Sustainability in Bio-Based Edible Films, Coatings, and Packaging for Small Fruits
Abstract
1. Introduction
2. Edible Films and Coatings: Definitions and Regulations
3. Biological Sources of the Compounds Used in Films, Coatings, and Packaging
3.1. Marine Processing By-Products
Materials from Fish and Shellfish
3.2. Agricultural Processing Byproducts
3.2.1. Materials from Vegetables and Fruits
3.2.2. Materials from Cereals
3.3. Animal Processing Byproducts
3.3.1. Materials from the Meat Industry
3.3.2. Materials from the Dairy Industry
3.4. Microorganisms-Based Materials
4. The Incorporation of Nanomaterials in Bio-Based Films and Sustainability
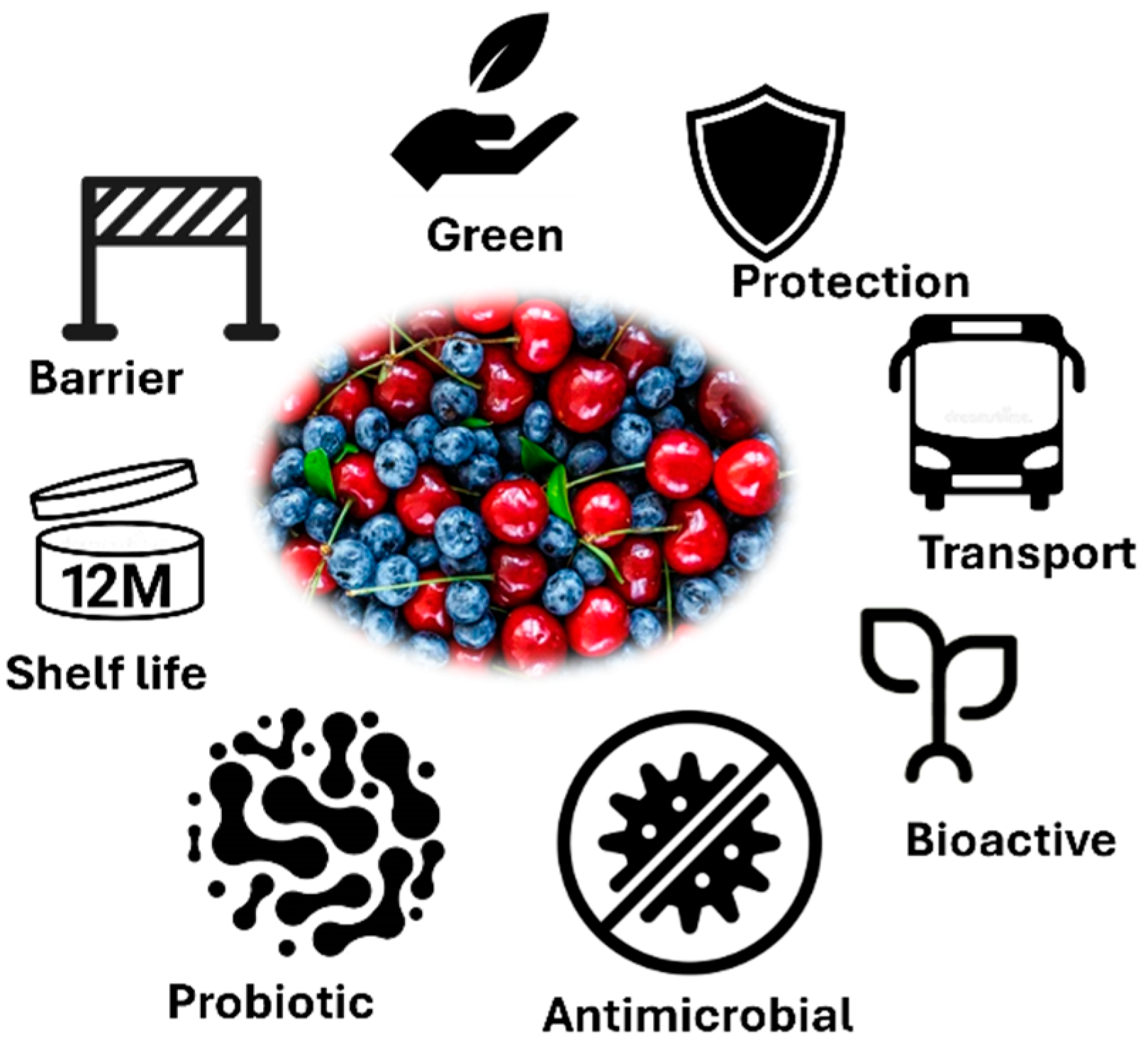
5. Edible Film and Coating Functionalities Important for the Small Fruit Industry
5.1. Bioactivity of the Edible Films and Coatings
| Fruit | Coating/Film Compounds | Activity | Reference |
|---|---|---|---|
| Blackberries | chitosan, lactic acid starch-nystose | anti-mold | [126,127] |
| Blueberry | chitosan and alginate-bases with inulin, oligofructose, and apple or orange fibers | antioxidative anti-fungal | [128] |
| chitosan and quinoa proteins with thymol oil | antimicrobial | [129] | |
| chitosan enriched with blueberry leaf extract | antimicrobial | [130] | |
| Blue honeysuckle | chitosan and Aloe vera gel | antioxidative | [131] |
| Highbush blueberry | starch and gelatin bases with cinnamon oil | antioxidative | [132] |
| Grapes | Aloe vera gel | antioxidative | [133] |
| Brazilian pine seeds starch, citric pectin, and feijoa fruit extracts | antimicrobial | [134] | |
| Raspberries | zein, Argentinian propolis extracts | antimicrobial | [135] |
| Strawberries | chia seed mucilage and bacterial cellulose nano-fiber | antioxidative | [136] |
| gum Arabic and bergamot pomace extract and oil | antioxidative | [137] | |
| xanthan gum and sodium nitroprusside | antioxidative | [138] | |
| chitosan and peony extracts | antimicrobial | [139] | |
| chitosan | antioxidative | [128] | |
| chitosan and leaf and olive pomace extracts | antifungal | [140] | |
| gum Arabic carrageenan and xanthan with lemongrass essential oil | anti-yeast and anti-mold | [141] | |
| chitosan with gelatin, starch, and sorbitol with or without monoterpenes (geraniol and thymol) | antioxidative | [142] | |
| Perishable fruits | chitosan derivatives conjugated with gallic acid | antioxidative and antimicrobial | [143] |
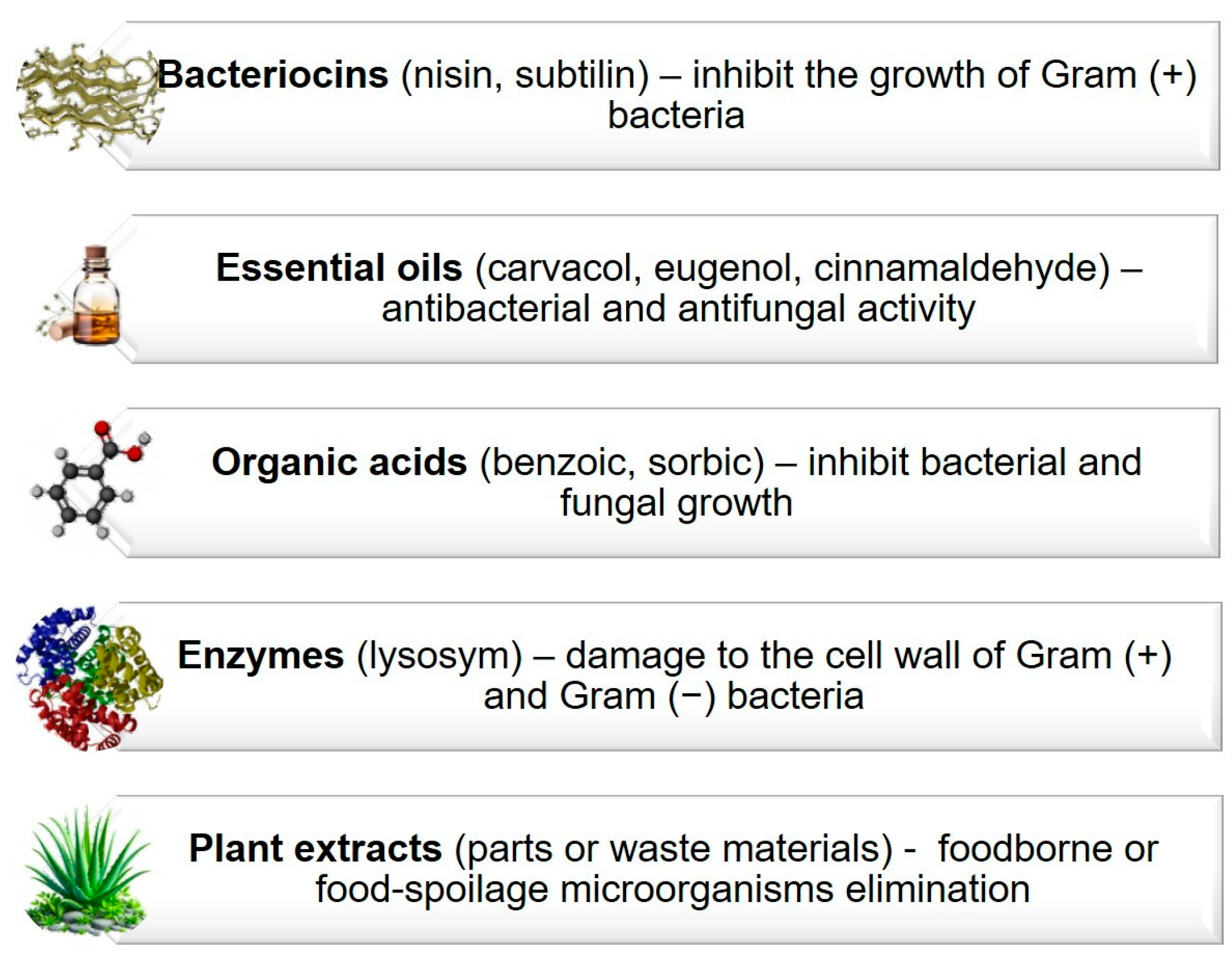
5.1.1. Antimicrobial Properties
5.1.2. Antioxidant Properties
5.1.3. Anti-Enzymatic Capacity
5.1.4. Anti-Cracking
5.2. Encapsulation Techniques and Mechanisms of Shelf-Life Extension Through Encapsulation Techniques
5.3. Pitfalls of the Bioactives Present in Edible Films and Coatings
5.4. Health Effects and Biodegradability of the Films
5.5. Innovations in the Edible Film, Coating and Packaging Industry
6. Final Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Martins, V.F.R.; Pintado, M.E.; Morais, R.M.S.C.; Morais, A.M.M.B. Recent Highlights in Sustainable Bio-Based Edible Films and Coatings for Fruit and Vegetable Applications. Foods 2024, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Palanisamy, C.P.; Srinivasan, G.P.; Panagal, M.; Kumar, S.S.D.; Mironescu, M. A comprehensive review on starch-based sustainable edible films loaded with bioactive components for food packaging. Int. J. Biol. Macromol. 2024, 274 Pt 1, 133332. [Google Scholar] [CrossRef]
- Ahmed, M.; Saini, P.; Iqbal, U.; Sahu, K. Edible microbial cellulose-based antimicrobial coatings and films containing clove extract. Food Prod. Process Nut. 2024, 6, 65. [Google Scholar] [CrossRef]
- Cosme, F.; Vilela, A. Chitin and Chitosan in the Alcoholic and Non-Alcoholic Beverage Industry: An Overview. Appl. Sci. 2021, 11, 11427. [Google Scholar] [CrossRef]
