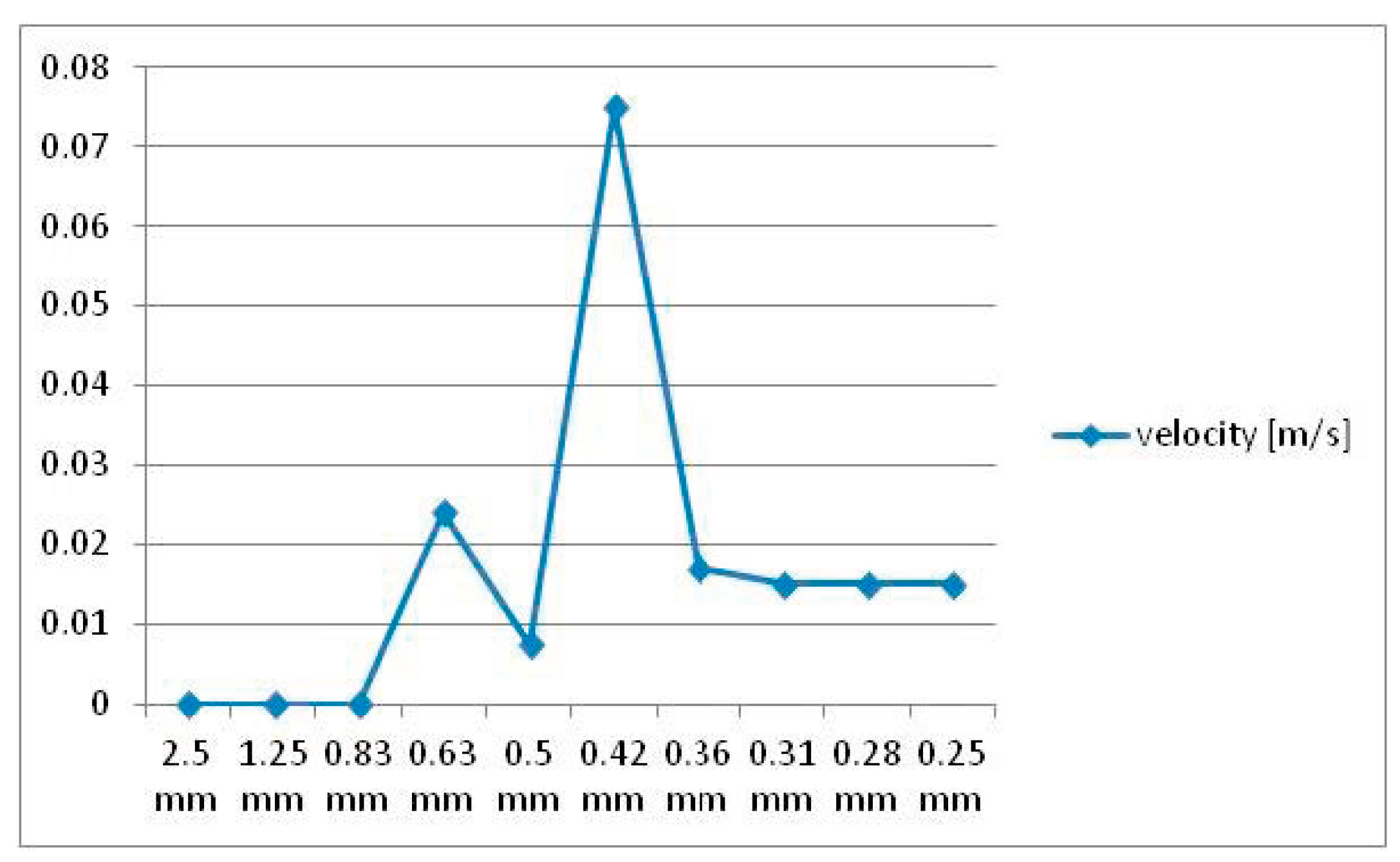
- Narrower (Models 6 and 9);
- Longer (Model 8);
- Eccentrically positioned (Models 7, 10, and 13);
- Irregularly shaped (Models 5 and 12).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cirovic, S.; Walsh, C.; Fraser, W.D.; Gulino, A. The effect of posture and positive pressure breathing on the hemodynamics of the internal jugular vein. Aviat Space Environ. Med. 2003, 74, 125–131. [Google Scholar] [PubMed]
- Ciuti, G.; Righi, D.; Forzoni, L.; Fabbri, A.; Pignone, A.M. differences between internal jugular vein and vertebral vein flow examined in real time with the use of multigate ultrasound color doppler. Am. J. Neuroradiol. 2013, 34, 2000–2004. [Google Scholar] [CrossRef] [PubMed]
- Doepp, F.; Schreiber, S.J.; von Műnster, T.; Rademacher, J.; Klingebiel, R.; Valdueza, J.M. How does the blood leave the brain? A systematic ultrasound analysis of cerebral venous drainage patterns. Neuroradiology 2004, 46, 565–570. [Google Scholar] [CrossRef]
- Schaller, B. Physiology of cerebral venous blood flow: From experimental data in animals to normal function in humans. Brain Res. Rev. 2004, 46, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.J.; Lürtzing, F.; Götze, R.; Doepp, F.; Klingbiel, R.; Valdueza, J.M. Extrajugular pathways of human cerebral venous blood drainage assessed by duplex ultrasound. J. Appl. Physiol. 2003, 94, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Simka, M.; Czaja, J.; Kowalczyk, D. Collapsibility of the internal jugular veins in the lateral decubitus body position: A potential role of the cerebral venous outflow against neurodegeneration. Med. Hypotheses 2019, 133, 109397. [Google Scholar] [CrossRef] [PubMed]
- Zaniewski, M.; Simka, M. Biophysics of venous return from the brain from the perspective of the pathophysiology of chronic cerebrospinal venous insufficiency. Rev. Recent Clin. Trials 2012, 7, 88–92. [Google Scholar] [CrossRef]
- Alpini, D.C.; Bavera, P.M.; Hahn, A.; Mattei, V. Chronic cerebrospinal venous insufficiency (CCSVI) in Meniere disease: Case or cause? Sci. Med. 2013, 4, 9–15. [Google Scholar]
