Abstract
Bicuspid Aortic Valve (BAV) is a prevalent congenital heart defect, characterized by the presence of two cusps instead of three, leading to significant clinical implications such as aortic stenosis, regurgitation, and aneurysms. Understanding the genetic underpinnings of BAV is essential for early diagnosis and management, which can prevent severe complications like aortic dissection and heart failure. Recent studies have identified critical genes associated with BAV, including NOTCH1, GATA4, GATA5, SMAD6, NKX2.5, BMP2, and ROBO4, all of which play vital roles in aortic valve development and function. Imaging advancements, particularly in cardiac MRI and echocardiography, have enhanced the assessment of valve morphology and hemodynamics, with Wall Shear Stress emerging as a promising biomarker. This review consolidates current genetic and imaging research, elucidating the contributions of genetic variants to the etiology and progression of BAV, while emphasizing the importance of imaging biomarkers in clinical management. The findings aim to improve genetic screening strategies, facilitate early diagnosis, and guide the development of targeted therapies for individuals with BAV.
1. Introduction
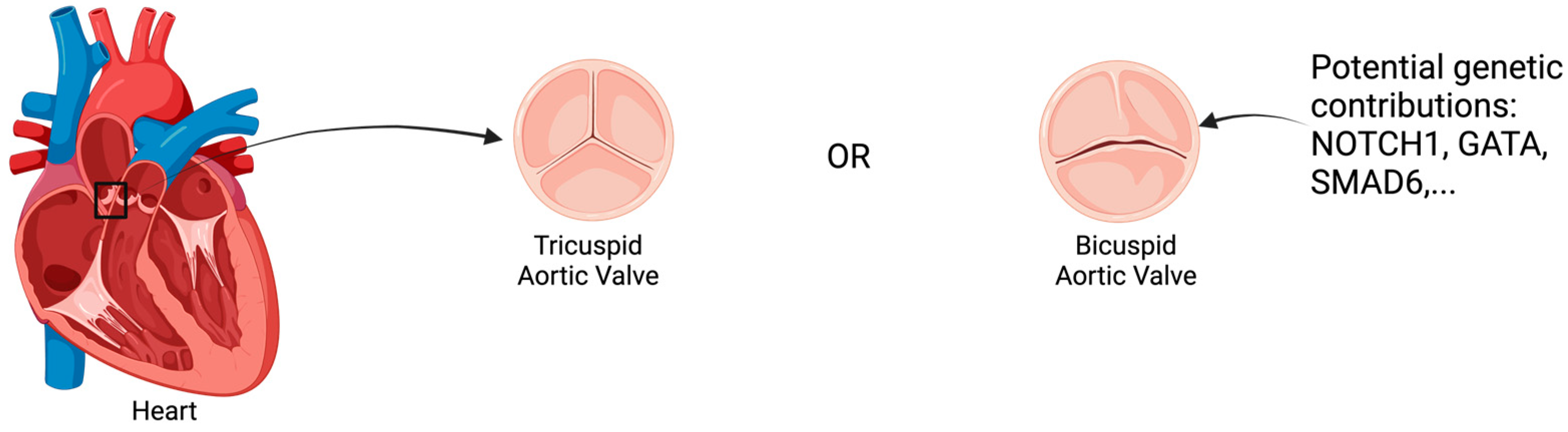
Bicuspid Aortic Valve (BAV) is the most common congenital heart defect, affecting approximately 1–2% of the population [1,2]. Characterized by the presence of two cusps in the aortic valve instead of the normal three, as shown in Figure 1, BAV can lead to significant clinical implications, including an increased risk of aortic stenosis, regurgitation, and aneurysms [3]. Although often diagnosed incidentally during imaging studies, its impact on cardiovascular health can be profound, making early diagnosis and management essential to prevent complications such as aortic dissection and heart failure. Genetic factors play a crucial role in BAV pathogenesis, as shown in Figure 2, offering insights into disease etiology and progression, while imaging provides a non-invasive means to assess structural and functional changes.
Figure 1.
Overview of Bicuspid Aortic Valve Disease Anatomy. Created in BioRender.com.
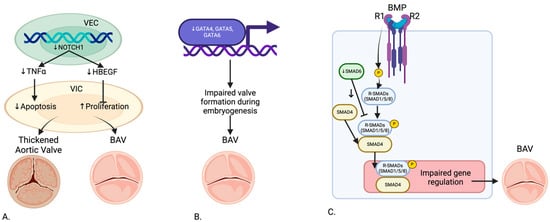
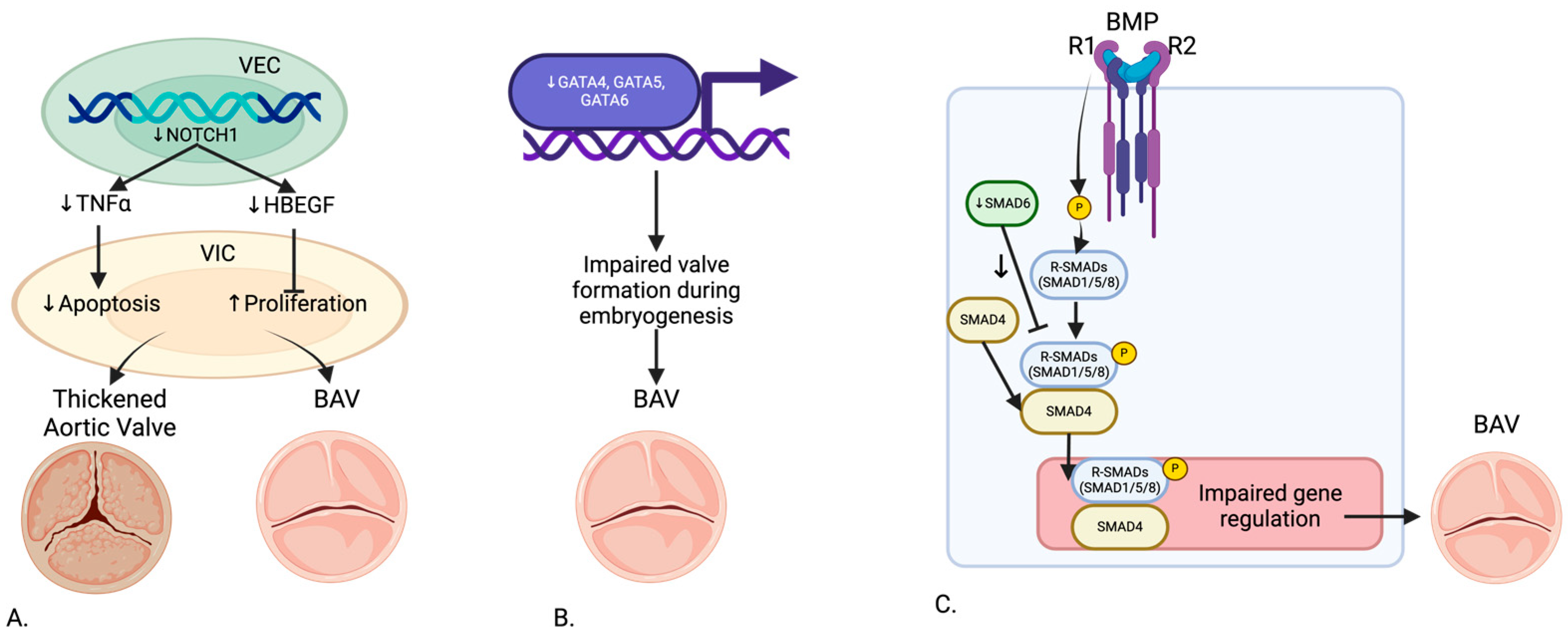
Figure 2.
