Exergaming-Based Rehabilitation for Lateral Trunk Flexion in Parkinson’s Disease: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical History
2.2. Clinical Assessment
2.3. Instrumental Assessment
2.3.1. Pressure Pain Threshold (PPT)
2.3.2. Tensiomyography (TMG)
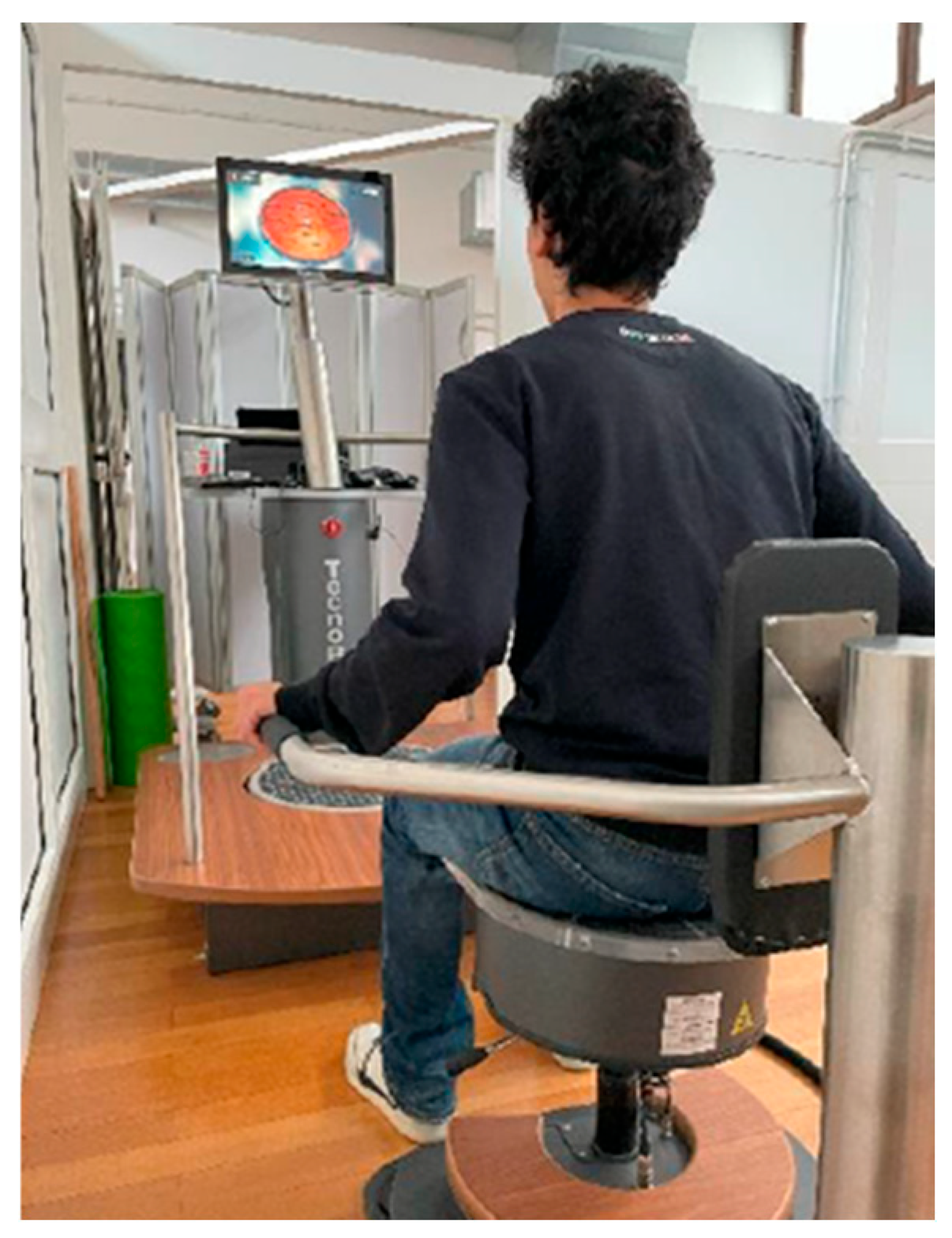
2.4. Treatment Protocol
2.5. Statistical Analysis
3. Results
3.1. Clinical Assessment
3.2. Neurophysiological Assessment
3.2.1. Pressure Pain Threshold (PPT)
3.2.2. Tensiomyography (TMG)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s Disease |
| HC | Healthy control |
| LTF | Lateral Trunk Flexion |
| BBS | Berg Balance Scale |
| TMG | Tensiomyography |
| PPT | Pressure Pain Threshold |
| TUG | Time Up andGo test |
| UPDRS-III | Unified Parkinson’s Disease Rating Scale—III |
| H&Y | Modified Hoehn and Yahr Scale |
| S&E ADL Scale | Modified Schwab and England Capacity for Daily Living Scale |
References
- Tinazzi, M.; Gandolfi, M.; Ceravolo, R.; Capecci, M.; Andrenelli, E.; Ceravolo, M.G.; Bonanni, L.; Onofrj, M.; Vitale, M.; Catalan, M.; et al. Postural Abnormalities in Parkinson’s Disease: An Epidemiological and Clinical Multicenter Study. Mov. Disord. Clin. Pract. 2019, 6, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Tinazzi, M.; Geroin, C.; Bhidayasiri, R.; Bloem, B.R.; Capato, T.; Djaldetti, R.; Doherty, K.; Fasano, A.; Tibar, H.; Lopiano, L.; et al. Task Force Consensus on Nosology and Cut-Off Values for Axial Postural Abnormalities in Parkinsonism. Mov. Disord. Clin. Pract. 2022, 9, 594–603. [Google Scholar] [CrossRef]
- Tinazzi, M.; Fasano, A.; Geroin, C.; Morgante, F.; Ceravolo, R.; Rossi, S.; Thomas, A.; Fabbrini, G.; Bentivoglio, A.; Tamma, F.; et al. Pisa Syndrome in Parkinson Disease: An Observational Multicenter Italian Study. Neurology 2015, 85, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Matteo, A.D.; Fasano, A.; Squintani, G.; Ricciardi, L.; Bovi, T.; Fiaschi, A.; Barone, P.; Tinazzi, M. Lateral Trunk Flexion in Parkinson’s Disease: EMG Features Disclose Two Different Underlying Pathophysiological Mechanisms. J. Neurol. 2011, 258, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Geroin, C.; Artusi, C.A.; Gandolfi, M.; Zanolin, E.; Ceravolo, R.; Capecci, M.; Andrenelli, E.; Ceravolo, M.G.; Bonanni, L.; Onofrj, M.; et al. Does the Degree of Trunk Bending Predict Patient Disability, Motor Impairment, Falls, and Back Pain in Parkinson’s Disease? Front. Neurol. 2020, 11, 207. [Google Scholar] [CrossRef]
