Advances in Fermentation Technology: A Focus on Health and Safety
Abstract
1. Introduction
2. Microbial Control of the Pathogens’ Growth—Biocontrol Strategies
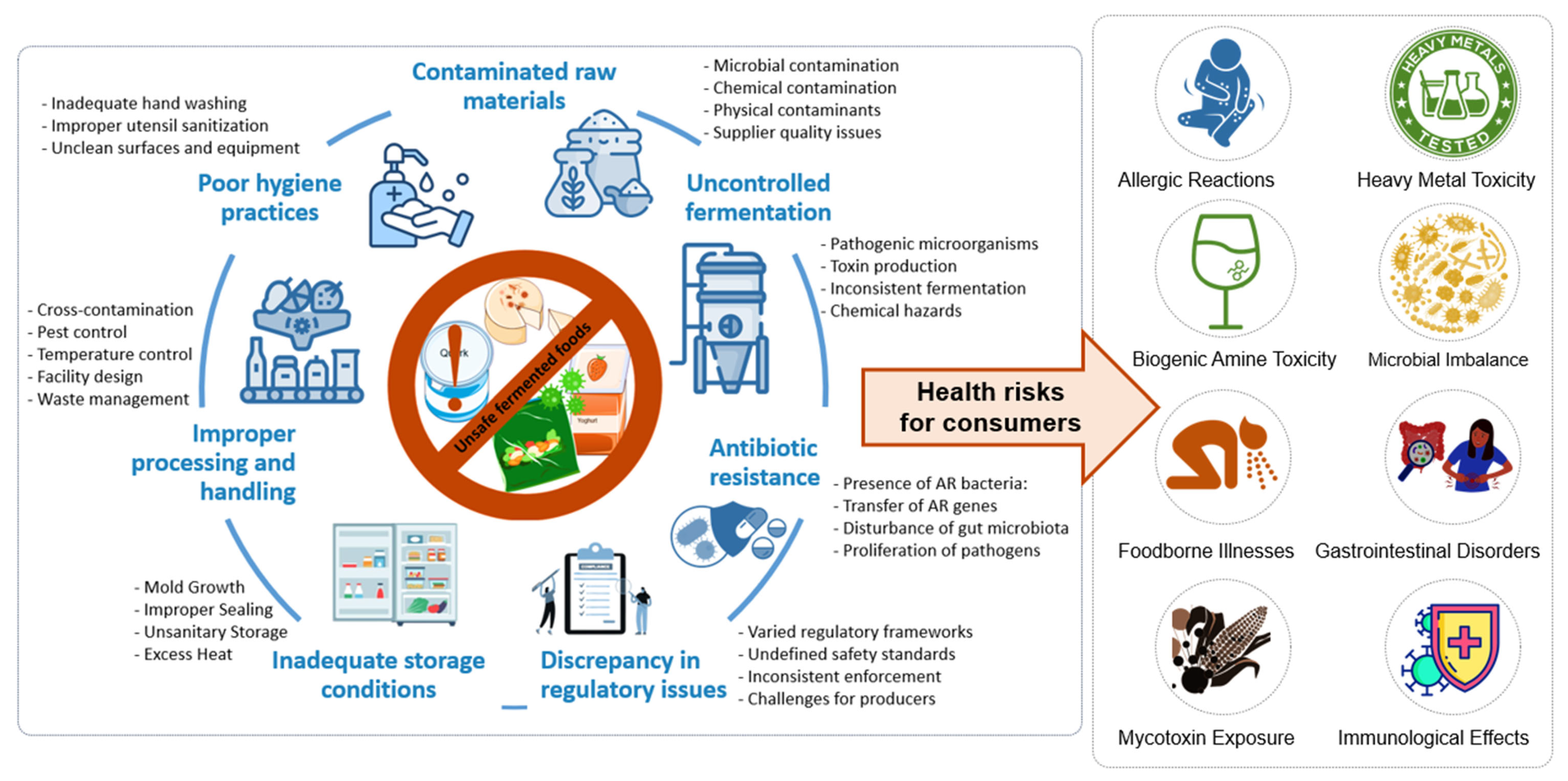
2.1. Potential Health Hazards in Traditional Fermentation
2.1.1. Pathogenic Contamination
2.1.2. Biogenic Amine Formation
2.1.3. Mycotoxin Contamination
2.1.4. Antimicrobial Resistance
2.2. Detection and Biopreservation for Food Fermentation and Safety
| Technology | Food | Contamination Type | Detection Limit | Application | Cost | References |
|---|---|---|---|---|---|---|
| HPLC with Fluorescence Detection | Fermented soybean products (ganjang, doenjang, gochujang) | Aflatoxins | Not detected to 6.06 μg/kg | Detection of aflatoxins in complex food matrices | Moderate | [63] |
| Multiplex PCR with Macroarray | Milk, meat products | Pathogenic bacteria (E. coli, Listeria, Salmonella) | 100 cells/mL or g | Accurate and time-saving simultaneous detection of multiple bacteria | Moderate | [64] |
| MALDI-TOF MS | Dairy products, fermented beverages, seafood, pork | Bacteria, fungi, yeasts | Not specified | Rapid and accurate identification of microorganisms | Moderate to high | [65] |
| Dispersive Liquid-Liquid Microextraction with HPLC | Fermented fish, wine, beer | Biogenic amines | 0.0010 to 0.0026 mg/L | Simultaneous determination of multiple biogenic amines | Low | [66] |
| CdTe Quantum Dots/Nano-TPP-OCH3 Fluorescence Sensor | Chinese spirits, yellow rice wine, Pu-erh tea, soy sauce | Toxic carcinogen (ethyl carbamate) | 7.14 μg/L | Sensitive and accurate detection of ethyl carbamate | Low to moderate | [9] |
| Potentiometric Stripping Analysis (PSA) | Dairy products | Heavy metals (Pb, Cd, Cu) | Pb: 1.7 μg/L, Cd: 0.30 μg/L, Cu: 3.8 μg/L | Direct determination of heavy metals | Low | [67] |
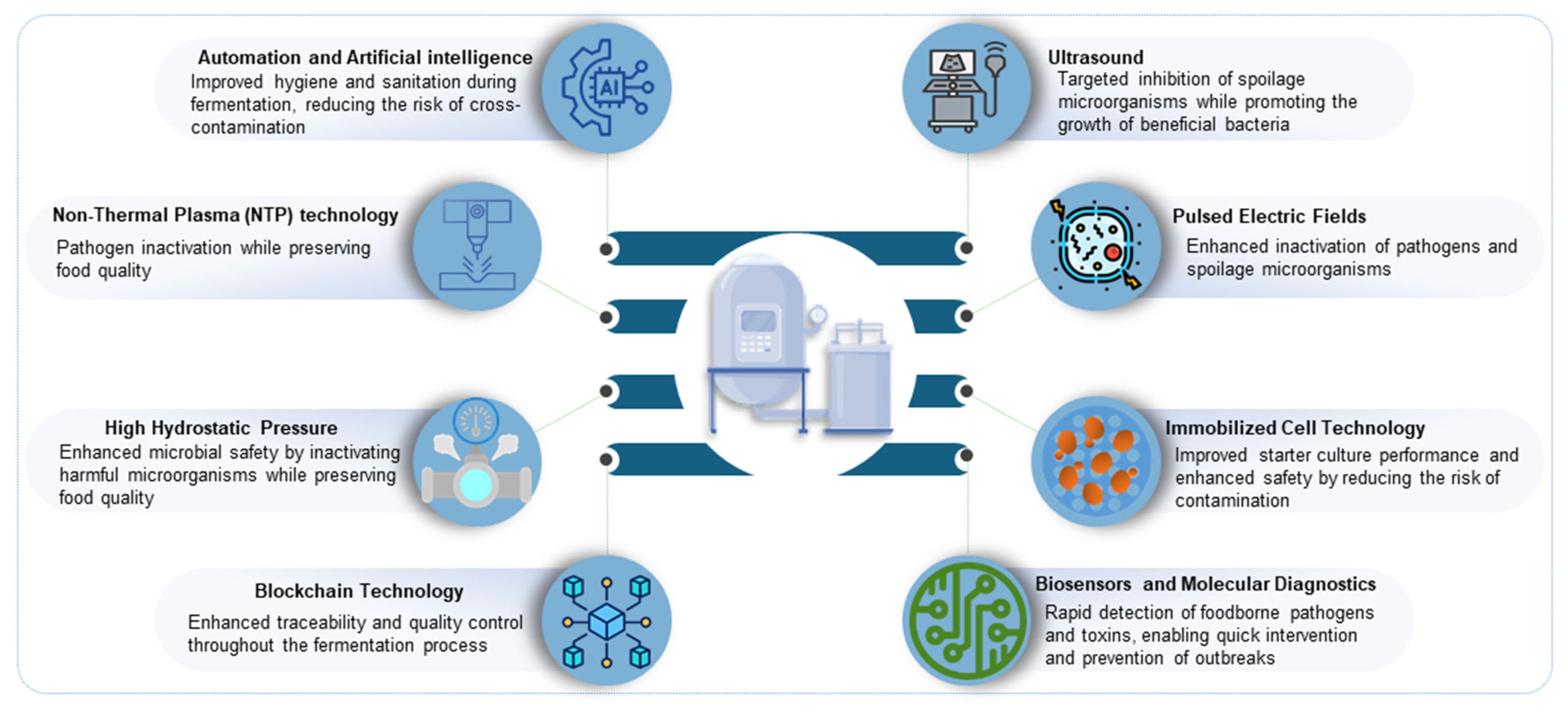
3. The Effectiveness of Innovative Methods in Fermented Food Safety Assurance
3.1. Biological and Technological Approaches
| Aspect | Traditional Methods | Modern Methods | References |
|---|---|---|---|
| Detection techniques | Culture-based methods using selective and non-selective enrichment; sensory evaluation by humans | High-throughput “omics” technologies; molecular profiling (e.g., DNA sequencing); biosensor and nanosensor-based techniques; instrumental techniques (e.g., E-nose, HPLC, gas chromatography–mass spectrometry). | [84,85,86] |
| Microbial control | Conditions such as temperature and time; use of starter cultures such as LAB, yeasts, and moulds; controlled Fermentation | Non-thermal processing technologies (e.g., pulsed electric fields, high-pressure processing); enzymatic approaches for monitoring chemicals; starter cultures for controlled fermentation. | [87,88,89,90] |