- Beggs, C.; Chung, C.P.; Bergsland, N.; Wang, P.-N.; Shepherd, S.; Cheng, C.-Y.; Dwyer, M.G.; Hu, H.-H.; Zivadinov, R. Jugular venous reflux and brain parenchyma volumes in elderly patients with mild cognitive impairment and Alzheimer’s disease. BMC Neurol. 2013, 13, 157. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Chang, F.C.; Chao, A.C.; Chung, C.-P.; Hu, H.-H. Internal jugular venous abnormalities in transient monocular blindness. BMC Neurol. 2013, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.P.; Beggs, C.; Wang, P.N.; Bergsland, N.; Shepherd, S.; Cheng, C.Y.; Ramasamy, D.P.; Dwyer, M.G.; Hu, H.H.; Zivadinov, R. Jugular venous reflux and white matter abnormalities in Alzheimer’s disease: A pilot study. J. Alzheimer Dis. 2014, 39, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.Y.; Zhou, D.; Pan, L.Q.; Ya, J.; Liu, C.; Yan, F.; Fan, C.; Ding, Y.; Ji, X.; Meng, R. Cervical spondylotic internal jugular venous compression syndrome. CNS Neurosci. Ther. 2020, 26, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.K.; Utriainen, D.T.; Daugherty, A.M.; Feng, W.; Hewett, J.J.; Raz, N.; Haacke, E.M. Jugular venous flow abnormalities in multiple sclerosis patients compared to normal controls. J. Neuroimaging 2015, 25, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Simka, M.; Latacz, P.; Ludyga, T.; Kazibudzki, M.; Świerad, M.; Janas, P.; Piegza, J. Prevalence of extracranial venous abnormalities: Results from a sample of 586 multiple sclerosis patients. Funct. Neurol. 2011, 26, 197–203. [Google Scholar] [PubMed]
- Zivadinov, R.; Marr, K.; Cutter, G.; Ramanathan, M.; Benedict, R.H.; Kennedy, C.; Elfadil, M.; Yeh, A.E.; Reuther, J.; Brooks, C.; et al. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS. Neurology 2011, 77, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Simka, M.; Hubbard, D.; Siddiqui, A.H.; Dake, M.D.; Sclafani, S.J.; Al-Omari, M.; Eisele, C.G.; Haskal, Z.J.; Ludyga, T.; Miloševič, Z.V.; et al. Catheter venography for the assessment of internal jugular veins and azygous vein: Position