- Cao, S.; Cui, Y.; Jin, J.; Li, F.; Liu, X.; Feng, T. Prevalence of Axial Postural Abnormalities and Their Subtypes in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Neurol. 2023, 270, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Artusi, C.A.; Geroin, C.; Imbalzano, G.; Camozzi, S.; Aldegheri, S.; Lopiano, L.; Tinazzi, M.; Bombieri, N. Assessment of Axial Postural Abnormalities in Parkinsonism: Automatic Picture Analysis Software. Mov. Disord. Clin. Pract. 2023, 10, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Doherty, K.M.; van de Warrenburg, B.P.; Peralta, M.C.; Silveira-Moriyama, L.; Azulay, J.P.; Gershanik, O.S.; Bloem, B.R. Postural Deformities in Parkinson’s Disease. Lancet Neurol. 2011, 10, 538–549. [Google Scholar] [CrossRef]
- Ledda, C.; Panero, E.; Dimanico, U.; Parisi, M.; Gandolfi, M.; Tinazzi, M.; Geroin, C.; Marchet, F.; Massazza, G.; Lopiano, L.; et al. Longitudinal Assessment of Botulinum Toxin Treatment for Lateral Trunk Flexion and Pisa Syndrome in Parkinson’s Disease: Real-Life, Long-Term Study. Toxins 2023, 15, 566. [Google Scholar] [CrossRef]
- Gandolfi, M.; Artusi, C.A.; Imbalzano, G.; Camozzi, S.; Crestani, M.; Lopiano, L.; Tinazzi, M.; Geroin, C. Botulinum Toxin for Axial Postural Abnormalities in Parkinson’s Disease: A Systematic Review. Toxins 2024, 16, 228. [Google Scholar] [CrossRef]
- Zak, M.; Sikorski, T.; Wasik, M.; Krupnik, S.; Andrychowski, J.; Brola, W. Pisa Syndrome: Pathophysiology, Physical Rehabilitation and Falls Risk. NeuroRehabilitation 2021, 49, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Alvisi, E.; Bossio, F.; Caremani, L.; Maestri, R.; Palamara, G.; Ferrazzoli, D.; Ortelli, P.; Frazzitta, G. Effectiveness of incobotulinumtoxinA Injection and Multidisciplinary Intensive Rehabilitation Treatment in Parkinsonian Patients with Pisa Syndrome. Toxicon 2018, 156, S3–S4. [Google Scholar] [CrossRef]
- Tinazzi, M.; Geroin, C.; Gandolfi, M.; Smania, N.; Tamburin, S.; Morgante, F.; Fasano, A. Pisa Syndrome in Parkinson’s Disease: An Integrated Approach from Pathophysiology to Management. Mov. Disord. 2016, 31, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Deci, E.L.; Ryan, R.M. The “What” and “Why” of Goal Pursuits: Human Needs and the Self-Determination of Behavior. Psychol. Inq. 2000, 11, 227–268. [Google Scholar] [CrossRef]
- Pacheco, T.B.; Medeiros, C.S.; Oliveira, V.H.; Vieira, E.R.; Cavalcanti, F.A. Effectiveness of exergames for improving mobility and balance in older adults: A systematic review and meta-analysis. Syst. Rev. 2020, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Tough, D.; Robinson, J.; Gowling, S.; Raby, P.; Dixon, J.; Harrison, S.L. The feasibility, acceptability and outcomes of exergaming among individuals with cancer: A systematic review. BMC Cancer 2018, 18, 1151. [Google Scholar] [CrossRef] [PubMed]
