A (Comprehensive) Review of the Application of Quantitative Structure–Activity Relationship (QSAR) in the Prediction of New Compounds with Anti-Breast Cancer Activity
Abstract
:1. Introduction
2. QSAR—A Brief Overview
2.1. Chemical Space
2.2. Activities in QSAR and QSPR
- Physicochemical end-points: these include properties such as logP, logD, pKa, logS, and water stability.
- ADMET end-points: these involve parameters like permeability through passive transport measured via PAMPA (Parallel Artificial Membrane Permeability Assay) [44], blood–brain barrier permeability (PAMPA-BBB) [45], absorption [46], drug transporters assessed through P-glycoprotein transporter assays [47], and metabolites [48].
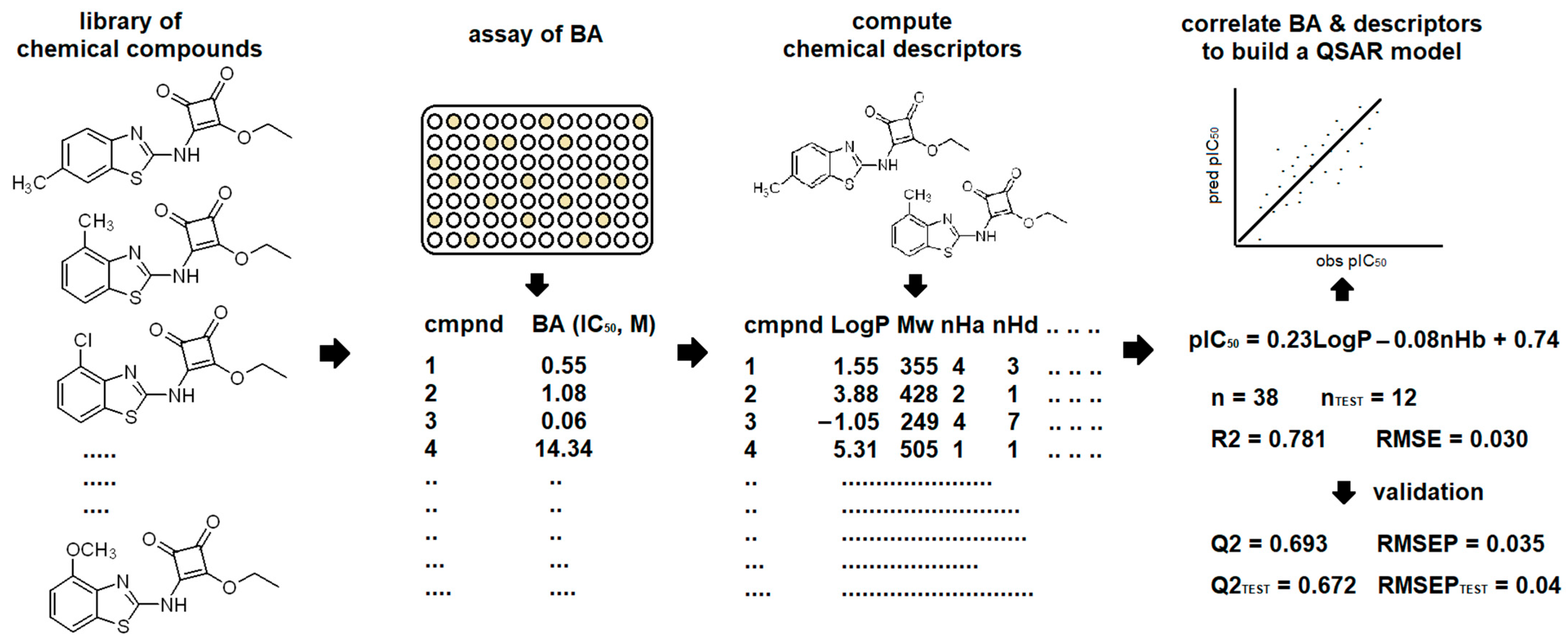
3. QSAR Today
- 1.
- Selection of Chemical Compounds: identify relevant compounds for analysis.
- 2.
- Assessment of Biological Activity: evaluate the biological activity of the selected compounds.
- 3.
- Descriptors Calculation: calculate molecular descriptors.
- 4.
- Data Matrix Setup: preprocess data into a uniform matrix.
- 5.
- Data Partitioning: set aside a portion of the data for external validation.
- 6.
- Feature Selection and Model Building: use chemometric methods to select relevant features and develop a QSAR model.
- 7.
- Model Validation: internal using the training dataset and external using the test dataset.
- 8.
- Model Interpretation and Prediction: interpret the model’s results and apply the model for predictions.
3.1. Selection of Chemical Compounds: Identify Relevant Compounds for Analysis
3.2. Assessment of Biological Activity: Evaluate the Biological Activity of the Selected Compounds
- Mechanism Consistency: chemical compounds should have the same mechanism of action and binding mode.
- Congeneric Series: compounds should belong to a congeneric series.
- Correlation with Binding Affinity: biological activity should correlate with binding affinity and be measurable.
- Uniform Data Acquisition: Biological data should be obtained using consistent protocols, preferably from a single source (cells, tissues, or organs) and a single laboratory. Inter-assay and inter-laboratory variations can be managed by using assay and laboratory descriptors.
- Standardized Units: activity data should be measured using the same units (e.g., Ki, IC50, or binding) expressed in mol/L.
- Sufficient Activity Range: the activity range should cover more than three logarithmic units with an even data distribution.
3.3. Descriptors Calculation: Calculate Molecular Descriptors
3.4. Data Matrix Setup: Preprocessing Data into a Uniform Matrix
3.5. Data Partitioning: Set Aside a Portion of the Data for External Validation
3.6. Feature Selection and Model Building: Use Chemometric Methods to Select Relevant Features and Develop a QSAR Model
3.7. Model Validation: Internal Using the Training Dataset and External Using the Test Sataset
- The QSAR model should be associated with a defined endpoint.
- An unambiguous algorithm.
- A defined domain of application.
- Appropriate measures for goodness-of-fit, robustness, and predictivity.
- Mechanistic interpretation, if possible.
- The Square of the Correlation Coefficient (R2): R2 measures the strength of the linear relationship between predicted and experimental activity values (y). It is calculated using the following equation:
- Predictive Squared Correlation Coefficient (Q2): Q2, also known as the cross-validation correlation coefficient when LGO (Q2LGO) or LOO (Q2LOO) cross-validation is applied, is calculated as follows:
- Root Mean Squared Error (RMSE): RMSE measures the standard deviation of residuals, indicating model accuracy:
- Root Mean Squared Error of Prediction (RMSEP): RMSEP has the same meaning as RMSE but corresponds to Q2. It is calculated as follows:
- Q2test (or Q2ext): calculated using the same equation as Q2 (Equation (6)) but applied to the test dataset.
- RMSEPtest: Similarly to RMSEP (Equation (8)) but applied to the test dataset.
3.8. Model Interpretation and Prediction: Interpret the Model Results and Apply the Model for Predictions
3.9. Applicability Domain and Methods for Its Identification
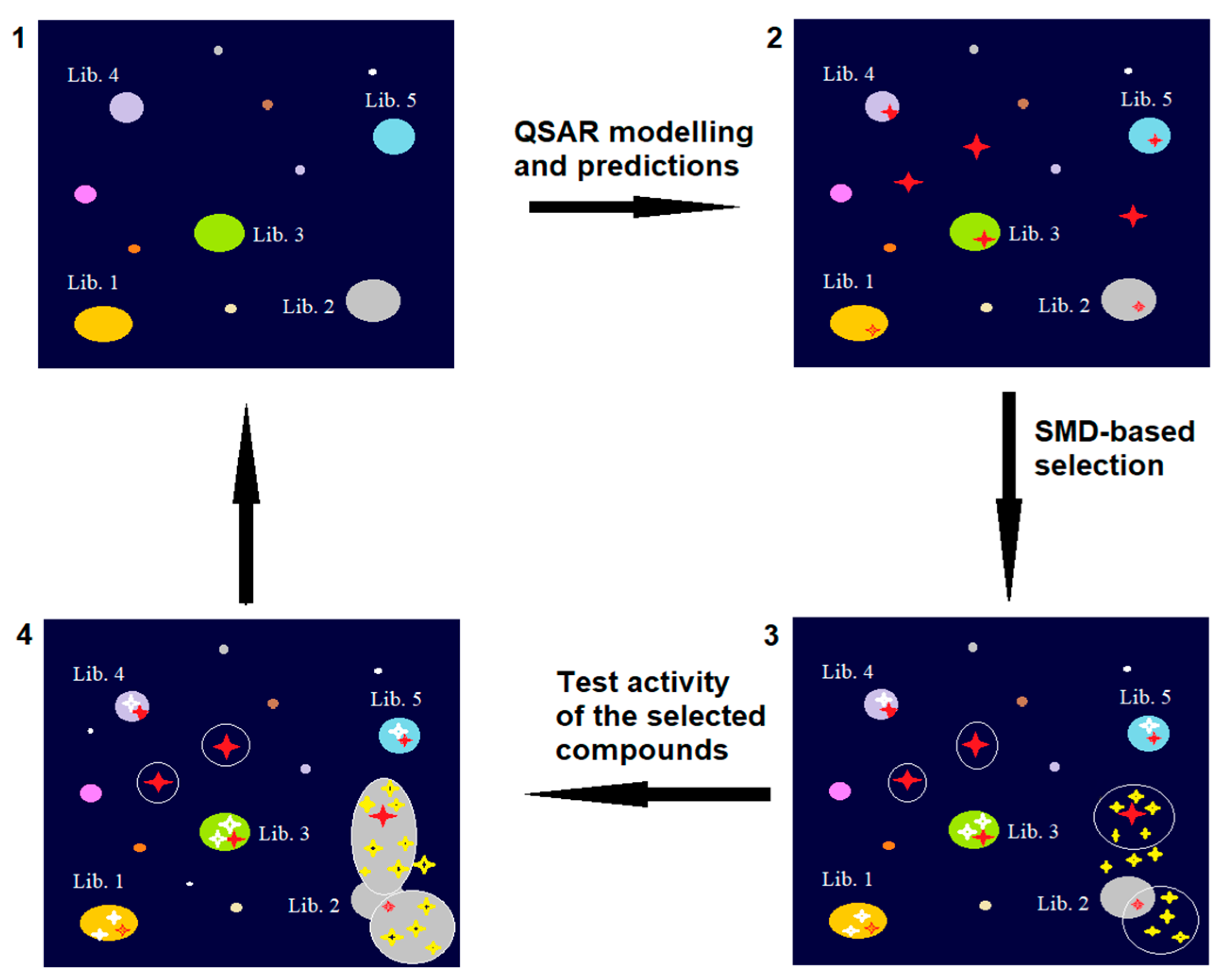
4. Lead Identification and Optimization
5. Breast Cancer and Current Treatment Strategies
6. Case Studies of QSAR Modeling of Anti-Breast Cancer Agents
7. QSAR: Benefits, Challenges, and Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | applicability domain |
| ADMET | Absorption, Distribution, Metabolism, Excretion and Toxicity |
| ANNs | Artificial Neural Networks |
| AI | Artificial Intelligence |
| AIs | aromatase inhibitors |
| BC | breast cancer |
| BFA | Brefeldin A |
| Caco-2 | Cancer coli, “colon cancer” |
| CADD | computer-aided drug design |
| CoMFA | Comparative Molecular Field Analysis |
| CoMSIA | Comparative Molecular Similarity Indices Analysis |
| CoMBINE | Comparative Binding Energy Analysis |
| CoMMA | Comparative Molecular Moment Analysis |
| CoRIA | Comparative Residue Interaction Analysis |
| CoSA | Comparative Spectral Analysis |
| DHFR | Dihydrofolate reductase |
| DL | Deep Learning |
| DNA | Deoxyribonucleic acid |
| DNMTs | DNA methyltransferases |
| DTs | Decision Trees |
| ER | estrogen receptor |
| EGFR | epidermal growth factor receptor also known as ErbB |
| EGFR-TKI | EGFR tyrosine kinase inhibitors |
| FDA | Food and Drug Administration |
| HDACs | histone deacetylases |
| HDAC6 | histone deacetylase 6 |
| HER2 | human epidermal growth factor receptor 2 |
| HIFA | Hint Interaction Field Analysis |
| HMTs | histone methyltransferases |
| Holo-QSAR | Hologram Quantitative Structure–Activity Relationship |
| HQSAR | Hologram QSAR |
| HOMO | Highest Occupied Molecular Orbital |
| HTS | high-throughput screening |
| HTVS | high-throughput virtual screening |
| JAK2 | Janus kinase 2 |
| k-NN | k-nearest neighbor |
| LAP | Leucine aminopeptidase |
| LBDD | ligand-based drug design |
| LUMO | Lowest Unoccupied Molecular Orbital |
| LOO | leave-one-out |
| LGO | leave-group-out |
| LSD1/LSD2 | histone lysine-specific demethylase |
| MAO | Monoamine oxidases |
| ML | machine learning |
| MLR | Multiple Linear Regression |
| MTAs | microtubule-targeting agents |
| NCEs | new chemical entities |
| NCI | National Cancer Institute |
| NSCLC | Non-small cell lung cancer |
| OECD | the Organization for Economic Co-operation and Development |
| PAMPA | Parallel Artificial Membrane Permeability Assay |
| PAMPA-BBB | Parallel Artificial Membrane Permeability Assay across the Blood–Brain Barrier |
| PCA | Principal Component Analysis |
| PCM | Proteochemometrics |
| PCR | Principal Component Regression |
| PDB | Protein Data Bank |
| PKIs | Protein kinase inhibitors |
| PLS | Partial Least Squares (Regression) |
| PPs | principal properties |
| PR | Progesterone Receptor |
| PI3K/AKT | Phosphatidylinositol 3-kinase and Akt (protein kinase B) |
| QSARs | Quantitative Structure–Activity Relationships |
| RMSE | Root Mean Squared Error |
| RMSEP | Root Mean Squared Error of Prediction |
| ROCKs | Rho-associated coiled-coil-containing protein kinases |
| SBDD | structure-based drug design |
| SERMs | selective estrogen receptor modulators |
| SMD | Statistical Molecular Design |
| SSRs | sum of squares of the residuals |
| SS | total sum of squares |
| SVMs | Support Vector Machines |
| TOPO I/II | topoisomerase inhibitor 1/2 |
| TNBC | Triple-Negative Breast Cancer |
| VERFG | Receptors for vascular endothelial growth factor |
| WHO | World Health Organization |
References
- Fischer, E. Einfluss Der Configuration Auf Die Wirkung Der Enzyme. Berichte Dtsch. Chem. Ges. 1894, 27, 2985–2993. [Google Scholar] [CrossRef]
- Koshland, D.E. Application of a Theory of Enzyme Specificity to Protein Synthesis. Proc. Natl. Acad. Sci. USA 1958, 44, 98–104. [Google Scholar] [CrossRef]
- Ramanathan, A.; Savol, A.; Burger, V.; Chennubhotla, C.S.; Agarwal, P.K. Protein Conformational Populations and Functionally Relevant Substates. Acc. Chem. Res. 2014, 47, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Kar, G.; Keskin, O.; Gursoy, A.; Nussinov, R. Allostery and Population Shift in Drug Discovery. Curr. Opin. Pharmacol. 2010, 10, 715–722. [Google Scholar] [CrossRef]
