Traceability of Surgical Instruments: A Systematic Review
Abstract
1. Introduction
- What are the different levels of a traceability system?
- What are the different techniques for identifying surgical instruments?
- What are the benefits of a traceability system?
- What are the recommendations for future traceability work?
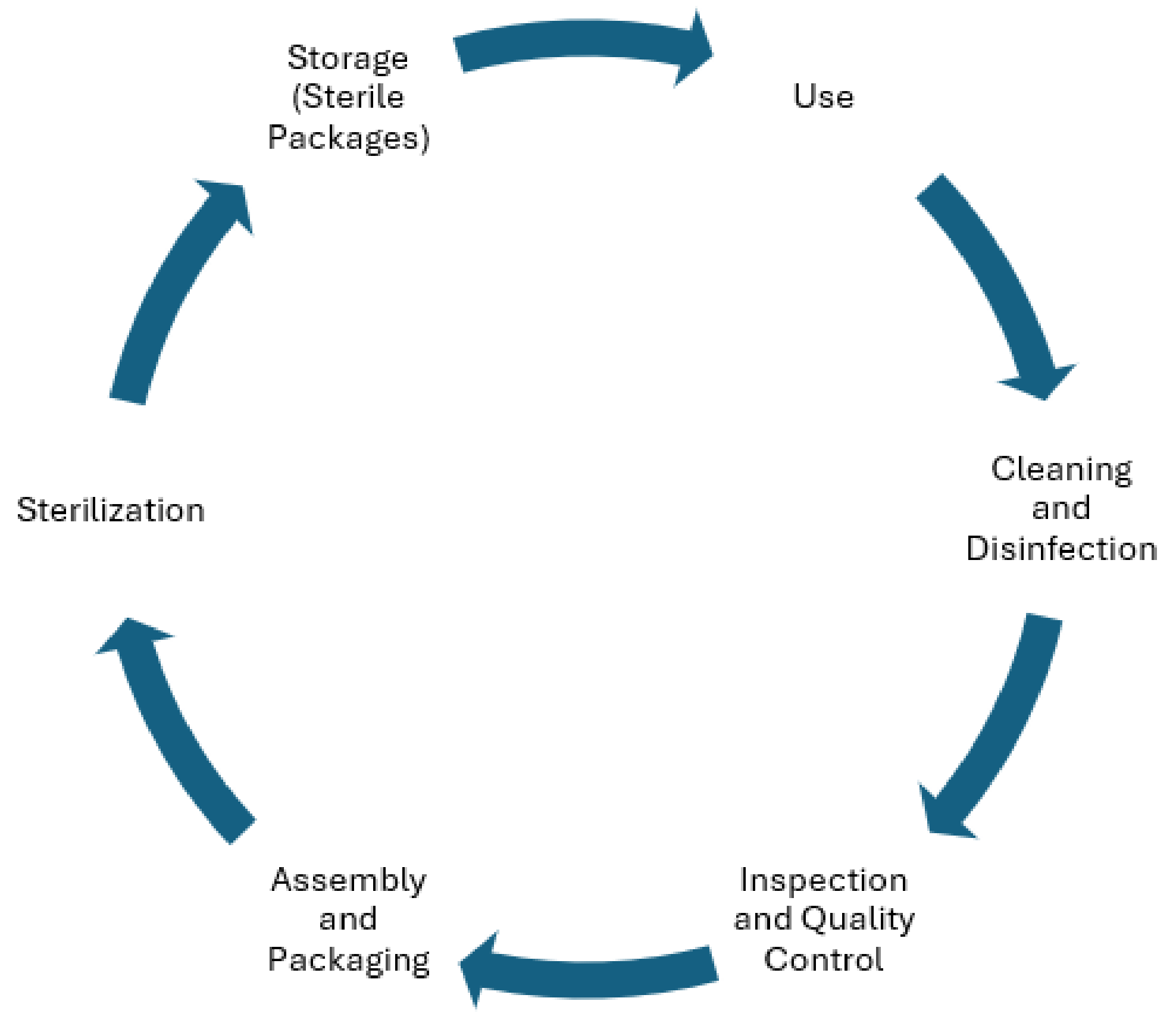
2. Traceability Surgical System Architecture Overview
2.1. Layer 1
- RFID: This wireless technology comprises an RFID tag, a reader, and an antenna [18]. It uses radio waves to transmit data from tagged objects to a reader, enabling automatic identification and real-time tracking [19]. This technology has advantages and disadvantages. Indeed, it does not disrupt workflow, which is crucial for maintaining the efficiency of medical procedures [18]. But RFID-tagged instruments may experience interference [20]. Also, RFID tags cannot be attached to all instruments, particularly those used in neurosurgery [21]. In addition, the integrated circuit chip of RFID tags is damaged after several washing cycles and sterilization at high temperatures, affecting the readability of crucial information [22]. Only a limited number of labeled instruments are identified simultaneously [23]. This can be restrictive in situations requiring the management of numerous surgical instruments.