- James, K.; Long, H.; O’Hara, I.; Williams, B.; Moghaddam, L. Bio-based film development: Harnessing alginate and fucoidan extracted from Ascophyllum nodosum with glycerol and choline chloride-based solvent. Food Hydrocoll. 2024, 153, 110013. [Google Scholar] [CrossRef]
- Morton, S.; Pencheon, D.; Squires, N. Sustainable Development Goals (SDGs), and their implementation: A national global framework for health, development and equity needs a systems approach at every level. Br. Med. Bull. 2017, 124, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Priya, K.; Thirunavookarasu, N.; Chidanand, D.V. Recent Advances in Edible Coating of Food Products and Its Legislations: A Review. J. Agric. Food Res. 2023, 12, 100623. [Google Scholar] [CrossRef]
- Bose, I.; Roy, S.; Pandey, V.; Singh, R. A comprehensive review on significance and advancements of antimicrobial agents in bio-degradable food packaging. Antibiotics 2023, 12, 968. [Google Scholar] [CrossRef] [PubMed]
- Rosenboom, J.G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef] [PubMed]
- Mazhandu, Z.S.; Muzenda, E.; Mamvura, T.A.; Belaid, M.; Nhubu, T. Integrated and Consolidated Review of Plastic Waste Management and Bio-Based Biodegradable Plastics: Challenges and Opportunities. Sustainability 2020, 12, 8360. [Google Scholar] [CrossRef]
- Schaafsma, M. Natural Environment and Human Well-Being. In Life on Land; Encyclopedia of the UN Sustainable Development Goals; Leal Filho, W., Azul, A.M., Brandli, L., Lange Salvia, A., Wall, T., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Morashti, J.A.; An, Y.; Jang, H. A Systematic Literature Review of Sustainable Packaging in Supply Chain Management. Sustainability 2022, 14, 4921. [Google Scholar] [CrossRef]
- Eissenberger, K.; Ballesteros, A.; De Bisschop, R.; Bugnicourt, E.; Cinelli, P.; Defoin, M.; Demeyer, E.; Fürtauer, S.; Gioia, C.; Gómez, L.; et al. Approaches in Sustainable, Biobased Multilayer Packaging Solutions. Polymers 2023, 15, 1184. [Google Scholar] [CrossRef]
- Ungureanu, C.; Tihan, G.; Zgârian, R.; Pandelea, G. Bio-Coatings for Preservation of Fresh Fruits and Vegetables. Coatings 2023, 13, 1420. [Google Scholar] [CrossRef]
- Cocetta, G.; Natalini, A. Ethylene: Management and breeding for postharvest quality in vegetable crops. A review. Front. Plant Sci. 2022, 13, 968315. [Google Scholar] [CrossRef]
- Lufu, R.; Ambaw, A.; Opara, U.L. Water loss of fresh fruit: Influencing pre-harvest, harvest and postharvest factors. Sci. Hortic. 2020, 272, 109519. [Google Scholar] [CrossRef]
- Afonso, S.; Oliveira, I.; Ribeiro, C.; Vilela, A.; Meyer, A.S.; Gonçalves, B. Innovative edible coatings for postharvest storage of sweet cherries. Sci. Hort. 2023, 310, 111738. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Prata, C.; Freschi, M.; Barbalace, M.; Hrelia, S. Mechanisms Underlying Neurodegenerative Disorders and Potential Neuroprotective Activity of Agrifood By-Products. Antioxidants 2023, 12, 94. [Google Scholar] [CrossRef]
- Vilela, A.; Cruz, I.; Oliveira, I.; Pinto, A.; Pinto, T. Sensory and Nutraceutical Properties of Infusions Prepared with Grape Pomace and Edible-Coated Dried–Minced Grapes. Coatings 2022, 12, 443. [Google Scholar] [CrossRef]
- Burdock, G.; Carabin, I.G. Generally recognized as safe (GRAS): History and description. Toxicol. Lett. 2004, 150, 3–18. [Google Scholar] [CrossRef]
- Regulation (EC). No 1935/2004. REGULATION (EC) No 1935/2004 OF THE EUROPEAN PARLIAMENT AND OF THE COUN-CIL of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC. Off. J. Eur. Union 2004, 338, 4–17.
- Codex Alimentarius 2024. Available online: https://www.fao.org/fao-who-codexalimentarius/en/ (accessed on 5 October 2024).