- Kenakin, T. Principles: Receptor Theory in Pharmacology. Trends Pharmacol. Sci. 2004, 25, 186–192. [Google Scholar] [CrossRef]
- Belorkar, S.A.; Jogaiah, S. Enzymes—Past, Present, and Future. In Protocols and Applications in Enzymology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–15. [Google Scholar]
- Bardal, S.K.; Waechter, J.E.; Martin, D.S. Basic Principles and Pharmacodynamics. In Applied Pharmacology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 3–16. [Google Scholar]
- Mannhold, R.; Kubinyi, H.; Folkers, G. Protein-Ligand Interactions; Böhm, H.-J., Schneider, G., Eds.; Methods and Principles in Medicinal Chemistry; Wiley: Hoboken, NJ, USA, 2003; ISBN 9783527305216. [Google Scholar]
- Seidel, T.; Schuetz, D.A.; Garon, A.; Langer, T. The Pharmacophore Concept and Its Applications in Computer-Aided Drug Design. In Progress in the Chemistry of Organic Natural Products 110: Cheminformatics in Natural Product Research; Springer: Berlin/Heidelberg, Germany, 2019; pp. 99–141. [Google Scholar]
- Tripathi, N.M.; Bandyopadhyay, A. High Throughput Virtual Screening (HTVS) of Peptide Library: Technological Advancement in Ligand Discovery. Eur. J. Med. Chem. 2022, 243, 114766. [Google Scholar] [CrossRef] [PubMed]
- Rester, U. From Virtuality to Reality–Virtual Screening in Lead Discovery and Lead Optimization: A Medicinal Chemistry Perspective. Curr. Opin. Drug Discov. Devel. 2008, 11, 559–568. [Google Scholar] [PubMed]
- Finan, C.; Gaulton, A.; Kruger, F.A.; Lumbers, R.T.; Shah, T.; Engmann, J.; Galver, L.; Kelley, R.; Karlsson, A.; Santos, R.; et al. The Druggable Genome and Support for Target Identification and Validation in Drug Development. Sci. Transl. Med. 2017, 9, eaag1166. [Google Scholar] [CrossRef]
- Oprea, T.I.; Hasselgren, C. Predicting Target and Chemical Druggability. In Comprehensive Medicinal Chemistry III.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 429–439. [Google Scholar]
- Carvalho, A.L.; Trincão, J.; Romão, M.J. X-Ray Crystallography in Drug Discovery. In Ligand-Macromolecular Interactions in Drug Discovery: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2010; pp. 31–56. [Google Scholar]
- Emwas, A.-H.; Szczepski, K.; Poulson, B.G.; Chandra, K.; McKay, R.T.; Dhahri, M.; Alahmari, F.; Jaremko, L.; Lachowicz, J.I.; Jaremko, M. NMR as a “Gold Standard” Method in Drug Design and Discovery. Molecules 2020, 25, 4597. [Google Scholar] [CrossRef] [PubMed]
- Nogales, E.; Mahamid, J. Bridging Structural and Cell Biology with Cryo-Electron Microscopy. Nature 2024, 628, 47–56. [Google Scholar] [CrossRef]
- Appasani, K. Cryo-Electron Microscopy in Structural Biology; CRC Press: Boca Raton, FL, USA, 2024; ISBN 9781003326106. [Google Scholar]
- Wishart, D.S. Identifying Putative Drug Targets and Potential Drug Leads. In Molecular Modeling of Proteins; Springer: Berlin/Heidelberg, Germany, 2008; pp. 333–351. [Google Scholar]
- Muhammed, M.T.; Aki-Yalcin, E. Pharmacophore Modeling in Drug Discovery: Methodology and Current Status. J. Turk. Chem. Soc. Sect. A Chem. 2021, 8, 749–762. [Google Scholar] [CrossRef]
- Lee, J.Y.; Krieger, J.M.; Li, H.; Bahar, I. Pharmmaker: Pharmacophore Modeling and Hit Identification Based on Druggability Simulations. Protein Sci. 2020, 29, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D.; Biancaniello, C.; Argenio, M.A.; Facchiano, A. Drug Design by Pharmacophore and Virtual Screening Approach. Pharmaceuticals 2022, 15, 646. [Google Scholar] [CrossRef]
- López-Pérez, K.; Avellaneda-Tamayo, J.F.; Chen, L.; López-López, E.; Juárez-Mercado, K.E.; Medina-Franco, J.L.; Miranda-Quintana, R.A. Molecular Similarity: Theory, Applications, and Perspectives. Artif. Intell. Chem. 2024, 2, 100077. [Google Scholar] [CrossRef]
- Stumpfe, D.; Bajorath, J. Similarity Searching. WIREs Comput. Mol. Sci. 2011, 1, 260–282. [Google Scholar] [CrossRef]
- Yu, W.; MacKerell, A.D. Computer-Aided Drug Design Methods. In Antibiotics: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2017; pp. 85–106. [Google Scholar]
- Shim, J.; MacKerell, A.D., Jr. Computational Ligand-Based Rational Design: Role of Conformational Sampling and Force Fields in Model Development. Medchemcomm 2011, 2, 356. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y. Pharmacophore Modeling and Applications in Drug Discovery: Challenges and Recent Advances. Drug Discov. Today 2010, 15, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H. Zur Theorie Der Alkoholnarkose. Arch. Für Exp. Pathol. Pharmakol. 1899, 42, 109–118. [Google Scholar] [CrossRef]
- Meyer, H. Zur Theorie Der Alkoholnarkose. Arch. Für Exp. Pathol. Pharmakol. 1901, 46, 338–346. [Google Scholar] [CrossRef]
- Meyer, K.H. Contributions to the Theory of Narcosis. Trans. Faraday Soc. 1937, 33, 1062. [Google Scholar] [CrossRef]
- Overton, C.E. Studien Über Die Narkose Zugleich Ein Beitrag Zur Allgemeinen Pharmakologie; Fischer: Jena, Germany, 1901. [Google Scholar]
- Hammett, L.P. The Effect of Structure upon the Reactions of Organic Compounds. Benzene Derivatives. J. Am. Chem. Soc. 1937, 59, 96–103. [Google Scholar] [CrossRef]
- Hansch, C.; Fujita, T. P-σ-π Analysis. A Method for the Correlation of Biological Activity and Chemical Structure. J. Am. Chem. Soc. 1964, 86, 1616–1626. [Google Scholar] [CrossRef]
- Fujita, T.; Iwasa, J.; Hansch, C. A New Substituent Constant, π, Derived from Partition Coefficients. J. Am. Chem. Soc. 1964, 86, 5175–5180. [Google Scholar] [CrossRef]
- Free, S.M.; Wilson, J.W. A Mathematical Contribution to Structure-Activity Studies. J. Med. Chem. 1964, 7, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.; Gleeson, M.P. The Importance of the Domain of Applicability in QSAR Modeling. J. Mol. Graph. Model. 2008, 26, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Drews, J. Drug Discovery: A Historical Perspective. Science 2000, 287, 1960–1964. [Google Scholar] [CrossRef]
- Franco, L.S.; de Jesus, B.d.S.M.; Pinheiro, P.d.S.M.; Fraga, C.A.M. Remapping the Chemical Space and the Pharmacological Space of Drugs: What Can We Expect from the Road Ahead? Pharmaceuticals 2024, 17, 742. [Google Scholar] [CrossRef] [PubMed]
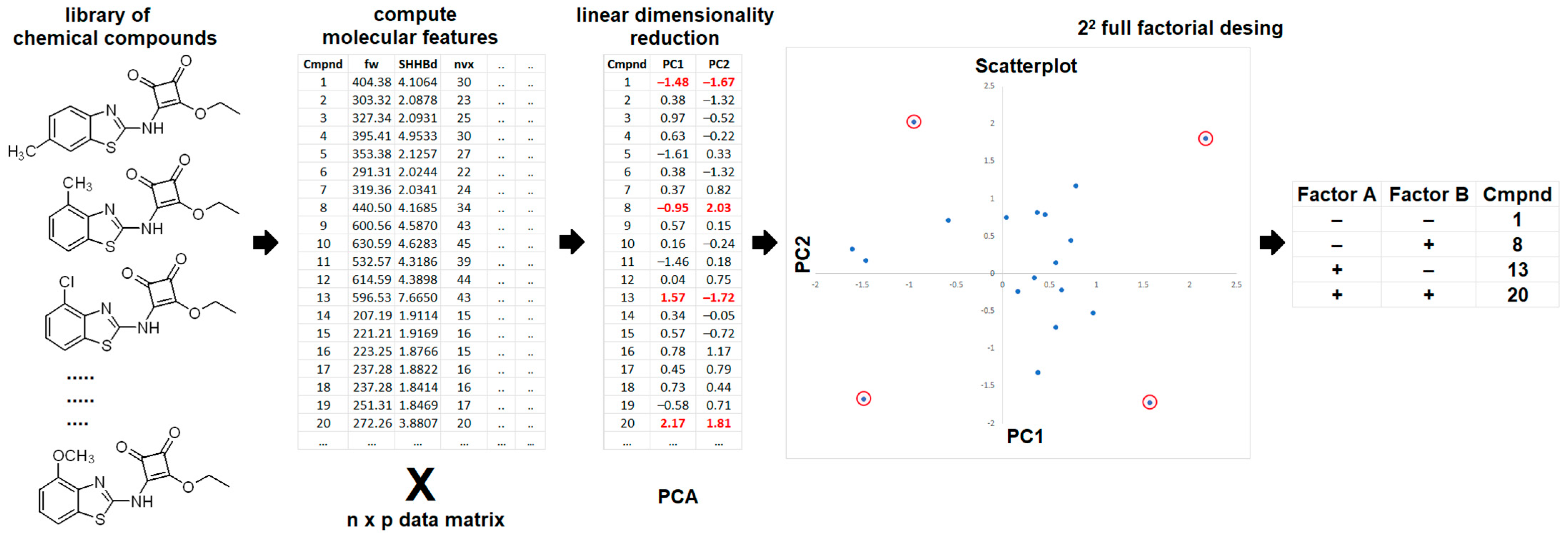
- Linusson, A.; Elofsson, M.; Andersson, I.E.; Dahlgren, M.K. Statistical Molecular Design of Balanced Compound Libraries for QSAR Modeling. Curr. Med. Chem. 2010, 17, 2001–2016. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Salem, N.; Hussein, S. Data Dimensional Reduction and Principal Components Analysis. Procedia Comput. Sci. 2019, 163, 292–299. [Google Scholar] [CrossRef]
- Ramachandran, K.M.; Tsokos, C.P. Design of Experiments. In Mathematical Statistics with Applications in R.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 459–494. [Google Scholar]
- Lundstedt, T.; Seifert, E.; Abramo, L.; Thelin, B.; Nyström, Å.; Pettersen, J.; Bergman, R. Experimental Design and Optimization. Chemom. Intell. Lab. Syst. 1998, 42, 3–40. [Google Scholar] [CrossRef]
- Linusson, A.; Gottfries, J.; Lindgren, F.; Wold, S. Statistical Molecular Design of Building Blocks for Combinatorial Chemistry. J. Med. Chem. 2000, 43, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhiti, A.; Barba-Bon, A.; Hennig, A.; Nau, W.M. Real-Time Parallel Artificial Membrane Permeability Assay Based on Supramolecular Fluorescent Artificial Receptors. Front. Chem. 2020, 8, 597927. [Google Scholar] [CrossRef]
- Mensch, J.; Melis, A.; Mackie, C.; Verreck, G.; Brewster, M.E.; Augustijns, P. Evaluation of Various PAMPA Models to Identify the Most Discriminating Method for the Prediction of BBB Permeability. Eur. J. Pharm. Biopharm. 2010, 74, 495–502. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Li, Y. Caco-2 Cell Permeability Assays to Measure Drug Absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef]