- Dots: are pre-printed adhesive dots with a unique DataMatrix code [24,25]. They can have different sizes, from 2 to 9.5 mm. The size versatility allows application on various instruments, including small ones such as microsurgery instruments. Additionally, the dots adhere strongly when exposed to heat during autoclave. However, these technologies may be subject to code alteration through deformation or fragmentation, which could affect the traceability process in the long term.
- Micro-percussion: registration by micro-percussion consists of pressing the surface of surgical instruments. This mechanical process uses a fine tip that strikes the surface at high speed. Implementing micro-percussion marking requires several pieces of equipment, including a micro-percussion machine, a peripheral PC equipped with specialized software for registration management, and a controller responsible for encoding management [25]. Micro-percussion markings are less sensitive to corrosion but have poorer contrast [14].
- Laser engraving: Laser marking is based on eliminating material through a laser beam directed toward the surface of the surgical instrument. Laser marks have excellent visibility but are quickly attacked by corrosion [14]. This technique also allows the registration of circular surface instruments.
2.2. Layer 2
2.3. Layer 3
- The number of uses [21] indicates the total number of times an instrument has been used until a fault is observed. This parameter helps predict the lifespan of each instrument based on the number of uses.
- Identification Accuracy [15]: Evaluates accuracy by reporting the system’s reliability in correctly identifying instruments.
- Usage Percentage [18]: Calculates the usage percentage of an instrument based on the total number of operations in which it has been recorded. This parameter is crucial in the process of rationalizing surgical platforms.
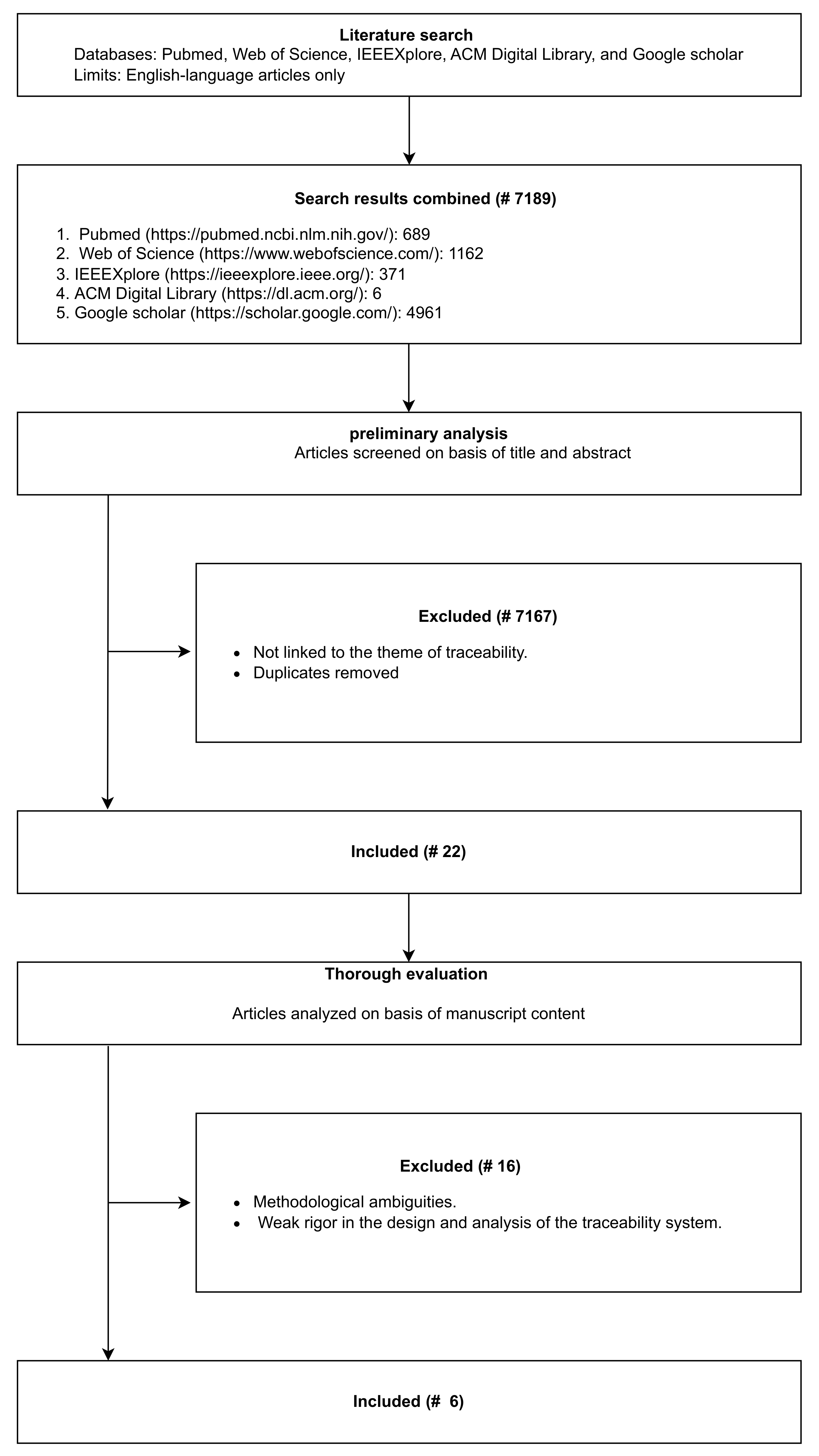
3. Methodology
3.1. Search Strategy
3.2. Eligibility Criteria
- Relevance: We included articles that explicitly detail the various stages of the life cycle of surgical instrument traceability systems, including design, implementation, and evaluation.
- Peer-reviewed sources: We prioritized articles published in peer-reviewed journals, conferences, or reputable academic sources to ensure quality and rigor.
- Accessible Full Texts: We selected studies with full-text availability to enable thorough analysis and replication of findings.
- Lack of Specific Focus: We excluded studies in which surgical instrument traceability was not a central objective or in which study outcomes (such as patient safety, environmental impact, economic considerations, or logistical ergonomics) were ambiguous or poorly evaluated after real-time deployment.
3.3. Selection Process
4. Results
4.1. Short-Term Benefits: Patient Safety and Logistical Ergonomics
4.1.1. Patient Safety
- Similarly, Duke University Hospital (2022) [18] demonstrated high sensitivity (93.8%) and specificity (80.8%) in measuring surgical instrument usage, reducing risks associated with missing or defective tools.