Genetic Pathway of (A) NOTCH1, (B) GATA, and (C) SMAD6 in the development of BAV. Created in BioRender.com.
In recent years, significant progress has been made in understanding the genetic basis of BAV. Key mutations in genes such as NOTCH1, GATA, and SMAD6 have been linked to abnormal valve development, calcification, and aortopathy. However, genetics alone cannot fully capture disease progression. Advances in imaging modalities, including echocardiography, cardiac MRI, and 4D Flow MRI, offer detailed visualization of valve morphology, flow patterns, and hemodynamic forces such as Wall Shear Stress (WSS). BAV displays a unique interplay between genetic mutations and hemodynamic changes contributing to disease progression. Mutations in genes like NOTCH1 can accelerate disease processes when paired with altered hemodynamic forces observed through imaging. Techniques such as 4D Flow MRI provide a detailed view of flow abnormalities, WSS, and energy loss, helping connect these genetic changes to measurable structural and functional outcomes. Integrating genetic findings with imaging biomarkers has emerged as a promising strategy to improve risk stratification, identify high-risk phenotypes, and personalize treatment approaches for BAV patients [4,5,6,7,8,9,10,11,12,13,14,15,16].
This review aims to consolidate and critically evaluate the current state of genetic and imaging research in BAV. We aim to identify key genetic variants and elucidate their functional implications in disease development and progression. Additionally, we explore the role of imaging biomarkers in linking genetic predispositions to observed hemodynamic and structural changes in BAV. By highlighting these connections, this review emphasizes the clinical utility of integrating genetic screening and advanced imaging modalities to enable earlier diagnosis, optimize risk stratification, and guide tailored management strategies.
2. Genetic Components of BAV
The role of genetics in BAV development has been extensively studied, revealing mutations in key genes such as NOTCH1, GATA, SMAD6, and others. Identifying genetic determinants not only enhances our understanding of BAV pathogenesis but also provides opportunities to integrate genetic findings with advanced imaging biomarkers. This integration may allow for improved risk stratification and earlier diagnosis, which is currently limited in clinical practice.
2.1. NOTCH1
NOTCH1 mutations have been extensively studied due to their significant role in aortic valve development. Garg et al. (2005) identified mutations in NOTCH1 that cause a spectrum of developmental aortic valve anomalies and severe valve calcification [5]. Theodoris et al. (2015) demonstrated that NOTCH1 affects calcium deposition through inhibition of Runx2 activation, with haploinsufficiency leading to calcification of endocardial cells [6]. MacGrogan et al. (2018) highlighted the sequential ligand-dependent NOTCH signaling activation that regulates valve primordium formation and development [4]. Kerstjens-Frederikse et al. (2016) reported that NOTCH1 mutations were highly penetrant (75%), most presenting as BAV in a cohort of 428 probands with left-sided congenital heart disease (LS-CHD) and their families [17]. Preuss et al. (2016) linked other members of the NOTCH1 pathway to BAV and left-ventricular outflow tract obstruction pathologies [18]. Balistreri et al. (2018) found reduced expression of the NOTCH gene in BAV patients, affecting endothelial progenitor cells [19]. Lee et al. (2019) indicated that familial studies show loss of function mutations in NOTCH1 responsible for less than 1% of non-syndromic BAV cases [20]. Roifman et al. (2021) and Debiec et al. (2020) further demonstrated heterozygous NOTCH1 deletion and novel loss of function mutations associated with a spectrum of congenital heart defects including BAV [21,22]. Mutations in NOTCH1, which regulate calcium deposition, align with imaging findings of calcified valves observed through echocardiography and CMR. Future studies could explore how NOTCH1 mutations influence hemodynamic forces such as WSS and contribute to aortic dilation.
2.2. GATA
GATA4, GATA5, and GATA6 genes have been implicated in BAV development. Laforest et al. (2011) showed that GATA5 deletion in mice led to RN type BAV, highlighting its role in valve development [8]. Padang et al. (2012) reported rare variants of the GATA5 gene, highly expressed in the endocardium, linked to non-syndromic BAV in a cohort of 40 BAV patients and 40 controls [23]. Michelena et al. (2014) found that GATA6 heterozygous mice exhibited highly penetrant RL type BAV, with haploinsufficiency affecting valve remodeling and extracellular matrix composition [3]. Ruo-Gu Li et al. (2017) identified a novel heterozygous GATA4 mutation (p.E147X) in 150 patients and 300 controls, disrupting the synergistic transcriptional activation necessary for proper valve formation [7]. Yang et al. (2017) identified GATA4 as a predisposing gene for BAV in a genome-wide association study involving 466 BAV cases and 4660 controls [24]. Xu et al. (2018) discovered a disruptive GATA6 variant in a non-syndromic BAV family [25].
2.3. SMAD6
SMAD6 has been recognized as a crucial inhibitor in the BMP signaling pathway, significantly contributing to BAV pathology. Galvin et al. (2000) identified SMAD6 variants leading to defects in cardiac septation and valve hyperplasia [26]. Gillis et al. (2017) further demonstrated SMAD6 as an important contributor to BAV-associated thoracic aortic aneurysm in a large cohort study [10]. Jong Eun Park et al. (2019) reported a novel SMAD6 variant (c.1168_1173dup; p.Gly390_Ile391dup) in a case report, associated with severely calcified BAV and thoracic aortic aneurysm [11]. Luyckx et al. (2019) identified recurrent rare missense and loss of function variants in SMAD4 and SMAD6 in 22 non-syndromic probands with BAV and thoracic aortic aneurysm [27].
2.4. NKX2.5
NKX2.5 mutations are also linked to BAV. Qu et al. (2014) identified a novel heterozygous NKX2.5 mutation (p.K192X) in 142 patients and 200 controls, showing no transcriptional activity and disrupting interaction with GATA5, which is crucial for heart development [28]. The interaction between NKX2.5 and GATA5 highlights the complex transcriptional regulation of aortic valve development. Imaging these structural abnormalities in patients carrying NKX2.5 mutations could aid in early diagnosis and risk stratification.
2.5. BMP2
BMP2 plays a vital role in bone morphogenesis and has been linked to craniofacial and skeletal abnormalities in BAV patients. Ahluwalia et al. (2021) reported a de novo pathogenic BMP2 variant in a case study, associated with craniofacial dysmorphisms, skeletal abnormalities, BAV, and aortic root aneurysm [13].
2.6. ROBO4
ROBO4 and its associated pathways are crucial in endothelial cell signaling and aortic valve development. Gould et al. (2019) conducted exome sequencing on two families and identified pathogenic ROBO4 variants associated with BAV, AVS, and thoracic aortic aneurysms [14]. Jaouadi et al. (2022) identified pathogenic variants in ROBO1 in familial BAV, although the exact number of participants was not specified [15].