- Klompstra, L.; Jaarsma, T.; Strömberg, A.; Evangelista, L.S.; van der Wal, M.H.L. Exercise Motivation and Self-Efficacy Vary Among Patients with Heart Failure—An Explorative Analysis Using Data from the HF-Wii Study. Patient Prefer. Adherence 2021, 15, 2353–2362. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Jiang, Y.; Wang, M.; Ang, W.H.D.; Lau, Y. Rehabilitation Training Based on Virtual Reality for Patients with Parkinson’s Disease in Improving Balance, Quality of Life, Activities of Daily Living, and Depressive Symptoms: A Systematic Review and Meta-Regression Analysis. Clin. Rehabil. 2021, 35, 1089–1102. [Google Scholar] [CrossRef]
- Triegaardt, J.; Han, T.S.; Sada, C.; Sharma, S.; Sharma, P. The Role of Virtual Reality on Outcomes in Rehabilitation of Parkinson’s Disease: Meta-Analysis and Systematic Review in 1031 Participants. Neurol. Sci. 2020, 41, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, C.; Imbimbo, I.; Tranchita, E.; Minganti, C.; Ricciardi, D.; Monaco, R.L.; Parisi, A.; Padua, L. Comparison of Virtual Reality Rehabilitation and Conventional Rehabilitation in Parkinson’s Disease: A Randomised Controlled Trial. Physiotherapy 2020, 106, 36–42. [Google Scholar] [CrossRef]
- Barry, G.; Galna, B.; Rochester, L. The Role of Exergaming in Parkinson’s Disease Rehabilitation: A Systematic Review of the Evidence. J. NeuroEngineering Rehabil. 2014, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.C.; Wang, R.Y.; Cheng, S.J.; Yang, Y.R. Effects of a Balance-Based Exergaming Intervention Using the Kinect Sensor on Posture Stability in Individuals with Parkinson’s Disease: A Single-Blinded Randomized Controlled Trial. J. NeuroEngineering Rehabil. 2016, 13, 78. [Google Scholar] [CrossRef]
- Skrzatek, A.; Nuic, D.; Cherif, S.; Beranger, B.; Gallea, C.; Bardinet, E.; Welter, M.L. Brain Modulation after Exergaming Training in Advanced Forms of Parkinson’s Disease: A Randomized Controlled Study. J. NeuroEngineering Rehabil. 2024, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Nuic, D.; van de Weijer, S.; Cherif, S.; Skrzatek, A.; Zeeboer, E.; Olivier, C.; Corvol, J.C.; Foulon, P.; Pastor, J.Z.; Mercier, G.; et al. Home-Based Exergaming to Treat Gait and Balance Disorders in Patients with Parkinson’s Disease: A Phase II Randomized Controlled Trial. Eur. J. Neurol. 2024, 31, e16055. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Poewe, W.; Litvan, I.; Lewis, S.; Lang, A.E.; Halliday, G.; Goetz, C.G.; Chan, P.; Slow, E.; Seppi, K.; et al. Validation of the MDS Clinical Diagnostic Criteria for Parkinson’s Disease. Mov. Disord. 2018, 33, 1601–1608. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, Progression, and Mortality. Neurology 1967, 17, 427. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, Progression, and Mortality. Neurology 2001, 57, S11–S26. [Google Scholar] [CrossRef]