- Li, N.; Kulkarni, P.; Badrinarayanan, A.; Kefelegn, A.; Manoukian, R.; Li, X.; Prasad, B.; Karasu, M.; McCarty, W.J.; Knutson, C.G.; et al. P-Glycoprotein Substrate Assessment in Drug Discovery: Application of Modeling to Bridge Differential Protein Expression Across In Vitro Tools. J. Pharm. Sci. 2021, 110, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Ladumor, M.K.; Tiwari, S.; Patil, A.; Bhavsar, K.; Jhajra, S.; Prasad, B.; Singh, S. High-Resolution Mass Spectrometry in Metabolite Identification. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; pp. 199–229. [Google Scholar]
- Niles, A.L.; Moravec, R.A.; Riss, T.L. Update on in Vitro Cytotoxicity Assays for Drug Development. Expert Opin. Drug Discov. 2008, 3, 655–669. [Google Scholar] [CrossRef]
- van der Laan, J.-W.; Spindler, P. The in Vivo Rodent Test Systems for Assessment of Carcinogenic Potential. Regul. Toxicol. Pharmacol. 2002, 35, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Guy, R.C. Ames Test. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 377–379. [Google Scholar]
- Ishidate, M.; Miura, K.F.; Sofuni, T. Chromosome Aberration Assays in Genetic Toxicology Testing in Vitro. Mutat. Res. Mol. Mech. Mutagen. 1998, 404, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Nettleton, D.O.; Einolf, H.J. Assessment of Cytochrome P450 Enzyme Inhibition and Inactivation in Drug Discovery and Development. Curr. Top. Med. Chem. 2011, 11, 382–403. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H. CYP Induction-Mediated Drug Interactions: In Vitro Assessment and Clinical Implications. Pharm. Res. 2006, 23, 1089–1116. [Google Scholar] [CrossRef]
- Sugimoto, H.; Matsumoto, S.-I.; Tachibana, M.; Niwa, S.-I.; Hirabayashi, H.; Amano, N.; Moriwaki, T. Establishment of In Vitro P-Glycoprotein Inhibition Assay and Its Exclusion Criteria to Assess the Risk Of Drug–Drug Interaction at the Drug Discovery Stage. J. Pharm. Sci. 2011, 100, 4013–4023. [Google Scholar] [CrossRef] [PubMed]
- Lanevskij, K.; Didziapetris, R. Physicochemical QSAR Analysis of Passive Permeability Across Caco-2 Monolayers. J. Pharm. Sci. 2019, 108, 78–86. [Google Scholar] [CrossRef]
- Furuhama, A.; Kitazawa, A.; Yao, J.; Matos dos Santos, C.E.; Rathman, J.; Yang, C.; Ribeiro, J.V.; Cross, K.; Myatt, G.; Raitano, G.; et al. Evaluation of QSAR Models for Predicting Mutagenicity: Outcome of the Second Ames/QSAR International Challenge Project. SAR QSAR Environ. Res. 2023, 34, 983–1001. [Google Scholar] [CrossRef]
- Sun, L.; Yang, H.; Li, J.; Wang, T.; Li, W.; Liu, G.; Tang, Y. In Silico Prediction of Compounds Binding to Human Plasma Proteins by QSAR Models. ChemMedChem 2018, 13, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, C.I.; Benfenati, E.; Cester, J. Evaluation of QSAR Models for Predicting the Partition Coefficient (LogP) of Chemicals under the REACH Regulation. Environ. Res. 2015, 143, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, K.; Cariello, N.F.; Korotcov, A.; Tkachenko, V.; Grulke, C.M.; Sprankle, C.S.; Allen, D.; Casey, W.M.; Kleinstreuer, N.C.; Williams, A.J. Open-Source QSAR Models for PKa Prediction Using Multiple Machine Learning Approaches. J. Cheminform. 2019, 11, 60. [Google Scholar] [CrossRef]
- Gozalbes, R.; Pineda-Lucena, A. QSAR-Based Solubility Model for Drug-like Compounds. Bioorg. Med. Chem. 2010, 18, 7078–7084. [Google Scholar] [CrossRef]
- Srimathi, R.; Kathiravan, M. Lead Optimization of 4-(Thio)-Chromenone 6-O-Sulfamate Analogs Using QSAR, Molecular Docking and DFT—A Combined Approach as Steroidal Sulfatase Inhibitors. J. Recept. Signal Transduct. 2021, 41, 123–137. [Google Scholar] [CrossRef]
- Arian, R.; Hariri, A.; Mehridehnavi, A.; Fassihi, A.; Ghasemi, F. Protein Kinase Inhibitors’ Classification Using K-Nearest Neighbor Algorithm. Comput. Biol. Chem. 2020, 86, 107269. [Google Scholar] [CrossRef] [PubMed]
- Myshkin, E.; Brennan, R.; Khasanova, T.; Sitnik, T.; Serebriyskaya, T.; Litvinova, E.; Guryanov, A.; Nikolsky, Y.; Nikolskaya, T.; Bureeva, S. Prediction of Organ Toxicity Endpoints by QSAR Modeling Based on Precise Chemical-Histopathology Annotations. Chem. Biol. Drug Des. 2012, 80, 406–416. [Google Scholar] [CrossRef]
- Lowe, C.N.; Charest, N.; Ramsland, C.; Chang, D.T.; Martin, T.M.; Williams, A.J. Transparency in Modeling through Careful Application of OECD’s QSAR/QSPR Principles via a Curated Water Solubility Data Set. Chem. Res. Toxicol. 2023, 36, 465–478. [Google Scholar] [CrossRef]
- Tropsha, A. Best Practices for QSAR Model Development, Validation, and Exploitation. Mol. Inform. 2010, 29, 476–488. [Google Scholar] [CrossRef]
- Bassani, D.; Moro, S. Past, Present, and Future Perspectives on Computer-Aided Drug Design Methodologies. Molecules 2023, 28, 3906. [Google Scholar] [CrossRef]
- Golbraikh, A.; Wang, X.S.; Zhu, H.; Tropsha, A. Predictive QSAR Modeling: Methods and Applications in Drug Discovery and Chemical Risk Assessment. In Handbook of Computational Chemistry; Springer International Publishing: Cham, Switzerland, 2017; pp. 2303–2340. [Google Scholar]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 24 December 2024).
- ChemSpider. Available online: https://www.chemspider.com (accessed on 24 December 2024).
- DrugBank. Available online: https://go.drugbank.com (accessed on 24 December 2024).
- ChEMBL. Available online: https://www.ebi.ac.uk/chembl (accessed on 24 December 2024).
- NCI60. Available online: https://dtp.cancer.gov/discovery_development/nci-60/ (accessed on 24 December 2024).
- STITCH. Available online: http://stitch.embl.de/ (accessed on 18 January 2025).
- BioAssay. Available online: https://www.ncbi.nlm.nih.gov/guide/chemicals-bioassays/ (accessed on 24 December 2024).
- Andersson, C.D.; Hillgren, J.M.; Lindgren, C.; Qian, W.; Akfur, C.; Berg, L.; Ekström, F.; Linusson, A. Benefits of Statistical Molecular Design, Covariance Analysis, and Reference Models in QSAR: A Case Study on Acetylcholinesterase. J. Comput. Aided. Mol. Des. 2015, 29, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Brereton, R.G. Orthogonality, Uncorrelatedness, and Linear Independence of Vectors. J. Chemom. 2016, 30, 564–566. [Google Scholar] [CrossRef]
- Gómez-Jiménez, G.; Gonzalez-Ponce, K.; Castillo-Pazos, D.J.; Madariaga-Mazon, A.; Barroso-Flores, J.; Cortes-Guzman, F.; Martinez-Mayorga, K. The OECD Principles for (Q)SAR Models in the Context of Knowledge Discovery in Databases (KDD). In Advances in Protein Chemistry and Structural Biology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 85–117. [Google Scholar]
- Burge, S.; Attwood, T.K.; Bateman, A.; Berardini, T.Z.; Cherry, M.; O’Donovan, C.; Xenarios, L.; Gaudet, P. Biocurators and Biocuration: Surveying the 21st Century Challenges. Database 2012, 2012, bar059. [Google Scholar] [CrossRef] [PubMed]
- Todeschini, R.; Consonni, V. Handbook of Molecular Descriptors; Methods and Principles in Medicinal Chemistry; Wiley: Hoboken, NJ, USA, 2000; ISBN 9783527299133. [Google Scholar]
- ACD/Labs. Available online: www.acdlabs.com (accessed on 24 December 2024).
- DRAGON. Available online: https://www.talete.mi.it/products/dragon_description.htm (accessed on 24 December 2024).
- E-DRAGON. Available online: https://vcclab.org/lab/edragon/ (accessed on 24 December 2024).
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual Computational Chemistry Laboratory—Design and Description. J. Comput. Aided. Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef]
- CDK. Available online: https://cdk.github.io/ (accessed on 24 December 2024).
- CODESSA. Available online: https://www.codessa-pro.com/index.htm (accessed on 24 December 2024).
- Chemical Computing Group Inc Molecular Operating Environment (MOE). 2016. Available online: https://www.chemcomp.com/en/Products.htm (accessed on 24 December 2024).
- MOE. Available online: www.chemcomp.com (accessed on 24 December 2024).
- MOLD2. Available online: https://www.fda.gov/science-research/bioinformatics-tools/mold2 (accessed on 24 December 2024).
- Hong, H.; Xie, Q.; Ge, W.; Qian, F.; Fang, H.; Shi, L.; Su, Z.; Perkins, R.; Tong, W. Mold 2, Molecular Descriptors from 2D Structures for Chemoinformatics and Toxicoinformatics. J. Chem. Inf. Model. 2008, 48, 1337–1344. [Google Scholar] [CrossRef]
- PowerMV. Available online: https://www.niss.org/research/software/powermv (accessed on 24 December 2024).
- PreADMET. Available online: https://preadmet.webservice.bmdrc.org/ (accessed on 24 December 2024).