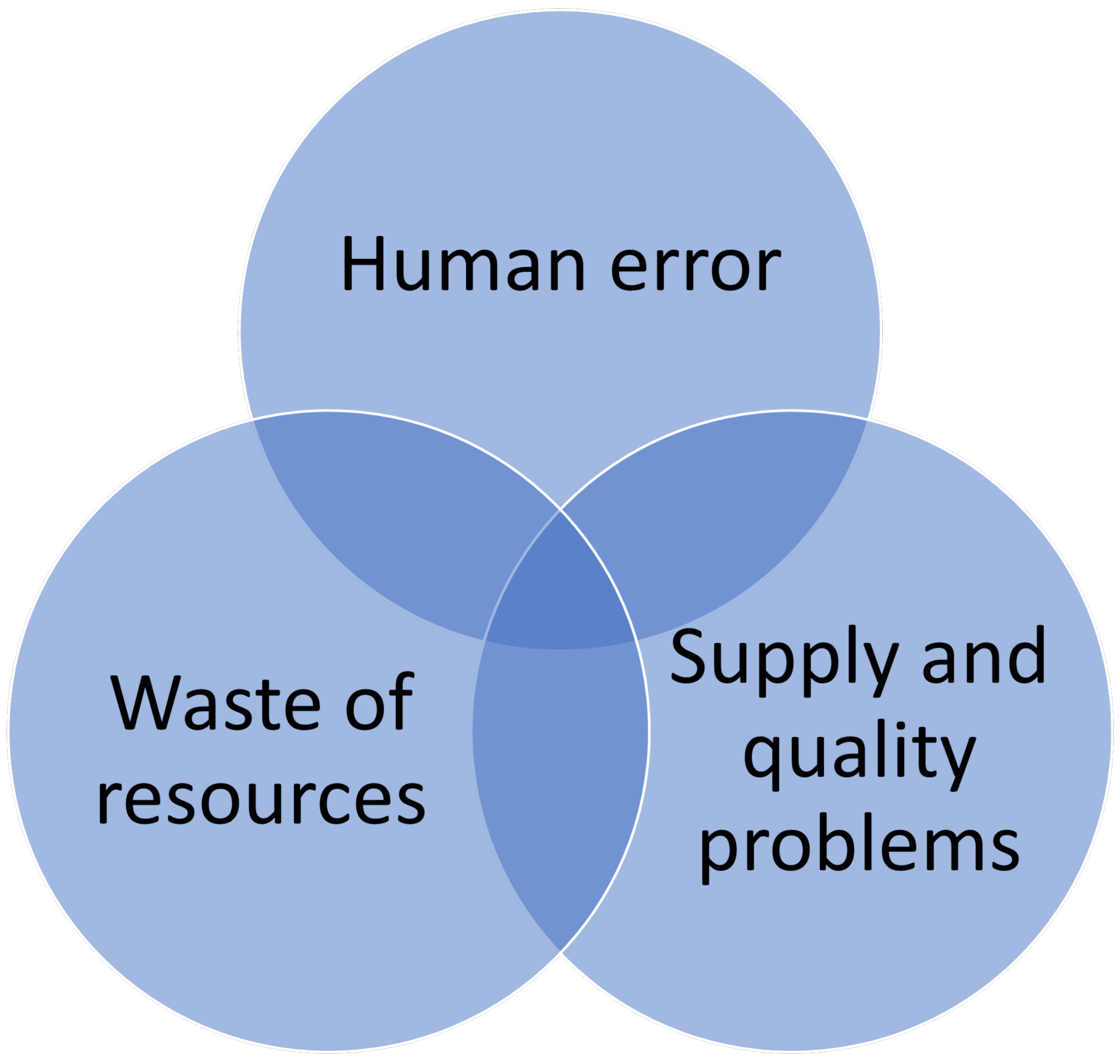
- Surgical instrument errors, such as forgotten instruments or use of unsterilized tools, can lead to severe outcomes, including post-operative infections or patient death [13,33,34]. According to Public Health France [35], nosocomial infections affect 1 in 18 patients, with an estimated 4200 deaths annually. Addressing these errors through traceability is critical to improving patient outcomes and strengthening health security.
4.1.2. Logistical Ergonomics
- At Davis Ambulatory Surgical Center (2021) [31], RFID technology reduced setup times from 23 min to 17 min, demonstrating the efficiency of real-time monitoring and automatic counting.
4.2. Medium-to-Long-Term Benefits: Economy and Environment
4.2.1. Economic Benefits
- At the Japanese Red Cross Wakayama Medical Center (2019) [22], traceability systems predicted instrument service life, enabling better resource planning and cost savings.
- Duke University Hospital [18] reported that logistical improvements, such as reduced setup time, contributed to a more cost-effective workflow.
4.2.2. Environmental Benefits
- Rationalizing surgical tray contents ensures that only used instruments are sterilized, reducing unnecessary sterilization processes. For instance, the Davis Ambulatory Surgical Center (2021) [31] achieved a 40.3% reduction in tray size, leading to decreased sterilization needs and associated energy consumption.
- Similarly, the Japanese Red Cross Wakayama Medical Center (2019) [22] reported sustainable waste management practices, where only necessary instruments were cleaned and reused, reducing overall waste.
5. Discussion
- Health Security: By reducing the risk of surgical site infections through meticulous tracking and sterilization validation.
- Sustainable Development: Promoting resource efficiency, reducing waste, and supporting eco-friendly practices in healthcare.
