Effect of Heat Treatment Methods on Color, Bioactive Compound Content, and Antioxidant Capacity of Carrot Root
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Research Materials
2.3. Color Analysis
2.4. Determination of Phenolic Compounds
2.4.1. Phenolic Extract Preparation
2.4.2. Total Phenolic Content (TPC) Analysis
2.4.3. Total Flavonoid Content (TFC) Analysis
2.4.4. Phenolic Profile Analysis by UHPLC-DAD-MS
2.5. Determination of Carotenoids
2.5.1. Carotenoid Extraction
2.5.2. Carotenoid Profile Analysis by RP-HPLC
2.6. Determination of Antioxidant Capacity
2.6.1. DPPH Assay
2.6.2. ABTS Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Color Parameters
3.2. Total Phenolic and Total Flavonoid Contents
3.3. Phenolic Compound Profile
3.4. Carotenoid Profile
3.5. Antioxidant Capacity
3.6. Multivariate Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paparella, A.; Kongala, P.R.; Serio, A.; Rossi, C.; Shaltiel-Harpaza, L.; Husaini, A.M.; Ibdah, M. Challenges and opportunities in the sustainable improvement of carrot production. Plants 2024, 13, 2092. [Google Scholar] [CrossRef] [PubMed]
- Nagraj, G.S.; Jaiswal, S.; Harper, N.; Jaiswal, A.K. Chapter 20—Carrot. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 323–337. [Google Scholar]
- Matějková, J.; Petříková, K. Variation in content of carotenoids and vitamin C in carrots. Not. Sci. Biol. 2010, 2, 88–91. [Google Scholar] [CrossRef]
- Chiavaro, E.; Mazzeo, T.; Visconti, A.; Manzi, C.; Fogliano, V.; Pellegrini, N. Nutritional quality of sous vide cooked carrots and brussels sprouts. J. Agric. Food Chem. 2012, 60, 6019–6025. [Google Scholar] [CrossRef] [PubMed]
- Boadi, N.O.; Badu, M.; Kortei, N.K.; Saah, S.A.; Annor, B.; Mensah, M.B.; Okyere, H.; Fiebor, A. Nutritional composition and antioxidant properties of three varieties of carrot (Daucus carota). Sci. Afr. 2021, 12, e00801. [Google Scholar] [CrossRef]
- Leja, M.; Kamińska, I.; Kramer, M.; Maksylewicz-Kaul, A.; Kammerer, D.; Carle, R.; Barański, R. The content of phenolic compounds and radical scavenging activity varies with carrot origin and root color. Plant Foods Hum. Nutr. 2013, 68, 163–170. [Google Scholar] [CrossRef]
- Kramer, M.; Bufler, G.; Nothnagel, T.; Carle, R.; Kammerer, D.R. Effects of cultivation conditions and cold storage on the polyacetylene contents of carrot (Daucus carota L.) and parsnip (Pastinaca sativa L.). J. Hortic. Sci. Biotech. 2012, 87, 101–106. [Google Scholar] [CrossRef]
- Mărăzan, V.; Anghel, I.M.; Rotariu, L.; Cozma, A. Comparative study on essential elements distribution in carrots and juice carrots. Res. J. Agric. Sci. 2021, 4, 91–96. [Google Scholar]