- Sylvester, J.J. Chemistry and Algebra. Nature 1878, 17, 284. [Google Scholar] [CrossRef]
- Randic, M. Characterization of Molecular Branching. J. Am. Chem. Soc. 1975, 97, 6609–6615. [Google Scholar] [CrossRef]
- Randić, M. The Connectivity Index 25 Years After. J. Mol. Graph. Model. 2001, 20, 19–35. [Google Scholar] [CrossRef]
- Kier, L.B.; Hall, L.H. Molecular Connectivity VII: Specific Treatment of Heteroatoms. J. Pharm. Sci. 1976, 65, 1806–1809. [Google Scholar] [CrossRef]
- Wiener, H. Structural Determination of Paraffin Boiling Points. J. Am. Chem. Soc. 1947, 69, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Bonche, D.; Trinajstič, N. Overall Molecular Descriptors. 3. Overall Zagreb Indices. SAR QSAR Environ. Res. 2001, 12, 213–236. [Google Scholar] [CrossRef]
- Balaban, A.T. Highly Discriminating Distance-Based Topological Index. Chem. Phys. Lett. 1982, 89, 399–404. [Google Scholar] [CrossRef]
- Kier, L.B. A Shape Index from Molecular Graphs. Quant. Struct. Relatsh. 1985, 4, 109–116. [Google Scholar] [CrossRef]
- Cramer, R.D.; Patterson, D.E.; Bunce, J.D. Comparative Molecular Field Analysis (CoMFA). 1. Effect of Shape on Binding of Steroids to Carrier Proteins. J. Am. Chem. Soc. 1988, 110, 5959–5967. [Google Scholar] [CrossRef] [PubMed]
- Klebe, G.; Abraham, U.; Mietzner, T. Molecular Similarity Indices in a Comparative Analysis (CoMSIA) of Drug Molecules to Correlate and Predict Their Biological Activity. J. Med. Chem. 1994, 37, 4130–4146. [Google Scholar] [CrossRef]
- Ortiz, A.R.; Pisabarro, M.T.; Gago, F.; Wade, R.C. Prediction of Drug Binding Affinities by Comparative Binding Energy Analysis. J. Med. Chem. 1995, 38, 2681–2691. [Google Scholar] [CrossRef] [PubMed]
- Datar, P.A.; Khedkar, S.A.; Malde, A.K.; Coutinho, E.C. Comparative Residue Interaction Analysis (CoRIA): A 3D-QSAR Approach to Explore the Binding Contributions of Active Site Residues with Ligands. J. Comput. Aided. Mol. Des. 2006, 20, 343–360. [Google Scholar] [CrossRef]
- Kellogg, G.E.; Semus, S.F.; Abraham, D.J. HINT: A New Method of Empirical Hydrophobic Field Calculation for CoMFA. J. Comput. Aided. Mol. Des. 1991, 5, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Silverman, B.D.; Platt, D.E. Comparative Molecular Moment Analysis (CoMMA): 3D-QSAR without Molecular Superposition. J. Med. Chem. 1996, 39, 2129–2140. [Google Scholar] [CrossRef] [PubMed]
- Bursi, R.; Dao, T.; van Wijk, T.; de Gooyer, M.; Kellenbach, E.; Verwer, P. Comparative Spectra Analysis (CoSA): Spectra as Three-Dimensional Molecular Descriptors for the Prediction of Biological Activities. J. Chem. Inf. Comput. Sci. 1999, 39, 861–867. [Google Scholar] [CrossRef] [PubMed]
- David, R.L. HQSAR: A New, Highly Predictive QSAR Technique. Available online: https://pdfs.semanticscholar.org/efb9/de3a7d30dc445dfc0904da4ff225237be50c.pdf (accessed on 24 December 2024).
- Cartier, A.; Rivail, J.-L. Electronic Descriptors in Quantitative Structure—Activity Relationships. Chemom. Intell. Lab. Syst. 1987, 1, 335–347. [Google Scholar] [CrossRef]
- Wang, L.; Ding, J.; Pan, L.; Cao, D.; Jiang, H.; Ding, X. Quantum Chemical Descriptors in Quantitative Structure–Activity Relationship Models and Their Applications. Chemom. Intell. Lab. Syst. 2021, 217, 104384. [Google Scholar] [CrossRef]
- Danishuddin; Khan, A.U. Descriptors and Their Selection Methods in QSAR Analysis: Paradigm for Drug Design. Drug Discov. Today 2016, 21, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Roy, K. Topological Descriptors in Drug Design and Modeling Studies. Mol. Divers. 2004, 8, 321–323. [Google Scholar] [CrossRef]
- Mapari, S.; Camarda, K. V Use of Three-Dimensional Descriptors in Molecular Design for Biologically Active Compounds. Curr. Opin. Chem. Eng. 2020, 27, 60–64. [Google Scholar] [CrossRef]
- van Speybroeck, V.; Gani, R.; Meier, R.J. The Calculation of Thermodynamic Properties of Molecules. Chem. Soc. Rev. 2010, 39, 1764. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Coquery, J.; Lê Cao, K.-A. Independent Principal Component Analysis for Biologically Meaningful Dimension Reduction of Large Biological Data Sets. BMC Bioinform. 2012, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Andrada, M.F.; Vega-Hissi, E.G.; Estrada, M.R.; Garro Martinez, J.C. Application of K-Means Clustering, Linear Discriminant Analysis and Multivariate Linear Regression for the Development of a Predictive QSAR Model on 5-Lipoxygenase Inhibitors. Chemom. Intell. Lab. Syst. 2015, 143, 122–129. [Google Scholar] [CrossRef]
- Ikotun, A.M.; Ezugwu, A.E.; Abualigah, L.; Abuhaija, B.; Heming, J. K-Means Clustering Algorithms: A Comprehensive Review, Variants Analysis, and Advances in the Era of Big Data. Inf. Sci. 2023, 622, 178–210. [Google Scholar] [CrossRef]
- Andrada, M.F.; Vega-Hissi, E.G.; Estrada, M.R.; Garro Martinez, J.C. Impact Assessment of the Rational Selection of Training and Test Sets on the Predictive Ability of QSAR Models. SAR QSAR Environ. Res. 2017, 28, 1011–1023. [Google Scholar] [CrossRef]
- Kohonen, T. The Self-Organizing Map. Proc. IEEE 1990, 78, 1464–1480. [Google Scholar] [CrossRef]
- Kohonen, T. Exploration of Very Large Databases by Self-Organizing Maps. In Proceedings of the Proceedings of International Conference on Neural Networks (ICNN’97), Houston, TX, USA, 12 June 1997; IEEE: Piscataway, NJ, USA; Volume 1, pp. PL1–PL6. [Google Scholar]
- Krzywinski, M.; Altman, N. Multiple Linear Regression. Nat. Methods 2015, 12, 1103–1104. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-Regression: A Basic Tool of Chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Abdi, H. Partial Least Squares Regression and Projection on Latent Structure Regression (PLS Regression). WIREs Comput. Stat. 2010, 2, 97–106. [Google Scholar] [CrossRef]
- Jolliffe, I.T. A Note on the Use of Principal Components in Regression. Appl. Stat. 1982, 31, 300. [Google Scholar] [CrossRef]
- Nielsen, F. Hierarchical Clustering. In Introduction to HPC with MPI for Data Science; Springer: Berlin/Heidelberg, Germany, 2016; pp. 195–211. [Google Scholar]
- Qu, L.; Pei, Y. A Comprehensive Review on Discriminant Analysis for Addressing Challenges of Class-Level Limitations, Small Sample Size, and Robustness. Processes 2024, 12, 1382. [Google Scholar] [CrossRef]
- Lachenbruch, P.A. Discriminant Analysis. In Encyclopedia of Statistical Sciences; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Bewick, V.; Cheek, L.; Ball, J. Statistics Review 14: Logistic Regression. Crit. Care 2005, 9, 112. [Google Scholar] [CrossRef]
- Allenbrand, C. Supervised and Unsupervised Learning Models for Pharmaceutical Drug Rating and Classification Using Consumer Generated Reviews. Healthc. Anal. 2024, 5, 100288. [Google Scholar] [CrossRef]
- Kaneko, H. Clustering Method for the Construction of Machine Learning Model with High Predictive Ability. Chemom. Intell. Lab. Syst. 2024, 246, 105084. [Google Scholar] [CrossRef]
- Ma, C.Y.; Buontempo, F.V.; Wang, X.Z. Inductive Data Mining: Automatic Generation of Decision Trees from Data for QSAR Modelling and Process Historical Data Analysis. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2008; pp. 581–586. [Google Scholar]
- Kingsford, C.; Salzberg, S.L. What Are Decision Trees? Nat. Biotechnol. 2008, 26, 1011–1013. [Google Scholar] [CrossRef]
- Hecht-Nielsen, R. Counterpropagation Networks. Appl. Opt. 1987, 26, 4979. [Google Scholar] [CrossRef]
- Mitchell, M. An Introduction to Genetic Algorithms; The MIT Press: Cambridge, MA, USA, 1996; ISBN 9780262280013. [Google Scholar]
- Baskin, I.I.; Ait, A.O.; Halberstam, N.M.; Palyulin, V.A.; Zefirov, N.S. An Approach to the Interpretation of Backpropagation Neural Network Models in QSAR Studies. SAR QSAR Environ. Res. 2002, 13, 35–41. [Google Scholar] [CrossRef]
- Cover, T.; Hart, P. Nearest Neighbor Pattern Classification. IEEE Trans. Inf. Theory 1967, 13, 21–27. [Google Scholar] [CrossRef]
- Kohonen, T. An Introduction to Neural Computing. Neural Netw. 1988, 1, 3–16. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-Vector Networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Vapnik, V.N. The Support Vector Method. In International Conference on Artificial Neural Networks; Springer: Berlin/Heidelberg, Germany, 1997; pp. 261–271. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost. In Proceedings of the Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; ACM: New York, NY, USA, 2016; pp. 785–794. [Google Scholar]
- Blanco-González, A.; Cabezón, A.; Seco-González, A.; Conde-Torres, D.; Antelo-Riveiro, P.; Piñeiro, Á.; Garcia-Fandino, R. The Role of AI in Drug Discovery: Challenges, Opportunities, and Strategies. Pharmaceuticals 2023, 16, 891. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, C.; Chandra, I.; Singh, S.K. Artificial Intelligence and Machine Learning Approaches for Drug Design: Challenges and Opportunities for the Pharmaceutical Industries. Mol. Divers. 2022, 26, 1893–1913. [Google Scholar] [CrossRef]
- Askr, H.; Elgeldawi, E.; Aboul Ella, H.; Elshaier, Y.A.M.M.; Gomaa, M.M.; Hassanien, A.E. Deep Learning in Drug Discovery: An Integrative Review and Future Challenges. Artif. Intell. Rev. 2023, 56, 5975–6037. [Google Scholar] [CrossRef]
- Kawakami, J.K.; Martinez, Y.; Sasaki, B.; Harris, M.; Kurata, W.E.; Lau, A.F. Investigation of a Novel Molecular Descriptor for the Lead Optimization of 4-Aminoquinazolines as Vascular Endothelial Growth Factor Receptor-2 Inhibitors: Application for Quantitative Structure–Activity Relationship Analysis in Lead Optimization. Bioorg. Med. Chem. Lett. 2011, 21, 1371–1375. [Google Scholar] [CrossRef]
- Saeys, Y.; Inza, I.; Larrañaga, P. A Review of Feature Selection Techniques in Bioinformatics. Bioinformatics 2007, 23, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Hira, Z.M.; Gillies, D.F. A Review of Feature Selection and Feature Extraction Methods Applied on Microarray Data. Adv. Bioinform. 2015, 2015, 198363. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J. A Novel Wrapper Method for Feature Selection and Its Applications. Neurocomputing 2015, 159, 219–226. [Google Scholar] [CrossRef]