- Professional Well-being: Enhancing ergonomics and reducing stress for healthcare professionals through streamlined workflows and error mitigation.
6. Conclusions
- Creation of public databases: The creation of public databases, rich in information on surgical instruments and details relating to maintenance and sterilization, has become necessary. This resource will serve as the basis for in-depth longitudinal studies, providing valuable information on the effectiveness and durability of the instruments.
- Integration of automation into the traceability system: The future lies in the increased integration of automation to minimize human errors and improve ergonomics for healthcare workers. Automation of the assembly phase of the sterilization cycle, combined with automated instrument tracking and sorting, promises more efficient management. Considering factors such as priority of use and actuation scenarios is essential.
- Investment in artificial intelligence and computer vision: Significant investment in artificial intelligence and computer vision is required for robust instrument identification with omnidirectional marking recognition. This technology also helps automatically detect faults, provide proactive maintenance, and generate daily reports.
- Risk analysis in the traceability system: Risk analysis at all stages of the traceability system, assessing generic severity and consequences, is crucial. Defining reaction protocols adapted to each scenario will guarantee the robustness and performance of the system.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bag, S. Overview of Surgical Instruments for the Operation Theatre. In Design and Development of Affordable Healthcare Technologies; IGI Global: Hershey, PA, USA, 2018; pp. 23–56. [Google Scholar]
- Vries, D. Aligning the Work Processes of the Medical Instrument Sterilization Cycle at the OLVG Hospital in Amsterdam: A Holistic Approach. Master’s Thesis, University of Twente, Enschede, The Netherlands, 2017. [Google Scholar]
- Zhu, X.; Yuan, L.; Li, T.; Cheng, P. Errors in packaging surgical instruments based on a surgical instrument tracking system: An observational study. BMC Health Serv. Res. 2019, 19, 176. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Model Regulatory Framework for Medical Devices Including In Vitro Diagnostic Medical Devices; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Jaison, F.; Ramaiah, N.S. A survey on traceability in food safety system using blockchain. J. Discret. Math. Sci. Cryptogr. 2022, 25, 793–799. [Google Scholar] [CrossRef]
- Demestichas, K.; Peppes, N.; Alexakis, T.; Adamopoulou, E. Blockchain in agriculture traceability systems: A review. Appl. Sci. 2020, 10, 4113. [Google Scholar] [CrossRef]
- Costa, C.; Antonucci, F.; Pallottino, F.; Aguzzi, J.; Sarriá, D.; Menesatti, P. A review on agri-food supply chain traceability by means of RFID technology. Food Bioprocess Technol. 2013, 6, 353–366. [Google Scholar] [CrossRef]
- Schuitemaker, R.; Xu, X. Product traceability in manufacturing: A technical review. Procedia CIRP 2020, 93, 700–705. [Google Scholar] [CrossRef]
- Kumar, G. Pharmaceutical Drug Packaging and Traceability: A Comprehensive Review. Univers. J. Pharm. Pharmacol. 2023, 2, 19–25. [Google Scholar] [CrossRef]
- Ahmadi, E.; Masel, D.T.; Metcalf, A.Y.; Schuller, K. Inventory management of surgical supplies and sterile instruments in hospitals: A literature review. Health Syst. 2019, 8, 134–151. [Google Scholar] [CrossRef]
- Dekonenko, C.; Oyetunji, T.A.; Rentea, R.M. Surgical tray reduction for cost saving in pediatric surgical cases: A qualitative systematic review. J. Pediatr. Surg. 2020, 55, 2435–2441. [Google Scholar] [CrossRef]
- Dos Santos, B.M.; Fogliatto, F.S.; Zani, C.M.; Peres, F.A.P. Approaches to the rationalization of surgical instrument trays: Scoping review and research agenda. BMC Health Serv. Res. 2021, 21, 163. [Google Scholar] [CrossRef]