- Varshney, K.; Mishra, K. An analysis of health benefits of carrot. Int. J. Innov. Res. Eng. Mgmt. 2022, 9, 211–214. [Google Scholar] [CrossRef]
- Tian, Z.; Dong, T.; Wang, S.; Sun, J.; Chen, H.; Zhang, N.; Wang, S. A compre-hensive review on chemical composition, flavors, and the impacts of heat processing on the aroma formation of fresh carrot. Food Chem. X 2024, 22, 101201. [Google Scholar] [CrossRef]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef]
- Nunn, M.D.; Giraud, D.W.; Parkhurst, A.M.; Hamouz, F.L.; Driskell, J.A. Effects of cooking methods on sensory qualities and carotenoid retention in selected vegetables. J. Food Qual. 2006, 29, 445–457. [Google Scholar] [CrossRef]
- Bongoni, R.; Stieger, M.; Dekker, M.; Steenbekkers, B.; Verkerk, R. Sensory and health properties of steamed and boiled carrots (Daucus carota ssp. Sativus). Int. J. Food Sci. Nutr. 2014, 65, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, Y.; Jeong, H.S.; Lee, J.; Sung, J. Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Sci. Biotechnol. 2018, 27, 333–342. [Google Scholar] [PubMed]
- Kosewski, G.; Kowalówka, M.; Drzymała-Czyż, S.; Przysławski, J. The impact of culinary processing, including sous-vide, on polyphenols, vitamin C content and antioxidant status in selected vegetables—Methods and results: A critical review. Foods 2023, 12, 2121. [Google Scholar] [CrossRef]
- Andersson, J.; Garrido-Banuelos, G.; Bergdoll, M.; Vilaplana, F.; Menzel, C.; Mihnea, M.; Lopez-Sanchez, P. Comparison of steaming and boiling of root vegetables for enhancing carbohydrate content and sensory profile. J. Food Eng. 2022, 312, 110754. [Google Scholar] [CrossRef]
- Zavadlav, S.; Blažić, M.; Van de Velde, F.; Vignatti, C.; Fenoglio, C.; Piagentini, A.M.; Pirovani, M.E.; Perotti, C.M.; Bursać Kovačević, D.; Putnik, P. Sous-vide as a technique for preparing healthy and high-quality vegetable and seafood products. Foods 2020, 9, 1537. [Google Scholar] [CrossRef]
- Singh, P.; Sultan, Z.; Pandey, V.K.; Singh, R. Sous vide processing for food quality enhancement: A review. Food Hum. 2023, 1, 543–552. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Iqbal, S. Effect of different cooking methods on the antioxidant activity of some vegetables from Pakistan. Int. J. Food Sci. Technol. 2008, 43, 560–567. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; Butler, F. Effect of water immersion and sous-vide processing on antioxidant activity, phenolic, carotenoid content and color of carrot disks. J. Food Process. Preserv. 2010, 34, 1009–1023. [Google Scholar] [CrossRef]
- Bembem, K.; Sadana, B. Effect of different cooking methods on the antioxidant components of carrot. Biosci. Discov. 2014, 5, 112–116. [Google Scholar]