statement by expert panel of the International Society for Neurovascular Disease. Vasa 2013, 42, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, P.; Morovic, P.; Menegatti, E.; Viselner, G.; Nicolaides, A.N. Screening for chronic cerebrospinal venous insufficiency (CCSVI) using ultrasound—Recommendations for a protocol. Int. Angiol. 2011, 30, 571–597. [Google Scholar] [PubMed]
- Zivadinov, R.; Bastianello, S.; Dake, M.D.; Ferral, H.; Haacke, E.M.; Haskal, Z.J.; Hubbard, D.; Liasis, N.; Mandato, K.; Sclafani, S.; et al. Recommendations for multimodal noninvasive and invasive screening for detection of extracranial venous abnormalities indicative of chronic cerebrospinal venous insufficiency: A position statement of the International Society for Neurovascular Disease. J. Vasc. Interv. Radiol. 2014, 25, 1785–1794.e17. [Google Scholar] [CrossRef]
- Simka, M.; Latacz, P. Numerical modeling of blood flow in the internal jugular vein with the use of computational fluid mechanics software. Phlebology 2021, 36, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Utriainen, D.; Trifan, G.; Elias, S.; Sethi, S.; Hewett, J.; Haacke, E.M. Characteristics of flow through the internal jugular veins at cervical C2/C3 and C5/C6 levels for multiple sclerosis patients using MR phase contrast imaging. Neurol. Res. 2012, 34, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Haacke, E.M.; Feng, W.; Utriainen, D.; Trifan, G.; Wu, Z.; Latif, Z.; Katkuri, Y.; Hewett, J.; Hubbard, D. Patients with multiple sclerosis with structural venous abnormalities on MR Imaging exhibit an abnormal flow distribution of the internal jugular veins. J. Vasc. Interv. Radiol. 2012, 23, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Simka, M.; Skuła, M.; Bielaczyc, G. Validation of models of venous outflow from the cranial cavity in the supine and upright body positions. Phlebol. Rev. 2022, 30, 8–12. [Google Scholar] [CrossRef]
- Phillips, R.A.; Van Slyke, D.D.; Hamilton, P.B.; Dole, V.P.; Emerson, K.; Archibald, R.M. Measurement of specific gravities of whole blood and plasma by standard copper sulfate solutions. J. Biol. Chem. 1950, 183, 305–330. [Google Scholar] [CrossRef]