- Moatari-Kazerouni, A.; Bendavid, Y. Improving logistics processes of surgical instruments: Case of RFID technology. Bus. Process Manag. J. 2017, 23, 448–466. [Google Scholar] [CrossRef]
- Rioblanc, F.; Cambier, C.; Le Grand, J. Traçabilité individuelle à l’instrument: évolution des marquages laser et micropercussion au fil des cycles de stérilisation. In Proceedings of the Annales Pharmaceutiques Françaises; Elsevier: Paris, France, 2023. [Google Scholar]
- Ishiyama, R.; Frøiland, P.H.L.; Øvrebotn, S.A. Automated Identification of Surgical Instruments without Tagging: Implementation in Real Hospital Work Environment. In Proceedings of the 2023 18th International Conference on Machine Vision and Applications (MVA), Hamamatsu, Japan, 23–25 July 2023; pp. 1–4. [Google Scholar]
- Glaser, B.; Schellenberg, T.; Franke, S.; Dänzer, S.; Neumuth, T. Surgical instrument similarity metrics and tray analysis for multi-sensor instrument identification. In Proceedings of the Medical Imaging 2015: Image-Guided Procedures, Robotic Interventions, and Modeling; SPIE: Bellingham, WA, USA, 2015; Volume 9415, pp. 534–541. [Google Scholar]
- Nicolaos, G.; Tournoud, M.; Hassani, Y.; Mignon, J.; Frémont, F.; Fabreguettes, A. Unique Device Identification of surgical instruments by DataMatrix 2D barcodes. In 2009/2010 GS1 Healthcare Reference Book; GS1: Brussels, Belgium, 2009. [Google Scholar]
- Hill, I.; Olivere, L.; Helmkamp, J.; Le, E.; Hill, W.; Wahlstedt, J.; Khoury, P.; Gloria, J.; Richard, M.J.; Rosenberger, L.H.; et al. Measuring intraoperative surgical instrument use with radio-frequency identification. JAMIA Open 2022, 5, ooac003. [Google Scholar] [CrossRef] [PubMed]
- Schwaitzberg, S. The emergence of radiofrequency identification tags: Applications in surgery. Surg. Endosc. Other Interv. Tech. 2006, 20, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Kusuda, K.; Yamashita, K.; Ohnishi, A.; Tanaka, K.; Komino, M.; Honda, H.; Tanaka, S.; Okubo, T.; Tripette, J.; Ohta, Y. Management of surgical instruments with radio frequency identification tags: A 27-month in hospital trial. Int. J. Health Care Qual. Assur. 2016, 29, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Kusuda, K.; Ito, Y.; Komino, M.; Tanaka, K.; Kurokawa, S.; Ameya, M.; Eba, D.; Masamune, K.; Muragaki, Y.; et al. Evaluation of surgical instruments with radiofrequency identification tags in the operating room. Surg. Innov. 2018, 25, 374–379. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Kimura, E.; Akama, E.; Nakao, H.; Yorozuya, T.; Ishihara, K. Prediction of the service life of surgical instruments from the surgical instrument management system log using radio frequency identification. BMC Health Serv. Res. 2019, 19, 695. [Google Scholar] [CrossRef]
- Hosaka, R.; Noji, R. Automatic identification for surgical instruments using UHF band passive RFID. In Proceedings of the EMBEC & NBC 2017: Joint Conference of the European Medical and Biological Engineering Conference (EMBEC) and the Nordic-Baltic Conference on Biomedical Engineering and Medical Physics (NBC), Tampere, Finland, 11-15 June 2017; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1061–1064. [Google Scholar]
- Talon, D. Gestion des Risques dans une Stérilisation Centrale d’un Établissement Hospitalier: Apport de la Traçabilité à L’instrument. Ph.D. Thesis, Ecole Centrale Paris, Gif-sur-Yvette, France, 2011. [Google Scholar]
- Jouvien, A. Préparation de la Mise en Place D’une TraçAbilité Individuelle à L’instrument au CHU de Bordeaux. Master’s Thesis, Université de Bordeaux, Bordeaux, France, 2022. [Google Scholar]
- FAORO. Brigitte Traçabilité Individuelle des Instruments. 2011. Available online: https://www.sssh.ch/uploads/media/f0311_guide_F.pdf (accessed on 30 January 2025).