| Safety and quality assurance | Natural preservation methods (pickling, curing, and smoking); traditional disinfection methods (e.g., heat, chemical preservatives); good manufacturing practices (GMP) and hazard analysis | Intelligent bionic sensing technologies (e.g., visual, olfactory, tactile, gustatory); hurdle technologies combining multiple methods; advanced disinfection technologies (e.g., UV-light, cold plasma); quality assurance protocols. | [91,92,93,94,95] |
| Food Product | Key Development | Key Findings and Safety Implications | References |
|---|---|---|---|
| Fermented beverages | Isolation and application of beneficial microorganisms | - Isolation of Clavispora lusitaniae for ethyl carbamate degradation. - Effective reduction of carcinogenic compounds in beverages, enhancing safety. | [96] |
| Fermented mullet fish | Application of controlled fermentation processes | - Using Lactiplantibacillus plantarum and Saccharomyces cerevisiae for improved safety and quality. - Enhanced safety through controlled fermentation, reducing spoilage risks. | [97] |
| Korean traditional soybean paste | Use of selected starter cultures | - Comparing microbial contamination with and without starter cultures. - Starter cultures significantly improved safety by controlling microbial populations. | [98] |
| Buffalo fermented milk | Using next-Generation 16S rRNA Amplicon Sequencing | - Use of Lactobacillus fermentum NMCC-14. - Higher protein content, water-holding capacity, and dynamic viscosity; safe for consumption with no histological dysfunctions in mice. | [99] |
| Fermented fruits | - Bacterial community structure and functional prediction using sequencing. - Improved understanding of beneficial microbial diversity enhances safety assessments. - The results showed that fermentation is a safe and reliable process since pathogenic bacteria were absent in the fermentation products. | [100] | |
| Cattle and poultry feed | XPC™ (pathogen mitigation tool) | - Use of Saccharomyces cerevisiae fermentation products. - Reduced prevalence, load, virulence, and antibiotic resistance of Salmonella and E. coli O157:H7; enhanced immunocyte killing of Salmonella. | [101] |
| Kefir | Novel functional starter development | - Development of non-yeast kefir using Lactobacillus acidophilus KCNU and Lactobacillus brevis Bmb6. - Stable microbial composition, improved disease activity index score in mice, and enhanced sensory properties. | [102] |
| Traditional Chinese fermented vegetables (Jiangshui) | - Isolation and safety assessment of Lactiplantibacillus plantarum WYH. - Inhibition of Aspergillus flavus growth, no hemolysin activity, absence of antimicrobial resistance genes, and no toxicity in mice. | [103] | |
| Fermented soy products | Microbiome analysis using shotgun metagenomics | - Identified harmful bacteria (e.g., Klebsiella) and antibiotic resistance gene transfer risks. | [104] |