2.7. Other Relevant Genes
The review also identified other significant genes associated with BAV. ADAMTS16 was studied by Lin et al. (2024), who found that extracellular matrix disorganization caused by ADAMTS16 deficiency leads to Bicuspid Aortic Valve with raphe formation [29]. FBN1, typically associated with Marfan Syndrome, was also implicated in BAV. Pepe et al. (2014) identified common and rare variants in non-syndromic BAV cases [30]. MMP-2 was studied by Haunschild et al. (2019), who found increased MMP-2 levels and decreased miR-29A in BAV patients, indicating a role in extracellular matrix destruction and aneurysm development [31]. ACTA2 was studied by Tortora et al. (2017), who found that ACTA2 mutations were not significant contributors to BAV aortopathy [32]. Table 1 further indicates all the genetic components involved.

Table 1.
Studies Identifying Genetic Components of BAV Disease.
Understanding these genetic determinants provides a foundation for linking molecular mechanisms to imaging findings. Future research combining genetic screening with advanced imaging modalities, such as 4D Flow MRI and high-resolution CMR, could enable predictive models to identify at-risk individuals earlier, improve risk stratification, and guide personalized management strategies for BAV patients.
3. Imaging in BAV
3.1. Techniques
Recent advances in imaging techniques have significantly enhanced the ability to diagnose and manage BAV and its associated complications. The most used imaging modalities include echocardiography, cardiac MRI (CMR), and CT angiography (CTA). The advances in detecting hemodynamic parameters have also been essential to exploring BAV further [33,34,35,36,37,38,39,40,41,42,43].
3.2. Cardiac MRI (CMR)
Cardiac MRI (CMR) is considered the gold standard for comprehensive assessment of BAV and aortopathy. CMR provides high-resolution images of the aortic valve and the entire aorta without the use of ionizing radiation. It allows for detailed evaluation of valve morphology, aortic dimensions, and functional assessment of the left ventricle. Additionally, CMR can measure blood flow dynamics, which are crucial for understanding the hemodynamic impact of BAV [16].
4D Flow MRI: 4D Flow MRI is a novel technique that captures three-dimensional blood flow within the heart and great vessels over time. This comprehensive visualization of blood flow patterns allows for detailed analysis of hemodynamics and wall shear stress (WSS), providing valuable insights into the pathophysiology of BAV and associated aortopathy [41].
3.3. CT Angiography (CTA)
CT Angiography (CTA) is another valuable imaging modality, particularly useful for assessing the aorta. CTA provides excellent spatial resolution, enabling detailed visualization of the aortic anatomy, including the presence and extent of aortic aneurysms, coarctation, and other vascular abnormalities. Its multiplanar reconstructions allow precise measurement of aortic diameters and detection of valve calcification, making it highly accurate in evaluating aortic stenosis severity [44,45]. In cases of severe valve calcification, CTA has been shown to outperform echocardiography in identifying bicuspid morphology, achieving a sensitivity of up to 94% [44].
CTA also enables the assessment of coronary artery disease and the ascending aorta, both of which are critical for preoperative planning in BAV patients undergoing surgical interventions [46]. Additionally, CTA’s ability to detect plaque and assess aortic wall integrity makes it particularly valuable for evaluating aortic stenosis and coexisting vascular abnormalities [45]. However, its use is limited by exposure to ionizing radiation and contrast agents, which may not be suitable for younger or high-risk patient populations [36]. Newer low-dose CT protocols are being developed to mitigate these risks while maintaining diagnostic accuracy.
3.4. Echocardiography
Echocardiography remains the cornerstone of BAV imaging, offering real-time, dynamic assessment of the valve and surrounding structures. Transthoracic echocardiography (TTE) is typically the first-line imaging tool due to its availability, low cost, and non-invasive nature. It is highly effective in identifying bicuspid morphology, particularly in young patients or when calcification is minimal [46]. TTE can also provide detailed information on valvular function, including stenosis severity (pressure gradients, valve area) and the presence of regurgitation [44].
Despite its strengths, TTE has limitations in cases of extensive calcification, poor acoustic windows, or challenging patient anatomy. In such instances, transesophageal echocardiography (TEE) is used to provide higher-resolution images of the aortic valve and root. TEE is particularly useful for detecting leaflet fusion, mini-raphe, and eccentric jets of aortic regurgitation [34,47]. Additionally, TEE has demonstrated utility in surgical planning by allowing precise measurements of the aortic root and proximal ascending aorta [46].
Echocardiography can also evaluate the functional consequences of BAV, including left ventricular hypertrophy and systolic dysfunction, which may result from chronic pressure or volume overload [47]. Advances in 3D echocardiography and strain imaging have further enhanced its ability to quantify valve anatomy and function. However, for definitive preoperative evaluation or in cases with diagnostic uncertainty, complementary imaging modalities such as CMR or CTA may be required [45].
3.5. Key Hemodynamic Parameters
Recent studies have highlighted additional hemodynamic parameters that provide valuable insights into BAV-related aortopathy:
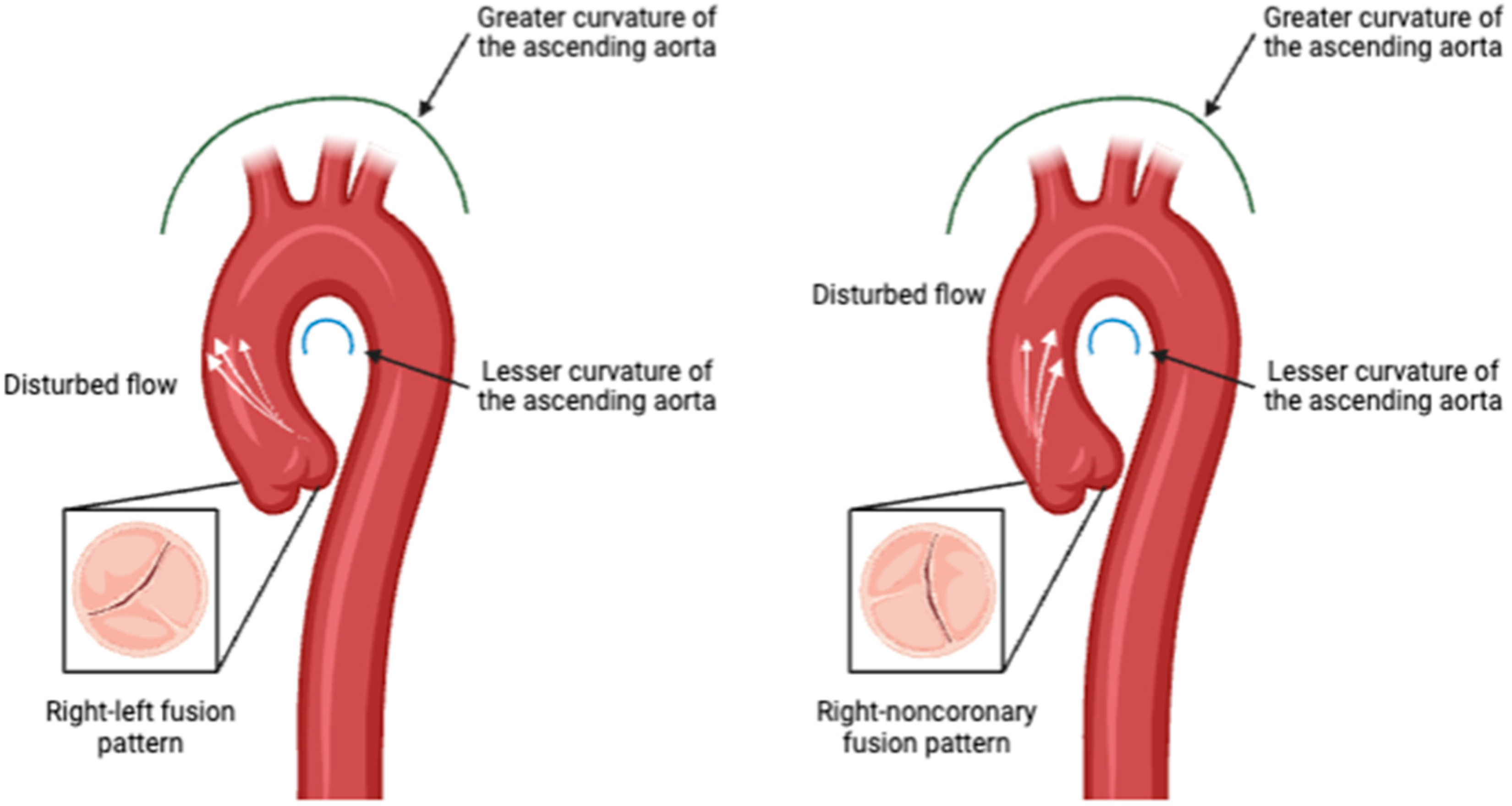
Flow Eccentricity and Helicity: BAV is associated with altered blood flow patterns, including increased flow eccentricity and helicity in the ascending aorta. These parameters are distinct from those observed in healthy individuals and are indicative of the abnormal flow dynamics caused by the bicuspid valve structure and phenotype [48,49,50], Figure 3.