- Gulzar, S.; Tagrida, M.; Prodpran, T.; Li, L.; Benjakul, S. Packaging films based on biopolymers from sea-food processing wastes: Preparation, properties, and their applications for shelf-life extension of seafoods—A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4451–4483. [Google Scholar] [CrossRef]
- Mahmud, N.; Islam, J.; Tahergorabi, R. Marine Biopolymers: Applications in Food Packaging. Processes 2021, 9, 2245. [Google Scholar] [CrossRef]
- Gürdal, A.; Çetinkaya, T. Advancements in edible films for aquatic product preservation and packaging. Rev. Aquac. 2024, 16, 997–1020. [Google Scholar] [CrossRef]
- Kumar, L.; Ramakanth, D.; Akhila, K. Edible films and coatings for food packaging applications: A review. Environ. Chem. Lett. 2022, 20, 875–900. [Google Scholar] [CrossRef]
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Sustainable use of fruit and vegetable by-products to enhance food packaging performance. Foods 2020, 9, 857. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.; Pérez-Mateos, M.; Gómez-Estaca, J.; Lopez-Caballero, E.; Giménez, B.; Montero, P. Fish gelatin: A renewable material for developing active biodegradable films. Trend Food Sci. Technol. 2019, 20, 3–16. [Google Scholar] [CrossRef]
- Park, J.; Nam, J.; Yun, H.; Jin, H.; Kwak, H. Aquatic polymer-based edible films of fish gelatin crosslinked with alginate dialdehyde having enhanced physicochemical properties. Carbohydr. Polym. 2020, 254, 117317. [Google Scholar] [CrossRef] [PubMed]
- Etxabide, A.; Uranga, J.; Guerrero, P.; Caba, K. Development of active gelatin films by means of valorisation of food processing waste: A review. Food Hydrocoll. 2017, 68, 192–198. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.; Verma, A.; Barba, F.; Singh, V.; Kumar, P.; Lorenzo, J. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Cardoso, L.; Santos, J.; Camilloto, G.; Miranda, A.; Druzian, J.; Guimarães, A. Development of active films poly (butylene adipate co-terephthalate)—PBAT incorporated with oregano essential oil and application in fish fillet preservation. Ind. Crops Prod. 2017, 108, 388–397. [Google Scholar] [CrossRef]
- Socaciu, M.-I.; Semeniuc, C.A.; Vodnar, D.C. Edible Films and Coatings for Fresh Fish Packaging: Focus on Quality Changes and Shelf-life Extension. Coatings 2018, 8, 366. [Google Scholar] [CrossRef]
- Neves, E.; Pereira, R.; Pereira, G.; Pereira, G.; Vieira, L.; Lourenço, L. Effect of polymer mixture on bio-plastic development from fish waste. Bol. Inst. Pesca. 2019, 45, e518. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Adegoke, I.I.; Oberholster, P.J.; Erasmus, M. From garbage to treasure: A review on biorefinery of organic solid wastes into valuable biobased products. Bioresour. Technol. Rep. 2023, 24, 101610. [Google Scholar] [CrossRef]
- Rațu, R.N.; Veleșcu, I.D.; Stoica, F.; Usturoi, A.; Arsenoaia, V.N.; Crivei, I.C.; Postolache, A.N.; Lipșa, F.D.; Filipov, F.; Florea, A.M.; et al. Application of agri-food by-products in the food industry. Agriculture 2023, 13, 1559. [Google Scholar] [CrossRef]
- Silva, V.; Oliveira, I.; Pereira, J.A.; Gonçalves, B. Almond by-products substrates as sustainable amendments for green bean cultivation. Plants 2024, 13, 540. [Google Scholar] [CrossRef]
- Almaraz-Sánchez, I.; Amaro-Reyes, A.; Acosta-Gallegos, J.A.; Mendoza-Sánchez, M. Processing agroindustry by-products for obtaining value-added products and reducing environmental impact. J. Chem. 2022, 2022, 3656932. [Google Scholar] [CrossRef]
- Gupta, D.; Lall, A.; Kumar, S.; Patil, T.D.; Gaikwad, K.K. Plant-based edible films and coatings for food-packaging applications: Recent advances, applications, and trends. Sustain. Food Technol. 2024, 2, 1428–1455. [Google Scholar] [CrossRef]
- Olawade, D.B.; Wada, O.Z.; Ige, A.O. Advances and recent trends in plant-based materials and edible films: A mini-review. Front. Chem. 2024, 12, 1441650. [Google Scholar] [CrossRef]
- Cazon, P.; Velázquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Pang, J.; Liu, X.; Zhang, X.; Wu, Y.; Sun, R. Fabrication of cellulose film with enhanced mechanical properties in ionic liquid 1-allyl-3-methylimidazolium chloride (AmimCl). Materials 2013, 6, 1270–1284. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Smith, D.M.; Li, Z.; Chang, T.; Xia, W.; Davis, C.S. Particle alignment effects on mechanical properties of cellulose nano-crystal thin films. Mater. Adv. 2023, 4, 1053–1064. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as edible films and coatings: Characteristics and influence on fruit and vegetable quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Pillai, A.R.S.; Eapen, A.S.; Zhang, W.; Roy, S. Polysaccharide-based edible biopolymer-based coatings for fruit preservation: A Review. Foods 2024, 13, 1529. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Kaveh, S.; Abedi, E.; Phimolsiripol, Y. Polysaccharide-based edible films/coatings for the preservation of meat and fish products: Emphasis on incorporation of lipid-based nanosystems loaded with bioactive compounds. Foods 2023, 12, 3268. [Google Scholar] [CrossRef]
- Mihalca, V.; Kerezsi, A.D.; Weber, A.; Gruber-Traub, C.; Schmucker, J.; Vodnar, D.C.; Dulf, F.V.; Socaci, S.A.; Fărcaș, A.; Mureșan, C.I.; et al. Protein-based films and coatings for food industry applications. Polymers 2021, 13, 769. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of protein-based films and coatings for food packaging: A review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Liu, D.; Zhang, C.; Yi, H.; Liu, D. Preparation, characterization, and application of edible antibacterial three-layer films based on gelatin–chitosan–corn starch–incorporated nisin. Food Pack. Shelf Life 2022, 34, 100980. [Google Scholar] [CrossRef]
- Vicente, A.A.; Cerqueira, M.A.; Hilliou, L.; Rocha, C.M.R. Protein-Based Resins for Food Packaging. In Multifunctional and Nanore-Inforced Polymers for Food Packaging; Lagarón, J.-M., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 610–648. [Google Scholar] [CrossRef]
- Jahangiri, F.; Mohanty, A.K.; Misra, M. Sustainable biodegradable coatings for food packaging: Challenges and opportunities. Green Chem. 2024, 26, 4934–4974. [Google Scholar] [CrossRef]
- Kitsiou, M.; Purk, L.; Ioannou, C.; Wantock, T.; Sandison, G.; Harle, T.; Gutierrez-Merino, J.; Klymenko, O.V.; Velliou, E. On the evaluation of the antimicrobial effect of grape seed extract and cold atmospheric plasma on the dynamics of Listeria monocyto-genes in novel multiphase 3D viscoelastic models. Int. J. Food Microbiol. 2023, 406, 110395. [Google Scholar] [CrossRef]
- Ivanov, Y.; Godjevargova, T. Antimicrobial polymer films with grape seed and skin extracts for food packaging. Microorganisms 2024, 12, 1378. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Hettiarachchy, N.; Luthra, K.; Chen, J.; Seo, H.-S.; Atungulu, G.G.; Ubeyitogullari, A. Effects of polyphenol-rich grape seed and green tea extracts on the physicochemical properties of 3D-printed edible soy protein films. Food Pack. Shelf Life 2023, 40, 101184. [Google Scholar] [CrossRef]
- Figueroa López, K.; Villabona-Ortíz, Á.; Ortega-Toro, R. Sustainable starch-based films from cereals and tubers: A comparative study on cherry tomato preservation. Polymers 2024, 16, 2913. [Google Scholar] [CrossRef]
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in applications and prospects of bioplastics and biopolymers: A review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar] [CrossRef]
- Bouchard, J.; Malalgoda, M.; Storsley, J.; Malunga, L.; Netticadan, T.; Thandapilly, S.J. Health benefits of cereal grain- and pulse-derived proteins. Molecules 2022, 27, 3746. [Google Scholar] [CrossRef]
- Wang, C.; An, X.; Lu, Y.; Li, Z.; Gao, Z.; Tian, S. Biodegradable active packaging material containing grape seed ethanol extract and corn starch/κ-carrageenan composite film. Polymers 2022, 14, 4857. [Google Scholar] [CrossRef]
- Yekta, R.; Dabbagh Moghaddam, A.; Hosseini, H.; Sharifan, A.; Hadi, S.; Hosseini-Shokouh, S.-J. Effect of using biodegradable film constituting red grape anthocyanins as a novel packaging on the qualitative attributes of emergency food bars during storage. Food Sci. Nutr. 2024, 12, 2702–2723. [Google Scholar] [CrossRef]
- Martins, B.A.; de Albuquerque, P.B.S.; de Souza, M.P. Bio-based films and coatings: Sustainable polysaccharide packaging alternatives for the food industry. J. Polym. Environ. 2022, 30, 4023–4039. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.E.; Xiaobo, Z.; Mahunu, G.K.; Arslan, M.; Abdalhai, M.; Zhihua, L. Recent developments in gum edible coating applications for fruits and vegetables preservation: A review. Carbohydr. Polym. 2019, 224, 115141. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, C.; Zhang, W.; Lei, W.; Wang, D.; Zhou, X. Novel grasshopper protein/soy protein isolate/pullulan ternary blend with hesperidin derivative for antimicrobial edible film. Arab. J. Chem. 2023, 16, 104563. [Google Scholar] [CrossRef]