- Qutubuddin, A.A.; Pegg, P.O.; Cifu, D.X.; Brown, R.; McNamee, S.; Carne, W. Validating the Berg Balance Scale for Patients with Parkinson’s Disease: A Key to Rehabilitation Evaluation. Arch. Phys. Med. Rehabil. 2005, 86, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Nocera, J.R.; Stegemöller, E.L.; Malaty, I.A.; Okun, M.S.; Marsiske, M.; Hass, C.J. Using the Timed up & Go Test in a Clinical Setting to Predict Falling in Parkinson’s Disease. Arch. Phys. Med. Rehabil. 2013, 94, 1300–1305. [Google Scholar] [CrossRef]
- Blanchet, P.J.; Brefel-Courbon, C. Chronic Pain and Pain Processing in Parkinson’s Disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 87, 200–206. [Google Scholar] [CrossRef]
- Lu, J.S.; Chen, Q.Y.; Chen, X.; Li, X.H.; Zhou, Z.; Liu, Q.; Lin, Y.; Zhou, M.; Xu, P.Y.; Zhuo, M. Cellular and Synaptic Mechanisms for Parkinson’s Disease-Related Chronic Pain. Mol. Pain 2021, 17, 1744806921999025. [Google Scholar] [CrossRef]
- Deodato, M.; Granato, A.; Ceschin, M.; Galmonte, A.; Manganotti, P. Algometer Assessment of Pressure Pain Threshold After Onabotulinumtoxin-A and Physical Therapy Treatments in Patients With Chronic Migraine: An Observational Study. Front. Pain Res. 2022, 3, 770397. [Google Scholar] [CrossRef]
- Deodato, M.; Granato, A.; Martini, M.; Sabot, R.; Buoite Stella, A.; Manganotti, P. Instrumental Assessment of Pressure Pain Threshold over Trigeminal and Extra-Trigeminal Area in People with Episodic and Chronic Migraine: A Cross-Sectional Observational Study. Neurol. Sci. 2024, 45, 3923–3929. [Google Scholar] [CrossRef] [PubMed]
- Deodato, M.; Granato, A.; Martini, M.; Stella, A.B.; Galmonte, A.; Murena, L.; Manganotti, P. Neurophysiological and Clinical Outcomes in Episodic Migraine Without Aura: A Cross-Sectional Study. J. Clin. Neurophysiol. 2024, 41, 388–395. [Google Scholar] [CrossRef]
- Čular, D.; Babić, M.; Zubac, D.; Kezić, A.; Macan, I.; Peyré-Tartaruga, L.A.; Ceccarini, F.; Padulo, J. Tensiomyography: From Muscle Assessment to Talent Identification Tool. Front. Physiol. 2023, 14, 1163078. [Google Scholar] [CrossRef]
- Pus, K.; Paravlic, A.H.; Šimunič, B. The Use of Tensiomyography in Older Adults: A Systematic Review. Front. Physiol. 2023, 14, 1213993. [Google Scholar] [CrossRef]
- Stella, A.B.; Galimi, A.; Martini, M.; Lenarda, L.D.; Murena, L.; Deodato, M. Muscle Asymmetries in the Lower Limbs of Male Soccer Players: Preliminary Findings on the Association between Countermovement Jump and Tensiomyography. Sports 2022, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Deodato, M.; Saponaro, S.; Šimunič, B.; Martini, M.; Murena, L.; Stella, A.B. Trunk Muscles’ Characteristics in Adolescent Gymnasts with Low Back Pain: A Pilot Study on the Effects of a Physiotherapy Intervention Including a Postural Reeducation Program. J. Man. Manip. Ther. 2024, 32, 310–324. [Google Scholar] [CrossRef]