| Sausage | Application of PCR, plasmid profiling and sequencing to identify antibiotic resistance genes | - Antibiotic resistance in sausages showed a very moderate risk in Staphylococcus xylosus. - Staphylococcus xylosus was recommended to be considered as a European QPS approach. | [105] |
| Low-Salt Fermented Chilies | Using high-throughput sequencing, controlled fermentation | - Reduced spoilage bacteria and biogenic amines; improved flavour and safety through LAB interactions. - The inhibition rate of Enterobacter hormaechei has increased by 80.31%. | [106] |
| Traditional Chinese Pu-erh tea | Integrated meta-omics approaches for characterizing the microbiome | - The analysis showed that microbiota played an essential part in fermentation by producing enzymes involved in polysaccharide degradation and phenolic compound metabolism, resulting in changes in metabolite content, which impacted the safety and quality of Pu-erh tea. | [107] |
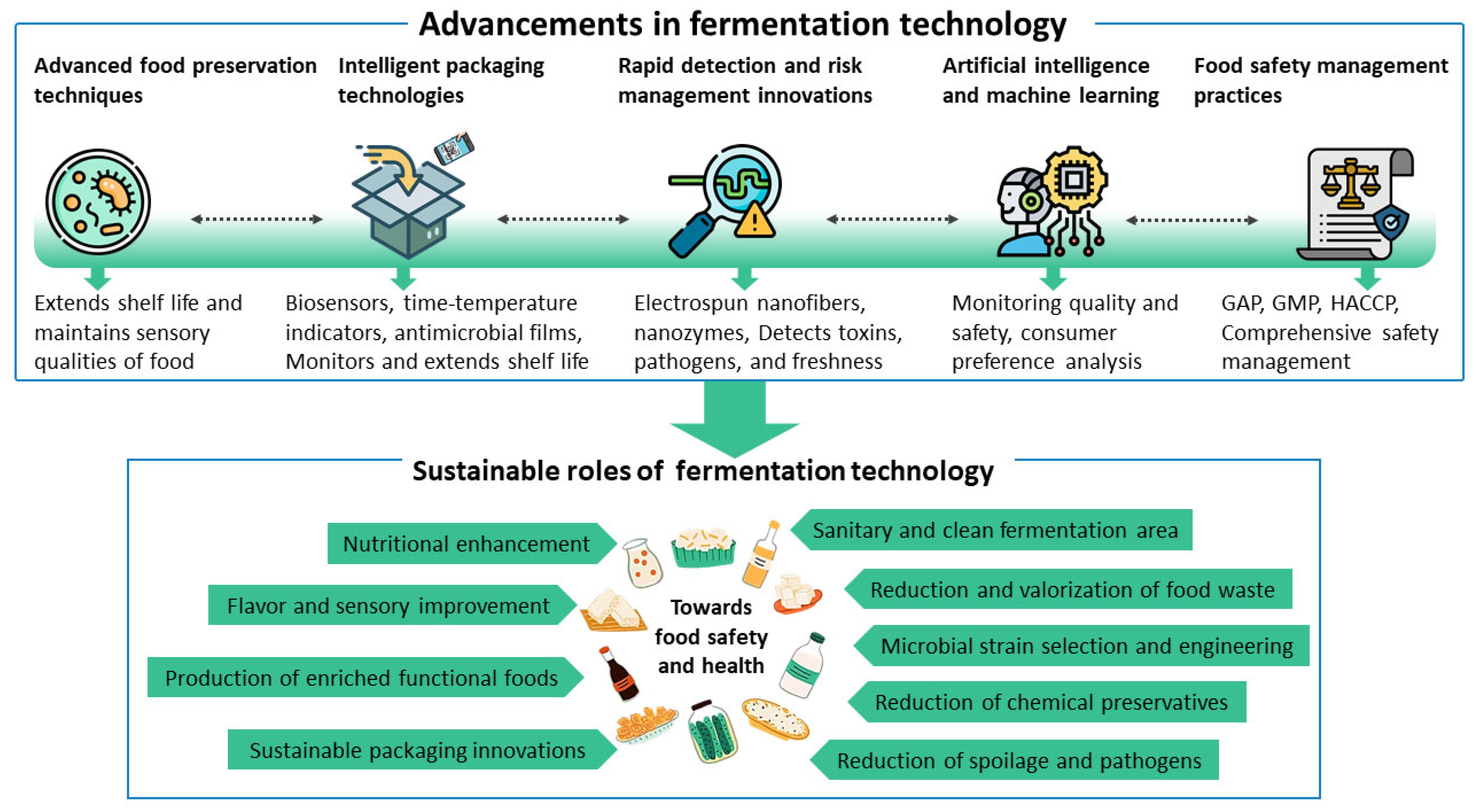
3.2. Advanced Bioreactor Technologies with Real-Time Monitoring
3.3. Smart Packaging and Sensor Technology for Supply Chain Monitoring
3.4. Artificial Intelligence and Machine Learning in Smart Fermentation Systems
3.5. Precision Fermentation for Targeted Compound Production
4. Regulatory Frameworks and Global Standards of Fermented Foods
5. International Harmonization of Safety Standards of Fermented Foods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | Antibiotic resistance |
| ARGs | Antibiotic resistance genes |
| CAGR | Compound annual growth |
| EFSA | European Food Safety Authority |
| FAO | Food and Agriculture Organization |
| FDA | U.S. Food and Drug Administration |
| GMOs | Genetically modified organisms |
| GRAS | Generally recognized as safe |
| HACCP | Hazard analysis and critical control points |
| HPLC | High-performance liquid chromatography |
| HTS | High-throughput sequencing |
| QPS | Qualified presumption of safety |
References
- De Vuyst, L.; Leroy, F. Functional Role of Yeasts, Lactic Acid Bacteria and Acetic Acid Bacteria in Cocoa Fermentation Processes. FEMS Microbiol. Rev. 2020, 44, 432–453. [Google Scholar] [CrossRef]
- Xu, M.; Su, S.; Zhang, Z.; Jiang, S.; Zhang, J.; Xu, Y.; Hu, X. Two Sides of the Same Coin: Meta-Analysis Uncovered the Potential Benefits and Risks of Traditional Fermented Foods at a Large Geographical Scale. Front. Microbiol. 2022, 13, 1045096. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.Z.; See, J.Y.; Ramazanu, S.; Chan, J.C.Y.; Wu, X.V. Effect of Lactic Acid Fermented Foods on Glycemic Control in Diabetic Adults: A Systemic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2024, 64, 2863–2878. [Google Scholar] [CrossRef] [PubMed]
- Research, N.D. Cognitive Market The Global Fermented Foods Market Size Is USD 584658.2 Million in 2024. Available online: https://www.cognitivemarketresearch.com/fermented-foods-market-report (accessed on 20 November 2024).