- Thongsrikhem, N.; Taokaew, S.; Sriariyanun, M.; Kirdponpattara, S. Antibacterial activity in gelatin-bacterial cellulose composite film by thermally crosslinking with cinnamaldehyde towards food packaging application. Food Pack. Shelf Life. 2022, 31, 100766. [Google Scholar] [CrossRef]
- Khan, M.R.; Sadiq, M.B. Importance of gelatin, nanoparticles and their interactions in the formulation of biodegradable composite films: A review. Polym. Bull. 2021, 78, 4047–4073. [Google Scholar] [CrossRef]
- Dhakal, D.; Koomsap, P.; Lamichhane, A.; Sadiq, M.B.; Anal, A.K. Optimization of collagen extraction from chicken feet by papain hydrolysis and synthesis of chicken feet collagen based biopolymeric fibres. Food Biosci. 2018, 23, 23–30. [Google Scholar] [CrossRef]
- Alipal, J.; Puad, N.M.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gavara, R.; Catalá, R.; Hernández-Muñoz, P. The potential of proteins for producing food packaging materials: A review. Packag. Technol. Sci. 2016, 29, 203–224. [Google Scholar] [CrossRef]
- Al-Kahtani, H.A.; Jaswir, I.; Ismail, E.A.; Ahmed, M.A.; Monsur Hammed, A.; Olorunnisola, S.; Octavianti, F. Structural characteristics of camel-bone gelatin by demineralization and extraction. Int. J. Food Prop. 2017, 20, 2559–2568. [Google Scholar] [CrossRef]
- Amani, F.; Rezaei, A.; Akbari, H.; Dima, C.; Jafari, S. Active packaging films made by complex coacervation of tragacanth gum and gelatin loaded with curcumin, characterization and antioxidant activity. Foods 2022, 11, 3168. [Google Scholar] [CrossRef]
- Ștefănescu, B.E.; Socaciu, C.; Vodnar, D.C. Recent progress in functional edible food packaging based on gelatin and chitosan. Coatings 2022, 12, 1815. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Biosci. 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Fatima, S.; Mir, M.; Khan, M.; Sayyed, R.; Masih, R. The Optimization of Gelatin Extraction from Chicken Feet and the Development of Gelatin Based Active Packaging for the Shelf-Life Extension of Fresh Grapes. Sustainability 2022, 14, 7881. [Google Scholar] [CrossRef]
- Cheng, L.; Ye, A.; Yang, Z.; Gilbert, E.P.; Knott, R.; de Campo, L.; Singh, H. Small-angle X-ray scattering (SAXS) and small-angle neutron scattering (SANS) study on the structure of sodium caseinate in dispersions and at the oil-water interface: Effect of calcium ions. Food Struct. 2022, 32, 100276. [Google Scholar] [CrossRef]
- Ahmed, M.; Hameed, B.; Hummadi, E. Review of recent progress in Chitosan/chitin-carbonaceous material composites for the adsorption of water pollutants. Carbohydr. Polym. 2020, 247, 116690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of multifunctional food packaging films based on chitosan, TiO2 nanoparticles and anthocyanin-rich black plum peel extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Yildirim-Aksoy, M.; Beck, B.H. Antimicrobial activity of Chitosan and a chitosan oligomer against bacterial pathogens of Warmwater Fish. J. Appl. Microbiol. 2017, 122, 1570–1578. [Google Scholar] [CrossRef]
- Dey, A.; Neogi, S. Oxygen scavengers for Food Packaging Applications: A Review. Trends Food Sci. Technol. 2019, 90, 26–34. [Google Scholar] [CrossRef]
- Gerna, S.; D’Incecco, P.; Limbo, S.; Sindaco, M.; Pellegrino, L. Strategies for Exploiting Milk Protein Properties in Making Films and Coatings for Food Packaging: A Review. Foods 2023, 12, 1271. [Google Scholar] [CrossRef] [PubMed]
- Liyanapathiranage, A.; Dassanayake, R.S.; Gamage, A.; Karri, R.R.; Manamperi, A.; Evon, P.; Jayakodi, Y.; Madhujith, T.; Merah, O. Recent developments in edible films and coatings for fruits and vegetables. Coatings 2023, 13, 1177. [Google Scholar] [CrossRef]
- Shendurse, A.; Gopikrishna, G.; Patel, A.; Pandya, A. Milk protein-based edible films and coatings–preparation, properties, and food applications. J. Nutr. Health Food Eng. 2018, 8, 219–226. [Google Scholar] [CrossRef]
- Khan, M.; Volpe, S.; Valentino, M.; Miele, N.; Cavella, S.; Torrieri, E. Active Casein Coatings and Films for Perishable Foods: Structural Properties and Shelf-Life Extension. Coatings 2021, 11, 899. [Google Scholar] [CrossRef]
- Yangılar, F.; Oğuzhan Yıldız, P. Casein/natamycin edible films efficiency for controlling mould growth and on microbiological, chemical and sensory properties during the ripening of Kashar cheese. J. Sci. Food Agric. 2016, 96, 2328–2336. [Google Scholar] [CrossRef] [PubMed]
- Avena-Bustillos, R.J.; Cisneros-Zevallos, L.A.; Krochta, J.M.; Saltveit, M.E. Application of casein-lipid edible film emulsions to reduce white blush on minimally processed carrots. Postharvest Biol. Technol. 1994, 4, 319–329. [Google Scholar] [CrossRef]
- Chevalier, E.; Chaabani, A.; Assezat, G.; Prochazka, F.; Oulahal, N. Casein/wax blend extrusion for production of edible films as carriers of potassium sorbate—A comparative study of waxes and potassium sorbate effect. Food Pack. Shelf Life 2018, 16, 41–50. [Google Scholar] [CrossRef]
- Brzoska, N.; Müller, M.; Nasui, L.; Schmid, M. Effects of film constituents on packaging-relevant properties of sodium caseinate-based emulsion films. Prog. Org. Coat. 2018, 114, 250–258. [Google Scholar] [CrossRef]
- Pereda, M.; Marcovich, N.E.; Mosiewicki, M.A. Sodium caseinate films containing linseed oil resin as oily modifier. Food Hydrocoll. 2015, 44, 407–415. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Kia, E.M.; Ghasempour, Z.; Ehsani, A. Preparation of Active Nanocomposite Film Consisting of Sodium Caseinate, ZnO Nanoparticles and Rosemary Essential Oil for Food Packaging Applications. J. Polym. Environ. 2020, 29, 588–598. [Google Scholar] [CrossRef]
- Ranjbaryan, S.; Pourfathi, B.; Almasi, H. Reinforcing and release controlling effect of cellulose nanofiber in sodium caseinate films activated by nanoemulsified cinnamon essential oil. Food Pack. Shelf Life. 2019, 21, 100341. [Google Scholar] [CrossRef]
- Sukyai, P.; Anongjanya, P.; Bunyahwuthakul, N.; Kongsin, K.; Harnkarnsujarit, N.; Sukatta, U.; Chollakup, R.J.F.R.I. Effect of cellulose nanocrystals from sugarcane bagasse on whey protein isolate-based films. Food Res. Int. 2018, 107, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Kajthunyakarn, W.; Sakloetsakun, D.; Pongjanyakul, T. Sodium caseinate-magnesium aluminum silicate nanocomposite films for modified-release tablets. Mater. Sci. Eng. C 2018, 92, 827–839. [Google Scholar] [CrossRef]
- Schmid, M.; Prinz, T.K.; Stäbler, A.; Sängerlaub, S. Effect of Sodium Sulfite, Sodium Dodecyl Sulfate, and Urea on the Molecular Interactions and Properties of Whey Protein Isolate-Based Films. Front. Chem. 2017, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarzogh, M.; Misaghi, A.; Shahbazi, Y.; Kamkar, A. Evaluation of probiotic carboxymethyl cellulose-sodium caseinate films and their application in extending shelf life quality of fresh trout fillets. LWT 2020, 126, 109305. [Google Scholar] [CrossRef]
- Fernandes, L.M.; Guimarães, J.T.; Silva, R.; Rocha, R.S.; Coutinho, N.M.; Balthazar, C.F.; Calvalcanti, R.N.; Piler, C.W.; Pimentel, T.C.; Neto, R.P.; et al. Whey protein films added with galactooligosaccharide and xylooligosaccharide. Food Hydrocoll. 2020, 104, 105755. [Google Scholar] [CrossRef]
- Nunes, C.; Silva, M.; Farinha, D.; Sales, H.; Pontes, R.; Nunes, J. Edible Coatings and Future Trends in Active Food Packaging–Fruits’ and Traditional Sausages’ Shelf Life Increasing. Foods 2023, 12, 3308. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, L.; Wang, Q.; Wu, G.; Liu, C.; Jiang, B.; Xu, J. Effect of Antioxidant and Antimicrobial Coating Based on Whey Protein Nanofibrils with TiO2 Nanotubes on the Quality and Shelf Life of Chilled Meat. Int. J. Mol. Sci. 2019, 20, 1184. [Google Scholar] [CrossRef]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk Derived Bioactive Peptides and Their Impact on Human Health—A Review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, F.; Siroli, L.; Gardini, F.; Lanciotti, R. Contribution of Two Different Packaging Material to Microbial Contamination of Peaches: Implications in Their Microbiological Quality. Front. Microbiol. 2016, 7, 938. [Google Scholar] [CrossRef]
- Palma, A.; Mangia, N.; Fadda, A.; Barberis, A.; Schirra, M.; D’aquino, S. Effect of different film packaging on microbial growth in minimally processed cactus pear (Opuntia ficus-indica). Commun. Agric. Appl. Biol. Sci. 2013, 78, 73–82. [Google Scholar] [PubMed]