- Koç, M.; Baysan, U.; Devseren, E.; Okut, D.; Atak, Z.; Karataş, H.; Kaymak-Ertekin, F. Effects of different cooking methods on the chemical and physical properties of carrot and green peas. Innov. Food Sci. Emerg. Technol. 2017, 42, 109–119. [Google Scholar] [CrossRef]
- Thanuja, S.; Sivakanthan, S.; Vasantharuba, S. The influence of various cooking methods on the antioxidant compounds of local carrot variety (Daucus carota) cultivated in Jaffna district. In Proceedings of the Jaffna University International Research Conference, Jaffa, Sri Lanka, 27–28 September 2018; Track: Agriculture & Food Sciences. pp. 37–39. [Google Scholar]
- Buratti, S.; Cappa, C.; Benedetti, S.; Giovanelli, G. Influence of cooking conditions on nutritional properties and sensory characteristics interpreter by e-senses: Case-study on selected vegetables. Foods 2020, 9, 607. [Google Scholar] [CrossRef] [PubMed]
- Calabretti, A.; Calabrese, M. Comparison of antioxidant properties in foods from organic and conventional agriculture. Int. J. Food Sci. Nutr. 2021, 6, 117–121. [Google Scholar]
- Stanikowski, P.; Michalak-Majewska, M.; Jabłońska-Ryś, E.; Gustaw, W.; Gruszecki, R. Influence of sous-vide thermal treatment, boiling, and steaming on the colour, texture and content of bioactive compounds in root vegetables. Ukr. Food J. 2021, 10, 77–89. [Google Scholar] [CrossRef]
- Kosewski, G.; Górna, I.; Bolesławska, I.; Kowalówka, M.; Więckowska, B.; Główka, A.K.; Morawska, A.; Jakubowski, K.; Dobrzyńska, M.; Miszczuk, P.; et al. Comparison of antioxidative properties of raw vegetables and thermally processed ones using the conventional and sous-vide methods. Food Chem. 2018, 240, 1092–1096. [Google Scholar] [CrossRef]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef]
- Ashour, M.M.S.; El-Hamzy, E.M.A. Influence of different cooking methods on physicochemical characteristics, phytochemical profile, antioxidant capacity and chromatic parameters of selected vegetables. Middle East. J. Appl. Sci. 2017, 7, 1127–1147. [Google Scholar]
- Lim, C.J.; Kim, H.Y.; Lee, C.H.; Kim, Y.U.; Back, K.W.; Bae, J.M.; Lee, S.W.; Ahn, M.J. Variation in carotenoid composition in carrots during storage and cooking. Prev. Nutr. Food Sci. 2009, 14, 240–245. [Google Scholar] [CrossRef][Green Version]
- Karafyllaki, D.; Narwojsz, A.; Kurp, L.; Sawicki, T. Effects of different processing methods on the polyphenolic compounds profile and the antioxidant and anti-glycaemic properties of horseradish roots (Armoracia rusticana). Eur. Food Res. Technol. 2023, 249, 1739–1747. [Google Scholar] [CrossRef]
- Horszwald, A.; Andlauer, W. Characterisation of bioactive compounds in berry juices by traditional photometric and modern microplate methods. J. Berry Res. 2011, 1, 189–199. [Google Scholar] [CrossRef]