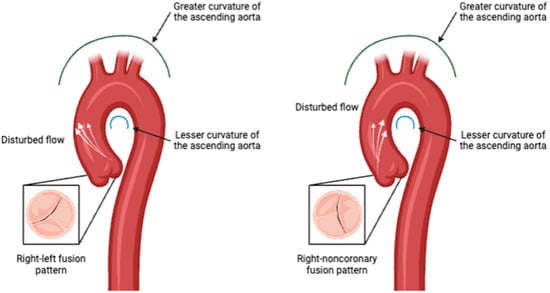
Figure 3.
Aortic valve phenotype impact in disturbed flow direction pattern. Created in BioRender.com.
Wall Shear Stress (WSS): WSS has emerged as a critical biomarker in the understanding and management of BAV-associated aortopathy. WSS refers to the tangential force exerted by blood flow on the endothelial surface of the vessel wall. Abnormal WSS patterns have been implicated in the development and progression of aortic aneurysms and other vascular complications in BAV patients. Studies have shown that BAV leads to altered flow dynamics within the aorta, resulting in regions of increased or decreased WSS. These abnormal WSS patterns can contribute to the heterogeneous nature of aortopathy observed in BAV patients. For instance, regions of high WSS are associated with the development of aortic dilation and aneurysm formation, while low WSS can lead to areas of stagnation and potential thrombus formation [37,38,39,40]. Elevated WSS is commonly observed in BAV patients, particularly along the greater curvature of the ascending aorta. This increased stress is linked to the development of aortic dilation and wall thinning, making it a critical marker for disease progression [49,51].
Clinical Utility of WSS: Hassanabad et al. (2019) highlighted the potential of using WSS as a non-invasive imaging biomarker for risk stratification in BAV patients. By creating individualized WSS ‘heat maps’, clinicians can identify areas of the aorta at higher risk of pathological changes, guiding the timing and extent of surgical interventions [16]. This approach allows for personalized management strategies, optimizing patient outcomes.
Forward and Reverse Flow: BAV patients exhibit significant alterations in forward and reverse flow dynamics. Studies using advanced 4D-flow MRI techniques have shown reduced forward flow and increased reverse flow in the ascending aorta, which are associated with aortic dilation and may predict the need for surgical intervention [48,50].
Energy Loss and Vorticity: Parameters such as energy loss, vorticity, and relative pressure provide further insights into BAV hemodynamics. Increased vorticity and energy loss have been linked to inefficient flow patterns and higher mechanical stress on the aortic wall, contributing to disease progression [49,51].
3.6. Elastography
Elastography is an imaging modality that measures tissue stiffness, including the aortic wall. Increased aortic stiffness is a marker of vascular pathology and can indicate underlying aortopathy in BAV patients. Elastography can be performed using ultrasound or MRI, providing another layer of diagnostic information for risk assessment and management.
Recent studies have demonstrated the utility of both ultrasound elastography and magnetic resonance elastography (MRE) for quantifying aortic stiffness. Ultrasound elastography uses sound waves to assess tissue stiffness and has been effectively used in various clinical settings, including cardiovascular imaging [42,43]. MRE combines MRI with low-frequency vibrations to create a visual map of tissue stiffness, offering a non-invasive method to detect changes in the aortic wall associated with diseases such as BAV [42].
3.7. Molecular Imaging
Molecular imaging is an emerging field that involves using targeted imaging agents to bind to specific molecular markers of disease. In BAV, molecular imaging can help visualize processes such as inflammation, calcification, and matrix remodeling at a cellular level. This technique holds promise for early detection of pathological changes and monitoring the efficacy of targeted therapies.
Molecular imaging provides insights into the biochemical and molecular processes underlying BAV. For example, targeted agents can bind to inflammatory markers or calcific deposits, allowing for precise imaging of these pathological changes. This approach has the potential to significantly enhance the early diagnosis and treatment monitoring in BAV patients, providing a more detailed understanding of disease progression at the molecular level [42,43].
4. Linking Genetics and Imaging
Integrating genetic and imaging data offers a comprehensive approach to understanding BAV and its complications. Genetic mutations in BAV, such as those in NOTCH1, GATA, and SMAD6, can influence imaging findings. For example, NOTCH1 mutations are often associated with calcification and valve abnormalities that can be visualized using echocardiography or cardiac MRI (CMR) [37,38,39]. These imaging modalities can detect structural changes in the valve and aorta, providing insights into the extent and severity of the disease caused by specific genetic mutations.
4.1. Role of Genetic Mutations in Imaging Findings
Genetic mutations can lead to specific phenotypic manifestations observable through imaging. For instance, NOTCH1 mutations have been linked to early valve calcification and abnormal valve morphology. Echocardiography can reveal thickened and calcified valve leaflets, while CMR can provide detailed images of the calcification extent and associated aortopathy [37].
GATA family genes, particularly GATA4 and GATA5, are crucial in cardiac development and can influence valve formation. Mutations in these genes might lead to valve dysfunctions detectable through echocardiography, such as bicuspid valves with abnormal cusp morphology or associated aortic dilatation [38]. SMAD6 mutations, which affect the TGF-beta signaling pathway, are associated with aortic aneurysms and valve abnormalities that can be precisely measured using CMR to monitor aortic dimensions and detect aneurysm formation [39].