- Sanchis, E.; Ghidelli, C.; Sheth, C.; Mateos, M.; Palou, L.; Pérez-Gago, M. Integration of antimicrobial pectin-based edible coating and active modified atmosphere packaging to preserve the quality and microbial safety of fresh-cut persimmon (Diospyros kaki Thunb. cv. Rojo Brillante). J. Sci. Food Agric. 2017, 97, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Banerjee, A.; Saurabh, C.; Tye, Y.; Suriani, A.; Mohamed, A.; Karim, A.; Rizal, S.; Paridah, M. Biodegradable Films for Fruits and Vegetables Packaging Application: Preparation and Properties. Food Eng. Rev. 2018, 10, 139–153. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, C.; Xu, Y.; Huang, H.; Zhao, H.; Wang, J.; Wang, S. Synthesis and characterization of antibacterial polylactic acid film incorporated with cinnamaldehyde inclusions for fruit packaging. Int. J. Biol. Macromol. 2020, 164, 4547–4555. [Google Scholar] [CrossRef]
- Campos-Requena, V.; Rivas, B.; Pérez, M.; Figueroa, C.; Figueroa, N.; Sanfuentes, E. Thermoplastic starch/clay nano-composites loaded with essential oil constituents as packaging for strawberries—In vivo antimicrobial synergy over Botrytis cinerea. Postharvest Biol. Technol. 2017, 129, 29–36. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Zhou, W. Safety assessment of nanocomposite for food packaging application. Trends Food Sci. Technol. 2015, 45, 187–199. [Google Scholar] [CrossRef]
- Primožič, M.; Knez, Ž.; Leitgeb, M. (Bio)Nanotechnology in Food Science—Food Packaging. Nanomaterials 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Hammed, A.M. Chap.8—Nanomaterial for food packaging. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Preparation and Processing of Religious and Cultural Foods; Eaqub Ali, E., Ahmad Nizar, N.N., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 163–171. [Google Scholar] [CrossRef]
- Emamhadi, M.; Sarafraz, M.; Akbari, M.; Thai, V.; Fakhri, Y.; Linh, N.; Khaneghah, A. Nanomaterials for food packaging applications: A systematic review. Food Chem. Toxicol. 2020, 146, 111825. [Google Scholar] [CrossRef]
- Jain, A.; Ranjan, S.; Dasgupta, N.; Ramalingam, C. Nanomaterials in food and agriculture: An overview on their safety concerns and regulatory issues. Crit. Rev. Food Sci. Nut. 2018, 58, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Kaphle, A.; Navya, P.; Umapathi, A.; Daima, H.; Daima, H. Nanomaterials for agriculture, food and environment: Applications, toxicity and regulation. Environ. Chem. Let. 2018, 16, 43–58. [Google Scholar] [CrossRef]
- Ranjan, S.; Dasgupta, N.; Singh, S.; Gandhi, M. Toxicity and regulations of food nanomaterials. Environ. Chem. Lett. 2019, 17, 929–944. [Google Scholar] [CrossRef]
- Saqqa, G. Nanotechnology in Food Packaging and Food Safety. Food Sci. Nut. 2020, 3, 24–33. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; et al. Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA J. 2021, 19, 6769. [Google Scholar] [CrossRef]
- Gazsó, A.; Rose, G.; Pavlicek, A.; Gressler, S. Regulating Nanotechnological Applications for Food Contact Materials. Eur. J. Risk Regul. 2019, 10, 219–226. [Google Scholar] [CrossRef]
- Amenta, V.; Aschberger, K.; Arena, M.; Bouwmeester, H.; Moniz, F.B.; Brandhoff, M.P.; Gottardo, S.; Marvin, H.J.P.; Mech, A.; Pesudo, L.Q.; et al. Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regul. Toxicol. Pharmacol. 2015, 73, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Baghel, M.; Yadav, A.; Dhakar, M.K. Postharvest biology and technology of berries. In Postharvest Biology and Technology of Temperate Fruits; Springer: Berlin/Heidelberg, Germany, 2018; pp. 349–370. [Google Scholar]
- Moghadas, H.C.; Smith, J.S.; Tahergorabi, R. Recent Advances in the Application of Edible Coatings for Shelf-Life Extension of Strawberries: A Review. Food Bioprocess. Technol. 2024, 18, 1079–1103. [Google Scholar] [CrossRef]
- King, E.S.; Noll, A.; Glenn, S.; Bolling, B.W. Refrigerated and frozen storage impact aronia berry quality. Food Prod. Process. Nutr. 2022, 4, 3. [Google Scholar] [CrossRef]
- Gidado, M.J.; Gunny, A.A.N.; Gopinath, S.C.B.; Ali, A.; Wongs-Aree, C.; Salleh, N.H.M. Challenges of postharvest water loss in fruits: Mechanisms, influencing factors, and effective control strategies—A comprehensive review. J. Agric. Food Res. 2024, 17, 101249. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and Incorporation of Functional Ingredients in Edible Films and Coatings. Food Bioprocess. Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Kaur, j.; Singh, J.; Rasane, P.; Gupta, P.; Kaur, S.; Sharma, N.; Sowdhanya, D. Natural additives as active components in edible films and coatings. Food Biosci. 2023, 53, 102689. [Google Scholar] [CrossRef]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial edible films in food packaging: Current scenario and recent nanotechnological advancements—A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Vilaplana, R.; Guerrero, K.; Guevara, J.; Valencia-Chamorro, S. Chitosan Coatings to Control Soft Mold on Fresh Blackberries (Rubus Glaucus Benth.) during Postharvest Period. Sci. Hortic. 2020, 262, 109049. [Google Scholar] [CrossRef]
- Bersaneti, G.T.; Prudencio, S.H.; Mali, S.; Pedrine Colabone Celligoi, M.A. Assessment of a new edible film biodegradable based on starch-nystose to increase quality and the shelf life of blackberries. Food Biosci. 2021, 42, 101173. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ponce, A.G.; Moreira, M.R. Influence of polysaccharide-based edible coatings as carriers of prebiotic fibers on quality attributes of ready-to-eat fresh blueberries. J. Sci. Food Agric. 2018, 98, 2587–2597. [Google Scholar] [CrossRef]
- Medina, E.; Caro, N.; Abugoch, L.; Gamboa, A.; Díaz-Dosque, M.; Tapia, C. Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J. Food Eng. 2019, 240, 191–198. [Google Scholar] [CrossRef]
- Yang, G.; Yue, J.; Gong, X.; Qian, B.; Wang, H.; Deng, Y.; Zhao, Y. Blueberry leaf extracts incorporated chitosan coatings for preserving postharvest quality of fresh blueberries. Postharvest Biol. Technol. 2014, 92, 46–53. [Google Scholar] [CrossRef]
- Qiao, J.; Li, D.; Guo, L.; Hong, X.; He, S.; Huo, J.; Sui, X. Enhancing Postharvest Quality and Antioxidant Capacity of Blue Honeysuckle with Chitosan and Aloe vera Gel Edible Coatings During Storage. Foods 2024, 13, 630. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Sójka, M.; Skóra, B.; Balawejder, M. Quality and antioxidant activity of highbush blueberry fruit coated with starch-based and gelatine-based film enriched with cinnamon oil. Food Control 2022, 138, 109015. [Google Scholar] [CrossRef]
- Nia, A.E.; Taghipour, S.; Siahmansour, S. Pre-harvest application of chitosan and postharvest Aloe vera gel coating enhances quality of table grape (Vitis vinifera L. cv.‘Yaghouti’) during postharvest period. Food Chem. 2021, 347, 129012. [Google Scholar]
- Sganzerla, W.G.; da Rosa, C.G.; da Silva, A.P.G.; Ferrareze, J.P.; Azevedo, M.S.; Forster-Carneiro, T.; Nunes, M.R.; de Lima Veeck, A.P. Application in situ of biodegradable films produced with starch, citric pectin and functionalized with feijoa (Acca sellowiana (Berg) Burret) extracts: An effective proposal for food conservation. Int. J. Biol. Macromol. 2021, 189, 544–553. [Google Scholar] [CrossRef]
- Moreno, M.A.; Vallejo, A.M.; Ballester, A.-R.; Zampini, C.; Isla, M.I.; López-Rubio, A.; Fabra, M.J. Antifungal Edible Coatings Containing Argentinian Propolis Extract and Their Application in Raspberries. Food Hydrocoll. 2020, 107, 105973. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Rahmati-Joneidabad, M.; Noshad, M. Effect of chia seed mucilage/bacterial cellulose edible coating on bioactive compounds and antioxidant activity of strawberries during cold storage. Int. J. Biol. Macromol. 2021, 190, 618–623. [Google Scholar] [CrossRef]
- De Bruno, A.; Gattuso, A.; Ritorto, D.; Piscopo, A.; Poiana, M. Effect of edible coating enriched with natural antioxidant extract and bergamot essential oil on the shelf life of strawberries. Foods 2023, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Gill, P.P.S.; Singh, N.; Jawandha, S.K.; Arora, R.; Singh, A. Composite coating of xanthan gum with sodium nitroprusside alleviates the quality deterioration in strawberry fruit. Food Hydrocoll. 2024, 155, 110208. [Google Scholar] [CrossRef]
- Pagliarulo, C.; Sansone, F.; Moccia, S.; Russo, G.L.; Aquino, R.P.; Salvatore, P.; Di Stasio, M.; Volpe, M.G. Preservation of Strawberries with an Antifungal Edible Coating Using Peony Extracts in Chitosan. Food Bioprocess. Technol. 2016, 9, 1951–1960. [Google Scholar] [CrossRef]
- Khalifa, I.; Barakat, H.; El-Mansy, H.A.; Soliman, S.A. Improving the shelf-life stability of apple and strawberry fruits applying chitosan-incorporated olive oil processing residues coating. Food Pack. Shelf Life. 2016, 9, 10–19. [Google Scholar] [CrossRef]