- Trudnowski, R.J.; Rico, R.C. Specific gravity of blood and plasma at 4 and 37 °C. Clin. Chem. 1974, 20, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Caiazzo, A.; Montecinos, G.; Müller, L.O.; Haacke, E.M.; Toro, E.F. Computational haemodynamics in stenotic internal jugular veins. J. Math. Biol. 2015, 70, 745–772. [Google Scholar] [CrossRef]
- Carini, V. A Computational Fluid Structure Interaction Study in Internal Jugular Veins Subjected to Gravity. Ph.D. Thesis, Politecnico di Milano, Milan, Italy, 2017. [Google Scholar]
- Zivadinov, R.; Chung, C.P. Potential involvement of the extracranial venous system in central nervous system disorders and aging. BMC Med. 2013, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Simka, M. Activation of the glymphatic system during sleep—Is the cerebral venous outflow a missing piece of the puzzle? Phlebol. Rev. 2019, 7, 1–2. [Google Scholar] [CrossRef]
- Zamboni, P.; Tesio, L.; Galimberti, S.; Massacesi, L.; Salvi, F.; D’alessandro, R.; Cenni, P.; Galeotti, R.; Papini, D.; D’amico, R.; et al. Efficacy and safety of extracranial vein angioplasty in multiple sclerosis. JAMA Neurol. 2018, 75, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, P.; Galeotti, R.; Salvi, F.; Giaquinta, A.; Setacci, C.; Alborino, S.; Guzzardi, G.; Sclafani, S.J.; Maietti, E.; Veroux, P.; et al. Effects of Venous Angioplasty on Cerebral Lesions in Multiple Sclerosis: Expanded Analysis of the Brave Dreams Double-Blind, Sham-Controlled Randomized Trial. J. Endovasc. Ther. 2020, 27, 1526602819890110. [Google Scholar] [CrossRef] [PubMed]
- Simka, M. An overview of randomized controlled trials on endovascular treatment for chronic cerebrospinal venous insufficiency in multiple sclerosis patients. Phlebologie 2021, 50, 76–80. [Google Scholar] [CrossRef]