- Campbell, R.; Hauptmann, A.; Campbell, K.; Fox, S.; Marco, M.L. Better Understanding of Food and Human Microbiomes through Collaborative Research on Inuit Fermented Foods. Microbiome Res. Rep. 2022, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Butani, K.; Kumar, A.; Singh, S.; Prajapati, B.G. Effects of Fermented Food Consumption on Non-Communicable Diseases. Foods 2023, 12, 687. [Google Scholar] [CrossRef]
- Food Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 20 November 2024).
- Ryu, J.-A.; Kim, E.; Yang, S.-M.; Lee, S.; Yoon, S.-R.; Jang, K.-S.; Kim, H.-Y. High-Throughput Sequencing of the Microbial Community Associated with the Physicochemical Properties of Meju (Dried Fermented Soybean) and Doenjang (Traditional Korean Fermented Soybean Paste). LWT 2021, 146, 111473. [Google Scholar] [CrossRef]
- Wei, L.; Chen, H.; Liu, R.; Wang, S.; Liu, T.; Hu, Z.; Lan, W.; Yu, Y.; She, Y.; Fu, H. Fluorescent Sensor Based on Quantum Dots and Nano-porphyrin for Highly Sensitive and Specific Determination of Ethyl Carbamate in Fermented Food. J. Sci. Food Agric. 2021, 101, 6193–6201. [Google Scholar] [CrossRef]
- Reyes, S.J.; Durocher, Y.; Pham, P.L.; Henry, O. Modern Sensor Tools and Techniques for Monitoring, Controlling, and Improving Cell Culture Processes. Processes 2022, 10, 189. [Google Scholar] [CrossRef]
- CDC Summary of Possible Multistate Enteric (Intestinal) Disease Outbreaks in 2021. Available online: https://www.cdc.gov/foodborne-outbreaks/php/data-research/summary-2021.html (accessed on 26 February 2025).
- Smith, C.R.; Bond, H.; Kearney, A.; Chau, K.; Chui, L.; Gerrie, M.; Honish, L.; Lowé, Y.O.; Mah, V.; Manore, A.J. Fermenting a Place in History: The First Outbreak of Escherichia coli O157 Associated with Kimchi in Canada. Epidemiol. Infect. 2023, 151, e106. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on Fermented Foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Mengistu, D.A.; Tolera, S.T. Prevalence of Microorganisms of Public Health Significance in Ready-to-eat Foods Sold in Developing Countries: Systematic Review and Meta-analysis. Int. J. Food Sci. 2020, 2020, 8867250. [Google Scholar] [CrossRef] [PubMed]
- Hakim, G.H.; Behnam, J.Y.; Hashemi, M.; Disfani, M.A.; Moghaddam, T.M.R.; Afshari, A. Prevalence of Pathogenic Microorganisms in Traditional Dairy Products of Mashhad, Iran. J. Hum. Environ. Health Promot. 2021, 7, 152–158. [Google Scholar]
- Schoustra, S.; van der Zon, C.; Groenenboom, A.; Moonga, H.B.; Shindano, J.; Smid, E.J.; Hazeleger, W. Microbiological Safety of Traditionally Processed Fermented Foods Based on Raw Milk, the Case of Mabisi from Zambia. LWT 2022, 171, 113997. [Google Scholar] [CrossRef]
- Praça, J.; Furtado, R.; Coelho, A.; Correia, C.B.; Borges, V.; Gomes, J.P.; Pista, A.; Batista, R. Listeria monocytogenes, Escherichia coli and Coagulase Positive Staphylococci in Cured Raw Milk Cheese from Alentejo Region, Portugal. Microorganisms 2023, 11, 322. [Google Scholar] [CrossRef]
- Lemma, F.; Alemayehu, H.; Stringer, A.; Eguale, T. Prevalence and Antimicrobial Susceptibility Profile of Staphylococcus aureus in Milk and Traditionally Processed Dairy Products in Addis Ababa, Ethiopia. BioMed Res. Int. 2021, 2021, 5576873. [Google Scholar] [CrossRef]
- Gonzales-Barron, U.; Cadavez, V.; Pereira, A.P.; Gomes, A.; Araújo, J.P.; Saavedra, M.J.; Estevinho, L.; Butler, F.; Pires, P.; Dias, T. Relating Physicochemical and Microbiological Safety Indicators during Processing of Linguiça, a Portuguese Traditional Dry-Fermented Sausage. Food Res. Int. 2015, 78, 50–61. [Google Scholar] [CrossRef][Green Version]
- Kim, C.; Cho, S.; Kang, S.; Park, Y.; Yoon, M.; Lee, J.; No, W.; Kim, J. Prevalence, Genetic Diversity, and Antibiotic Resistance of Bacillus cereus Isolated from Korean Fermented Soybean Products. J. Food Sci. 2015, 80, M123–M128. [Google Scholar] [CrossRef]
- Keisam, S.; Tuikhar, N.; Ahmed, G.; Jeyaram, K. Toxigenic and Pathogenic Potential of Enteric Bacterial Pathogens Prevalent in the Traditional Fermented Foods Marketed in the Northeast Region of India. Int. J. Food Microbiol. 2019, 296, 21–30. [Google Scholar] [CrossRef]
- Chrun, R.; Hosotani, Y.; Kawasaki, S.; Inatsu, Y. Microbioligical Hazard Contamination in Fermented Vegetables Sold in Local Markets in Cambodia. Biocontrol Sci. 2017, 22, 181–185. [Google Scholar] [CrossRef]
- Peña-Gómez, N.; Ruiz-Rico, M.; Pérez-Esteve, É.; Fernández-Segovia, I.; Barat, J.M. Microbial Stabilization of Craft Beer by Filtration through Silica Supports Functionalized with Essential Oil Components. LWT 2020, 117, 108626. [Google Scholar] [CrossRef]
- Kim, S.; Kim, N.; Lee, S.; Hwang, I.; Rhee, M. Survival of Foodborne Pathogenic Bacteria (Bacillus cereus, Escherichia coli O157: H7, Salmonella enterica Serovar Typhimurium, Staphylococcus aureus, and Listeria monocytogenes) and Bacillus cereus Spores in Fermented Alcoholic Beverages (Beer and Refined Rice Wine). J. Food Prot. 2014, 77, 419–426. [Google Scholar] [PubMed]