- Naik, A.K.; Kuppili, V. An Embedded Feature Selection Method Based on Generalized Classifier Neural Network for Cancer Classification. Comput. Biol. Med. 2024, 168, 107677. [Google Scholar] [CrossRef]
- Eriksson, L.; Jaworska, J.; Worth, A.P.; Cronin, M.T.D.; McDowell, R.M.; Gramatica, P. Methods for Reliability and Uncertainty Assessment and for Applicability Evaluations of Classification- and Regression-Based QSARs. Environ. Health Perspect. 2003, 111, 1361–1375. [Google Scholar] [CrossRef]
- Berrar, D. Cross-Validation. In Encyclopedia of Bioinformatics and Computational Biology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 542–545. [Google Scholar]
- Stone, M. Cross-Validatory Choice and Assessment of Statistical Predictions. J. R. Stat. Soc. Ser. B Stat. Methodol. 1974, 36, 111–133. [Google Scholar] [CrossRef]
- Golbraikh, A.; Tropsha, A. Beware of Q2! J. Mol. Graph. Model. 2002, 20, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Tropsha, A.; Gramatica, P.; Gombar, V.K. The Importance of Being Earnest: Validation Is the Absolute Essential for Successful Application and Interpretation of QSPR Models. QSAR Comb. Sci. 2003, 22, 69–77. [Google Scholar] [CrossRef]
- Alexander, D.L.J.; Tropsha, A.; Winkler, D.A. Beware of R 2: Simple, Unambiguous Assessment of the Prediction Accuracy of QSAR and QSPR Models. J. Chem. Inf. Model. 2015, 55, 1316–1322. [Google Scholar] [CrossRef]
- Schüürmann, G.; Ebert, R.-U.; Chen, J.; Wang, B.; Kühne, R. External Validation and Prediction Employing the Predictive Squared Correlation Coefficient—Test Set Activity Mean vs Training Set Activity Mean. J. Chem. Inf. Model. 2008, 48, 2140–2145. [Google Scholar] [CrossRef] [PubMed]
- Consonni, V.; Ballabio, D.; Todeschini, R. Evaluation of Model Predictive Ability by External Validation Techniques. J. Chemom. 2010, 24, 194–201. [Google Scholar] [CrossRef]
- Király, P.; Kiss, R.; Kovács, D.; Ballaj, A.; Tóth, G. The Relevance of Goodness-of-Fit, Robustness and Prediction Validation Categories of OECD-QSAR Principles with Respect to Sample Size and Model Type. Mol. Inform. 2022, 41, 2200072. [Google Scholar] [CrossRef] [PubMed]
- Mienye, I.D.; Swart, T.G.; Obaido, G. Recurrent Neural Networks: A Comprehensive Review of Architectures, Variants, and Applications. Information 2024, 15, 517. [Google Scholar] [CrossRef]
- Shi, Y. Support Vector Regression-Based QSAR Models for Prediction of Antioxidant Activity of Phenolic Compounds. Sci. Rep. 2021, 11, 8806. [Google Scholar] [CrossRef]
- Xu, Y. Deep Neural Networks for QSAR. In Artificial Intelligence in Drug Design; Springer: Berlin/Heidelberg, Germany, 2022; pp. 233–260. [Google Scholar]
- Stanton, D.T. QSAR and QSPR Model Interpretation Using Partial Least Squares (PLS) Analysis. Curr. Comput. Aided Drug Des. 2012, 8, 107–127. [Google Scholar] [CrossRef] [PubMed]
- Sahigara, F.; Mansouri, K.; Ballabio, D.; Mauri, A.; Consonni, V.; Todeschini, R. Comparison of Different Approaches to Define the Applicability Domain of QSAR Models. Molecules 2012, 17, 4791–4810. [Google Scholar] [CrossRef] [PubMed]
- Fernández Pierna, J.A.; Jin, L.; Daszykowski, M.; Wahl, F.; Massart, D.L. A Methodology to Detect Outliers/Inliers in Prediction with PLS. Chemom. Intell. Lab. Syst. 2003, 68, 17–28. [Google Scholar] [CrossRef]
- Liu, R.; Wallqvist, A. Molecular Similarity-Based Domain Applicability Metric Efficiently Identifies Out-of-Domain Compounds. J. Chem. Inf. Model. 2019, 59, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Alvarsson, J.; Arvidsson McShane, S.; Norinder, U.; Spjuth, O. Predicting With Confidence: Using Conformal Prediction in Drug Discovery. J. Pharm. Sci. 2021, 110, 42–49. [Google Scholar] [CrossRef]
- Lampa, S.; Alvarsson, J.; Arvidsson Mc Shane, S.; Berg, A.; Ahlberg, E.; Spjuth, O. Predicting Off-Target Binding Profiles With Confidence Using Conformal Prediction. Front. Pharmacol. 2018, 9, 1256. [Google Scholar] [CrossRef] [PubMed]
- Mavromoustakos, T.; Durdagi, S.; Koukoulitsa, C.; Simcic, M.; Papadopoulos, M.G.; Hodoscek, M.; Grdadolnik, S.G. Strategies in the Rational Drug Design. Curr. Med. Chem. 2011, 18, 2517–2530. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Andersson, P.L.; Johansson, E.; Tysklind, M. Megavariate Analysis of Environmental QSAR Data. Part I—A Basic Framework Founded on Principal Component Analysis (PCA), Partial Least Squares (PLS), and Statistical Molecular Design (SMD). Mol. Divers. 2006, 10, 169–186. [Google Scholar] [CrossRef]
- Keserű, G.M.; Makara, G.M. Hit Discovery and Hit-to-Lead Approaches. Drug Discov. Today 2006, 11, 741–748. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and Drug-like Compounds: The Rule-of-Five Revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple Selection Criteria for Drug-like Chemical Matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Lai, X.; Pan, Y.; Zheng, H.; Vera, J.; Liu, S.; Deng, S.; Zhang, L. Artificial Intelligence in Cancer Target Identification and Drug Discovery. Signal Transduct. Target. Ther. 2022, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Wallach, I.; Bernard, D.; Nguyen, K.; Ho, G.; Morrison, A.; Stecula, A.; Rosnik, A.; O’Sullivan, A.M.; Davtyan, A.; Samudio, B.; et al. AI Is a Viable Alternative to High Throughput Screening: A 318-Target Study. Sci. Rep. 2024, 14, 7526. [Google Scholar] [CrossRef]
- Isert, C.; Atz, K.; Schneider, G. Structure-Based Drug Design with Geometric Deep Learning. Curr. Opin. Struct. Biol. 2023, 79, 102548. [Google Scholar] [CrossRef]
- Janet, J.P.; Mervin, L.; Engkvist, O. Artificial Intelligence in Molecular de Novo Design: Integration with Experiment. Curr. Opin. Struct. Biol. 2023, 80, 102575. [Google Scholar] [CrossRef]
- Gomes, P.S.F.C.; Gomes, D.E.B.; Bernardi, R.C. Protein Structure Prediction in the Era of AI: Challenges and Limitations When Applying to in Silico Force Spectroscopy. Front. Bioinforma. 2022, 2, 983306. [Google Scholar] [CrossRef]
- Siramshetty, V.B.; Xu, X.; Shah, P. Artificial Intelligence in ADME Property Prediction. In Computational Drug Discovery and Design; Springer: New York, NY, USA, 2024; pp. 307–327. [Google Scholar]
- Raies, A.; Tulodziecka, E.; Stainer, J.; Middleton, L.; Dhindsa, R.S.; Hill, P.; Engkvist, O.; Harper, A.R.; Petrovski, S.; Vitsios, D. DrugnomeAI Is an Ensemble Machine-Learning Framework for Predicting Druggability of Candidate Drug Targets. Commun. Biol. 2022, 5, 1291. [Google Scholar] [CrossRef]
- Karampuri, A.; Perugu, S. A Breast Cancer-Specific Combinational QSAR Model Development Using Machine Learning and Deep Learning Approaches. Front. Bioinforma. 2024, 3, 1328262. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Jiang, S.; Xiao, W. Optimization Method of an Antibreast Cancer Drug Candidate Based on Machine Learning. Comput. Math. Methods Med. 2022, 2022, 4133663. [Google Scholar] [CrossRef] [PubMed]
- Shahab, M.; Zheng, G.; Khan, A.; Wei, D.; Novikov, A.S. Machine Learning-Based Virtual Screening and Molecular Simulation Approaches Identified Novel Potential Inhibitors for Cancer Therapy. Biomedicines 2023, 11, 2251. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Zhou, F.; Liu, X.; Zhi, L. Artificial Intelligence in Small Molecule Drug Discovery from 2018 to 2023: Does It Really Work? Bioorg. Chem. 2023, 141, 106894. [Google Scholar] [CrossRef]
- Rehman, A.U.; Li, M.; Wu, B.; Ali, Y.; Rasheed, S.; Shaheen, S.; Liu, X.; Luo, R.; Zhang, J. Role of Artificial Intelligence in Revolutionizing Drug Discovery. Fundam. Res. 2024, in press. [Google Scholar] [CrossRef]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cancer Stat Facts: Female Breast Cancer. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 13 March 2024).
- Xu, H.; Xu, B. Breast Cancer: Epidemiology, Risk Factors and Screening. Chinese J. Cancer Res. 2023, 35, 565–583. [Google Scholar] [CrossRef] [PubMed]
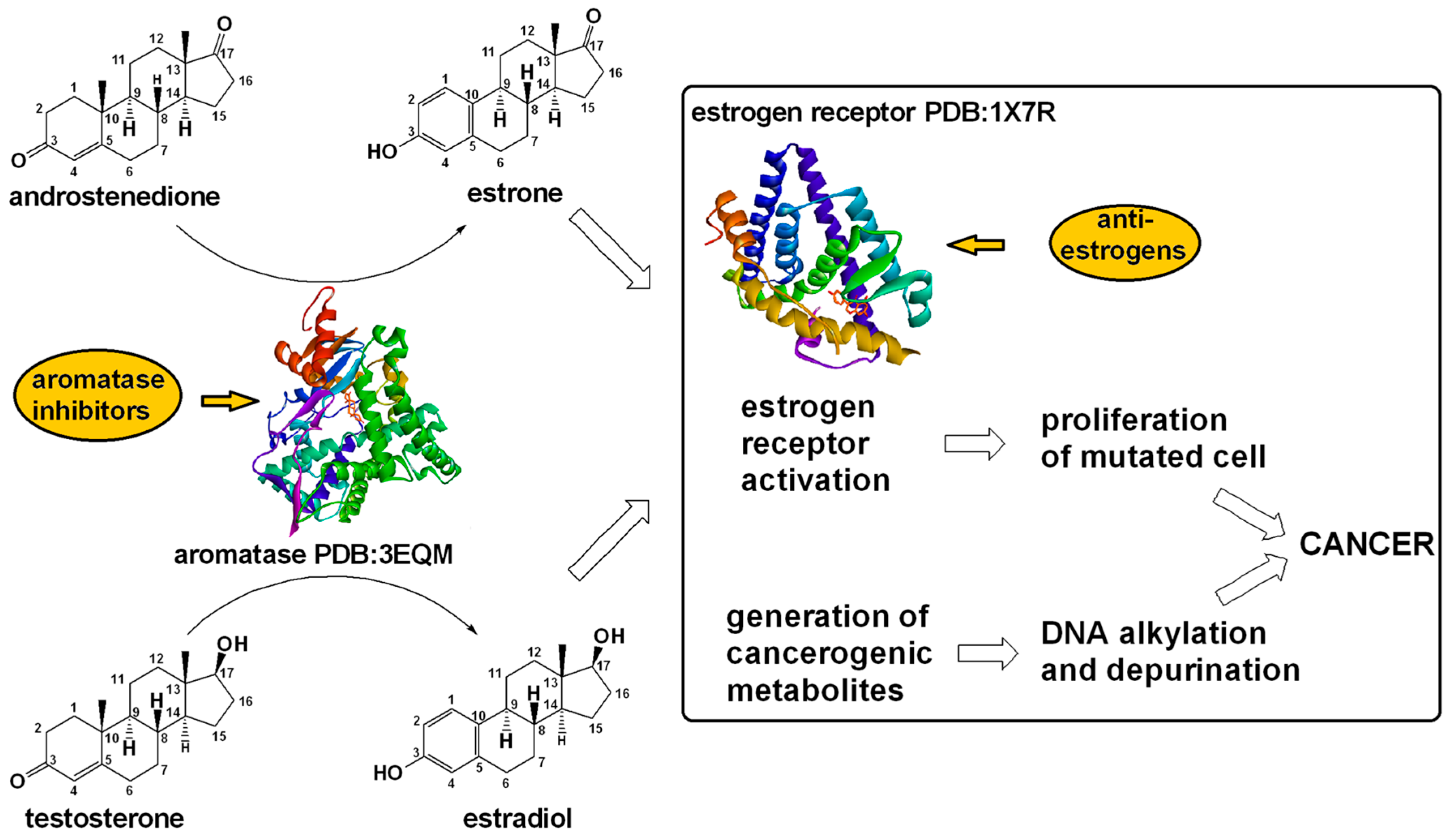
- Saha, T.; Makar, S.; Swetha, R.; Gutti, G.; Singh, S.K. Estrogen Signaling: An Emanating Therapeutic Target for Breast Cancer Treatment. Eur. J. Med. Chem. 2019, 177, 116–143. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt Signal Transduction for Cancer Therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Kümler, I.; Knoop, A.S.; Jessing, C.A.R.; Ejlertsen, B.; Nielsen, D.L. Review of Hormone-Based Treatments in Postmenopausal Patients with Advanced Breast Cancer Focusing on Aromatase Inhibitors and Fulvestrant. ESMO Open 2016, 1, e000062. [Google Scholar] [CrossRef]
- Ali, S.; Buluwela, L.; Coombes, R.C. Antiestrogens and Their Therapeutic Applications in Breast Cancer and Other Diseases. Annu. Rev. Med. 2011, 62, 217–232. [Google Scholar] [CrossRef]
- Generali, D.; Berardi, R.; Caruso, M.; Cazzaniga, M.; Garrone, O.; Minchella, I.; Paris, I.; Pinto, C.; De Placido, S. Aromatase Inhibitors: The Journey from the State of the Art to Clinical Open Questions. Front. Oncol. 2023, 13, 1249160. [Google Scholar] [CrossRef]
- Seruga, B.; Tannock, I.F. Up-Front Use of Aromatase Inhibitors As Adjuvant Therapy for Breast Cancer: The Emperor Has No Clothes. J. Clin. Oncol. 2009, 27, 840–842. [Google Scholar] [CrossRef] [PubMed]
- Riemsma, R.; Forbes, C.A.; Kessels, A.; Lykopoulos, K.; Amonkar, M.M.; Rea, D.W.; Kleijnen, J. Systematic Review of Aromatase Inhibitors in the First-Line Treatment for Hormone Sensitive Advanced or Metastatic Breast Cancer. Breast Cancer Res. Treat. 2010, 123, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Hartkopf, A.D.; Grischke, E.-M.; Brucker, S.Y. Endocrine-Resistant Breast Cancer: Mechanisms and Treatment. Breast Care 2020, 15, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Lannigan, D.A. Estrogen Receptor Phosphorylation. Steroids 2003, 68, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Anbalagan, M.; Rowan, B.G. Estrogen Receptor Alpha Phosphorylation and Its Functional Impact in Human Breast Cancer. Mol. Cell. Endocrinol. 2015, 418, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Rude Voldborg, B.; Damstrup, L.; Spang-Thomsen, M.; Skovgaard Poulsen, H. Epidermal Growth Factor Receptor (EGFR) and EGFR Mutations, Function and Possible Role in Clinical Trials. Ann. Oncol. 1997, 8, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Chang, J.; Fu, P. Endocrine Therapy Resistance in Breast Cancer: Current Status, Possible Mechanisms and Overcoming Strategies. Future Med. Chem. 2015, 7, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Behl, A.; Wani, Z.A.; Das, N.N.; Parmar, V.S.; Len, C.; Malhotra, S.; Chhillar, A.K. Monoclonal Antibodies in Breast Cancer: A Critical Appraisal. Crit. Rev. Oncol. Hematol. 2023, 183, 103915. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-Positive Breast Cancer: Advances and Future Directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef]