- Simka, M.; Ludyga, T.; Latacz, P.; Kazibudzki, M. Diagnostic accuracy of current sonographic criteria for the detection of outflow abnormalities in the internal jugular veins. Phlebology 2013, 28, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Simka, M. Chronic cerebrospinal venous insufficiency: Current perspectives. J. Vasc. Diagn. 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Rend, R.R.; Sparrow, E.M.; Bettenhausen, D.W.; Abraham, J. Parasitic pressure losses in diffusers and in their downstream piping systems for fluid flow and heat transfer. Int. J. Heat Mass Transf. 2013, 61, 56–61. [Google Scholar] [CrossRef]
- Li, L.; Walker, A.M.; Rival, D.E. The characterization of a non-Newtonian blood analog in natural- and shear-layer-induced transitional flow. Biorheology 2014, 51, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, A.A.; Boccadifuoco, A.; Celi, S.; Salvetti, M.V. Hemodynamics and stresses in numerical simulations of the thoracic aorta: Stochastic sensitivity analysis to inlet flow-rate waveform. Comput. Fluids 2021, 230, 105123. [Google Scholar] [CrossRef]
- Morbiducci, U.; Ponzini, R.; Gallo, D.; Bignardi, C.; Rizzo, G. Inflow boundary conditions for image-based computational hemodynamics: Impact of idealized versus measured velocity profiles in the human aorta. J. Biomech. 2013, 46, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, D.; Di Achille, P.; Humphre, Y.D. Strongly coupled morphological features of aortic aneurysms drive intraluminal thrombus. Sci. Rep. 2018, 8, 13273. [Google Scholar] [CrossRef] [PubMed]
- Joly, F.; Soulez, G.; Garcia, D.; Lessard, S.; Kauffmann, C. Flow stagnation volume and abdominal aortic aneurysm growth: Insights from patient-specific computational flow dynamics of Lagrangian-coherent structures. Comput. Biol. Med. 2018, 92, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Lozowy, R.J.; Kuhn, D.C.; Ducas, A.A.; Boyd, A.J. The relationship between pulsatile flow impingement and intraluminal thrombus deposition in abdominal aortic aneurysms. Cardiovasc. Eng. Technol. 2017, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tian, Z.; Liu, J.; Jing, L.; Paliwal, N.; Wang, S.; Zhang, Y.; Xiang, J.; Siddiqui, A.H.; Meng, H.; et al. Flow diverter effect of LVIS stent on cerebral aneurysm hemodynamics: A comparison with Enterprise stents and the Pipeline device. J. Transl. Med. 2016, 14, 199. [Google Scholar] [CrossRef]