- Siracusa, V.; Romani, S.; Gigli, M.; Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Lotti, N. Characterization of active edible films based on citral essential oil, alginate and pectin. Materials 2018, 11, 1980. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.; Rabea, E.I.; AM El-Nouby, M.; Ismail, R.I.; Taktak, N.E. Strawberry shelf life, composition, and enzymes activity in response to edible chitosan coatings. Int. J. Fruit Sci. 2017, 17, 117–136. [Google Scholar] [CrossRef]
- Lee, C.R.; Lee, S.J.; Kim, T.I.; Chathuranga, K.; Lee, J.S.; Kim, S.; Park, W.H. Chitosan-gallic acid conjugate edible coating film for perishable fruits. Food Chem. 2025, 463, 141322. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; González-González, R.B.; Flores-Contreras, E.A.; Araújo, R.G.; Chen, W.N.; Alfaro-Ponce, M.; Iqbal, H.M.N.; Melchor-Martínez, E.M.; Parra-Saldívar, R. Nano and Technological Frontiers as a Sustainable Platform for Postharvest Preservation of Berry Fruits. Foods 2023, 12, 3159. [Google Scholar] [CrossRef]
- Raybaudi Massilia, R.M.; Mosqueda Melgar, J.; Soliva Fortuny, R.; Martín Belloso, O. Control of pathogenic and spoilage microorganisms in fresh cut fruits and fruit juices by traditional and alternative natural antimicrobials. Compr. Rev. Food Sci. Food Saf. 2009, 8, 157–180. [Google Scholar] [CrossRef] [PubMed]
- Barth, M.; Hankinson, T.R.; Zhuang, H.; Breidt, F. Microbiological spoilage of fruits and vegetables. In Compendium of the Microbiological Spoilage of Foods and Beverages; Springer: Berlin/Heidelberg, Germany, 2009; pp. 135–183. [Google Scholar]
- Ragaert, P.; Devlieghere, F.; Debevere, J. Role of microbiological and physiological spoilage mechanisms during storage of minimally processed vegetables. Postharvest Biol. Technol. 2007, 44, 185–194. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Edible coatings and antimicrobial nanoemulsions for enhancing shelf life and reducing foodborne pathogens of fruits and vegetables: A review. Sustain. Mater. Technol. 2020, 26, e00215. [Google Scholar] [CrossRef]
- Li, X.; Song, Y.; Yang, X.; Xu, J.; Zhang, X.; Sun, H.; Barth, M.; Hankinson, T.R.; Zhuang, H.; Breidt, F.; et al. Advances in formulation, functionality, and application of edible coatings on fresh produce and fresh-cut products: A review. Food Bioprocess. Technol. 2023, 239, 1951–1960. [Google Scholar] [CrossRef]
- Cruz-Monterrosa, R.G.; Rayas-Amor, A.A.; González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Aguilar-Toalá, J.E.; Liceaga, A.M. Application of Polysaccharide-Based Edible Coatings on Fruits and Vegetables: Improvement of Food Quality and Bioactivities. Polysaccharides 2023, 4, 99–115. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent advances in chitosan-based applications—A review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef] [PubMed]
- Riseh, R.S.; Vatankhah, M.; Hassanisaadi, M.; Shafiei-Hematabad, Z.; Kennedy, J.F. Advancements in coating technologies: Unveiling the potential of chitosan for the preservation of fruits and vegetables. Int. J. Biol. Macromol. 2024, 254, 127677. [Google Scholar]
- Jiang, H.; Zhang, W.; Chen, L.; Liu, J.; Cao, J.; Jiang, W. Recent Advances in Guar Gum-Based Films or Coatings: Diverse Property Enhancement Strategies and Applications in Foods. Food Hydrocoll. 2023, 136, 108278. [Google Scholar] [CrossRef]
- Tiamiyu, Q.O.; Adebayo, S.E.; Yusuf, A.A. Gum Arabic Edible Coating and Its Application in Preservation of Fresh Fruits and Vegetables: A Review. Food Chem. Adv. 2023, 2, 100251. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, A.R.L.; Basumatary, I.B.; Nayak, A.; Dutta, D.; Konwar, J.; Purkayastha, M.; Das Mukherjee, A. Recent progress in pectin extraction and their applications in developing films and coatings for sustainable food packaging: A review. Int. J. Biol. Macromol. 2023, 239, 124281. [Google Scholar] [CrossRef]
- Xu, B.; Wu, S. Preservation of Mango Fruit Quality Using Fucoidan Coatings. LWT 2021, 143, 111150. [Google Scholar] [CrossRef]
- Anisha, G.S.; Augustianath, T.; Padmakumari, S.; Singhania, R.R.; Pandey, A.; Patel, A.K. Ulvan from Green Macroalgae: Bioactive Properties Advancing Tissue Engineering, Drug Delivery Systems, Food Industry, Agriculture and Water Treatment. Bioresour. Technol. Rep. 2023, 22, 101457. [Google Scholar] [CrossRef]
- Daniloski, D.; Petkoska, A.T.; Lee, N.A.; Bekhit, A.E.D.; Carne, A.; Vaskoska, R.; Vasiljevic, T. Active edible packaging based on milk proteins: A route to carry and deliver nutraceuticals. Trends Food Sci. Technol. 2021, 111, 688–705. [Google Scholar] [CrossRef]
- Aranda-Ledesma, N.E.; Bautista-Hernández, I.; Rojas, R.; Aguilar-Zárate, P.; del Pilar Medina-Herrera, N.; Castro-López, C.; Guadalupe Martínez-Ávila, G.C. Candelilla wax: Prospective suitable applications within the food field. LWT 2022, 159, 113170. [Google Scholar] [CrossRef]
- Iseppi, R.; Stefani, S.; de Niederhausern, S.; Bondi, M.; Sabia, C.; Messi, P. Characterization of Anti-Listeria monocytogenes Properties of two Bacteriocin-Producing Enterococcus mundtii Isolated from Fresh Fish and Seafood. Curr. Microbiol. 2019, 76, 1010–1019. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Yagafarov, N.Z.; Kurasova, M.N.; Dysin, A.P.; Kurliuk, A.V.; Shakola, T.V.; et al. Active antibacterial food coatings based on blends of succinyl chitosan and triazole betaine chitosan derivatives. Food Pack. Shelf Life. 2020, 25, 100534. [Google Scholar] [CrossRef]
- Çakmak, H.; Özselek, Y.; Turan, O.Y.; Fıratlıgil, E.; Karbancioğlu-Güler, F. Whey protein isolate edible films incorporated with essential oils: Antimicrobial activity and barrier properties. Polym. Degrad. Stab. 2020, 179, 109285. [Google Scholar] [CrossRef]
- Oliveira Filho, J.G.; Duarte, L.G.R.; Silva, Y.B.B.; Milan, E.P.; Santos, H.V.; Moura, T.C.; Bandini, V.P.; Vitolano, L.E.S.; Nobre, J.J.C.; Moreira, C.T.; et al. Novel Approach for Improving Papaya Fruit Storage with CarnaubaWax Nanoemulsion in Combination with Syzigium aromaticum and Mentha spicata Essential Oils. Coatings 2023, 13, 847. [Google Scholar] [CrossRef]
- Das, S.; Vishakha, K.; Banerjee, S.; Mondal, S.; Ganguli, A. Sodium Alginate-Based Edible Coating Containing Nanoemulsion of Citrus sinensis Essential Oil Eradicates Planktonic and Sessile Cells of Food-Borne Pathogens and Increased Quality Attributes of Tomatoes. Int. J. Biol. Macromol. 2020, 162, 1770–1779. [Google Scholar] [CrossRef]
- Wani, S.M.; Gull, A.; Ahad, T.; Malik, A.R.; Ganaie, T.A.; Masoodi, F.A.; Gani, A. Effect of gum Arabic, xanthan and carrageenan coatings containing antimicrobial agent on postharvest quality of strawberry: Assessing the physicochemical, enzyme activity and bioactive properties. Int. J. Biol. Macromol. 2021, 183, 2100–2108. [Google Scholar] [CrossRef]
- De Freitas, C.A.S.; de Sousa, P.H.M.; Soares, D.J.; da Silva, J.Y.G.; Benjamin, S.R.; Guedes, M.I.F. CarnaubaWax Uses in Food—A Review. Food Chem. 2019, 291, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Chen, T.; Liu, Y. The physical properties, antioxidant and antimicrobial activity of chitosan–gelatin edible films incorporated with the extract from hop plant. Polym. Bull. 2021, 78, 3607–3624. [Google Scholar] [CrossRef]
- Gaspar, M.C.; Braga, M.E. Edible films and coatings based on agrifood residues: A new trend in the food packaging research. Curr. Opin. Food Sci. 2023, 50, 101006. [Google Scholar] [CrossRef]
- Bodana, V.; Swer, T.L.; Kumar, N.; Singh, A.; Samtiya, M.; Sari, T.P.; Babar, O.A. Development and characterization of pomegranate peel extract-functionalized jackfruit seed starch-based edible films and coatings for prolonging the shelf life of white grapes. Int. J. Biol. Macromol. 2024, 254, 127234. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, Y.; Bi, J.-L.; Yang, X.-Q.; Huang, Y.; Zhao, X.; Hu, Y.; Cai, Q.-N. Constitutive and induced activities of defense-related enzymes in aphid-resistant and aphid-susceptible cultivars of wheat. J. Chem. Ecol. 2009, 35, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Defilippi, B.G.; Ejsmentewicz, T.; Covarrubias, M.P.; Gudenschwager, O.; Campos-Vargas, R. Changes in cell wall pectins and their relation to postharvest mesocarp softening of “Hass” avocados (Persea americana Mill.). Plant Physiol. Biochem. 2018, 128, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions, and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Santos, M.; Egea-Cortines, M.; Gonçalves, B.; Matos, M. Molecular mechanisms involved in fruit cracking: A review. Front. Plant Sci. 2023, 14, 1130857. [Google Scholar] [CrossRef]
- Tufan, E.G.; Borazan, A.A.; Koçkar, Ö.M. A review on edible film and coating applications for fresh and dried fruits and vegetables. BŞEÜ Fen Bilimleri Dergisi (BSEU J. Sci.) 2021, 8, 1073–1085. [Google Scholar] [CrossRef]