- Deodato, M.; Saponaro, S.; Šimunič, B.; Martini, M.; Galmonte, A.; Murena, L.; Stella, A.B. Sex-Based Comparison of Trunk Flexors and Extensors Functional and Contractile Characteristics in Young Gymnasts. Sport Sci. Health 2024, 20, 147–155. [Google Scholar] [CrossRef]
- Stella, A.B.; Cargnel, A.; Raffini, A.; Mazzari, L.; Martini, M.; Ajčević, M.; Accardo, A.; Deodato, M.; Murena, L. Shoulder Tensiomyography and Isometric Strength in Swimmers Before and After a Fatiguing Protocol. J. Athl. Train. 2024, 59, 738–744. [Google Scholar] [CrossRef]
- Bravi, M.; Massaroni, C.; Santacaterina, F.; Di Tocco, J.; Schena, E.; Sterzi, S.; Bressi, F.; Miccinilli, S. Validity Analysis of WalkerViewTM Instrumented Treadmill for Measuring Spatiotemporal and Kinematic Gait Parameters. Sensor 2021, 21, 4795. [Google Scholar] [CrossRef]
- Bravi, M.; Santacaterina, F.; Bressi, F.; Morrone, M.; Renzi, A.; Di Tocco, J.; Schena, E.; Sterzi, S.; Massaroni, C. Instrumented Treadmill for Run Biomechanics Analysis: A Comparative Study. Biomed. Tech. 2023, 68, 563–571. [Google Scholar] [CrossRef]
- Garcia-Agundez, A.; Folkerts, A.K.; Konrad, R.; Caserman, P.; Tregel, T.; Goosses, M.; Göbel, S.; Kalbe, E. Recent Advances in Rehabilitation for Parkinson’s Disease with Exergames: A Systematic Review. J. NeuroEngineering Rehabil. 2019, 16, 17. [Google Scholar] [CrossRef]
- Harris, D.M.; Rantalainen, T.; Muthalib, M.; Johnson, L.; Duckham, R.L.; Smith, S.T.; Daly, R.M.; Teo, W.-P. Concurrent Exergaming and Transcranial Direct Current Stimulation to Improve Balance in People with Parkinson’s Disease: Study Protocol for a Randomised Controlled Trial. Trials 2018, 19, 387. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-S.; Chen, Y.-W.; Zeng, B.-Y.; Hung, C.-M.; Tu, Y.-K.; Tai, Y.-C.; Wu, Y.-C.; Hsu, C.-W.; Lei, W.-T.; Wu, S.-L.; et al. Effects of Modern Technology (Exergame and Virtual Reality) Assisted Rehabilitation vs Conventional Rehabilitation in Patients with Parkinson’s Disease: A Network Meta-Analysis of Randomised Controlled Trials. Physiotherapy 2022, 117, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Çetin, B.; Kılınç, M.; Çakmaklı, G.Y. The Effects of Exergames on Upper Extremity Performance, Trunk Mobility, Gait, Balance, and Cognition in Parkinson’s Disease: A Randomized Controlled Study. Acta Neurol. Belg. 2024, 124, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Deodato, M.; Granato, A.; Stella, A.B.; Martini, M.; Marchetti, E.; Lise, I.; Galmonte, A.; Murena, L.; Manganotti, P. Efficacy of a Dual Task Protocol on Neurophysiological and Clinical Outcomes in Migraine: A Randomized Control Trial. Neurol. Sci. 2024, 45, 4015–4026. [Google Scholar] [CrossRef]