- Li, G.; Song, X.; Wang, H.; Liu, S.; Ji, J.; Guo, Y.; Qiao, A.; Liu, Y.; Wang, X. Prediction of cerebral aneurysm hemodynamics with porous-medium models of flow-diverting stents via deep learning. Front. Physiol. 2021, 12, 733444. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yang, H.; Hong, I.; Oh, J.H.; Kim, Y.B. computational study of hemodynamic changes induced by overlapping and compacting of stents and flow diverter in cerebral aneurysms. Front. Neurol. 2021, 12, 705841. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Sugiu, K.; Hiramatsu, M.; Haruma, J.; Date, I. Evaluation of the shrinkage process of a neck remnant after stent-coil treatment of a cerebral aneurysm using silent magnetic resonance angiography and computational fluid dynamics analysis: Illustrative case. J. Neurosurg. Case Lessons 2024, 7, CASE24141. [Google Scholar] [CrossRef] [PubMed]
- Simka, M.; Latacz, P.; Redelbach, W. Blood flow in the internal jugular veins during the spaceflight—Is it actually bidirectional? Life Sci. Space Res. 2020, 25, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Iqrar, S.A.; Rashid, A.; Simka, M. Results of numerical modeling of blood flow in the internal jugular vein exhibiting different types of strictures. Diagnostics 2022, 12, 2862. [Google Scholar] [CrossRef] [PubMed]
- Zivadinov, R.; Karmon, Y.; Dolic, K.; Hagemeier, J.; Marr, K.; Valnarov, V.; Kennedy, C.L.; Hojnacki, D.; Carl, E.M.; Hopkins, L.N.; et al. Multimodal noninvasive and invasive imaging of extracranial venous abnormalities indicative of CCSVI: Results of the PREMiSe pilot study. BMC Neurol. 2013, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Sethi, S.K.; Utriainen, D.T.; Hewett, J.J.; Haacke, E.M. A comparative study of magnetic resonance venography techniques for the evaluation of the internal jugular veins in multiple sclerosis patients. Magn. Reson. Imaging 2013, 31, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Zaharchuk, G.; Fischbein, N.J.; Rosenberg, J.; Herfkens, R.J.; Dake, M.D. Comparison of MR and contrast venography of the cervical venous system in multiple sclerosis. Am. J. Neuroradiol. 2011, 32, 1482–1489. [Google Scholar] [CrossRef] [PubMed]