- Hanada, E.; Ohira, A.; Hayashi, M.; Sawa, T. Improving efficiency through analysis of data obtained from an RFID tag system for surgical instruments. In Proceedings of the 2015 IEEE 5th International Conference on Consumer Electronics-Berlin (ICCE-Berlin), Berlin, Germany, 6–9 September 2015; pp. 84–87. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W-65. [Google Scholar] [CrossRef]
- Sawa, T.; Komatsu, H. Shimane university hospital implements RFID technology to manage surgical instruments. In Proceedings of the 2013 7th International Symposium on Medical Information and Communication Technology (ISMICT), Tokyo, Japan, 6–8 March 2013; pp. 90–92. [Google Scholar]
- Olivere, L.A.; Hill, I.T.; Thomas, S.M.; Codd, P.J.; Rosenberger, L.H. Radiofrequency identification track for tray optimization: An instrument utilization pilot study in surgical oncology. J. Surg. Res. 2021, 264, 490–498. [Google Scholar] [CrossRef]
- Stockert, E.W.; Langerman, A. Assessing the magnitude and costs of intraoperative inefficiencies attributable to surgical instrument trays. J. Am. Coll. Surg. 2014, 219, 646–655. [Google Scholar] [CrossRef]
- Cheng, V.C.C.; Wong, S.C.Y.; Sridhar, S.; Chan, J.F.W.; Ng, M.L.M.; Lau, S.K.P.; Woo, P.C.Y.; Lo, E.C.M.; Chan, K.K.C.; Yuen, K.Y. Management of an incident of failed sterilization of surgical instruments in a dental clinic in Hong Kong. J. Formos. Med. Assoc. 2013, 112, 666–675. [Google Scholar] [CrossRef][Green Version]
- Benamara, M. Traçabilité RFID à l’aide de Petites Antennes: Application au cas des Instruments Chirurgicaux: Étude et Validation d’une Solution Prototype. Ph.D. Thesis, Université Paris-Est, Créteil, Paris, 2017. [Google Scholar]
- SPF. Principaux Résultats de L’enquête Nationale de Prévalence 2022 des Infections Nosocomiales et des Traitements Anti-Infectieux En établissement de Santé. 2023. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/infections-associees-aux-soins-et-resistance-aux-antibiotiques/infections-associees-aux-soins/documents/enquetes-etudes/principaux-resultats-de-l-enquete-nationale-de-prevalence-2022-des-infections-nosocomiales-et-des-traitements-anti-infectieux-en-etablissement-de-s (accessed on 23 July 2024).
- Mhlaba, J.M.; Stockert, E.W.; Coronel, M.; Langerman, A.J. Surgical instrumentation: The true cost of instrument trays and a potential strategy for optimization. J. Hosp. Adm. 2015, 4, 82–88. [Google Scholar] [CrossRef]
- Dobson, G.P. Trauma of major surgery: A global problem that is not going away. Int. J. Surg. 2020, 81, 47–54. [Google Scholar] [CrossRef] [PubMed]

| Paper | Year | Type of Research Paper | Content | Summary of Results |
|---|---|---|---|---|
| Ahmadi et al. [10] | 2019 | Literature review | Economic | (1) Economic Order Quantity, Simple rules, Constraint programming, Integer programming, Markov chain, Simulation and Semi-Markov process are the current methods and strategies used in the management of surgical supplies and sterile instruments inventory to achieve savings in operating rooms. (2) Healthcare professionals highlight improving preference cards and quantifying usage/waste as key practices for reducing operating room expenses. |
| Dekonenko et al. [11] | 2020 | Qualitative systematic review | Economic | (1) Doctor preference cards and individual feedback are the techniques deployed for standardization and cost effectiveness on pediatric surgical. |
| Dos Santos et al. [12] | 2021 | Scoping review | Economic | (1) The majority of authors and publications discussing surgical tray rationalization (STR) originate from the United States. (2) The primary methodologies and strategies mentioned for STR include checklists, focus groups, observation, and standardization. (3) STR positively influences operational and economic performance, with its main impact observed in operating rooms and the sterilization process. (4) Future research could focus on two promising areas: instrument traceability and cross-sectional analysis. |