- Silveira, A.C.; Aguayo, E.; Chisari, M.; Artés, F. Calcium salts and heat treatment for quality retention of fresh-cut ‘Galia’ melon. Postharvest Biol. Technol. 2011, 62, 77–84. [Google Scholar] [CrossRef]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- Kupervaser, M.G.; Traffano-Schiffo, M.V.; Dellamea, M.L.; Flores, S.K.; Sosa, C.A. Trends in starch-based edible films and coatings enriched with tropical fruit extracts: A review. Food Hydrocoll. Health 2023, 4, 100138. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Marasciulo, C.; Maggi, F.; Caprioli, G.; Mustafa, A.M.; Fini, P.; De Vietro, N.; Aresta, A.M.; Cosma, P. Realizing eco-friendly water-resistant sodium-alginate-based films blended with a polyphenolic aqueous extract from grape pomace waste for potential food packaging applications. Int. J. Mol. Sci. 2023, 24, 11462. [Google Scholar] [CrossRef]
- Metha, C.; Pawar, S.; Suvarna, V. Recent advancements in alginate-based films for active food packaging applications. Sustain. Food Technol. 2024, 2, 1246–1265. [Google Scholar] [CrossRef]
- Rodríguez, G.M.; Sibaja, J.C.; Espitia, P.J.; Otoni, C.G. Antioxidant active packaging based on papaya edible films incorporated with Moringa oleifera and ascorbic acid for food preservation. Food Hydrocoll. 2020, 103, 105630. [Google Scholar] [CrossRef]
- Muhammad, D.R.A.; Doost, A.S.; Gupta, V.; bin Sintang, M.D.; Van de Walle, D.; Van der Meeren, P. Stability and functionality of xanthan gum–shellac nanoparticles for the encapsulation of cinnamon bark extract. Food Hydrocoll. 2020, 100, 105377. [Google Scholar] [CrossRef]
- Dahiya, D.; Terpou, A.; Dasenaki, M.; Nigam, P.S. Current status and future prospects of bioactive molecules delivered through sustainable encapsulation techniques for food fortification. Sustain. Food Technol. 2023, 1, 500–510. [Google Scholar] [CrossRef]
- Yan, C.; Kim, S.R.; Ruiz, D.R.; Farmer, J.R. Microencapsulation for food applications: A review. ACS Appl. Bio Mater. 2022, 5, 5497–5512. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Dima, E.; Assadpour, S.; Dima, S.; Jafari, M. Bioactive-loaded nanocarriers for functional foods: From designing to bioavailability. Curr. Opin. Food Sci. 2020, 33, 21–29. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K. Scope of nanotechnology in nutraceuticals. In Nanotechnology Applications in Food: Flavor, Stability, Nutrition and Safety; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 43–63. [Google Scholar]
- Shavronskaya, D.O.; Noskova, A.O.; Skvortsova, N.N.; Adadi, P.; Nazarova, E.A. Encapsulation of Hydrophobic Bioactive Substances for Food Applications: Carriers, Techniques, and Biosafety. J. Food Process. Preserv. 2023, 2023, 5578382. [Google Scholar] [CrossRef]
- Ayyaril, S.S.; Shanableh, A.; Bhattacharjee, S.; Rawas-Qalaji, M.; Cagliani, R.; Shabib, A. Recent progress in micro and nano-encapsulation techniques for environmental applications: A review. Results Eng. 2023, 18, 101094. [Google Scholar] [CrossRef]
- Lima, N.G.; Almeida, E.L.; Carvalho, H.J.; Lopes, P.H.; Nelson, D.L. Advances in the encapsulation of bioactive compounds present in Brazilian fruits. Int. J. Pharm. Excip. 2023, 14, 85–95. [Google Scholar]
- Bu, Y.; He, W.; Zhu, L.; Zhu, W.; Li, J.; Liu, H.; Li, X. Effects of different wall materials on stability and umami release of microcapsules of Maillard reaction products derived from Aloididae aloidi. Int. J. Food Sci. 2021, 56, 6484–6496. [Google Scholar] [CrossRef]
- Kopecna, K.; Secanin, B.; Tešević, V.; Yelic, M. Encapsulation techniques for improving stability and delivery of vitamins and minerals. Int. J. Mol. Sci. 2020, 21, 1303. [Google Scholar] [CrossRef]
- Shahid, S.; Leghari, A.A.; Farid, M.S.; Saeed, M.; Anwar, S.; Anjum, R.; Abbas, Z. Role of active food packaging developed from microencapsulated bioactive ingredients in quality and shelf life enhancement: A review. Am. J. Sci. 2021, 17, 12–28. [Google Scholar] [CrossRef]
- Dos Santos, P.P.; Flôres, S.H.; de Oliveira Rios, A.; Chiste, R.C. Biodegradable polymers as wall materials to the synthesis of bioactive compound nanocapsules. Trends Food Sci. Technol. 2016, 53, 23–33. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Heinrich, J. Encapsulation of aroma. In Encapsulation Technologies for Food Active Ingredients and Food Processing; Zuidam, N.J., Nedovic, V.A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 127–160. [Google Scholar]
- Milanovic, J.; Manojlovic, V.; Levic, S.; Rajic, N.; Nedovic, V.; Bugarski, B. Microencapsulation of Flavors in Carnauba Wax. Sensors 2010, 10, 901–912. [Google Scholar] [CrossRef]
- Porzio, M.A. Flavor delivery and product development. Food Technol. 2007, 1, 22–29. [Google Scholar]
- Zuidam, N.J.; Shimoni, E. Overview of Microencapsulates for Use in Food Products or Processes and Methods to Make Them. In Encapsulation Technologies for Food Active Ingredients and Food Processing; Zuidam, N.J., Nedovic, V.A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 3–31. [Google Scholar]
- Dewettinck, K.; Huyghebaert, A. Fluidized bed coating in food technology. Trend Food Sci. Technol. 1999, 10, 163–168. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, X.; Zheng, H.; Li, J.; Wu, X.; Xu, J.; Zhen, Z.; Du, C. The application of encapsulation technology in the food Industry: Classifications, recent advances, and perspectives. Food Chem. X 2024, 21, 101240. [Google Scholar] [CrossRef]
- Gouin, S. Microencapulation: Industrial appraisal of existing technologies and trends. Trends Food Sci. Technol. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Boostani, S.; Jafari, S.M. A comprehensive review on the controlled release of encapsulated food ingredients, fundamental concepts to design and applications. Tends Food Sci. Technol. 2021, 109, 303–321. [Google Scholar] [CrossRef]
- Boostani, S.; Jafari, S.M. Controller release of nanoencapsulated food ingredients. In Nanoencapsulation in the Food Industry, Release and Bioavailability of Nanoencapsulated Food Ingredients; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; Chapter 2; Volume 5, pp. 27–78. [Google Scholar]
- Peanparkdee, M.; Iwamoto, S. Encapsulation for Improving in Vitro Gastrointestinal Digestion of Plant Polyphenols and Their Application in Food Products. Food Rev. Int. 2022, 38, 335–353. [Google Scholar] [CrossRef]
- Duguma, H. Potential applications and limitations of edible coatings for maintaining tomato quality and shelf life. Int. J. Food Sci. 2020, 57, 1353–1366. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Thakur, N.; Kajla, P.; Kumar, M.; Trif, M. Natural antimicrobials as additives for edible food packaging applications: A Review. Foods 2021, 10, 2282. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, E.I.; Molehin, O.R.; Ajayi, O.O.; Adeloju, E.O.; Oladele, J.O. Chapter 28—Application of chitosan-coated foods, fruits and vegetables on inflammation in metabesity. In Next Generation Anochitosan; Adetunji, C., Hefft, D., Jeevanandam, J., Danquah, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 431–446. [Google Scholar]
- Tan, M.T.H.; Eshaghi Gorji, M.; Toh, J.Y.L.; Park, A.Y.; Li, Y.; Gong, Z.; Li, D. Fucoidan from Fucus versiculosus Can Inhibit Human Norovirus Replication by Enhancing the Host Innate Immune Response. J. Funct. Foods 2022, 95, 105149. [Google Scholar] [CrossRef]
- Pedreiro, S.; Figueirinha, A.; Silva, A.S.; Ramos, F. Bioactive edible films and coatings based in gums and starch: Phenolic enrichment and foods application. Coatings 2021, 11, 1393. [Google Scholar] [CrossRef]
- Mohamed Abdoul-Latif, F.; Ainane, A.; Houmed Aboubaker, I.; Mohamed, J.; Ainane, T. Exploring the Potent Anticancer Activity of Essential Oils and Their Bioactive Compounds: Mechanisms and Prospects for Future Cancer Therapy. Pharmaceuticals 2023, 16, 1086. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Batista, R.A.; Azeredo, H.M.C.; Otoni, C.G. Probiotics and their potential application in active edible films and coatings. Food Res. Int. 2016, 90, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Yonekura, L.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG in prebiotic edible films. Food Chem. 2014, 159, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Pavli, F.; Tassou, C.; Nychas, G.J.E.; Chorianopoulos, N. Probiotic incorporation in edible films and coatings: Bioactive solution for functional foods. Int. J. Mol. Sci. 2018, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Martín Pérez, L. Biodegradability of Antimicrobial Edible Films and Coatings: What’s the Real Thing? Nutr. Food Sci. Int. J. 2019, 8, 555748. [Google Scholar] [CrossRef]
- EN13432:2000; Packaging–Requirements for Packaging Recoverable Through Composting and Biodegradation–Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. European Commission Standard: Brussels, Belgium, 2000; pp. 1–26.