- Shepard, H.M. Trastuzumab: Dreams, Desperation and Hope. Nat. Rev. Cancer 2024, 24, 287–288. [Google Scholar] [CrossRef]
- Masuda, H.; Zhang, D.; Bartholomeusz, C.; Doihara, H.; Hortobagyi, G.N.; Ueno, N.T. Role of Epidermal Growth Factor Receptor in Breast Cancer. Breast Cancer Res. Treat. 2012, 136, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Maennling, A.E.; Tur, M.K.; Niebert, M.; Klockenbring, T.; Zeppernick, F.; Gattenlöhner, S.; Meinhold-Heerlein, I.; Hussain, A.F. Molecular Targeting Therapy against EGFR Family in Breast Cancer: Progress and Future Potentials. Cancers 2019, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, S.; Attina, G.; Triarico, S.; Romano, A.; Maurizi, P.; Ruggiero, A. The DNA-Topoisomerase Inhibitors in Cancer Therapy. Biomed. Pharmacol. J. 2022, 15, 553–562. [Google Scholar] [CrossRef]
- Yakkala, P.A.; Penumallu, N.R.; Shafi, S.; Kamal, A. Prospects of Topoisomerase Inhibitors as Promising Anti-Cancer Agents. Pharmaceuticals 2023, 16, 1456. [Google Scholar] [CrossRef] [PubMed]
- Vuger, A.T.; Tiscoski, K.; Apolinario, T.; Cardoso, F. Anthracyclines in the Treatment of Early Breast Cancer Friend or Foe? Breast 2022, 65, 67–76. [Google Scholar] [CrossRef]
- Venkatesh, P.; Kasi, A. Anthracyclines; StatPearls: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Malik, M.S.; Alsantali, R.I.; Jassas, R.S.; Alsimaree, A.A.; Syed, R.; Alsharif, M.A.; Kalpana, K.; Morad, M.; Althagafi, I.I.; Ahmed, S.A. Journey of Anthraquinones as Anticancer Agents—A Systematic Review of Recent Literature. RSC Adv. 2021, 11, 35806–35827. [Google Scholar] [CrossRef]
- Kozurkova, M. Acridine Derivatives as Inhibitors/Poisons of Topoisomerase II. J. Appl. Toxicol. 2022, 42, 544–552. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, Y.; Wang, Y.; Jiang, M.; Yao, L. The Recent Developments of Camptothecin and Its Derivatives as Potential Anti-Tumor Agents. Eur. J. Med. Chem. 2023, 260, 115710. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jiang, E. Recent Advances in the Application of Podophyllotoxin Derivatives to Fight Against Multidrug-Resistant Cancer Cells. Curr. Top. Med. Chem. 2021, 21, 1712–1724. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, S.; Kumar, K.; Das, R.; Negi, A.S. Microtubule Associated Proteins as Targets for Anticancer Drug Development. Bioorg. Chem. 2021, 116, 105320. [Google Scholar] [CrossRef] [PubMed]
- Čermák, V.; Dostál, V.; Jelínek, M.; Libusová, L.; Kovář, J.; Rösel, D.; Brábek, J. Microtubule-Targeting Agents and Their Impact on Cancer Treatment. Eur. J. Cell Biol. 2020, 99, 151075. [Google Scholar] [CrossRef] [PubMed]
- Churchill, C.D.M.; Klobukowski, M.; Tuszynski, J.A. The Unique Binding Mode of Laulimalide to Two Tubulin Protofilaments. Chem. Biol. Drug Des. 2015, 86, 190–199. [Google Scholar] [CrossRef]
- Willson, M.L.; Burke, L.; Ferguson, T.; Ghersi, D.; Nowak, A.K.; Wilcken, N. Taxanes for Adjuvant Treatment of Early Breast Cancer. Cochrane Database Syst. Rev. 2019, 9, CD004421. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Rui, M.; Siciliano, A.M.; Collina, S. Vinca Alkaloids and Analogues as Anti-Cancer Agents: Looking Back, Peering Ahead. Bioorg. Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef]
- Jung, H., II.; Shin, I.; Park, Y.M.; Kang, K.W.; Ha, K.-S. Colchicine Activates Actin Polymerization by Microtubule Depolymerization. Mol. Cells 1997, 7, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, P.; Quispe, C.; Sharma, E.; Bahukhandi, A.; Sati, P.; Attri, D.C.; Szopa, A.; Sharifi-Rad, J.; Docea, A.O.; Mardare, I.; et al. Anticancer Potential of Alkaloids: A Key Emphasis to Colchicine, Vinblastine, Vincristine, Vindesine, Vinorelbine and Vincamine. Cancer Cell Int. 2022, 22, 206. [Google Scholar] [CrossRef]
- Podolak, M.; Holota, S.; Deyak, Y.; Dziduch, K.; Dudchak, R.; Wujec, M.; Bielawski, K.; Lesyk, R.; Bielawska, A. Tubulin Inhibitors. Selected Scaffolds and Main Trends in the Design of Novel Anticancer and Antiparasitic Agents. Bioorg. Chem. 2024, 143, 107076. [Google Scholar] [CrossRef] [PubMed]
- Smolarczyk, R.; Czapla, J.; Jarosz-Biej, M.; Czerwinski, K.; Cichoń, T. Vascular Disrupting Agents in Cancer Therapy. Eur. J. Pharmacol. 2021, 891, 173692. [Google Scholar] [CrossRef]
- Fritz, A.J.; El Dika, M.; Toor, R.H.; Rodriguez, P.D.; Foley, S.J.; Ullah, R.; Nie, D.; Banerjee, B.; Lohese, D.; Tracy, K.M.; et al. Epigenetic-Mediated Regulation of Gene Expression for Biological Control and Cancer: Cell and Tissue Structure, Function, and Phenotype. In Nuclear, Chromosomal, and Genomic Architecture in Biology and Medicine; Springer: Berlin/Heidelberg, Germany, 2022; pp. 339–373. [Google Scholar]
- Vietri, M.; D’elia, G.; Benincasa, G.; Ferraro, G.; Caliendo, G.; Nicoletti, G.; Napoli, C. DNA Methylation and Breast Cancer: A Way Forward (Review). Int. J. Oncol. 2021, 59, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Huo, Q.; Yang, F.; Xie, N. Perspectives on the Role of Histone Modification in Breast Cancer Progression and the Advanced Technological Tools to Study Epigenetic Determinants of Metastasis. Front. Genet. 2020, 11, 603552. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Sonar, S.; Nyahatkar, S.; Kalele, K.; Adhikari, M.D. Role of DNA Methylation in Cancer Development and Its Clinical Applications. Clin. Transl. Discov. 2024, 4, e279. [Google Scholar] [CrossRef]
- Verde, G.; Querol-Paños, J.; Cebrià-Costa, J.; Pascual-Reguant, L.; Serra-Bardenys, G.; Iturbide, A.; Peiró, S. Lysine-Specific Histone Demethylases Contribute to Cellular Differentiation and Carcinogenesis. Epigenomes 2017, 1, 4. [Google Scholar] [CrossRef]
- Yang, G.-J.; Liu, Y.-J.; Ding, L.-J.; Tao, F.; Zhu, M.-H.; Shi, Z.-Y.; Wen, J.-M.; Niu, M.-Y.; Li, X.; Xu, Z.-S.; et al. A State-of-the-Art Review on LSD1 and Its Inhibitors in Breast Cancer: Molecular Mechanisms and Therapeutic Significance. Front. Pharmacol. 2022, 13, 989575. [Google Scholar] [CrossRef]
- Noce, B.; Di Bello, E.; Fioravanti, R.; Mai, A. LSD1 Inhibitors for Cancer Treatment: Focus on Multi-Target Agents and Compounds in Clinical Trials. Front. Pharmacol. 2023, 14, 1120911. [Google Scholar] [CrossRef]
- Yoon, S.; Eom, G.H. HDAC and HDAC Inhibitor: From Cancer to Cardiovascular Diseases. Chonnam Med. J. 2016, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Mottamal, M.; Zheng, S.; Huang, T.; Wang, G. Histone Deacetylase Inhibitors in Clinical Studies as Templates for New Anticancer Agents. Molecules 2015, 20, 3898–3941. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Hu, Z.; Xu, X.; Dai, X.; Liu, Z. Key Signal Transduction Pathways and Crosstalk in Cancer: Biological and Therapeutic Opportunities. Transl. Oncol. 2022, 26, 101510. [Google Scholar] [CrossRef] [PubMed]
- Brogowska, K.K.; Zajkowska, M.; Mroczko, B. Vascular Endothelial Growth Factor Ligands and Receptors in Breast Cancer. J. Clin. Med. 2023, 12, 2412. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Wang, Y.; Lin, C.; Zhang, D.; Chen, J.; Ouyang, L.; Wu, F.; Zhang, J.; Chen, L. Recent Progress on Vascular Endothelial Growth Factor Receptor Inhibitors with Dual Targeting Capabilities for Tumor Therapy. J. Hematol. Oncol. 2022, 15, 89. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/MTOR Signaling Transduction Pathway and Targeted Therapies in Cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wu, Y.; He, P.; Fan, Y.; Zhong, X.; Zheng, H.; Luo, T. PI3K/AKT/MTOR-Targeted Therapy for Breast Cancer. Cells 2022, 11, 2508. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, S.A.; Han, J.; Kim, I.-S. Rho-Kinase as a Target for Cancer Therapy and Its Immunotherapeutic Potential. Int. J. Mol. Sci. 2021, 22, 12916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, S.; Zhang, Z.; He, J.; Xu, Y.; Liu, S. ROCK Has a Crucial Role in Regulating Prostate Tumor Growth through Interaction with C-Myc. Oncogene 2014, 33, 5582–5591. [Google Scholar] [CrossRef]
- Du, R.; Huang, C.; Liu, K.; Li, X.; Dong, Z. Targeting AURKA in Cancer: Molecular Mechanisms and Opportunities for Cancer Therapy. Mol. Cancer 2021, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Borisa, A.C.; Bhatt, H.G. A Comprehensive Review on Aurora Kinase: Small Molecule Inhibitors and Clinical Trial Studies. Eur. J. Med. Chem. 2017, 140, 1–19. [Google Scholar] [CrossRef]
- Kovacs, A.H.; Zhao, D.; Hou, J. Aurora B Inhibitors as Cancer Therapeutics. Molecules 2023, 28, 3385. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Pang, X.; Baeg, G.H. Targeting the JAK/STAT Signaling Pathway for Breast Cancer. Curr. Med. Chem. 2021, 28, 5137–5151. [Google Scholar] [CrossRef] [PubMed]
- Shawky, A.M.; Almalki, F.A.; Abdalla, A.N.; Abdelazeem, A.H.; Gouda, A.M. A Comprehensive Overview of Globally Approved JAK Inhibitors. Pharmaceutics 2022, 14, 1001. [Google Scholar] [CrossRef] [PubMed]
- Kurtanović, N.; Tomašević, N.; Matić, S.; Proia, E.; Sabatino, M.; Antonini, L.; Mladenović, M.; Ragno, R. Human Estrogen Receptor Alpha Antagonists, Part 3: 3-D Pharmacophore and 3-D QSAR Guided Brefeldin A Hit-to-Lead Optimization toward New Breast Cancer Suppressants. Molecules 2022, 27, 2823. [Google Scholar] [CrossRef]
- Rajagopal, K.; Kalusalingam, A.; Bharathidasan, A.R.; Sivaprakash, A.; Shanmugam, K.; Sundaramoorthy, M.; Byran, G. In Silico Drug Design of Anti-Breast Cancer Agents. Molecules 2023, 28, 4175. [Google Scholar] [CrossRef]