| Ours | Literature review | Engineering | (1) A traceability system operates across three distinct levels. (2) Surgical instrument identification methods include unique instrument identification and general identification. (3) The advantages of a traceability system are evident in enhanced patient safety, environmental improvements, economic benefits, and better logistical ergonomics in the workplace. (4) Future research should prioritize developing public datasets, incorporating automation into traceability systems, utilizing artificial intelligence and computer vision techniques, and conducting risk analysis studies. |
| Query (Title) | “surgical instrument” OR “surgical instruments” OR “surgical tool” OR “surgical tools” OR “operating instruments” OR “operating instrument” OR “operating tools” OR “operating tool” OR “surgical trays” OR “surgical tray” |
| Paper | Hanada et al. [27], and Sawa et al. [30] | Yoshikawa et al. [22] | Oliver et al. [31] | Hill et al. [18] | Ishiyama et al. [15] |
|---|---|---|---|---|---|
| Year | 2013 | 2019 | 2021 | 2022 | 2023 |
| Country | Japan | Japan | USA | USA | Norway |
| Technology used | RFID (13.56 MHz) | RFID (13.56 MHz) | RFID | RFID (915 MHz) | Microscopic image of fingerprint |
| Location deployement | Shimane University Hospital | Japanese Red Cross Wakayama Medical Center | Davis Ambulatory Surgical Center | Duke University Hospital | Haukeland University Hospita |
| Types of Operation | - | General surgery, ophthalmology, otolaryngology, orthopedic surgery, gynecology, cardiovascular surgery, pediatric cardiac surgery, urology, neurosurgery, thoracic surgery, plastic surgery, pediatric surgery, breast surgery, emergency department, and dental and oral surgery | Lumpectomy and excisional breast biopsies | Craniotomies, CMC arthroplasties, and breast surgeries | - |
| Period | - | 1 September 2013 to 30 April2017 | 2019 October to 2020 March | - | February to June 2022 for 160 h |
| Aim of study | Inceases the safety of surgical procedure | Predict the precise service of instruments | Demonstrate the use of RFID technology as a data-driven method for instrument reduction | Develop and evaluate an automated system for measuring surgical instrument use | Present new solution to track and trace surgical instruments without tagging |
| Results | (1) Reduction of counting time from 7 to 5 min for approximately 130 instruments. (2) 50% reduction in container assembly time for general laparotomy (from 20 to 10 min). (3) Rationalization of containers by reducing the storage of more than 70 instruments. | (1) Not all sterilized instruments were used during the operation.
(2) The probability of failure exceeded 80% when the number of uses exceeded 224 times. | (1) 40.3% reduction in tray size. (2) Reduced setup time from 23 min to 17 min. (3) Agreement between automatic and manual counting with a kappa value of 0.824. (4) No instruments were added to the reduced trays in 10 additional cases. | (1) Sensitivity of 93.8% and specificity of 80.8%.
(2) Consistency between the RFID system and human ethnography with a Cohen’s kappa coefficient of 0.81. (3) Average reduction of 50.8% in surgical plateaus in breast and orthopedic surgery. (4) Logistical reduction of 6 min in preparation for breast surgery. | (1) Accuracy >99% in unique identification.
(2) Reduction of scanning time to approximately 1.5 to 2 min for 12 to 13 instruments. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayad, M.; Yahiaoui, R.; Auber, F.; Pidoux, H.; Hild, O.; Picaud, F.; Herlem, G.; Chaussy, Y. Traceability of Surgical Instruments: A Systematic Review. Appl. Sci. 2025, 15, 1592. https://doi.org/10.3390/app15031592
Fayad M, Yahiaoui R, Auber F, Pidoux H, Hild O, Picaud F, Herlem G, Chaussy Y. Traceability of Surgical Instruments: A Systematic Review. Applied Sciences. 2025; 15(3):1592. https://doi.org/10.3390/app15031592
Chicago/Turabian StyleFayad, Moustafa, Réda Yahiaoui, Frédéric Auber, Hervé Pidoux, Olivier Hild, Fabien Picaud, Guillaume Herlem, and Yann Chaussy. 2025. "Traceability of Surgical Instruments: A Systematic Review" Applied Sciences 15, no. 3: 1592. https://doi.org/10.3390/app15031592
APA StyleFayad, M., Yahiaoui, R., Auber, F., Pidoux, H., Hild, O., Picaud, F., Herlem, G., & Chaussy, Y. (2025). Traceability of Surgical Instruments: A Systematic Review. Applied Sciences, 15(3), 1592. https://doi.org/10.3390/app15031592