4.2. Imaging Biomarkers and Genetic Data Correlation
Imaging biomarkers, such as Wall Shear Stress (WSS), provide a non-invasive means to assess the functional consequences of genetic mutations. Abnormal WSS patterns, which can be quantified using 4D Flow MRI, are associated with different BAV phenotypes and related aortopathies [40]. High WSS regions correlate with aortic dilation and aneurysm formation, while low WSS regions might indicate areas prone to thrombosis [40]. In addition to WSS, other imaging biomarkers have emerged as valuable tools in understanding the genetic impact on BAV pathology. Flow helicity and eccentricity, measurable via 4D Flow MRI, reflect disturbed hemodynamics often associated with genetic mutations such as NOTCH1 and SMAD6. These biomarkers highlight asymmetric and turbulent blood flow, which can further exacerbate aortic wall stress and promote localized remodeling [52,53].
4.3. Genetic-Driven Hemodynamic Variations
Recent research highlights the interplay between genetic mutations and hemodynamic variations observable through advanced imaging techniques. For instance, mutations in NOTCH1 and SMAD6 have been associated with flow eccentricity and elevated WSS, particularly along the greater curvature of the ascending aorta [52,54]. Such abnormal hemodynamics contribute to localized aortic wall remodeling and increase the risk of aneurysm formation. Imaging tools like 4D Flow MRI can map these flow abnormalities, providing crucial data for correlating genetic variants with observed structural changes [50,53].
For example, NOTCH1 mutations not only result in early calcification but also exhibit characteristic flow disruptions on imaging, which may serve as early indicators of disease progression. Studies have shown that NOTCH1 mutations are associated with altered blood flow patterns, including increased flow eccentricity and turbulence in the ascending aorta, which can be effectively visualized using advanced imaging techniques like 4D Flow MRI [52,53]. Such findings highlight the role of NOTCH1 in contributing to hemodynamic changes that precede structural complications, emphasizing its potential as a combined genetic and imaging biomarker for disease progression.
Similarly, SMAD6-associated aortopathies show distinct flow abnormalities and elevated WSS, which can be effectively monitored using imaging tools. Studies have demonstrated that SMAD6 mutations, which disrupt TGF-beta signaling, result in altered hemodynamic forces, including elevated WSS and flow eccentricity, particularly in the ascending aorta. These abnormalities contribute to localized aortic wall remodeling and increase susceptibility to aneurysm formation. Advanced imaging tools such as 4D Flow MRI and computational fluid dynamics (CFD) analyses are essential in capturing these flow disruptions, providing a comprehensive link between genetic predisposition and observed structural changes [53,54].
4.4. Future Directions in Genetic and Imaging Integration
Advances in molecular imaging and next-generation sequencing (NGS) provide an opportunity to combine genetic and imaging biomarkers for risk stratification. Practically, this can be achieved by integrating genetic screening results with imaging-derived parameters into diagnostic algorithms or predictive models. For instance, patients identified with NOTCH1 or SMAD6 mutations can be prioritized for advanced imaging techniques such as 4D Flow MRI to quantify wall shear stress (WSS) and flow abnormalities. Combining these findings into machine learning-based risk stratification tools could allow earlier identification of high-risk patients, enabling timely interventions such as surgical planning or close monitoring. Additionally, predictive models incorporating genetic and imaging data can inform personalized management strategies, bridging the gap between research insights and clinical practice. Genetic screening for mutations such as NOTCH1, GATA, and SMAD6, combined with imaging-derived parameters like WSS, flow helicity, and energy loss, can help identify high-risk BAV phenotypes early. Emerging techniques, including elastography and molecular imaging, can further integrate with genetic findings to provide a personalized approach to BAV management [54,55,56].
This integrative approach not only improves diagnostic accuracy but also aids in monitoring disease progression and optimizing surgical planning. Future studies are needed to validate these combined methodologies and explore their clinical utility in larger BAV cohorts. Specific research directions could include longitudinal studies that track patients with known genetic mutations such as NOTCH1 and SMAD6, correlating these findings with imaging biomarkers like WSS, flow eccentricity, and aortic dimensions over time. Additionally, multi-center trials could focus on the development of integrative diagnostic algorithms combining genetic sequencing and imaging data to improve early detection and risk stratification. The use of artificial intelligence and machine learning models should be explored to identify patterns and predict outcomes based on genetic and imaging parameters. Clinically, integrating genetic screening with imaging workflows could streamline personalized treatment pathways, enabling earlier interventions for high-risk patients and optimizing surgical planning. Emphasizing the combined utility of genetic data and imaging-derived parameters will pave the way for more comprehensive, individualized approaches to managing BAV and its associated aortopathy.
5. Conclusions
This review consolidates recent advances in understanding the genetic underpinnings and imaging characteristics of BAV with a focus on how well-established genetic determinants such as NOTCH1, GATA4, GATA5, GATA6, and SMAD6 can be integrated with imaging data to better understand disease progression and clinical outcomes [37,38,39,40].
The integration of genetic data with imaging techniques provides a comprehensive approach to diagnosing and managing BAV. Wall Shear Stress (WSS) has emerged as a valuable imaging biomarker, offering insights into the hemodynamic forces that contribute to aortopathy in BAV patients. Studies have demonstrated the potential of WSS to guide clinical decisions, such as the timing of surgical interventions and the strategies for postoperative care.
5.1. Clinical Implications
The integration of genetic and imaging data is pivotal for the personalized management of BAV patients. Genetic screening for mutations in NOTCH1, GATA, and SMAD6 can identify individuals at risk for severe valve anomalies and aortopathy. Imaging biomarkers, such as WSS, can monitor disease progression and guide the timing of surgical interventions. This combined approach can improve outcomes by enabling early diagnosis, personalized treatment plans, and targeted therapies [37,38,39,40].
5.2. Limitations
This review has several limitations. The inclusion of only English-language studies may have excluded relevant research, introducing potential bias. The reliance on published studies could result in publication bias, as positive results are more likely to be published. Additionally, the heterogeneity of study designs and populations makes it challenging to generalize the findings universally. Future studies should aim for larger, more diverse cohorts and standardized methodologies to validate these results.
5.3. Future Directions
Future research should focus on larger multi-center studies to confirm the prevalence and impact of specific genetic mutations across diverse populations. Functional studies exploring the mechanistic pathways of genes like NOTCH1, GATA, and SMAD6 will provide deeper insights into their roles in BAV development. Investigating gene-environment interactions could reveal how external factors influence genetic predispositions to BAV. Moreover, integrating advanced genomic technologies, such as CRISPR and next-generation sequencing, could facilitate the discovery of novel genetic variants and their functional consequences. Additionally, the development of sophisticated imaging techniques and biomarkers, such as WSS, will enhance the precision of diagnosis and the effectiveness of treatment strategies [37,38,39,40].