- Frangopoulos, T.; Marinopoulou, A.; Goulas, A.; Likotrafiti, E.; Rhoades, J.; Petridis, D.; Kannidou, E.; Stamelos, A.; Theodoridou, M.; Arampatzidou, A.; et al. Optimizing the Functional Properties of Starch-Based Biodegradable Films. Foods 2023, 12, 2812. [Google Scholar] [CrossRef] [PubMed]
- Thakwani, Y.; Karwa, A.; Kumar, B.G.P.; Purkait, M.K.; Changmai, M. A composite starch-date seeds extract based biodegradable film for food packaging application. Food Biosci. 2023, 54, 102818. [Google Scholar] [CrossRef]
- Vargas, V.H.; Marczak, L.D.F.; Flôres, S.H.; Mercali, G.D. Morphology and functional properties of gelatin-based films modified by UV radiation and bacterial cellulose nanofibers. J. Food Process Eng. 2023, 46, e14399. [Google Scholar] [CrossRef]
- Rohadi, T.N.T.; Ridzuan, M.J.M.; Majid, M.S.A.; Sulaiman, M.H. Biodegradability of bioplastic film using different regions of Pennisetum purpureum incorporated with gelatine and chitosan. Int. J. Environ. Sci. Technol. 2023, 20, 10313–10324. [Google Scholar] [CrossRef]
- Gan, L.; Jiang, G.; Yang, Y.; Zheng, B.; Zhang, S.; Li, X.; Tian, Y.; Peng, B. Development and Characterization of Levan/Pullulan/Chitosan Edible Films Enriched with ε-Polylysine for Active Food Packaging. Food Chem. 2022, 388, 132989. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyaya, S.; Sahaa, N.; Brodnjakb, U.V.; Sáha, P. Bacterial cellulose and guar gum based modified PVP-CMC hydrogel films: Characterized for packaging fresh berries. Food Packag. Shelf Life. 2019, 22, 100402. [Google Scholar] [CrossRef]
- Chettri, S.; Sharma, N.; Mohite, M. Edible coatings and films for shelf-life extension of fruit and vegetables. Adv. Biomater. 2023, 154, 213632. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M. Review on nanoparticles and nanostructured materials: Bioimaging, biosensing, drug delivery, tissue engineering, Antimicrobial, and Agro-Food Applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Forough, M.; Amjadi, S.; Kouzegaran, V.J.; Almasi, H.; Garavand, F. Plant protein-based nanocomposite films: A review on the used nanomaterials, characteristics, and food packaging applications. Crit. Rev. Food Sci. Nutr. 2022, 63, 9667–9693. [Google Scholar] [CrossRef]
- Jafari, S. An overview of nanoencapsulation techniques and their classification. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Academic Press: Cambridge, MA, USA, 2017; pp. 1–34. [Google Scholar] [CrossRef]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of polysaccharide-based materials as advanced food packaging. Molecules 2019, 25, 135. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Bhuiyan, M.H.R.; Ngadi, M. Challenges and prospects of plant-protein-based 3D printing. Foods 2023, 12, 4490. [Google Scholar] [CrossRef] [PubMed]




| Material | Provenience | Applications | Properties | Ref. |
|---|---|---|---|---|
| Starch | Starch-rich crops (Vegetal origin) | Food and beverage, cosmetics, pharmaceuticals, and consumer goods | Enhanced shelf life and improved food safety; good printability and sealability, allowing for branding and labeling customization while ensuring product integrity and safety; | [3] |
| Cellulose | Microbial cellulose is obtained by cultivating Acetobacter aceti in fruit waste media (orange, kiwi, and guava fruit peel, blended in 200 mL distilled water). (Microbial origin) | Food, biomedical, biosensing, and environmental applications. | Biodegradability, biocompatibility, high crystallinity, non-toxicity, hydrophilicity, elevated tensile strength, extensive polymerization, in-situ moldability, and porosity; Extending the nutritional value and shelf life | [4] |
| Chitosan | A polysaccharide of N-acetyl D-glucosamine and D-glucosamine units, obtained by the partial deacetylation of chitin exoskeletons of insects, cephalopods, and crustaceans. (Animal origin) | Food, biomedical, biosensing, and environmental applications. | Antimicrobial activity, biocompatibility, biodegradability, and non-toxic profile | [5] |
| Alginate | Alginate, a polysaccharide derived from brown macroalgae (Seaweed origin) | Packaging and storing perishable food items | Extend the freshness and shelf-life of perishable food items; Inhibits lipid oxidation in meat and animal-based products; Allows respiration of fruit and vegetables; Antimicrobial properties. | [6] |
| Encapsulation System | Principle | Features |
|---|---|---|
| Spray–Drying Technology | Emulsion dried into granules using atomization in the hot air stream | Advantages: short drying process, good solubility, low cost and process operation, proper transportation and storage. Disadvantages: uneven particle dimension, partial rupture of core material. |
| Spray–Cooling–Drying | Material combined with emulsifier and wall material. Creation of microcapsules by freezing at a temperature of −20 °C and then placing freeze-dried | Advantages: minimal core damage. Disadvantages: it requires crushing and sieving and has high equipment requirements. |
| Fluidized Bed | Hot air-flow of fluidized bed is used to wrap core material with wall material solution | Advantage: uniform and moderate wall thickness. Disadvantages: the surface is easy to damage and has a low yield. |
| Oil-Phase Separating Method | The core material is added to a shell polymer and solvent solution, mixed and dispersed into a suspension, then added to a nonsolvent liquid and precipitation solvent, encapsulating the core material into microcapsules. | Advantages: simple equipment needs and a varied range of shell materials. Disadvantages: potential environmental pollution from solvents. |
| Extrusion | The core and wall material emulsion is extruded through pore membranes at low temperatures. Wall material in direct contact with a dehydrating agent forms microcapsules due to dehydration. | Advantages: improved closure of the capsule. Disadvantages: low surface oil content. |
| Interfacial Polymerization | Two monomers of different solubility are uniformly added to the continuous phase of wall material and the dispersed phase of core material. Polycondensation occurs at the phase interface, resulting in the formation of microcapsules. | Advantages: good densification and faster reaction rates. Disadvantage: retention of the monomers in microcapsules. |
| Air Suspension | An aqueous solution containing wall material is sprayed on the surface of the suspended core material. Solvent vaporization is performed using low hot air. | Advantages: a range of wall materials and uniform wall thickness of the capsule. Disadvantages: control factors and limitations regarding core material. |
| Electrostatic Spinning | Charged polymer solution flow deformed in an electrostatic field, followed by solvent evaporation or melt–cooling. | Advantages: easy to operate, no organic solvents, low cost, high efficiency. Disadvantages: difficulty in obtaining nanofiber filaments or separate staple fibers. |
| Phases Emulsion Method | Core material is combined with an emulsifier and mixed with wall material to form an emulsion. Microcapsules are obtained by curing after removing the solvent from the suspension emulsion. | Advantages: simple, high stability. Disadvantages: residual toxic organic solvents. |
| Tiny Hole-Coagulation Method | Microcapsules are formed by placing a mixture of core and wall materials into a tiny hole-coagulation device, followed by calcium chloride or aldehydes, for a cross-linking reaction. | Advantages: simple operation and absence of the use of organic solvents. Disadvantages: larger particle size and low encapsulation rate. |
| Liposome Encapsulation Technology | The wall material consists of spherical or approximately spherical vesicles with an biofilm structure composed of phospholipid bilayers or thin layers. | Advantages: reduced degradation in extreme environments. Disadvantages: difficult elimination of organic solvents, challenging storage conditions. |
| Emulsion Encapsulation | In oil–an oil-water system, emulsion is formed, creating colloidal particles to be encapsulated. | Advantages: simple process, improved digestibility, antibacterial and antioxidant activity. Disadvantages: low stability and demulsification in extreme environments. |
| Complex Coacervation | After dilution, pH value, or temperature adjustment, the reaction between the wall materials is condensed and precipitated. | Advantages: minor preparation process, high efficiency. Disadvantages: difficult control of reaction conditions and high production costs. |
| Nanoencapsulation Technology | Bioactive substances are encapsulated through nanocomposite, nanoemulsification, and nanoconstruction. | Advantages: stability, improved in vivo absorption, effective core quality improvement, safety, and functionality. Disadvantages: obliges high precision. |
| Supercritical Impinging Stream Technology | The solute is dissolved in supercritical fluid to saturate and then introduced into the low-pressure chamber, causing it to precipitate in tiny particles. | The advantages are the low-temperature process, minimal residual solvent in particles, recycling of solvent and antisolvent, and uniform particle size. The disadvantages are the nozzle anti-blocking, sealing simplification, and equipment investment. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, I.; Pinto, T.; Afonso, S.; Karaś, M.; Szymanowska, U.; Gonçalves, B.; Vilela, A. Sustainability in Bio-Based Edible Films, Coatings, and Packaging for Small Fruits. Appl. Sci. 2025, 15, 1462. https://doi.org/10.3390/app15031462
Oliveira I, Pinto T, Afonso S, Karaś M, Szymanowska U, Gonçalves B, Vilela A. Sustainability in Bio-Based Edible Films, Coatings, and Packaging for Small Fruits. Applied Sciences. 2025; 15(3):1462. https://doi.org/10.3390/app15031462
Chicago/Turabian StyleOliveira, Ivo, Teresa Pinto, Sílvia Afonso, Monika Karaś, Urszula Szymanowska, Berta Gonçalves, and Alice Vilela. 2025. "Sustainability in Bio-Based Edible Films, Coatings, and Packaging for Small Fruits" Applied Sciences 15, no. 3: 1462. https://doi.org/10.3390/app15031462
APA StyleOliveira, I., Pinto, T., Afonso, S., Karaś, M., Szymanowska, U., Gonçalves, B., & Vilela, A. (2025). Sustainability in Bio-Based Edible Films, Coatings, and Packaging for Small Fruits. Applied Sciences, 15(3), 1462. https://doi.org/10.3390/app15031462