- Khaled, D.M.; Elshakre, M.E.; Noamaan, M.A.; Butt, H.; Abdel Fattah, M.M.; Gaber, D.A. A Computational QSAR, Molecular Docking and In Vitro Cytotoxicity Study of Novel Thiouracil-Based Drugs with Anticancer Activity against Human-DNA Topoisomerase II. Int. J. Mol. Sci. 2022, 23, 11799. [Google Scholar] [CrossRef]
- Phanus-umporn, C.; Prachayasittikul, V.; Nantasenamat, C.; Prachayasittikul, S.; Prachayasittikul, V. QSAR-Driven Rational Design of Novel DNA Methyltransferase 1 Inhibitors. EXCLI J. 2020, 19, 458–475. [Google Scholar] [CrossRef]
- Jawarkar, R.D.; Bakal, R.L.; Mukherjee, N.; Ghosh, A.; Zaki, M.E.A.; AL-Hussain, S.A.; Al-Mutairi, A.A.; Samad, A.; Gandhi, A.; Masand, V.H. QSAR Evaluations to Unravel the Structural Features in Lysine-Specific Histone Demethylase 1A Inhibitors for Novel Anticancer Lead Development Supported by Molecular Docking, MD Simulation and MMGBSA. Molecules 2022, 27, 4758. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fan, B.; Gao, Y.; Chen, Y.; Han, D.; Lu, J.; Liu, T.; Gao, Q.; Zhang, J.Z.; Wang, M. Design Two Novel Tetrahydroquinoline Derivatives against Anticancer Target LSD1 with 3D-QSAR Model and Molecular Simulation. Molecules 2022, 27, 8358. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; He, Z.; Yang, M.; Gao, Y.; Jin, L.; Wang, M.; Zheng, Y.; Lu, X.; Zhang, S.; Wang, C.; et al. Investigating the Binding Mode of Reversible LSD1 Inhibitors Derived from Stilbene Derivatives by 3D-QSAR, Molecular Docking, and Molecular Dynamics Simulation. Molecules 2019, 24, 4479. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; He, Z.; Liu, H.; Chen, Y.; Gao, Y.; Zhang, S.; Wang, M.; Lu, X.; Wang, C.; Zhao, Z.; et al. 3D-QSAR, Molecular Docking, and Molecular Dynamics Simulation Study of Thieno[3,2-b]Pyrrole-5-Carboxamide Derivatives as LSD1 Inhibitors. RSC Adv. 2020, 10, 6927–6943. [Google Scholar] [CrossRef]
- Aljanabi, R.; Alsous, L.; Sabbah, D.A.; Gul, H.I.; Gul, M.; Bardaweel, S.K. Monoamine Oxidase (MAO) as a Potential Target for Anticancer Drug Design and Development. Molecules 2021, 26, 6019. [Google Scholar] [CrossRef] [PubMed]
- Balbuena-Rebolledo, I.; Rivera-Antonio, A.M.; Sixto-López, Y.; Correa-Basurto, J.; Rosales-Hernández, M.C.; Mendieta-Wejebe, J.E.; Martínez-Martínez, F.J.; Olivares-Corichi, I.M.; García-Sánchez, J.R.; Guevara-Salazar, J.A.; et al. Dihydropyrazole-Carbohydrazide Derivatives with Dual Activity as Antioxidant and Anti-Proliferative Drugs on Breast Cancer Targeting the HDAC6. Pharmaceuticals 2022, 15, 690. [Google Scholar] [CrossRef] [PubMed]
- Shirbhate, E.; Pandey, J.; Patel, V.K.; Veerasamy, R.; Rajak, H. Exploration of Structure-Activity Relationship Using Integrated Structure and Ligand Based Approach: Hydroxamic Acid-Based HDAC Inhibitors and Cytotoxic Agents. Turk. J. Pharm. Sci. 2023, 20, 270–284. [Google Scholar] [CrossRef]
- Bülbül, E.F.; Robaa, D.; Sun, P.; Mahmoudi, F.; Melesina, J.; Zessin, M.; Schutkowski, M.; Sippl, W. Application of Ligand- and Structure-Based Prediction Models for the Design of Alkylhydrazide-Based HDAC3 Inhibitors as Novel Anti-Cancer Compounds. Pharmaceuticals 2023, 16, 968. [Google Scholar] [CrossRef]
- Moussaoui, M.; Baammi, S.; Soufi, H.; Baassi, M.; El Allali, A.; Belghiti, M.E.; Daoud, R.; Belaaouad, S. QSAR, ADMET, Molecular Docking, and Dynamics Studies of 1,2,4-Triazine-3(2H)-One Derivatives as Tubulin Inhibitors for Breast Cancer Therapy. Sci. Rep. 2024, 14, 16418. [Google Scholar] [CrossRef]
- Mirzaei, S.; Ghodsi, R.; Hadizadeh, F.; Sahebkar, A. 3D-QSAR-Based Pharmacophore Modeling, Virtual Screening, and Molecular Docking Studies for Identification of Tubulin Inhibitors with Potential Anticancer Activity. Biomed Res. Int. 2021, 2021, 6480804. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mahmud, F.; Deng, S.; Ma, L.; Yun, M.-K.; Fakayode, S.O.; Arnst, K.E.; Yang, L.; Chen, H.; Wu, Z.; et al. X-Ray Crystallography-Guided Design, Antitumor Efficacy, and QSAR Analysis of Metabolically Stable Cyclopenta-Pyrimidinyl Dihydroquinoxalinone as a Potent Tubulin Polymerization Inhibitor. J. Med. Chem. 2021, 64, 13072–13095. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, S.H.; Uzairu, A.; Shallangwa, G.A.; Uba, S.; Umar, A.B. Pharmacokinetic Profiling of Quinazoline-4(3H)-One Analogs as EGFR Inhibitors: 3D-QSAR Modeling, Molecular Docking Studies and the Design of Therapeutic Agents. J. Taibah Univ. Med. Sci. 2023, 18, 1018–1029. [Google Scholar] [CrossRef]
- Anwar, S.; Alanazi, J.; Ahemad, N.; Raza, S.; Chohan, T.A.; Saleem, H. Deciphering Quinazoline Derivatives’ Interactions with EGFR: A Computational Quest for Advanced Cancer Therapy through 3D-QSAR, Virtual Screening, and MD Simulations. Front. Pharmacol. 2024, 15, 1399372. [Google Scholar] [CrossRef] [PubMed]
- Simeon, S.; Jongkon, N. Construction of Quantitative Structure Activity Relationship (QSAR) Models to Predict Potency of Structurally Diversed Janus Kinase 2 Inhibitors. Molecules 2019, 24, 4393. [Google Scholar] [CrossRef]
- Tian, Y.-Y.; Tong, J.-B.; Liu, Y.; Tian, Y. QSAR Study, Molecular Docking and Molecular Dynamic Simulation of Aurora Kinase Inhibitors Derived from Imidazo[4,5-b]Pyridine Derivatives. Molecules 2024, 29, 1772. [Google Scholar] [CrossRef] [PubMed]
- Bathula, S.; Sankaranarayanan, M.; Malgija, B.; Kaliappan, I.; Bhandare, R.R.; Shaik, A.B. 2-Amino Thiazole Derivatives as Prospective Aurora Kinase Inhibitors against Breast Cancer: QSAR, ADMET Prediction, Molecular Docking, and Molecular Dynamic Simulation Studies. ACS Omega 2023, 8, 44287–44311. [Google Scholar] [CrossRef] [PubMed]
- Beljkas, M.; Petkovic, M.; Vuletic, A.; Djuric, A.; Santibanez, J.F.; Srdic-Rajic, T.; Nikolic, K.; Oljacic, S. Development of Novel ROCK Inhibitors via 3D-QSAR and Molecular Docking Studies: A Framework for Multi-Target Drug Design. Pharmaceutics 2024, 16, 1250. [Google Scholar] [CrossRef]
- Ziemska, J.; Solecka, J.; Jarończyk, M. In Silico Screening for Novel Leucine Aminopeptidase Inhibitors with 3,4-Dihydroisoquinoline Scaffold. Molecules 2020, 25, 1753. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Jeong, J.-H. Structure-Activity Relationship Studies Based on 3D-QSAR CoMFA/CoMSIA for Thieno-Pyrimidine Derivatives as Triple Negative Breast Cancer Inhibitors. Molecules 2022, 27, 7974. [Google Scholar] [CrossRef] [PubMed]
- Subramani, A.K.; Sivaperuman, A.; Natarajan, R.; Bhandare, R.R.; Shaik, A.B. QSAR and Molecular Docking Studies of Pyrimidine-Coumarin-Triazole Conjugates as Prospective Anti-Breast Cancer Agents. Molecules 2022, 27, 1845. [Google Scholar] [CrossRef]
- Gandhi, A.; Masand, V.; Zaki, M.E.A.; Al-Hussain, S.A.; Ghorbal, A.B.; Chapolikar, A. Quantitative Structure–Activity Relationship Evaluation of MDA-MB-231 Cell Anti-Proliferative Leads. Molecules 2021, 26, 4795. [Google Scholar] [CrossRef]
- Szafrański, K.; Sławiński, J.; Tomorowicz, Ł.; Kawiak, A. Synthesis, Anticancer Evaluation and Structure-Activity Analysis of Novel (E)-5-(2-Arylvinyl)-1,3,4-Oxadiazol-2-Yl)Benzenesulfonamides. Int. J. Mol. Sci. 2020, 21, 2235. [Google Scholar] [CrossRef] [PubMed]
- Tomorowicz, Ł.; Sławiński, J.; Żołnowska, B.; Szafrański, K.; Kawiak, A. Synthesis, Antitumor Evaluation, Molecular Modeling and Quantitative Structure–Activity Relationship (QSAR) of Novel 2-[(4-Amino-6-N-Substituted-1,3,5-Triazin-2-Yl)Methylthio]-4-Chloro-5-Methyl-N-(1H-Benzo[d]Imidazol-2(3H)-Ylidene)Benzenesulfonamides. Int. J. Mol. Sci. 2020, 21, 2924. [Google Scholar] [CrossRef]
- Angelova, V.T.; Tatarova, T.; Mihaylova, R.; Vassilev, N.; Petrov, B.; Zhivkova, Z.; Doytchinova, I. Novel Arylsulfonylhydrazones as Breast Anticancer Agents Discovered by Quantitative Structure-Activity Relationships. Molecules 2023, 28, 2058. [Google Scholar] [CrossRef]
- Sarhan, M.O.; Abd El-Karim, S.S.; Anwar, M.M.; Gouda, R.H.; Zaghary, W.A.; Khedr, M.A. Discovery of New Coumarin-Based Lead with Potential Anticancer, CDK4 Inhibition and Selective Radiotheranostic Effect: Synthesis, 2D & 3D QSAR, Molecular Dynamics, In Vitro Cytotoxicity, Radioiodination, and Biodistribution Studies. Molecules 2021, 26, 2273. [Google Scholar] [CrossRef] [PubMed]
- Salas, C.O.; Zarate, A.M.; Kryštof, V.; Mella, J.; Faundez, M.; Brea, J.; Loza, M.I.; Brito, I.; Hendrychová, D.; Jorda, R.; et al. Promising 2,6,9-Trisubstituted Purine Derivatives for Anticancer Compounds: Synthesis, 3D-QSAR, and Preliminary Biological Assays. Int. J. Mol. Sci. 2019, 21, 161. [Google Scholar] [CrossRef]
- Nikolova-Mladenova, B.; Momekov, G.; Zhivkova, Z.; Doytchinova, I. Design, Synthesis and Cytotoxic Activity of Novel Salicylaldehyde Hydrazones against Leukemia and Breast Cancer. Int. J. Mol. Sci. 2023, 24, 7352. [Google Scholar] [CrossRef]
- Stanton, D.T.; Baker, J.R.; McCluskey, A.; Paula, S. Development and Interpretation of a QSAR Model for in Vitro Breast Cancer (MCF-7) Cytotoxicity of 2-Phenylacrylonitriles. J. Comput. Aided. Mol. Des. 2021, 35, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Lawal, H.A.; Uzairu, A.; Uba, S. QSAR, Molecular Docking, Design, and Pharmacokinetic Analysis of 2-(4-Fluorophenyl) Imidazol-5-Ones as Anti-Breast Cancer Drug Compounds against MCF-7 Cell Line. J. Bioenerg. Biomembr. 2020, 52, 475–494. [Google Scholar] [CrossRef] [PubMed]
- Bennani, F.E.; Doudach, L.; Karrouchi, K.; El rhayam, Y.; Rudd, C.E.; Ansar, M.; El Abbes Faouzi, M. Design and Prediction of Novel Pyrazole Derivatives as Potential Anti-Cancer Compounds Based on 2D-QSAR Study against PC-3, B16F10, K562, MDA-MB-231, A2780, ACHN and NUGC Cancer Cell Lines. Heliyon 2022, 8, e10003. [Google Scholar] [CrossRef] [PubMed]
- Altaf, R.; Nadeem, H.; Ilyas, U.; Iqbal, J.; Paracha, R.Z.; Zafar, H.; Paiva-Santos, A.C.; Sulaiman, M.; Raza, F. Cytotoxic Evaluation, Molecular Docking, and 2D-QSAR Studies of Dihydropyrimidinone Derivatives as Potential Anticancer Agents. J. Oncol. 2022, 2022, 7715689. [Google Scholar] [CrossRef] [PubMed]