- Yoon, J.-H.; Lee, S.; Lee, S.-Y. Control of Escherichia coli O157: H7, Salmonella enterica Serovar Typhimurium, and Listeria monocytogenes Inoculated in Beetroot or Watermelon Juice by Combined Treatments with Organic Acid or Lemon (Citrus limon) Extract and Mild Heat. Food Sci. Biotechnol. 2024, 33, 2887–2896. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, C.; He, R.; Zhang, Y.; Wang, B.; Zhang, Z.-H.; Ho, C.-T. Research Advances on Biogenic Amines in Traditional Fermented Foods: Emphasis on Formation Mechanism. Detection and Control Methods. Food Chem. 2023, 405, 134911. [Google Scholar] [CrossRef]
- Hu, M.; Dong, J.; Tan, G.; Li, X.; Zheng, Z.; Li, M. Metagenomic Insights into the Bacteria Responsible for Producing Biogenic Amines in Sufu. Food Microbiol. 2021, 98, 103762. [Google Scholar] [CrossRef]
- Sahu, L.; Panda, S.K.; Paramithiotis, S.; Zdolec, N.; Ray, R.C. Biogenic Amines in Fermented Foods: Overview. Fermented Foods Part 2015, 1, 318–332. [Google Scholar]
- Banicod, R.J.S.; Ntege, W.; Njiru, M.N.; Abubakar, W.H.; Kanthenga, H.T.; Javaid, A.; Khan, F. Production and Transformation of Biogenic Amines in Different Food Products by the Metabolic Activity of the Lactic Acid Bacteria. Int. J. Food Microbiol. 2024, 428, 110996. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, T.; Song, H.; Niu, C.; Wang, J.; Zheng, F.; Li, Q. Evaluation and Prediction of the Biogenic Amines in Chinese Traditional Broad Bean Paste. J. Food Sci. Technol. 2021, 58, 2734–2748. [Google Scholar] [CrossRef]
- Ma, X.; Bi, J.; Li, X.; Zhang, G.; Hao, H.; Hou, H. Contribution of Microorganisms to Biogenic Amine Accumulation during Fish Sauce Fermentation and Screening of Novel Starters. Foods 2021, 10, 2572. [Google Scholar] [CrossRef]
- Sokvibol, C.; Arunya, P.; Chuleeporn, C.; Wanticha, S.; Kriangkrai, P. Assessment of Biogenic Amine Level from Cambodia Fermented Fish Products. Food Res. 2022, 6, 294–302. [Google Scholar] [CrossRef]
- Algahtani, F.D.; Morshdy, A.E.; Hussein, M.A.; Abouelkheir, E.S.; Adeboye, A.; Valentine, A.; Elabbasy, M.T. Biogenic Amines and Aflatoxins in Some Imported Meat Products: Incidence, Occurrence, and Public Health Impacts. J. Food Qual. 2020, 2020, 8718179. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.B.; Seol, K.-H.; Ham, J.-S. Quantitative Analysis of Biogenic Amines in Different Cheese Varieties Obtained from the Korean Domestic and Retail Markets. Metabolites 2021, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Turna, N.S.; Chung, R.; McIntyre, L. A Review of Biogenic Amines in Fermented Foods: Occurrence and Health Effects. Heliyon 2024, 10, e24501. [Google Scholar] [CrossRef]
- Sun, X.; Sun, E.; Sun, L.; Su, L.; Jin, Y.; Ren, L.; Zhao, L. Effect of Biogenic Amine-Degrading Lactobacillus on the Biogenic Amines and Quality in Fermented Lamb Jerky. Foods 2022, 11, 2057. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Macias, S.; Martín-Garcia, A.; Ferrer-Bustins, N.; Comas-Basté, O.; Riu-Aumatell, M.; López-Tamames, E.; Jofré, A.; Latorre-Moratalla, M.L.; Bover-Cid, S.; Vidal-Carou, M.C. Inhibition of Biogenic Amines Formation in Fermented Foods by the Addition of Cava Lees. Front. Microbiol. 2022, 12, 818565. [Google Scholar] [CrossRef]
- Del Rio, B.; Sánchez-Llana, E.; Redruello, B.; Magadan, A.H.; Fernández, M.; Martin, M.C.; Ladero, V.; Alvarez, M.A. Enterococcus faecalis Bacteriophage 156 Is an Effective Biotechnological Tool for Reducing the Presence of Tyramine and Putrescine in an Experimental Cheese Model. Front. Microbiol. 2019, 10, 566. [Google Scholar] [CrossRef]
- Ganjeh, A.M.; Moreira, N.; Pinto, C.A.; Casal, S.; Saraiva, J.A. The Effects of High-Pressure Processing on Biogenic Amines in Food: A Review. Food Humanity 2024, 2, 100252. [Google Scholar] [CrossRef]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Park, S.B.; Im, J.H.; Chun, H.S. Mycotoxins in Soybean-based Foods Fermented with Filamentous Fungi: Occurrence and Preventive Strategies. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5131–5152. [Google Scholar] [CrossRef]
- Owolabi, I.O.; Kolawole, O.; Jantarabut, P.; Elliott, C.T.; Petchkongkaew, A. The Importance and Mitigation of Mycotoxins and Plant Toxins in Southeast Asian Fermented Foods. Npj Sci. Food 2022, 6, 39. [Google Scholar] [CrossRef]
- Rämö, S.; Kahala, M.; Joutsjoki, V. Aflatoxin B1 Binding by Lactic Acid Bacteria in Protein-Rich Plant Material Fermentation. Appl. Sci. 2022, 12, 12769. [Google Scholar] [CrossRef]
- Wolfe, B.E. Are Fermented Foods an Overlooked Reservoir of Antimicrobial Resistance? Curr. Opin. Food Sci. 2023, 51, 101018. [Google Scholar] [CrossRef]
- Vinayamohan, P.G.; Viju, L.S.; Joseph, D.; Venkitanarayanan, K. Fermented Foods as a Potential Vehicle of Antimicrobial-Resistant Bacteria and Genes. Fermentation 2023, 9, 688. [Google Scholar] [CrossRef]
- Jasiak, K.; Amund, D. Are Spontaneously Fermented Plant-based Foods Potential Sources of Transferable Antibiotic Resistance Genes? Food Front. 2022, 3, 46–55. [Google Scholar] [CrossRef]
- Wu-Wu, J.W.F.; Guadamuz-Mayorga, C.; Oviedo-Cerdas, D.; Zamora, W.J. Antibiotic Resistance and Food Safety: Perspectives on New Technologies and Molecules for Microbial Control in the Food Industry. Antibiotics 2023, 12, 550. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and Potential Use as Antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic Acid Bacteria as Antimicrobial Agents: Food Safety and Microbial Food Spoilage Prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef]