- Sawicki, T.; Błaszczak, W.; Latocha, P. In vitro anticholinergic and antiglycaemic properties of frost-hardy Actinidia fruit extracts and their polyphenol profile, L-ascorbic acid content and antioxidant capacity. Food Res. Int. 2023, 173, 113324. [Google Scholar] [CrossRef] [PubMed]
- Chociej, P.; Foss, K.; Jabłońska, M.; Ustarbowska, M.; Sawicki, T. The profile and content of polyphenolic compounds and antioxidant and anti-glycation properties of root extracts of selected medicinal herbs. Plant Foods Hum. Nutr. 2024, 79, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, G.; Czaplicki, S.; Szustak, M.; Cichońska, E.; Gendaszewska-Darmach, E.; Konopka, I. Composition of flesh lipids and oleosome yield optimization of selected sea buckthorn (Hippophae rhamnoides L.) cultivars grown in Poland. Food Chem. 2022, 369, 130921. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.A.A.; Lara, S.B.; Miranda, L.S.; Pires, I.S.C.; Pires, C.V.; Halboth, N.V. Influence of color on acceptance and identification of flavor of foods by adults. Ciênc. Tecnol. Aliment. 2012, 32, 296–301. [Google Scholar]
- Zielinska, M.; Markowski, M. Color characteristics of carrots: Effect of drying and rehydration. Int. J. Food Prop. 2012, 15, 450–466. [Google Scholar] [CrossRef]
- Bao, S.; Li, X.; Lan, T.; Wang, J.; Hu, Y.; Sun, X.; Ma, T. Effects of different cooking treatments on the sensory qualities and pigmented phytochemicals of carrots. Food Chem. 2023, 405, 135015. [Google Scholar] [CrossRef]
- Salehi, F. Color changes kinetics during deep fat frying of carrot slice. Heat Mass Transf. 2018, 54, 3421–3426. [Google Scholar] [CrossRef]
- Chen, B.H.; Peng, H.Y.; Chen, H.E. Changes of carotenoids, color, and vitamin A contents during processing of carrot juice. J. Agric. Food Chem. 1995, 43, 1912–1918. [Google Scholar] [CrossRef]
- Islam, M.S.; Igura, N.; Shimoda, M.; Hayakawa, I. Effects of low hydrostatic pressure and moderate heat on texture, pectic substances and color of carrot. Eur. Food Res. Technol. 2003, 217, 34–38. [Google Scholar]
- Chatatikun, M.; Chiabchalard, A. Phytochemical screening and free radical scavenging activities of orange baby carrot and carrot (Daucus carota Linn.) root crude extracts. J. Chem. Pharm. Res. 2013, 5, 97–102. [Google Scholar]
- Seljåsen, R.; Kristensen, H.L.; Lauridsen, C.; Wyss, G.S.; Kretzschmar, U.; Birlouez-Aragone, I.; Kahl, J. Quality of carrots as affected by pre- and postharvest factors and processing. J. Sci. Food Agric. 2013, 93, 2611–2626. [Google Scholar] [CrossRef] [PubMed]
- Cwalina-Ambroziak, B.; Amarowicz, R.; Tyburski, J.; Janiak, M.; Nowak, M.K. Effect of farming systems na pathogen infections and content of phenolic compounds in carrot (Daucus carota L. subsp sativus (Hoffm.) roots. J. Anim. Plant Sci. 2014, 24, 1183–1189. [Google Scholar]