- Marotta, N.; Calanfiore, D.; Curci, C.; Lippi, L.; Ammendolia, V.; Ferraro, F.; Invernizzi, M.; de Sire, A. Integrating Virtual Reality and Exergaming in Cognitive Rehabilitation of Patients with Parkinson Disease: A Systematic Review of Randomized Controlled Trials. Eur. J. Phys. Rehabil. Med. 2022, 58, 818–826. [Google Scholar] [CrossRef]
- Perini, R.; Bortoletto, M.; Capogrosso, M.; Fertonani, A.; Miniussi, C. Acute Effects of Aerobic Exercise Promote Learning. Sci. Rep. 2016, 6, 25440. [Google Scholar] [CrossRef] [PubMed]
- Rea, K.; Finn, D.P. The Role of Supraspinal GABA and Glutamate in the Mediation and Modulation of Pain. In Acute Pain: Causes, Effects and Treatment; Nova Science Publishers, Inc.: New York, USA, 2009; ISBN 978-1-60741-223-6. [Google Scholar]
- Enna, S.J.; McCarson, K.E. The Role of GABA in the Mediation and Perception of Pain. Adv. Pharmacol. 2006, 54, 1–27. [Google Scholar]
- Peyron, R.; Laurent, B.; García-Larrea, L. Functional Imaging of Brain Responses to Pain. A Review and Meta-Analysis (2000). Neurophysiol. Clin. 2000, 30, 263–288. [Google Scholar] [CrossRef] [PubMed]
- Adlakha, S.; Chhabra, D.; Shukla, P. Effectiveness of gamification for the rehabilitation of neurodegenerative disorders. Chaos Solitons Fractals 2020, 140, 110192. [Google Scholar] [CrossRef]



| PPT | LTF | HC | Differences t1 vs. t1 vs. HC | p-Value | |
|---|---|---|---|---|---|
| L3 ipsilateral | t1 365.76 (SD ± 181.12) | 713.30 (SD ± 216.34) | t1 vs. t2 | −11.56 | <0.01 ** |
| t2 704.17 (SD ± 134.24) | t1 vs HC | −10.44 | <0.05 * | ||
| t2 vs HC | 1.11 | >0.05 | |||
| L3 contralateral | t1 438.62 (SD ± 159.25) | 713.30 (SD ± 216.34) | t1 vs. t2 | −11.33 | <0.01 ** |
| t2 714.61 (SD ± 157.35) | t1 vs HC | −10.00 | <0.05 * | ||
| t2 vs HC | 1.33 | >0.05 | |||
| T6 ipsilateral | t1 423.99 (SD ± 91.11) | 598.02 (SD ± 80.12) | t1 vs. t2 | −8.78 | >0.05 |
| t2 561.13 (SD ± 143.01) | t1 vs. HC | −10.89 | <0.05 * | ||
| t2 vs. HC | −2.11 | >0.05 | |||
| T6 contralateral | t1 369.09 (SD ± 68.06) | 598.02 (SD ± 80.12) | t1 vs. t2 | −11.78 | <0.01 ** |
| t2 573.89 (SD ± 137.52) | t1 vs. HC | −13.22 | <0.01 ** | ||
| t2 vs. HC | −1.44 | >0.05 | |||
| C3 ipsilateral | t1 330.38 (SD ± 132.70) | 565.49 (SD ± 82.35) | t1 vs. t2 | −8.00 | >0.05 |
| t2 493.60 (SD ± 133.94) | t1 vs. HC | −12.33 | <0.01 ** | ||
| t2 vs HC | −4.33 | >0.05 | |||
| C3 contralateral | t1 356.61 (SD ± 136.25) | 565.49 (SD ± 82.35) | t1 vs. t2 | −4.33 | >0.05 |
| t2 446.81 (SD ± 102.14) | t1 vs. HC | −11.17 | <0.01 ** | ||
| t2 vs. HC | −6.83 | >0.05 | |||