- Tóth, A.G.; Csabai, I.; Maróti, G.; Jerzsele, Á.; Dubecz, A.; Patai, Á.V.; Judge, M.F.; Nagy, S.Á.; Makrai, L.; Bányai, K. A Glimpse of Antimicrobial Resistance Gene Diversity in Kefir and Yoghurt. Sci. Rep. 2020, 10, 22458. [Google Scholar] [CrossRef]
- Xue, Y.; He, S.; Li, M.; Qiu, Y. Development and Application of Four Foodborne Pathogens by TaqMan Multiplex Real-Time PCR. Foodborne Pathog. Dis. 2024, 22, 193–201. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jung, S.; Oh, S.-W. Combination of Bacteria Concentration and DNA Concentration for Rapid Detection of E. coli O157: H7, L. Monocytogenes, and S. Typhimurium without Microbial Enrichment. LWT 2020, 117, 108609. [Google Scholar] [CrossRef]
- Ngamwongsatit, N.; Chaturongakul, S.; Aunpad, R. Development and Validation of an Efficient Multiplex PCR Assay for Simultaneous Detection of Six Common Foodborne Pathogens and Hygiene Indicators. Foodborne Pathog. Dis. 2023, 20, 222–229. [Google Scholar] [CrossRef]
- Kim, E.; Yang, S.-M.; Won, J.-E.; Kim, D.-Y.; Kim, D.-S.; Kim, H.-Y. Real-Time PCR Method for the Rapid Detection and Quantification of Pathogenic Staphylococcus Species Based on Novel Molecular Target Genes. Foods 2021, 10, 2839. [Google Scholar] [CrossRef]
- Okoye, C.O.; Jiang, H.; Nazar, M.; Tan, X.-L.; Jiang, J. Redefining Modern Food Analysis: Significance of Omics Analytical Techniques Integration, Chemometrics and Bioinformatics. TrAC Trends Anal. Chem. 2024, 175, 117706. [Google Scholar] [CrossRef]
- Wen, L.; Yang, L.; Chen, C.; Li, J.; Fu, J.; Liu, G.; Kan, Q.; Ho, C.-T.; Huang, Q.; Lan, Y. Applications of Multi-Omics Techniques to Unravel the Fermentation Process and the Flavor Formation Mechanism in Fermented Foods. Crit. Rev. Food Sci. Nutr. 2024, 64, 8367–8383. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Briandet, R.; Callon, C.; Champomier-Vergès, M.-C.; Christieans, S.; Chuzeville, S.; Denis, C.; Desmasures, N.; Desmonts, M.-H.; Feurer, C. Contribution of Omics to Biopreservation: Toward Food Microbiome Engineering. Front. Microbiol. 2022, 13, 951182. [Google Scholar] [CrossRef] [PubMed]
- Cozzio, C.; Viglia, G.; Lemarie, L.; Cerutti, S. Toward an Integration of Blockchain Technology in the Food Supply Chain. J. Bus. Res. 2023, 162, 113909. [Google Scholar] [CrossRef]
- Niya, S.R.; Dordevic, D.; Hurschler, M.; Grossenbacher, S.; Stiller, B. A Blockchain-Based Supply Chain Tracing for the Swiss Dairy Use Case. In Proceedings of the 2nd International Conference on Societal Automation (SA), Madeira, Portugal, 26–28 May 2021; pp. 1–8. [Google Scholar]
- Casino, F.; Kanakaris, V.; Dasaklis, T.K.; Moschuris, S.; Stachtiaris, S.; Pagoni, M.; Rachaniotis, N.P. Blockchain-Based Food Supply Chain Traceability: A Case Study in the Dairy Sector. Int. J. Prod. Res. 2021, 59, 5758–5770. [Google Scholar] [CrossRef]
- Tan, A.; Ngan, P.T. A Proposed Framework Model for Dairy Supply Chain Traceability. Sustain. Futur. 2020, 2, 100034. [Google Scholar] [CrossRef]
- Cocco, L.; Mannaro, K.; Tonelli, R.; Mariani, L.; Lodi, M.B.; Melis, A.; Simone, M.; Fanti, A. A Blockchain-Based Traceability System in Agri-Food SME: Case Study of a Traditional Bakery. IEEE Access 2021, 9, 62899–62915. [Google Scholar] [CrossRef]
- Woo, S.Y.; Ok, H.E.; Lee, S.Y.; Jeong, A.-Y.; Jeong, T.K.; Chun, H.S. Simple Chromatographic Determination of Aflatoxins in Korean Fermented Soybean Products Doenjang, Ganjang, and Gochujang, with Comparison of Derivatization Methods. Food Sci. Biotechnol. 2022, 31, 475–482. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Tsen, H.-Y.; Chen, H.-Y.; Chang, Y.-H.; Lin, C.-K.; Chen, C.-Y.; Pai, W.-Y. Multiplex PCR and a Chromogenic DNA Macroarray for the Detection of Listeria monocytogens, Staphylococcus aureus, Streptococcus agalactiae, Enterobacter sakazakii, Escherichia coli O157: H7, Vibrio parahaemolyticus, Salmonella spp. and Pseudomonas fluorescens in Milk and Meat Samples. J. Microbiol. Methods 2012, 88, 110–116. [Google Scholar]
- Condina, M.R.; Dilmetz, B.A.; Bazaz, S.R.; Meneses, J.; Warkiani, M.E.; Hoffmann, P. Rapid Separation and Identification of Beer Spoilage Bacteria by Inertial Microfluidics and MALDI-TOF Mass Spectrometry. Lab. Chip 2019, 19, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Donthuan, J.; Yunchalard, S.; Srijaranai, S. Vortex-assisted Surfactant-enhanced-emulsification Liquid–Liquid Microextraction of Biogenic Amines in Fermented Foods before Their Simultaneous Analysis by High-performance Liquid Chromatography. J. Sep. Sci. 2014, 37, 3164–3173. [Google Scholar] [CrossRef] [PubMed]
- Suturović, Z.; Kravić, S.; Milanović, S.; Đurović, A.; Brezo, T. Determination of Heavy Metals in Milk and Fermented Milk Products by Potentiometric Stripping Analysis with Constant Inverse Current in the Analytical Step. Food Chem. 2014, 155, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Pu, H.; Sun, D. Emerging Spectroscopic and Spectral Imaging Techniques for the Rapid Detection of Microorganisms: An Overview. Compr. Rev. Food Sci. Food Saf. 2018, 17, 256–273. [Google Scholar] [CrossRef]