- Al-Subeihi, A.A.A.; Awal, A.; Ahmed, M.W.; Ahmed, M.; Alam, T.; Khan, M.S.I.; Islam, M.A. Effects of organic and chemical fertilizers on the content of major phenolic compounds of carrot. Poll Res. 2022, 41, 347–352. [Google Scholar] [CrossRef]
- Augšpole, I.; Kince, T.; Cinkmanis, I. Changes of polyphenol compound concentrations in hybrids of Nante type carrots during storage. Proc. Latv. Acad. Sci. Sect. B 2017, 71, 492–495. [Google Scholar] [CrossRef]
- Razzak, A.; Mahjabin, T.; Khan, M.R.M.; Hossain, M.; Sadia, U.; Zzaman, W. Effect of cooking methods on the nutritional quality of selected vegetables at Sylhet City. Heliyon 2023, 9, e21709. [Google Scholar] [CrossRef]
- Michala, A.S.; Pritsa, A. Quercetin: A molecule of great biochemical and clinical value and its beneficial effect on diabetes and cancer. Diseases 2022, 10, 37. [Google Scholar] [CrossRef]
- Barreca, D.; Trombetta, D.; Smeriglio, A.; Mandalari, G.; Romeo, O.; Felice, M.R.; Gattuso, G.; Nabavi, S.M. Food flavonols: Nutraceuticals with complex health benefits and functionalities. Trends Food Sci. Technol. 2021, 117, 194–204. [Google Scholar] [CrossRef]
- Gao, Y.; Xia, W.; Shao, P.; Wu, W.; Chen, H.; Fang, X.; Mu, H.; Xiao, J.; Gao, H. Impact of thermal processing on dietary flavonoids. Curr. Opin. Food Sci. 2022, 48, 100915. [Google Scholar] [CrossRef]
- Rohn, S.; Buchner, N.; Driemel, G.; Rauser, M.; Kroh, L.W. Thermal degradation of onion quercetin glucosides under roasting conditions. J. Agric. Food Chem. 2007, 55, 1568–1573. [Google Scholar] [CrossRef]
- Lukšič, L.; Bonafaccia, G.; Timoracka, M.; Vollmannova, A.; Trček, J.; Nyambe, T.K.; Melini, V.; Acquistucci, R.; Germ, M.; Kreft, I. Rutin and quercetin transformation during preparation of buckwheat sourdough bread. J. Cereal Sci. 2016, 69, 71–76. [Google Scholar] [CrossRef]
- Biesaga, M.; Pyrzynska, K. Analytical procedures for determination of quercetin and its glycosides in plant material. Crit. Rev. Anal. Chem. 2009, 39, 95–107. [Google Scholar] [CrossRef]
- Vázquez-Vázquez, J.; Barajas-Salazar, M.; Casas-Godoy, L.; Alcázar-Valle, M.; Arellano-García, L.; Barrera-Martínez, I. Enhanced extraction of antioxidant phenolics: Quercetin, kaempferol, gallic acid, syringic acid, and epicatechin from fresh berries and their waste using Ultra-Turrax and ultrasonication. Appl. Food Res. 2024, 4, 100539. [Google Scholar] [CrossRef]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Spectrom. 2006, 20, 3229–3235. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Martinez, E.J.; Flores-Hernández, F.Y.; Salazar-Montes, A.M.; Nario-Chaidez, H.F.; Hernández-Ortega, L.D. Quercetin, a flavonoid with great pharmacological capacity. Molecules 2024, 29, 1000. [Google Scholar] [CrossRef] [PubMed]