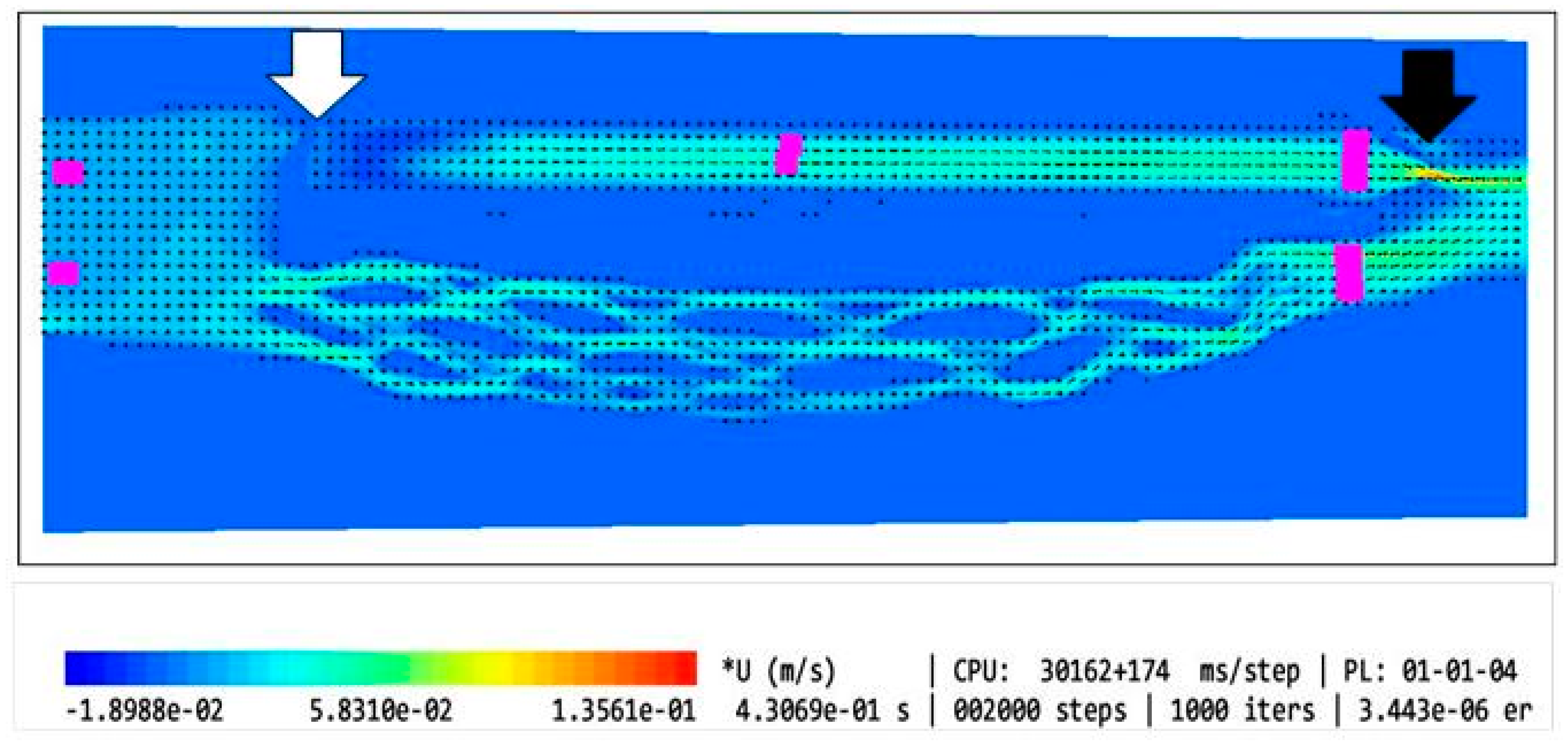
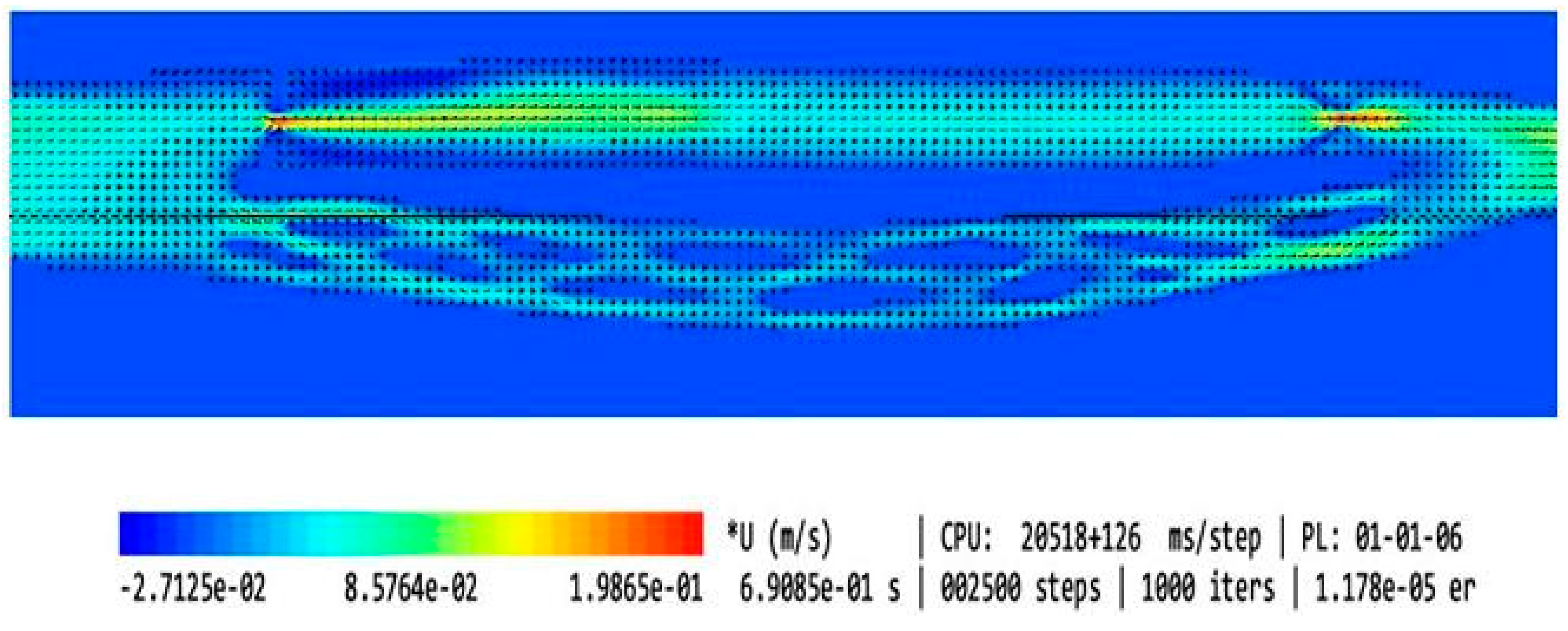
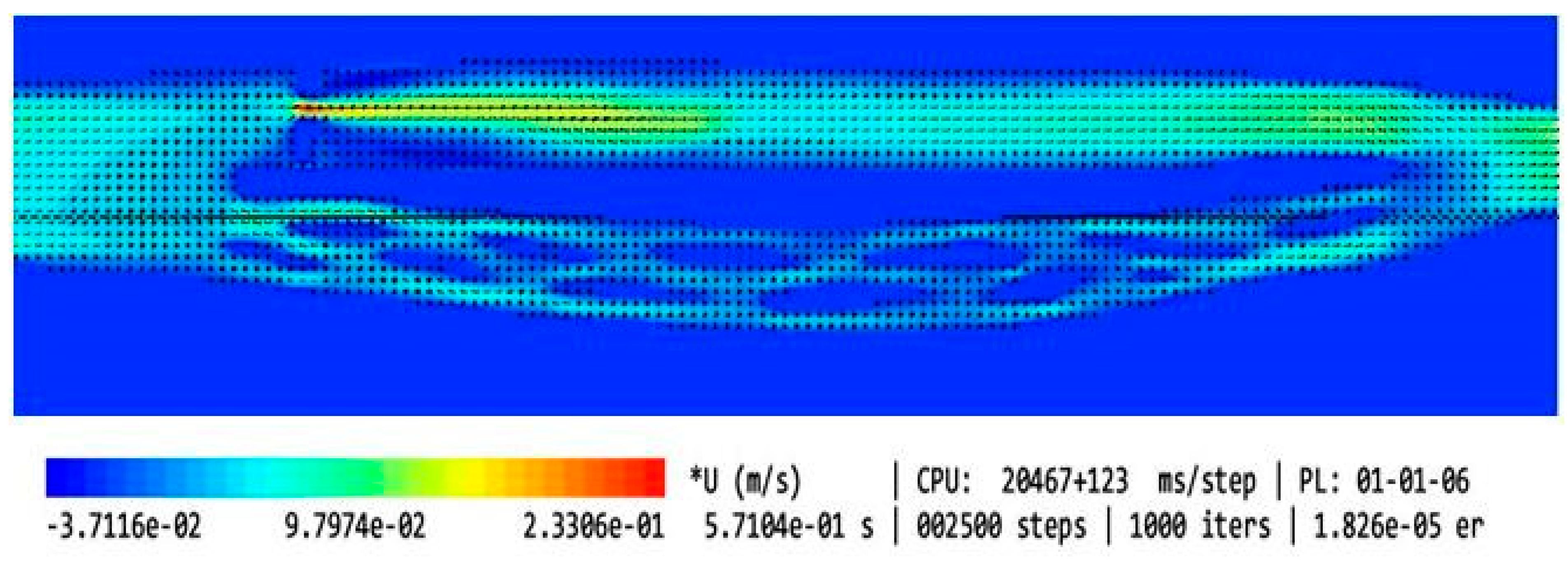
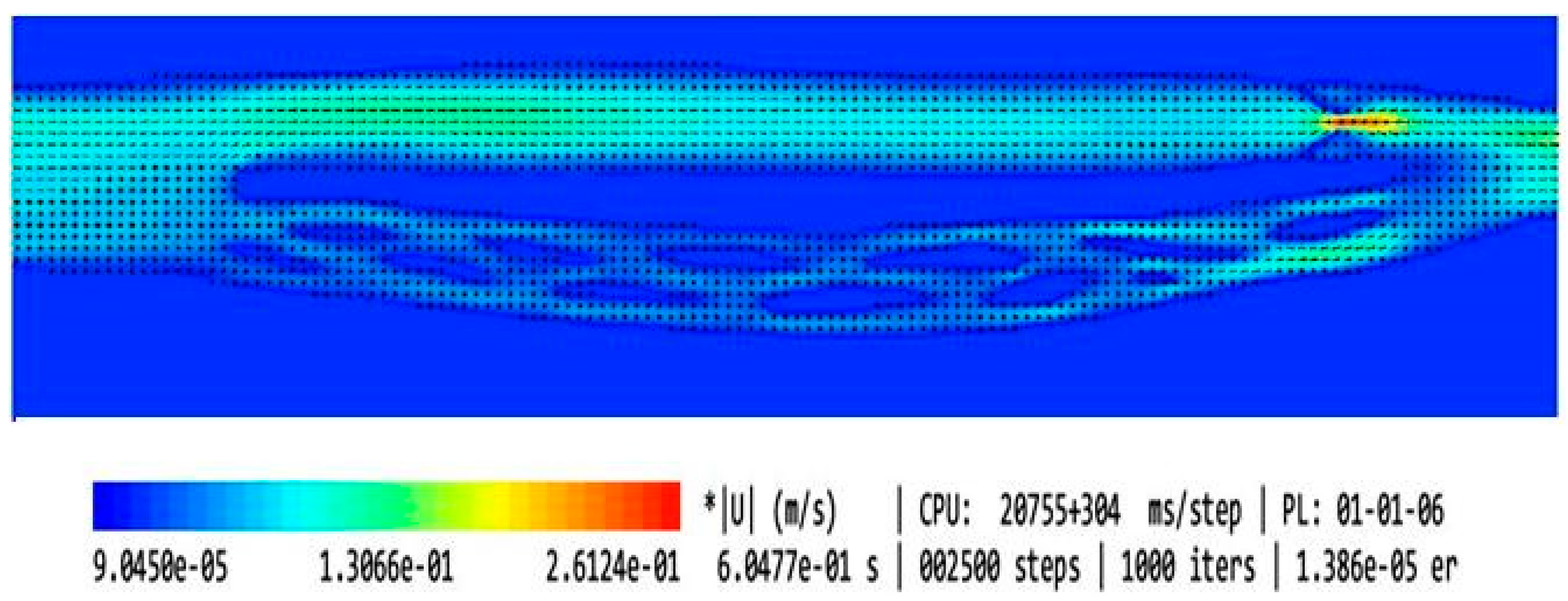
| Type of the Model | Shape of the Upper Nozzle-like Stricture | Cross-Sectional Area at the Level of Upper Stenosis | Length of the Upper Stricture | Valve in the Lower Part of IJV | Flow Volume in the Internal Jugular Vein [mL/min] | Flow Volume in the Vertebral Plexus [mL/min] | Total Flow in the Outflow Field [mL/min] | Jugular/ Vertebral Flows [%/%] | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Model without upper stenosis and no valve | no stricture | 0.94 cm2 | no stricture | no valve | 321 | 35 | 356 | 90/10 |
| 2 | Model with minor upper stenosis and no valve |  | 0.42 cm2 | 2.5 mm | no valve | 664 | 170 | 834 | 80/20 |
| 3 | Model without upper stenosis and wide valve | no stricture | 0.94 cm2 | no stricture | bicuspid valve, distance between leaflets: 4.3 mm | 579 | 187 | 766 | 76/24 |
| 4 | Model without upper stenosis, and narrow valve | no stricture | 0.94 cm2 | no stricture | bicuspid valve, distance between leaflets: 1.5 mm | 446 | 339 | 785 | 57/43 |
| 5 | Model with irregularly shaped minor stenosis, and narrow valve |  | 0.60 cm3 | 2.5 mm | bicuspid valve, distance between leaflets: 1.5 mm | 398 | 301 | 699 | 57/42 |
| 6 | Model with elliptically shaped, centrally positioned stenosis, and narrow valve |  | 0.24 cm3 | 2.5 mm | bicuspid valve, distance between leaflets: 1.5 mm | 282 | 294 | 576 | 49/51 |
| 7 | Model with elliptically shaped, eccentrically positioned stenosis, and narrow valve |  | 0.24 cm3 | 2.5 mm | bicuspid valve, distance between leaflets: 1.5 mm | 250 | 295 | 545 | 46/54 |
| 8 | Model with elliptically shaped, centrally positioned long stenosis, and narrow valve |  | 0.24 cm3 | 12 mm | bicuspid valve, distance between leaflets: 1.5 mm | 396 | 448 | 844 | 47/53 |