- Feghali, N.; Piras, N.; Serini, B.; Borghini, A.; Zara, G.; Bianco, A.; Budroni, M. A Deliberative Model for Preserving the Diversity of Lebanese Traditional Fermented Food and Beverages. Hum. Ecol. 2022, 50, 589–600. [Google Scholar] [CrossRef]
- Mujahid, M.; Wakeel, M.; Ali, A.M.; Saeed, S.; Nawaz, A.S.; Hafeez, K. Food Fermentation: Traditional Practices and Modern Applications in Food Industry. Int. J. Food Ferment. Technol. 2024, 14, 239–273. [Google Scholar] [CrossRef]
- Terefe, N.S. Recent Developments in Fermentation Technology: Toward the next Revolution in Food Production. In Food Engineering Innovations Across the Food Supply Chain; Academic Press: New York, NY, USA, 2022; pp. 89–106. [Google Scholar]
- Gao, L.; Zhou, J.; He, G. Effect of Microbial Interaction on Flavor Quality in Chinese Baijiu Fermentation. Front. Nutr. 2022, 9, 960712. [Google Scholar] [CrossRef]
- Ren, F.; Yan, D.; Liu, Y.; Wang, C.; Guo, C. Bacterial and Fungal Communities of Traditional Fermented Chinese Soybean Paste (Doujiang) and Their Properties. Food Sci. Nutr. 2021, 9, 5457–5466. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, M.; Zhang, Y.; Zhang, X.; Zhang, X.; Zhao, X.; Xu, H.; Huang, Y. Correlation between Bacterial Diversity and Flavor Substances in Longgang Soy Sauce. Biosci. Biotechnol. Biochem. 2023, 87, 541–554. [Google Scholar] [CrossRef]
- Fan, J.; Qu, G.; Wang, D.; Chen, J.; Du, G.; Fang, F. Synergistic Fermentation with Functional Microorganisms Improves Safety and Quality of Traditional Chinese Fermented Foods. Foods 2023, 12, 2892. [Google Scholar] [CrossRef]
- Dučić, M.; Barcenilla, C.; Cobo-Díaz, J.F.; López, M.; Álvarez-Ordóñez, A.; Prieto, M. High Pressure Processing at the Early Stages of Ripening Enhances the Safety and Quality of Dry Fermented Sausages Elaborated with or without Starter Culture. Food Res. Int. 2023, 163, 112162. [Google Scholar] [CrossRef] [PubMed]
- Austrich-Comas, A.; Serra-Castelló, C.; Jofré, A.; Gou, P.; Bover-Cid, S. Control of Listeria Monocytogenes in Chicken Dry-Fermented Sausages with Bioprotective Starter Culture and High-Pressure Processing. Front. Microbiol. 2022, 13, 983265. [Google Scholar] [CrossRef] [PubMed]
- Komora, N.; Maciel, C.; Amaral, R.A.; Fernandes, R.; Castro, S.M.; Saraiva, J.A.; Teixeira, P. Innovative Hurdle System towards Listeria monocytogenes Inactivation in a Fermented Meat Sausage Model-High Pressure Processing Assisted by Bacteriophage P100 and Bacteriocinogenic Pediococcus acidilactici. Food Res. Int. 2021, 148, 110628. [Google Scholar] [CrossRef] [PubMed]
- Komora, N.; Maciel, C.; Isidro, J.; Pinto, C.A.; Fortunato, G.; Saraiva, J.M.; Teixeira, P. The Impact of HPP-Assisted Biocontrol Approach on the Bacterial Communities’ Dynamics and Quality Parameters of a Fermented Meat Sausage Model. Biology 2023, 12, 1212. [Google Scholar] [CrossRef]
- Li, S.; Du, D.; Wang, J.; Wei, Z. Application Progress of Intelligent Flavor Sensing System in the Production Process of Fermented Foods Based on the Flavor Properties. Crit. Rev. Food Sci. Nutr. 2024, 64, 3764–3793. [Google Scholar] [CrossRef]
- Watson, N.J.; Bowler, A.L.; Rady, A.; Fisher, O.J.; Simeone, A.; Escrig, J.; Woolley, E.; Adedeji, A.A. Intelligent Sensors for Sustainable Food and Drink Manufacturing. Front. Sustain. Food Syst. 2021, 5, 642786. [Google Scholar] [CrossRef]
- Adeleke, I.; Nwulu, N.; Adebo, O.A. Internet of Things (IoT) in the Food Fermentation Process: A Bibliometric Review. J. Food Process Eng. 2023, 46, e14321. [Google Scholar] [CrossRef]
- Bleicher, F.; Ramsauer, C.; Leonhartsberger, M.; Lamprecht, M.; Stadler, P.; Strasser, D.; Wiedermann, C. Tooling Systems with Integrated Sensors Enabling Data Based Process Optimization. J. Mach. Eng. 2021, 21, 5–21. [Google Scholar] [CrossRef]
- Ferone, M.; Gowen, A.; Fanning, S.; Scannell, A.G. Microbial Detection and Identification Methods: Bench Top Assays to Omics Approaches. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3106–3129. [Google Scholar] [CrossRef]
- Ruiz, G.D.; Rodarte, C.W. Methods for the Study of Microbial Communities in Fermented Foods. Rev. Latinoam. Microbiol. 2003, 45, 30–40. [Google Scholar]
- Fayyaz, K.; Nawaz, A.; Olaimat, A.N.; Akram, K.; Farooq, U.; Fatima, M.; Siddiqui, S.A.; Rana, I.S.; Shahbaz, H.M. Microbial Toxins in Fermented Foods: Health Implications and Analytical Techniques for Detection. J. Food Drug Anal. 2022, 30, 523. [Google Scholar] [CrossRef] [PubMed]
- Mataragas, M.; Bosnea, L. Fermented Foods: New Concepts and Technologies for the Development of New Products, Quality Control. Foods 2022, 11, 441. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, H.M.; Pasquali, M.B.; dos Anjos, A.I.; Sarinho, A.M.; de Melo, E.D.; Andrade, R.; Batista, L.; Lima, J.; Diniz, Y.; Barros, A. Innovative and Sustainable Food Preservation Techniques: Enhancing Food Quality, Safety, and Environmental Sustainability. Sustainability 2024, 16, 8223. [Google Scholar] [CrossRef]