| Muscle | TLF T1 | TLF T2 | HC (Average Right and Left Side) | p-Value |
|---|---|---|---|---|
| Upper Trapezius ipsilateral | ||||
| Time of contraction, ms | 30.21 (SD ± 18.13) | 37.33 (SD ± 23.24) | 27.21 (SD ± 10.60) | 0.51 |
| Time of delay, ms | 21.78 (SD ± 2.81) | 23.01 (SD ± 3.04) | 21.10 (SD ± 2.10) | 0.31 |
| Time of relaxation, ms | 193.44 (SD ± 229.46) | 134.54 (SD ± 95.87) | 113.51 (SD ± 50.80) | 0.89 |
| Maximal radial displacement, mm | 4.06 (SD± 2.21) | 4.67 (SD ± 2.01) | 2.74 (SD ± 2.16) | 0.15 |
| Time of sustain, ms | 505.01 (SD± 181.14) | 468.23 (SD ± 211.26) | 498.96 (SD ± 284.42) | 0.72 |
| Upper Trapezius contralateral | ||||
| Time of contraction, ms | 35.33 (SD ± 23.23) | 44.52 (SD ± 26.62) | 27.21 (SD ± 10.60) | 0.14 |
| Time of delay, ms | 22.02 (SD ± 1.86) | 23.72 (SD ± 3.26) | 21.10 (SD ± 2.10) | 0.30 |
| Time of relaxation, ms | 135.30 (SD ± 87.78) | 136.01 (SD ± 61.67) | 113.51 (SD ± 50.80) | 0.88 |
| Maximal radial displacement, mm | 3.40 (SD ± 1.41) | 3.96 (SD ± 1.04) | 2.74 (SD ± 2.16) | 0.14 |
| Time of sustain, ms | 472.74 (SD ± 217.46) | 520.27 (SD ± 256.76) | 498.96 (SD ± 284.42) | 0.92 |
| Middle Trapezius ipsilateral | ||||
| Time of contraction, ms | 42.34 (SD ± 30.25) | 22.49 (SD ± 7.14) | 23.69 (SD ± 12.22) | 0.06 |
| Time of delay, ms | 21.17 (SD ± 5.50) | 19.20 (SD ± 2.27) | 20.30 (SD ± 2.36) | 0.58 |
| Time of relaxation, ms | 181.43 (SD ± 162.36) | 139.45 (SD ± 104.99) | 181.74 (SD ± 116.06) | 0.51 |
| Maximal radial displacement, mm | 2.26 (SD ± 1.42) | 2.24 (SD ± 1.08) | 1.27 (SD ± 0.40) | 0.06 |
| Time of sustain, ms | 337.53 (SD ± 236.10) | 365.94 (SD ± 150.03) | 389.75 (SD ± 168.88) | 0.22 |
| Middle Trapezius contralateral | ||||
| Time of contraction, ms | 21.92 (SD ± 3.73) | 19.09 (SD ± 2.14) | 23.69 (SD ± 12.22) | 0.37 |
| Time of delay, ms | 19.88 (SD ± 1.98) | 18.92 (SD ± 1.31) | 20.30 (SD ± 2.36) | 0.50 |
| Time of relaxation, ms | 129.39 (SD ± 121.15) | 107.67 (SD ± 73.10) | 181.74 (SD ± 116.06) | 0.32 |
| Maximal radial displacement, mm | 1.96 (SD ± 1.33) | 2.00 (SD ± 1.03) | 1.27 (SD ± 0.40) | 0.35 |
| Time of sustain, ms | 351.02 (SD ± 164.94) | 376.78 (SD ± 248.09) | 389.75 (SD ± 168.88) | 0.62 |
| Latissimus Dorsi ipsilateral | ||||
| Time of contraction, ms | 33.52 (SD ± 24.69) | 40.24 (SD ± 23.20) | 41.45 (SD ± 12.76) | 0.29 |
| Time of delay, ms | 26.7 (SD ± 7.3) | 27.86 (SD ± 9.06) | 38.30 (SD ± 23.32) | 0.09 * |
| Time of relaxation, ms | 96.24 (SD ± 189.25) | 115.92 (SD ± 80.26) | 112.97 (SD ± 110.98) | 0.14 |
| Maximal radial displacement, mm | 1.91 (SD ± 2.63) | 4.70 (SD ± 3.67) | 1.86 (SD ± 0.919) | 0.12 |
| Time of sustain, ms | 262.94 (SD ± 344.42) | 280.60 (SD ± 202.42) | 205.98 (SD ± 114.26) | 0.35 |