The next frontier in BAV research lies in redefining the paradigm of patient-centered care. Instead of focusing solely on the molecular or imaging aspects of the disease, future approaches should aim to connect the dots between these domains to offer comprehensive solutions. The future of BAV research should also emphasize the seamless integration of genetic findings with clinical applications. For example, developing risk prediction models and tests based on genetic and environmental factors could help identify high-risk individuals early, allowing for targeted surveillance and timely intervention. Advances in computational biology and machine learning could support the creation of dynamic, patient-specific simulations to predict the progression of BAV-related complications, offering a more proactive approach to management.
A greater emphasis should be placed on the predictive power of lifestyle and environmental modifications in mitigating the progression of BAV-related complications. Collaborative efforts should extend beyond the clinical setting to involve public health initiatives that educate and empower individuals about their genetic risks. By fostering innovation and collaboration, the field can move toward a precision medicine approach that improves the quality of life for individuals with BAV.
Author Contributions
Conceptualization, V.M. and J.G.; methodology, V.M. and J.G.; software, V.M. and J.G.; validation, V.M. and J.G.; formal analysis, V.M. and J.G.; investigation, V.M. and J.G.; resources, V.M. and J.G.; data curation, V.M. and J.G.; writing—original draft preparation, V.M. and J.G.; writing—review and editing, J.G.; visualization, V.M. and J.G.; supervision, J.G.; project administration, J.G.; funding acquisition, V.M. and J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The University of Calgary; J.G. start-up funding (#11022618 and #11021988). We acknowledge the support of the Natural Science and Engineering Research Council of Canada/Conseil de recherche en science naturelles et en génie du Canada, RGPIN-2020-04549 and DGECR-2020-00204. NSERC Alliance—Alberta Innovates Advance Program (#232403115). V.M was supported by the Mach-Gaensslen Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ward, C. Clinical Significance of the Bicuspid Aortic Valve. Heart 2000, 83, 81–85. [Google Scholar] [CrossRef]
- Bravo-Jaimes, K.; Prakash, S.K. Genetics in Bicuspid Aortic Valve Disease: Where Are We? Prog. Cardiovasc. Dis. 2020, 63, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Michelena, H.I.; Prakash, S.K.; Della Corte, A.; Bissell, M.M.; Anavekar, N.; Mathieu, P.; Bossé, Y.; Limongelli, G.; Bossone, E.; Benson, D.W.; et al. Bicuspid Aortic Valve: Identifying Knowledge Gaps and Rising to the Challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation 2014, 129, 2691–2704. [Google Scholar] [CrossRef] [PubMed]
- MacGrogan, D.; Münch, J.; de la Pompa, J.L. Notch and Interacting Signalling Pathways in Cardiac Development, Disease, and Regeneration. Nat. Rev. Cardiol. 2018, 15, 685–704. [Google Scholar] [CrossRef]
- Garg, V.; Muth, A.N.; Ransom, J.F.; Schluterman, M.K.; Barnes, R.; King, I.N.; Grossfeld, P.D.; Srivastava, D. Mutations in NOTCH1 Cause Aortic Valve Disease. Nature 2005, 437, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Theodoris, C.V.; Li, M.; White, M.P.; Liu, L.; He, D.; Pollard, K.S.; Bruneau, B.G.; Srivastava, D. Human Disease Modeling Reveals Integrated Transcriptional and Epigenetic Mechanisms of NOTCH1 Haploinsufficiency. Cell 2015, 160, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-G.; Xu, Y.-J.; Wang, J.; Liu, X.-Y.; Yuan, F.; Huang, R.-T.; Xue, S.; Li, L.; Liu, H.; Li, Y.-J.; et al. GATA4 Loss-of-Function Mutation and the Congenitally Bicuspid Aortic Valve. Am. J. Cardiol. 2018, 121, 469–474. [Google Scholar] [CrossRef]
- Laforest, B.; Andelfinger, G.; Nemer, M. Loss of Gata5 in Mice Leads to Bicuspid Aortic Valve. J. Clin. Investig. 2011, 121, 2876–2887. [Google Scholar] [CrossRef]
- Ackah, R.L.; Yasuhara, J.; Garg, V. Genetics of Aortic Valve Disease. Curr. Opin. Cardiol. 2023, 38, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Gillis, E.; Kumar, A.A.; Luyckx, I.; Preuss, C.; Cannaerts, E.; van de Beek, G.; Wieschendorf, B.; Alaerts, M.; Bolar, N.; Vandeweyer, G.; et al. Candidate Gene Resequencing in a Large Bicuspid Aortic Valve-Associated Thoracic Aortic Aneurysm Cohort: SMAD6 as an Important Contributor. Front. Physiol. 2017, 8, 400. [Google Scholar] [CrossRef]
- Park, J.E.; Park, J.S.; Jang, S.Y.; Park, S.H.; Kim, J.; Ki, C.; Kim, D. A Novel SMAD6 Variant in a Patient with Severely Calcified Bicuspid Aortic Valve and Thoracic Aortic Aneurysm. Mol. Genet. Genom. Med. 2019, 7, e620. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, I.; Verstraeten, A.; Goumans, M.-J.; Loeys, B. SMAD6-Deficiency in Human Genetic Disorders. NPJ Genom. Med. 2022, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, N.; Gelb, B.D. A de Novo Pathogenic BMP2 Variant-Related Phenotype with the Novel Finding of Bicuspid Aortic Valve. Am. J. Med. Genet. A 2021, 185, 575–578. [Google Scholar] [CrossRef]
- Gould, R.A.; Aziz, H.; Woods, C.E.; Seman-Senderos, M.A.; Sparks, E.; Preuss, C.; Wünnemann, F.; Bedja, D.; Moats, C.R.; McClymont, S.A.; et al. ROBO4 Variants Predispose Individuals to Bicuspid Aortic Valve and Thoracic Aortic Aneurysm. Nat. Genet. 2019, 51, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Jaouadi, H.; Gérard, H.; Théron, A.; Collod-Béroud, G.; Collart, F.; Avierinos, J.-F.; Zaffran, S. Identification of Non-Synonymous Variations in ROBO1 and GATA5 Genes in a Family with Bicuspid Aortic Valve Disease. J. Hum. Genet. 2022, 67, 515–518. [Google Scholar] [CrossRef]