| 9 | Model with elliptically shaped, centrally positioned severe stenosis, and narrow valve |  | 0.07 cm3 | 2.5 mm | bicuspid valve, distance between leaflets: 1.5 mm | 280 | 337 | 617 | 45/55 |
| 10 | Model with elliptically shaped, eccentrically positioned severe stenosis, and narrow valve |  | 0.07 cm3 | 2.5 mm | bicuspid valve, distance between leaflets: 1.5 mm | 126 | 167 | 293 | 43/57 |
| 11 | Model without upper stenosis, and septum instead of valve | no stricture | 0.94 cm2 | no stricture | septum | 26 | 35 | 61 | 43/57 |
| 12 | Model with two elliptically shaped, eccentrically positioned stenoses, and narrow valve |  | 0.48 cm3 (2 × 0.24 cm3) | 2.5 mm | bicuspid valve, distance between leaflets: 1.5 mm | 250 | 295 | 545 | 46/54 |
| 13 | Model with eccentrically positioned short flattening, and normal valve |  | 0.48 cm3 | 2.5 mm | bicuspid valve, distance between leaflets: 1.5 mm | 121 | 158 | 279 | 43/57 |
| 14 | Model with collapsed IJV | no stricture | collapsed internal jugular vein | no stricture | no valve | 49 | 87 | 136 | 36/64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simka, M.; Czaja, J.; Kawalec, A.; Latacz, P.; Kovalko, U. Numerical Modeling of Venous Outflow from the Cranial Cavity in the Supine Body Position. Appl. Sci. 2024, 14, 3878. https://doi.org/10.3390/app14093878
Simka M, Czaja J, Kawalec A, Latacz P, Kovalko U. Numerical Modeling of Venous Outflow from the Cranial Cavity in the Supine Body Position. Applied Sciences. 2024; 14(9):3878. https://doi.org/10.3390/app14093878
Chicago/Turabian StyleSimka, Marian, Joanna Czaja, Agata Kawalec, Paweł Latacz, and Uliana Kovalko. 2024. "Numerical Modeling of Venous Outflow from the Cranial Cavity in the Supine Body Position" Applied Sciences 14, no. 9: 3878. https://doi.org/10.3390/app14093878
APA StyleSimka, M., Czaja, J., Kawalec, A., Latacz, P., & Kovalko, U. (2024). Numerical Modeling of Venous Outflow from the Cranial Cavity in the Supine Body Position. Applied Sciences, 14(9), 3878. https://doi.org/10.3390/app14093878