| Latissimus Dorsi contralateral | ||||
| Time of contraction, ms | 31.08 (SD ± 21.72) | 44.79 (SD ± 17.06) | 41.45 (SD ± 12.76) | 0.16 |
| Time of delay, ms | 28.7 (SD ± 10.6) | 33.01 (SD ± 4.69) | 38.30 (SD ± 23.32) | 0.23 |
| Time of relaxation, ms | 207.12 (SD ± 278.98) | 109.82 (SD ± 83.22) | 112.97 (SD ± 110.98) | >0.99 |
| Maximal radial displacement, mm | 1.29 (SD ± 1.03) | 3.53 (SD ± 2.70) | 1.86 (SD ± 0.91) | 0.15 |
| Time of sustain, ms | 281.39 (SD ± 285.85) | 234.65 (SD ± 119.47) | 205.98 (SD ± 114.26) | 0.92 |
| Erector Spinae ipsilateral | ||||
| Time of contraction, ms | 22.00 (SD ± 25.13) | 33.36 (SD ± 40.58) | 60.86 (SD ± 49.80) | 0.16 |
| Time of delay, ms | 11.72 (SD ± 11.63) | 12.46 (SD ± 12.26) | 24.67 (SD ± 9.10) | 0.05 |
| Time of relaxation, ms | 48.21 (SD ± 69.09) | 39.45 (SD ± 79.20) | 168.98 (SD ± 242.02) | 0.96 |
| Maximal radial displacement, mm | 0.35 (SD ± 0.53) | 0.37 (SD ± 0.70) | 1.44 (SD ± 1.05) | <0.01 * |
| Time of sustain, ms | 147.92 (SD ± 207.26) | 252.44 (SD ± 340.25) | 501.27 (SD ± 199.03) | 0.03 |
| Erector Spinae contralateral | ||||
| Time of contraction, ms | 51.98 (SD ± 58.35) | 54.16 (SD ± 72.78) | 60.86 (SD ± 49.80) | 0.65 |
| Time of delay, ms | 21.38 (SD ± 23.24) | 23.97 (SD ± 31.73) | 24.67 (SD ± 9.10) | 0.62 |
| Time of relaxation, ms | 94.83 (SD ± 159.01) | 37.93 (SD ± 73.21) | 168.98 (SD ± 242.02) | 0.14 |
| Maximal radial displacement, mm | 0.81 (SD ± 1.22) | 0.61(SD ± 0.69) | 1.44 (SD ± 1.05) | 0.07 |
| Time of sustain, ms | 333.43 (SD ± 369.28) | 320.50 (SD ± 383.32) | 501.27 (SD ± 199.03) | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzari, L.; Zambon, E.; Tonzar, S.; Martini, M.; Sabot, R.; Galmonte, A.; Manganotti, P. Exergaming-Based Rehabilitation for Lateral Trunk Flexion in Parkinson’s Disease: A Pilot Study. Appl. Sci. 2025, 15, 1745. https://doi.org/10.3390/app15041745
Mazzari L, Zambon E, Tonzar S, Martini M, Sabot R, Galmonte A, Manganotti P. Exergaming-Based Rehabilitation for Lateral Trunk Flexion in Parkinson’s Disease: A Pilot Study. Applied Sciences. 2025; 15(4):1745. https://doi.org/10.3390/app15041745
Chicago/Turabian StyleMazzari, Laura, Elena Zambon, Serena Tonzar, Miriam Martini, Raffaele Sabot, Alessandra Galmonte, and Paolo Manganotti. 2025. "Exergaming-Based Rehabilitation for Lateral Trunk Flexion in Parkinson’s Disease: A Pilot Study" Applied Sciences 15, no. 4: 1745. https://doi.org/10.3390/app15041745
APA StyleMazzari, L., Zambon, E., Tonzar, S., Martini, M., Sabot, R., Galmonte, A., & Manganotti, P. (2025). Exergaming-Based Rehabilitation for Lateral Trunk Flexion in Parkinson’s Disease: A Pilot Study. Applied Sciences, 15(4), 1745. https://doi.org/10.3390/app15041745