- Palmero, P.; Lemmens, L.; Hendrickx, M.; Van Loey, A. Role of carotenoid type on the effect of thermal processing on bioaccessibility. Food Chem. 2014, 157, 275–282. [Google Scholar] [CrossRef]
- Hornero-Méndez, D.; Mínguez-Mosquera, M.I. Bioaccessibility of carotenes from carrots: Effect of cooking and addition of oil. Innov. Food Sci. Emerg. Technol. 2007, 8, 407–412. [Google Scholar] [CrossRef]
- Bozalan, N.K.; Karadeniz, F. Carotenoid profile, total phenolic content, and antioxidant activity of carrots. Int. J. Food Prop. 2011, 14, 1060–1068. [Google Scholar] [CrossRef]
- Gajewski, M.; Szymczak, P. The influence of controlled atmosphere storage on selected quality traits of carrot cultivars with different root colours. Ann. Warsaw Univ. Life Sci.—SGGW Horticult. Landsc. Architect. 2017, 38, 51–60. [Google Scholar]
- Arkoub-Djermoune, L.; Louaileche, H.; Benmeziane, F.; Madani, K.; Boulekbache-Makhlouf, L. Impact of refrigerated storage on the bioactive compounds and antioxidant capacity of two Algerian carrot varieties (Daucus carota L.). Acta Univ. Sapientiae Aliment. 2020, 13, 5–31. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Jiménez-Monreal, A.M.; García-Diz, L.; Martínez-Tomé, M.; Mariscal, M.; Murcia, M.A. Influence of cooking methods on antioxidant activity of vegetables. J. Food Sci. 2009, 74, H97–H103. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Correlations of antioxidant activity against phenolic content revisited: A new approach in data analysis for food and medicinal plants. J. Food Sci. 2009, 74, R107–R113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hamauzu, Y. Phenolic compounds and their antioxidant properties in different tissues of carrots (Daucus carota L.). J. Food Agric. Environ. 2004, 2, 95–100. [Google Scholar]
- Lee, S.W.; Kim, B.K.; Han, J.A. Physical and functional properties of carrots differently cooked within the same hardness-range. LWT-Food Sci. Technol. 2018, 93, 346–353. [Google Scholar] [CrossRef]

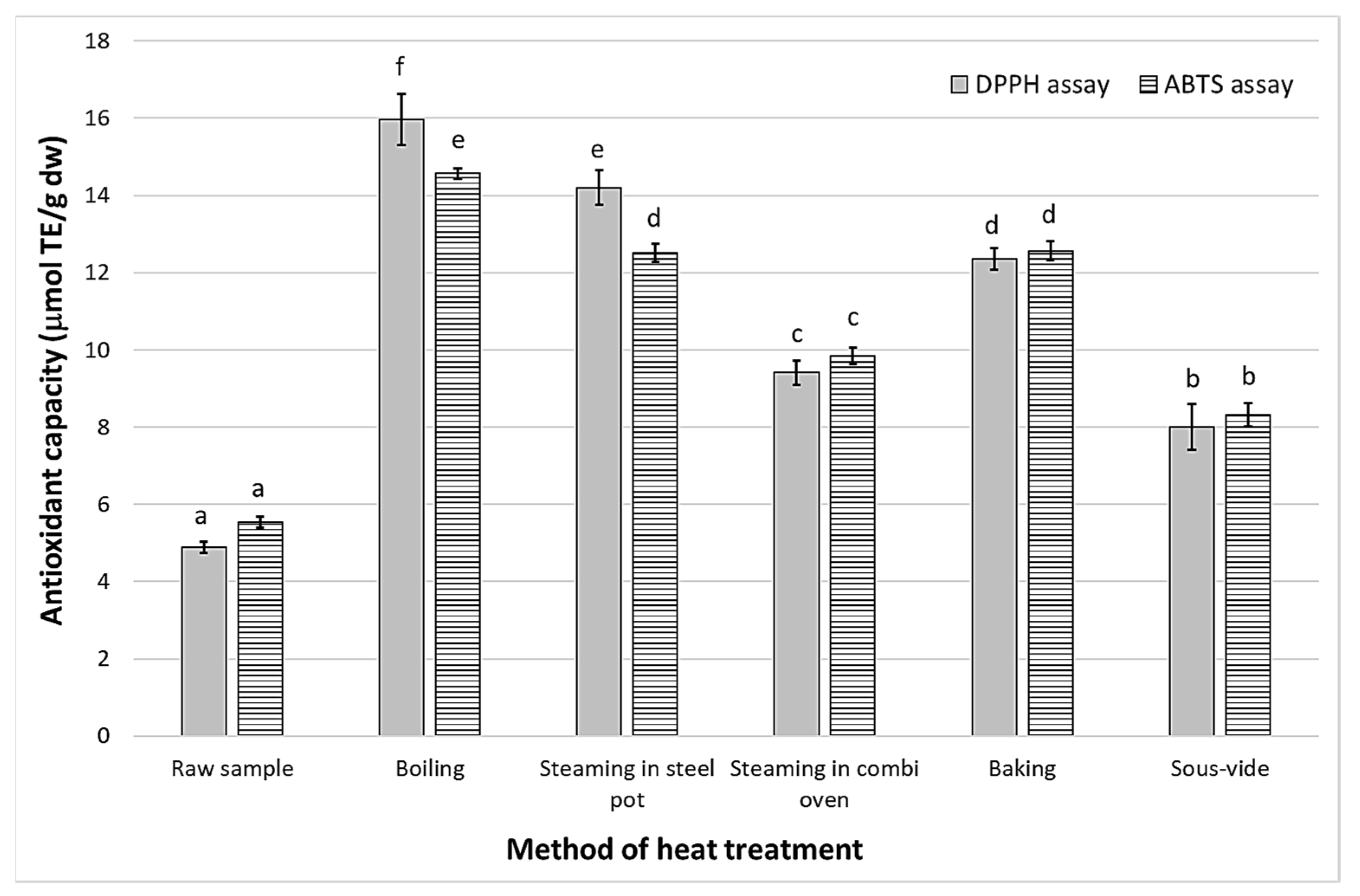
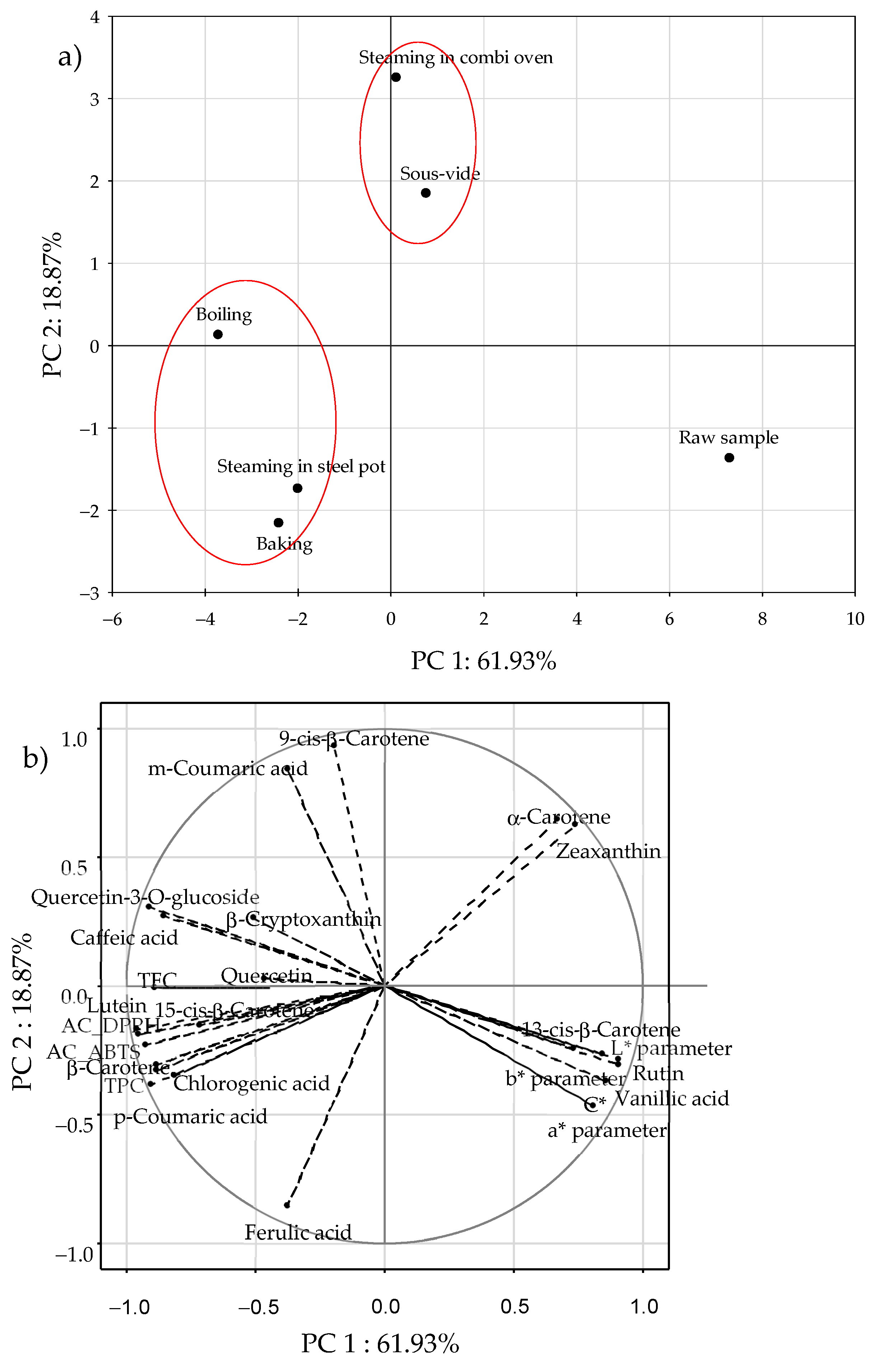
| Method of Heat Treatment | L* | a* | b* | C* | ΔE |
|---|---|---|---|---|---|
| Raw sample | 53.35 ± 1.41 c | 24.80 ± 2.46 d | 29.28 ± 1.05 c | 38.39 ± 2.25 c | - |
| Boiling | 40.74 ± 2.75 a | 10.66 ± 1.59 bc | 22.40 ± 2.60 a | 24.81 ± 3.02 a | 20.16 |
| Steaming in steel pot | 41.58 ± 1.03 ab | 10.09 ± 1.62 abc | 23.40 ± 0.84 ab | 25.54 ± 0.74 ab | 19.74 |
| Steaming in combi oven | 43.17 ± 1.44 b | 8.99 ± 0.90 ab | 24.92 ± 1.29 b | 26.50 ± 1.44 b | 19.30 |