- Hassanabad, A.F.; Garcia, J.; Verma, S.; White, J.A.; Fedak, P.W.M. Utilizing Wall Shear Stress as a Clinical Biomarker for Bicuspid Valve Associated Aortopathy. Curr. Opin. Cardiol. 2019, 34, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Kerstjens-Frederikse, W.S.; van de Laar, I.M.B.H.; Vos, Y.J.; Verhagen, J.M.A.; Berger, R.M.F.; Lichtenbelt, K.D.; Klein Wassink-Ruiter, J.S.; van der Zwaag, P.A.; du Marchie Sarvaas, G.J.; Bergman, K.A.; et al. Cardiovascular Malformations Caused by NOTCH1 Mutations Do Not Keep Left: Data on 428 Probands with Left-Sided CHD and Their Families. Genet. Med. 2016, 18, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Preuss, C.; Capredon, M.; Wünnemann, F.; Chetaille, P.; Prince, A.; Godard, B.; Leclerc, S.; Sobreira, N.; Ling, H.; Awadalla, P.; et al. Family Based Whole Exome Sequencing Reveals the Multifaceted Role of Notch Signaling in Congenital Heart Disease. PLoS Genet. 2016, 12, e1006335. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Crapanzano, F.; Schirone, L.; Allegra, A.; Pisano, C.; Ruvolo, G.; Forte, M.; Greco, E.; Cavarretta, E.; Marullo, A.G.M.; et al. Deregulation of Notch1 Pathway and Circulating Endothelial Progenitor Cell (EPC) Number in Patients with Bicuspid Aortic Valve with and without Ascending Aorta Aneurysm. Sci. Rep. 2018, 8, 13834. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Wei, S.; Schwertani, A. A Notch More: Molecular Players in Bicuspid Aortic Valve Disease. J. Mol. Cell. Cardiol. 2019, 134, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Roifman, M.; Chung, B.H.Y.; Reid, D.M.; Teitelbaum, R.; Martin, N.; Nield, L.E.; Thompson, M.; Shannon, P.; Chitayat, D. Heterozygous Deletion Associated with Variable Congenital Heart Defects. Clin. Genet. 2021, 99, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Debiec, R.; Hamby, S.E.; Jones, P.D.; Coolman, S.; Asiani, M.; Kharodia, S.; Skinner, G.J.; Samani, N.J.; Webb, T.R.; Bolger, A. Novel Loss of Function Mutation in NOTCH1 in a Family with Bicuspid Aortic Valve, Ventricular Septal Defect, Thoracic Aortic Aneurysm, and Aortic Valve Stenosis. Mol. Genet. Genom. Med. 2020, 8, e1437. [Google Scholar] [CrossRef]
- Padang, R.; Bagnall, R.D.; Richmond, D.R.; Bannon, P.G.; Semsarian, C. Rare Non-Synonymous Variations in the Transcriptional Activation Domains of GATA5 in Bicuspid Aortic Valve Disease. J. Mol. Cell. Cardiol. 2012, 53, 277–281. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, W.; Jiao, J.; Nielsen, J.B.; Mathis, M.R.; Heydarpour, M.; Lettre, G.; Folkersen, L.; Prakash, S.; Schurmann, C.; et al. Protein-Altering and Regulatory Genetic Variants near GATA4 Implicated in Bicuspid Aortic Valve. Nat. Commun. 2017, 8, 15481. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-J.; Di, R.-M.; Qiao, Q.; Li, X.-M.; Huang, R.-T.; Xue, S.; Liu, X.-Y.; Wang, J.; Yang, Y.-Q. GATA6 Loss-of-Function Mutation Contributes to Congenital Bicuspid Aortic Valve. Gene 2018, 663, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Galvin, K.M.; Donovan, M.J.; Lynch, C.A.; Meyer, R.I.; Paul, R.J.; Lorenz, J.N.; Fairchild-Huntress, V.; Dixon, K.L.; Dunmore, J.H.; Gimbrone, M.A., Jr.; et al. A Role for Smad6 in Development and Homeostasis of the Cardiovascular System. Nat. Genet. 2000, 24, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, I.; MacCarrick, G.; Kempers, M.; Meester, J.; Geryl, C.; Rombouts, O.; Peeters, N.; Claes, C.; Boeckx, N.; Sakalihasan, N.; et al. Confirmation of the Role of Pathogenic SMAD6 Variants in Bicuspid Aortic Valve-Related Aortopathy. Eur. J. Hum. Genet. 2019, 27, 1044–1053. [Google Scholar] [CrossRef]
- Qu, X.-K.; Qiu, X.-B.; Yuan, F.; Wang, J.; Zhao, C.-M.; Liu, X.-Y.; Zhang, X.-L.; Li, R.-G.; Xu, Y.-J.; Hou, X.-M.; et al. A Novel NKX2.5 Loss-of-Function Mutation Associated with Congenital Bicuspid Aortic Valve. Am. J. Cardiol. 2014, 114, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, Q.; Lin, X.; Liu, X.; Qian, Y.; Xu, D.; Cao, N.; Han, X.; Zhu, Y.; Hu, W.; et al. Extracellular Matrix Disorganization Caused by ADAMTS16 Deficiency Leads to Bicuspid Aortic Valve With Raphe Formation. Circulation 2024, 149, 605–626. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Nistri, S.; Giusti, B.; Sticchi, E.; Attanasio, M.; Porciani, C.; Abbate, R.; Bonow, R.O.; Yacoub, M.; Gensini, G.F. Identification of Fibrillin 1 Gene Mutations in Patients with Bicuspid Aortic Valve (BAV) without Marfan Syndrome. BMC Med. Genet. 2014, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Haunschild, J.; Schellinger, I.N.; Barnard, S.J.; von Aspern, K.; Davierwala, P.; Misfeld, M.; Petroff, D.; Borger, M.A.; Etz, C.D. Bicuspid Aortic Valve Patients Show Specific Epigenetic Tissue Signature Increasing Extracellular Matrix Destruction. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Tortora, G.; Wischmeijer, A.; Berretta, P.; Alfonsi, J.; Di Marco, L.; Barbieri, A.; Marconi, C.; Isidori, F.; Rossi, C.; Leone, O.; et al. Search for Genetic Factors in Bicuspid Aortic Valve Disease: ACTA2 Mutations Do Not Play a Major Role. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 813–817. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lim, M.S.-A.; Portelli, S.S.; Padang, R.; Bannon, P.G.; Hambly, B.D.; Jeremy, R.W.; Celermajer, D.S.; Robertson, E.N. Novel Insights into Bicuspid Aortic Valve (BAV) Aortopathy: Long Non-Coding RNAs TUG1 and MIAT Are Differentially Expressed in BAV Ascending Aortas. Cardiovasc. Pathol. 2022, 60, 107433. [Google Scholar] [CrossRef] [PubMed]
- Evangelista Masip, A.; Galian-Gay, L.; Guala, A.; Lopez-Sainz, A.; Teixido-Turà, G.; Ruiz Muñoz, A.; Valente, F.; Gutierrez, L.; Fernandez-Galera, R.; Casas, G.; et al. Unraveling Bicuspid Aortic Valve Enigmas by Multimodality Imaging: Clinical Implications. J. Clin. Med. 2022, 11, 456. [Google Scholar] [CrossRef]
- Frigiola, A.; Sophocleous, F.; Biglino, G. Aortic Disease: Bicuspid Aortic Valve, Aortic Coarctation, Marfan Syndrome. In Multimodality Imaging Innovations in Adult Congenital Heart Disease: Emerging Technologies and Novel Applications; Gallego, P., Valverde, I., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 243–273. ISBN 978-3-030-61927-5. [Google Scholar]