- Beč, A.; Mioč, M.; Bertoša, B.; Kos, M.; Debogović, P.; Kralj, M.; Starčević, K.; Hranjec, M. Design, Synthesis, Biological Evaluation and QSAR Analysis of Novel N -Substituted Benzimidazole Derived Carboxamides. J. Enzym. Inhib. Med. Chem. 2022, 37, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Tomorowicz, Ł.; Żołnowska, B.; Szafrański, K.; Chojnacki, J.; Konopiński, R.; Grzybowska, E.A.; Sławiński, J.; Kawiak, A. New 2-[(4-Amino-6-N-Substituted-1,3,5-Triazin-2-Yl)Methylthio]-N-(Imidazolidin-2-Ylidene)-4-Chloro-5-Methylbenzenesulfonamide Derivatives, Design, Synthesis and Anticancer Evaluation. Int. J. Mol. Sci. 2022, 23, 7178. [Google Scholar] [CrossRef]
- Aloui, M.; El fadili, M.; Mujwar, S.; Er-rahmani, S.; Abuelizz, H.A.; Er-rajy, M.; Zarougui, S.; Elhallaoui, M. Design of Novel Potent Selective Survivin Inhibitors Using 2D-QSAR Modeling, Molecular Docking, Molecular Dynamics, and ADMET Properties of New MX-106 Hydroxyquinoline Scaffold Derivatives. Heliyon 2024, 10, e38383. [Google Scholar] [CrossRef] [PubMed]
| Unsupervised ML | Supervised ML |
|---|---|
| PCA [39,40,115] Clustering [119] Kohonen networks (self-organizing maps) [130] | MLR [121] PLS [122,123] Counter-propagation networks [131] Genetic algorithms (GAs) [132] Decision Trees [133,134] Back-propagation network [135] |
| Method | Filter | Wrapper | Embedded |
|---|---|---|---|
| Description | selection is based on the feature relevance score, which identifies and prioritizes the most impactful features based on their contribution to the dependent variable (Y) by evaluating the intrinsic properties of the data | selection of a subset of relevant features involves generating all possible feature/descriptor subsets, training a machine learning model for each subset, and comparing their performance | feature selection is based on constructing a classifier using a specific learning method, training the model on all descriptors, and extracting the importance of each feature |
| Advantages | Can scale high-dimensional datasets; faster and computationally affordable compared to wrapper methods | consider feature/descriptor dependency; interaction with classifier; simple to implement | classifier interaction; consider feature dependencies |
| Disadvantages | no interaction with the classifier, do not consider feature dependencies/redundancy | overfitting, selection based on classifiers, computationally demanding | classifier dependencies |
| Applications | in various statistical tests that associate X and Y variables, such as the Chi squared test, correlation coefficient scores, information gain, t-test | genetic search, exhaustive search, sequential forward selection/backward elimination | Decision Tree, Weighted Naïve Bayes, Weighted Vector of SVM |
| Hit | Lead | Drug |
|---|---|---|
| bring the pharmacophore | Mw < 300 Da logP < 3 up to 3 H-bond donors up to 3 H-bond acceptors positions for replacement | Mw < 500 Da logP < 3 up to 5 H-bond donors up to 10 H-bond acceptors up to 10 rotatable bonds 10 |
| affinity < 50 mmol/L | affinity < 10 µmol/L | affinity < 10 nmol/L |
| Ligand\Target Structure | Unknown | Known |
|---|---|---|
| unknown | HTS Combinatorial Chemistry | De Novo Design Target-based Pharmacophore Identification Molecular Docking |
| known | Proteochemometrics (PCM) QSAR Pharmacophore Identification Similarity | SBDD including Molecular Docking Molecular Dynamics De Novo Design |
| Compounds’ Scaffold | Target | Action | Modeling | Software | Ref. |
|---|---|---|---|---|---|
| Heterogenic group (all available SERMs) | Human Estrogen Receptor Alpha (ERα) | Selective ER modulators (SERMs, mixed agonists/antagonists of ERα) | 3D-Pharmacophore 3D-QSAR | PHASE program from the Schrödinger suite 2015-2 | [247] * |
| Heterogenic group | Human Estrogen Receptor Alpha (ERα) | ERα inhibitors | 3D-QSAR | Schrodinger suite 2021-4 | [248] |
| Thiouracil-based indeno pyrido pyrimidines | Human-DNA topoisomerase II | Inhibition of Human-DNA topoisomerase II | 2D-QSAR | MINITAB v. 19 | [249] |
| Indole and Oxazoline/1,2-oxazole scaffolds | DNA methyltransferases | Inhibition of DNA methyltransferases (an epigenetic modification) | 2D-QSAR | Weka 3.6 | [250] |
| Heterogenic group | Lysine-specific histone demethylase 1A (LSD1) | LSD1 inhibition (an epigenetic modification) | 2D-QSAR | QSARINS-v2.2.4 | [251] |
| Tetrahydroquinoline derivatives | Lysine-specific histone demethylase 1A (LSD1) | LSD1 inhibition (an epigenetic modification) | 3D-QSAR | SYBYL-X2.0 | [252] |
| Stilbene derivatives | Lysine-specific histone demethylase 1A (LSD1) | LSD1 inhibition (an epigenetic modification) | 3D-QSAR | SYBYL-X2.0 | [253] |
| Thieno [3,2-b]pyrrole-5-carboxamide derivatives | Lysine-specific histone demethylase 1 (LSD1) | LSD1 inhibition (an epigenetic modification) | 3D-QSAR | SYBYL-X2.0 | [254] |
| Xanthone, Pyrrole, Pyridazine, and Phenyl alkylamine derivatives | MAO inhibitors/LSD inhibitors | Inhibition of MAO | 2D-3D QSAR | SYBYL software 6.3 | [255] |
| Dihydropyrazole-Carbohydrazide derivatives | Histone deacetylase 6 (HDAC6) | Inhibition of HDAC6 (an epigenetic modification) | 2D-QSAR | Sigma Stat 3.5 | [256] |
| Heterogenic group | Histone deacetylase (HDAC) | Inhibition of HDAC (an epigenetic modification) | 3D QSAR | Schrodinger suite (Maestro v 9.3, LLC, New York) | [257] |
| N-monosubstituted hydrazide derivatives | Histone deacetylase 3 | Inhibition of HDAC3 (an epigenetic modification) | 3D-QSAR | MOE program 2019 (Molecular database calculator–RAND) | [258] |
| 1,2,4-triazine-3(2H)-one derivatives | Tubulin protein | Tubulin Polymerization Inhibitor | 2D-QSAR | XLSTAT v2019 | [259] |
| Quinolines derivatives | Tubulin protein | Tubulin Polymerization Inhibitor | 3D-QSAR | Phase (v4.3) module of Schrodinger 2016-1 | [260] |
| 1H-Pyrazole-1-carbothioamide derivatives | Epidermal growth factor receptor (EGFR) | EGTK-TK inhibitors (tyrosine kinase inhibitor) | 2D-QSAR | IBM SPSS statistics v.23 | [261] |
| Quinazoline-4(3H)-one analogs | Epidermal growth factor receptor (EGFR) | EGFR-TK inhibitors (tyrosine kinase inhibitor) | 3D-QSAR | SYBYL-X 2.1.1 | [262] |
| Quinazoline analogs | Epidermal growth factor receptor (EGFR) | EGFR-TK inhibitors (tyrosine kinase inhibitor) | 3D-QSAR | Sybyl-X1.3. | [263] |
| Chemical Space of JAK2 Inhibitors | Janus kinase 2 (JAK2) | JAK2 inhibitors (tyrosine kinase inhibitor) | 2D-QSAR | R package 2018 | [264] |
| Imidazo [4,5-b]pyridine derivatives | Aurora kinase | Aurora kinase inhibitors (serine-threonine kinase inhibitor) | 3D-QSAR | SYBYL 2.0 | [265] |
| 2-Amino Thiazole derivatives | Aurora kinase | Aurora kinase inhibitors (serine-threonine kinase inhibitor) | 2D-QSAR | QSARINS | [266] |
| Heterogenic group isoquinoline, pyridine, indazole, and pyrazole derivatives | Rho-associated coiled-coil-containing protein kinases (ROCKs) | ROCK inhibitors (serine-threonine kinase inhibitor) | 3D-QSAR | Pentacle 1.07 | [267] * |
| Dihydroisoquinoline analogs | Leucine aminopeptidase (LAP) | Leucine aminopeptidase inhibitors | 3D-QSAR | Forge software 10.6.0 | [268] |
| Thieno-pyrimidine derivatives | Receptors for vascular endothelial growth factor (VERFG 3) | Inhibitors of VERFG 3 | 3D-QSAR | SYBYL-X2.1.1 | [269] |
| Pyrimidine–coumarin–triazole conjugates | Dihydrofolate reductase (DHFR) | Inhibitors of Dihydrofolate reductase (DHFR) | 2D-QSAR | QSARINS | [270] |
| Compounds’ Scaffold | Cell Line | Modeling | Software | Ref. |
|---|---|---|---|---|
| Heterogenic group | MDA-MB-231 | 2D-QSAR | QSARINS v2.2.4 | [271] |
| (E)-5-(2-Arylvinyl)-1,3,4-oxadiazol-2-yl)benzenesulfonamides | HCT-116, MCF-7 and HeLa | 2D-QSAR | Statistica v13, TIBCO | [272] |
| 2-[(4-Amino-6-N-substituted-1,3,5-triazin-2-yl)methylthio]-4-chloro-5-methyl-N-(1H-benzo[d]imidazol-2(3H)-ylidene)Benzenesulfonamides | HCT-116, MCF-7 and HeLa | 2D-QSAR | MOE 2016 | [273] * |
| Arylsulfonylhydrazones | MCF-7 and MDA-MB-231 | 2D-QSAR | MDL QSAR v.2.2 | [274] * |
| 6-bromo-coumarin-ethylidene-hydrazonyl-thiazolyl and 6-bromo-coumarin-thiazolyl-based derivatives | MCF-7, A-549, and CHO-K1 | 2D- and 3D-QSAR | MOE 2016.08 | [275] |
| 2,6,9-Trisubstituted Purine derivatives | CFAPC- 1, NCI-H460, HL-60, CACO2, HCT-116, K562, MCF-7, MRC-5 | 3D-QSAR | Sybyl X-1.2 | [276] |
| Salicylaldehyde hydrazones | HL-60, KE-37, K-562, BV-173, SaOS-2, MCF-7, MDA-MB-231, HEK-293 lines | 2D-QSAR | MDL QSAR v. 2.2 | [277] |
| 2-phenylacrylonitriles | MCF-7 | 2D-QSAR | winMolconn v. 1.0.2.1 | [278] |
| Novel series of 2-(4-fluorophenyl) imidazol-5-ones | MCF-7 | 2D-QSAR | Material studio v8 | [279] |
| Pyrazole derivatives | PC-3, B16F10, K562, MDA-MB-231, A2780, ACHN and NUGC | 2D-QSAR | XLSTAT 2014 | [280] |
| Dihydropyrimidinone derivatives | MCF-7 | 2D-QSAR | QSARINS | [281] |
| N-substituted benzimidazole derivatives | HCT 116, H 460, MCF-7 and HEK 293 | 3D-QSAR | VolSurf+ 3-D | [282] |
| 2-[(4-Amino-6-N-substituted-1,3,5-triazin-2-yl)methylthio]-N-(imidazolidin-2-ylidene)-4-chloro-5-methylbenzenesulfonamide derivatives | HCT-116, MCF-7, HeLa, HaCaT lines | 2D-QSAR | STATISTICA software v.13 | [283] * |
| Hydroxyquinoline scaffold derivatives | MDA-MB-435 | 2D-QSAR | na | [284] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilev, B.; Atanasova, M. A (Comprehensive) Review of the Application of Quantitative Structure–Activity Relationship (QSAR) in the Prediction of New Compounds with Anti-Breast Cancer Activity. Appl. Sci. 2025, 15, 1206. https://doi.org/10.3390/app15031206
Vasilev B, Atanasova M. A (Comprehensive) Review of the Application of Quantitative Structure–Activity Relationship (QSAR) in the Prediction of New Compounds with Anti-Breast Cancer Activity. Applied Sciences. 2025; 15(3):1206. https://doi.org/10.3390/app15031206
Chicago/Turabian StyleVasilev, Boris, and Mariyana Atanasova. 2025. "A (Comprehensive) Review of the Application of Quantitative Structure–Activity Relationship (QSAR) in the Prediction of New Compounds with Anti-Breast Cancer Activity" Applied Sciences 15, no. 3: 1206. https://doi.org/10.3390/app15031206
APA StyleVasilev, B., & Atanasova, M. (2025). A (Comprehensive) Review of the Application of Quantitative Structure–Activity Relationship (QSAR) in the Prediction of New Compounds with Anti-Breast Cancer Activity. Applied Sciences, 15(3), 1206. https://doi.org/10.3390/app15031206