| Baking | 42.72 ± 0.50 ab | 11.99 ± 2.22 c | 24.23 ± 0.34 b | 27.10 ± 1.04 b | 17.40 |
| Sous-vide | 40.83 ± 1.93 a | 8.12 ± 1.41 a | 22.00 ± 1.12 a | 23.47 ± 1.50 a | 22.09 |
| Method of Heat Treatment | TPC [mg GAE/g dw] | TFC [mg QE/g dw] |
|---|---|---|
| Raw sample | 0.81 ± 0.06 a | 0.43 ± 0.01 a |
| Boiling | 1.75 ± 0.10 cd | 0.97 ± 0.02 d |
| Steaming in steel pot | 1.89 ± 0.11 d | 0.83 ± 0.01 c |
| Steaming in combi oven | 1.33 ± 0.05 b | 0.69 ± 0.02 b |
| Baking | 1.68 ± 0.09 c | 0.97 ± 0.02 d |
| Sous-vide | 1.01 ± 0.04 a | 0.93 ± 0.02 d |
| Phenolic Compound | Method of Heat Treatment | |||||
|---|---|---|---|---|---|---|
| Raw Sample | Boiling | Steaming in Steel Pot | Steaming in Combi Oven | Baking | Sous-Vide | |
| Caffeic acid | 253.30 ± 0.03 a | 460.60 ± 5.73 f | 354.21 ± 0.83 c | 424.21 ± 1.06 e | 404.16 ± 3.79 d | 327.62 ± 1.39 b |
| Chlorogenic acid | 1026.10 ± 16.28 a | 2674.49 ± 26.55 e | 2223.27 ± 8.60 d | 1473.49 ± 19.01 b | 2002.19 ± 0.63 c | 992.40 ± 1.64 a |
| Ferulic acid | 162.56 ± 0.11 b | 163.93 ± 0.11 b | 177.15 ± 1.28 c | 154.75 ± 1.76 a | 172.75 ± 1.85 c | 160.11 ± 1.49 b |
| m-Coumaric acid | 183.83 ± 0.00 a | 192.15 ± 0.84 d | 185.75 ± 0.02 b | 192.06 ± 0.67 d | 183.83 ± 0.00 a | 190.00 ± 0.35 c |
| p-Coumaric acid | 71.80 ± 0.01 a | 82.08 ± 0.21 c | 80.74 ± 0.93 c | 76.13 ± 1.07 b | 82.95 ± 0.11 c | 74.54 ± 0.05 b |
| Vanillic acid | 20.59 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Sum of phenolic acids | 1718.18 ± 16.16 a | 3573.25 ± 31.12 e | 3021.11 ± 9.75 d | 2320.66 ± 20.04 b | 2845.88 ± 4.91 c | 1744.68 ± 4.22 a |
| Quercetin | 0.00 ± 0.00 a | 144.71 ± 0.07 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| Quercetin-3-O-glucoside | 0.00 ± 0.00 a | 145.60 ± 0.21 c | 143.85 ± 0.05 b | 145.48 ± 0.31 c | 145.54 ± 0.07 c | 143.75 ± 0.04 b |
| Rutin | 38.73 ± 0.05 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| Sum of flavonoids | 38.73 ± 0.05 a | 290.31 ± 0.14 d | 143.85 ± 0.05 b | 145.48 ± 0.31 c | 145.54 ± 0.07 c | 143.75 ± 0.04 b |
| Sum of phenolic compounds | 1756.91 ± 16.21 a | 3863.57 ± 31.26 f | 3164.97 ± 9.80 e | 2466.13 ± 20.35 c | 2991.42 ± 4.97 d | 1888.42 ± 4.25 b |
| Carotenoid | Method of Heat Treatment | |||||
|---|---|---|---|---|---|---|
| Raw Sample | Boiling | Steaming in Steel Pot | Steaming in Combi Oven | Baking | Sous-Vide | |
| Lutein | 29.87 ± 2.50 a | 51.03 ± 0.13 c | 53.15 ± 1.09 c | 43.59 ± 1.74 b | 49.84 ± 0.43 c | 40.58 ± 0.19 b |
| Zeaxanthin | 20.60 ± 0.64 c | 14.70 ± 1.58 ab | 14.69 ± 0.91 ab | 21.55 ± 0.51 c | 13.74 ± 0.11 a | 18.08 ± 1.33 bc |
| β-cryptoxanthin | 24.46 ± 0.32 a | 27.37 ± 0.54 ab | 32.33 ± 0.13 c | 32.87 ± 1.06 c | 28.25 ± 1.36 b | 26.36 ± 0.73 ab |