- Kumamaru, K.K.; Hoppel, B.E.; Mather, R.T.; Rybicki, F.J. CT Angiography: Current Technology and Clinical Use. Radiol. Clin. N. Am. 2010, 48, 213–235. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Palomares, J.F.; Dux-Santoy, L.; Guala, A.; Kale, R.; Maldonado, G.; Teixidó-Turà, G.; Galian, L.; Huguet, M.; Valente, F.; Gutiérrez, L.; et al. Aortic Flow Patterns and Wall Shear Stress Maps by 4D-Flow Cardiovascular Magnetic Resonance in the Assessment of Aortic Dilatation in Bicuspid Aortic Valve Disease. J. Cardiovasc. Magn. Reson. 2018, 20, 28. [Google Scholar] [CrossRef]
- van Ooij, P.; Farag, E.S.; Blanken, C.P.S.; Nederveen, A.J.; Groenink, M.; Planken, R.N.; Boekholdt, S.M. Fully Quantitative Mapping of Abnormal Aortic Velocity and Wall Shear Stress Direction in Patients with Bicuspid Aortic Valves and Repaired Coarctation Using 4D Flow Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2021, 23, 9. [Google Scholar] [CrossRef]
- Markl, M.; Fedak, P.W.M.; Barker, A. Impact of Aortopathy and Aortic Valve Disease on 3D Blood Flow and Wall Shear Stress in the Thoracic Aorta: As Assessed by 4D Flow MRI. In Surgical Management of Aortic Pathology: Current Fundamentals for the Clinical Management of Aortic Disease; Stanger, O.H., Pepper, J.R., Svensson, L.G., Eds.; Springer: Vienna, Austria, 2019; pp. 447–464. ISBN 978-3-7091-4874-7. [Google Scholar]
- Piatti, F.; Sturla, F.; Bissell, M.M.; Pirola, S.; Lombardi, M.; Nesteruk, I.; Della Corte, A.; Redaelli, A.C.L.; Votta, E. 4D Flow Analysis of BAV-Related Fluid-Dynamic Alterations: Evidences of Wall Shear Stress Alterations in Absence of Clinically-Relevant Aortic Anatomical Remodeling. Front. Physiol. 2017, 8, 441. [Google Scholar] [CrossRef]
- Stankovic, Z.; Allen, B.D.; Garcia, J.; Jarvis, K.B.; Markl, M. 4D Flow Imaging with MRI. Cardiovasc. Diagn. Ther. 2014, 4, 173–192. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, P.J. Advanced Ultrasound Applications: Elastography and Contrast-Enhanced Ultrasound. In Musculoskeletal Ultrasound in Rheumatology Review; Kohler, M.J., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 529–560. ISBN 978-3-030-73555-5. [Google Scholar]
- Tzschätzsch, H. Methods and Approaches in Ultrasound Elastography. In Quantification of Biophysical Parameters in Medical Imaging; Sack, I., Schaeffter, T., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 281–302. ISBN 978-3-319-65924-4. [Google Scholar]
- Tanaka, R.; Yoshioka, K.; Niinuma, H.; Ohsawa, S.; Okabayashi, H.; Ehara, S. Diagnostic value of cardiac CT in the evaluation of bicuspid aortic stenosis: Comparison with echocardiography and operative findings. Am. J. Roentgenology 2010, 195, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.J.; Maleszewski, J.J.; Araoz, P.A. CT and MR imaging of the aortic valve: Radiologic-pathologic correlation. Radiographics 2012, 32, 1399–1420. [Google Scholar] [CrossRef] [PubMed]
- Galian-Gay, L.; Rodríguez-Palomares, J.; Guala, A.; Michelena, H.I.; Evangelista, A. Multimodality imaging in bicuspid aortic valve. Prog. Cardiovasc. Dis. 2020, 63, 442–451. [Google Scholar] [CrossRef]
- Michelena, H.I.; Chandrasekaran, K.; Topilsky, Y.; Messika-Zeitoun, D.; Della Corte, A.; Evangelista, A.; Enriquez-Sarano, M. The bicuspid aortic valve condition: The critical role of echocardiography and the case for a standard nomenclature consensus. Prog. Cardiovasc. Dis. 2018, 61, 404–415. [Google Scholar] [CrossRef]
- Edlin, J.; Youssefi, P.; Bilkhu, R.; Figueroa, C.A.; Morgan, R.; Nowell, J.; Jahangiri, M. Haemodynamic Assessment of Bicuspid Aortic Valve Aortopathy: A Systematic Review of the Current Literature. Eur. J. Cardiothorac. Surg. 2019, 55, 610–617. [Google Scholar] [CrossRef]
- Geeraert, P.; Jamalidinan, F.; Burns, F.; Jarvis, K.; Bristow, M.S.; Lydell, C.; Hidalgo Tobon, S.S.; de Celis Alonso, B.; Fedak, P.W.M.; White, J.A.; et al. Hemodynamic Assessment in Bicuspid Aortic Valve Disease and Aortic Dilation: New Insights From Voxel-By-Voxel Analysis of Reverse Flow, Stasis, and Energetics. Front. Bioeng. Biotechnol. 2022, 9, 725113. [Google Scholar] [CrossRef]
- Sotelo, J.; Franco, P.; Guala, A.; Dux-Santoy, L.; Ruiz-Muñoz, A.; Evangelista, A.; Mella, H.; Mura, J.; Hurtado, D.E.; Rodríguez-Palomares, J.F.; et al. Fully Three-Dimensional Hemodynamic Characterization of Altered Blood Flow in Bicuspid Aortic Valve Patients With Respect to Aortic Dilatation: A Finite Element Approach. Front. Cardiovasc. Med. 2022, 9, 885338. [Google Scholar] [CrossRef] [PubMed]
- Bailoor, S.; Seo, J.-H.; Schena, S.; Mittal, R. Changes in Aorta Hemodynamics in Left-Right Type 1 Bicuspid Aortic Valve Patients After Replacement with Bioprosthetic Valves: An In-Silico Study. PLoS ONE 2024, 19, e0301350. [Google Scholar] [CrossRef] [PubMed]
- Aquila, I.; Frati, G.; Sciarretta, S.; Dellegrottaglie, S.; Torella, D.; Torella, M. New imaging techniques project the cellular and molecular alterations underlying bicuspid aortic valve development. J. Mol. Cell. Cardiol. 2019, 129, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Pasipoularides, A. Clinical-pathological correlations of BAV and the attendant thoracic aortopathies. Part 2: Pluridisciplinary perspective on their genetic and molecular origins. J. Mol. Cell. Cardiol. 2019, 133, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Sticchi, E.; De Cario, R.; Magi, A.; Giglio, S.; Provenzano, A.; Nistri, S.; Pepe, G.; Giusti, B. Bicuspid aortic valve: Role of multiple gene variants in influencing the clinical phenotype. Biomed Res. Int. 2018, 2018, 8386123. [Google Scholar] [CrossRef] [PubMed]
- Antequera-González, B.; Martínez-Micaelo, N.; Alegret, J.M. Bicuspid aortic valve and endothelial dysfunction: Current evidence and potential therapeutic targets. Front. Physiol. 2020, 11, 1015. [Google Scholar] [CrossRef] [PubMed]
- Bulut, H.I.; Arjomandi Rad, A.; Syrengela, A.A.; Ttofi, I.; Djordjevic, J.; Kaur, R.; Keiralla, A.; Krasopoulos, G. A comprehensive review of management strategies for bicuspid aortic valve (BAV): Exploring epidemiology, aetiology, aortopathy, and interventions in light of recent guidelines. J. Cardiovasc. Dev. Dis. 2023, 10, 398. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).