| 15-cis-β-carotene | 24.86 ± 0.33 a | 45.99 ± 0.18 d | 30.41 ± 0.81 b | 37.81 ± 0.74 c | 50.94 ± 1.38 e | 26.46 ± 1.11 a |
| 13-cis-β-carotene | 21.13 ± 0.01 b | 13.84 ± 0.23 a | 16.60 ± 0.57 ab | 12.53 ± 2.44 a | 14.60 ± 0.85 a | 20.05 ± 1.54 b |
| α-carotene | 20.14 ± 0.51 bc | 15.94 ± 1.88 a | 14.97 ± 1.14 a | 21.40 ± 0.44 c | 13.40 ± 0.42 a | 17.24 ± 0.43 ab |
| β-carotene | 152.29 ± 4.10 a | 270.08 ± 6.72 d | 218.01 ± 3.99 c | 190.05 ± 7.13 b | 272.22 ± 5.33 d | 193.09 ± 5.90 b |
| 9-cis-β-carotene | 20.71 ± 0.12 a | 23.40 ± 1.43 ab | 20.19 ± 0.62 a | 24.69 ± 0.37 b | 20.84 ± 0.68 a | 23.09 ± 1.60 ab |
| Sum of carotenoids | 314.07 ± 5.96 a | 462.35 ± 0.76 d | 400.34 ± 6.81 c | 384.50 ± 11.57 bc | 463.83 ± 5.38 d | 364.95 ± 0.22 b |
| Color Parameter/Content of Bioactive Compounds | Antioxidant Capacity | |
|---|---|---|
| DPPH Assay | ABTS Assay | |
| L* parameter | −0.73 | −0.75 |
| a* parameter | −0.58 | −0.61 |
| b* parameter | −0.68 | −0.68 |
| TPC | 0.96 * | 0.95 * |
| TFC | 0.74 | 0.78 |
| Caffeic acid | 0.78 | 0.84 * |
| Chlorogenic acid | 0.96 * | 0.95 * |
| Ferulic acid | 0.53 | 0.48 |
| m-Coumaric acid | 0.25 | 0.27 |
| p-Coumaric acid | 0.93 * | 0.96 * |
| Vanillic acid | −0.70 | −0.74 |
| Sum of phenolic acids | 0.97 * | 0.96 * |
| Quercetin | 0.61 | 0.59 |
| Quercetin-3-O-glucoside | 0.70 | 0.75 |
| Rutin | −0.70 | −0.74 |
| Sum of flavonoids | 0.83 * | 0.84 * |
| Lutein | 0.95 * | 0.95 * |
| Zeaxanthin | −0.80 | −0.80 |
| β-cryptoxanthin | 0.44 | 0.44 |
| 15-cis-β-carotene | 0.66 | 0.76 |
| 13-cis-β-carotene | −0.67 | −0.74 |
| α-carotene | −0.68 | −0.69 |
| β-carotene | 0.86 * | 0.92 * |
| 9-cis-β-carotene | 0.00 | 0.06 |
| Sum of carotenoids | 0.88 * | 0.94 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narwojsz, A.; Sawicki, T.; Piłat, B.; Tańska, M. Effect of Heat Treatment Methods on Color, Bioactive Compound Content, and Antioxidant Capacity of Carrot Root. Appl. Sci. 2025, 15, 254. https://doi.org/10.3390/app15010254
Narwojsz A, Sawicki T, Piłat B, Tańska M. Effect of Heat Treatment Methods on Color, Bioactive Compound Content, and Antioxidant Capacity of Carrot Root. Applied Sciences. 2025; 15(1):254. https://doi.org/10.3390/app15010254
Chicago/Turabian StyleNarwojsz, Agnieszka, Tomasz Sawicki, Beata Piłat, and Małgorzata Tańska. 2025. "Effect of Heat Treatment Methods on Color, Bioactive Compound Content, and Antioxidant Capacity of Carrot Root" Applied Sciences 15, no. 1: 254. https://doi.org/10.3390/app15010254
APA StyleNarwojsz, A., Sawicki, T., Piłat, B., & Tańska, M. (2025). Effect of Heat Treatment Methods on Color, Bioactive Compound Content, and Antioxidant Capacity of Carrot Root. Applied Sciences, 15(1), 254. https://doi.org/10.3390/app15010254