- Chen, L.; Wang, G.; Teng, M.; Wang, L.; Yang, F.; Jin, G.; Du, H.; Xu, Y. Non-gene-editing Microbiome Engineering of Spontaneous Food Fermentation Microbiota—Limitation Control. Design Control, and Integration. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1902–1932. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ferreira, V.; Magalhães, R.; Teixeira, P. Biocontrol Strategies for Mediterranean-Style Fermented Sausages. Food Res. Int. 2018, 103, 438–449. [Google Scholar] [CrossRef]
- Chen, W.; Lv, X.; Tran, V.-T.; Maruyama, J.; Han, K.-H.; Yu, J.-H. From Traditional to Modern: Progress of Molds and Yeasts in Fermented-Food Production. Front. Microbiol. 2022, 13, 876872. [Google Scholar]
- Rao, C.S.M.; Madhuri, M.L.; Swami, D.V.; Ashok, P.; Rao, B.B.; Suneetha, S.D.R. Preserving the Past, Embracing the Future: Exploring Traditional and Modern Methods of Food Preservation. In Futuristic Trends in Agriculture Engineering & Food Sciences; Senthilvalavan, P., Langyan, S., Anwar, A., Sharma, S., Eds.; Iterative International Publisher, Selfypage Developers Pvt Ltd.: Chikkamagaluru, India, 2024; Volume 3, pp. 39–65. ISBN 978-93-5747-760-4. [Google Scholar]
- Siddiqui, S.A.; Erol, Z.; Rugji, J.; Taşçı, F.; Kahraman, H.A.; Toppi, V.; Musa, L.; Di Giacinto, G.; Bahmid, N.A.; Mehdizadeh, M. An Overview of Fermentation in the Food Industry-Looking Back from a New Perspective. Bioresour. Bioprocess. 2023, 10, 85. [Google Scholar] [CrossRef]
- Anal, A. Quality Ingredients and Safety Concerns for Traditional Fermented Foods and Beverages from Asia: A Review. Fermentation 2019, 5, 8. [Google Scholar] [CrossRef]
- Meloni, D. High-Hydrostatic-Pressure (HHP) Processing Technology as a Novel Control Method for Listeria monocytogenes Occurrence in Mediterranean-Style Dry-Fermented Sausages. Foods 2019, 8, 672. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Wang, H.; Gou, F.; He, Y.; Yang, L. Advancements in Fermented Beverage Safety: Isolation and Application of Clavispora lusitaniae Cl-p for Ethyl Carbamate Degradation and Enhanced Flavor Profile. Microorganisms 2024, 12, 882. [Google Scholar] [CrossRef]
- Al, H.F.A.E. Enhancing the Value Added and Quality Characteristics of Fermented Mullet Fish (Feseekh) by Microbial Inoculation with Lactobacillus plantarum and Saccharomyces cerevisiae. Egypt. J. Aquat. Biol. Fish. 2023, 27, 1369–1391. [Google Scholar]
- Kim, J.; Jeong, J.; Jang, M.; Kim, J.-C.; Lee, H. Comparative Analysis of Microbial and Mycotoxin Contamination in Korean Traditional Soybean Paste and Soy Sauce Production with and without Starter. Fermentation 2023, 9, 621. [Google Scholar] [CrossRef]
- Abid, S.; Farid, A.; Abid, R.; Rehman, M.U.; Alsanie, W.F.; Alhomrani, M.; Alamri, A.S.; Asdaq, S.M.B.; Hefft, D.I.; Saqib, S.; et al. Identification, Biochemical Characterization, and Safety Attributes of Locally Isolated Lactobacillus fermentum from Bubalus bubalis (buffalo) Milk as a Probiotic. Microorganisms 2022, 10, 954. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Chen, J.-S.; Hsu, B.-M.; Chu, I.-T.; Koner, S.; Chen, T.-H.; Rathod, J.; Chan, M.W. Deciphering Bacterial Community Structure, Functional Prediction and Food Safety Assessment in Fermented Fruits Using next-Generation 16S rRNA Amplicon Sequencing. Microorganisms 2021, 9, 1574. [Google Scholar] [CrossRef] [PubMed]
- Feye, K.M.; Carroll, J.P.; Anderson, K.L.; Whittaker, J.H.; Schmidt-McCormack, G.R.; McIntyre, D.R.; Pavlidis, H.O.; Carlson, S.A. Saccharomyces cerevisiae Fermentation Products That Mitigate Foodborne Salmonella in Cattle and Poultry. Front. Vet. Sci. 2019, 6, 107. [Google Scholar] [CrossRef]
- Lee, B.; Yong, C.-C.; Yi, H.-C.; Kim, S.; Oh, S. A Non-Yeast Kefir-Like Fermented Milk Development with Lactobacillus acidophilus KCNU and Lactobacillus brevis Bmb6. Food Sci. Anim. Resour. 2020, 40, 541–550. [Google Scholar] [CrossRef]
- Ou, D.; Ling, N.; Wang, X.; Zou, Y.; Dong, J.; Zhang, D.; Shen, Y.; Ye, Y. Safety Assessment of One Lactiplantibacillus plantarum Isolated from the Traditional Chinese Fermented Vegetables—Jiangshui. Foods 2022, 11, 2177. [Google Scholar] [CrossRef]
- Xiang, X.; Li, Y.; Ye, J.; Li, B.; He, G.; Zhu, M.; Zhang, J.; Zhang, B.; Miao, M.; Yang, Y. Exploring the Microbiome of Fermented Soy Products: Implications for Gut Health in China. Research Square 2024. [Google Scholar] [CrossRef]
- Leroy, S.; Christieans, S.; Talon, R. Tetracycline Gene Transfer in Staphylococcus xylosus in Situ during Sausage Fermentation. Front. Microbiol. 2019, 10, 392. [Google Scholar] [CrossRef]
- Liao, H.; Luo, Y.; Asif, H.; Luo, Y.; Xia, X. Novel Insights into Safety and Quality Enhancement of Low-Salt Fermented Chilies: High-Order Positively Interacting Lactic Acid Bacteria Co-Fermentation Regulates Microflora Structure, Metabolomics, and Volatilomics Profiles. Food Biosci. 2024, 59, 103861. [Google Scholar] [CrossRef]
- Zhao, M.; Su, X.Q.; Nian, B.; Chen, L.J.; Zhang, D.L.; Duan, S.M.; Wang, L.Y.; Shi, X.Y.; Jiang, B.; Jiang, W.W. Integrated Meta-omics Approaches to Understand the Microbiome of Spontaneous Fermentation of Traditional Chinese Pu-Erh Tea. Msystems 2019, 4, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Monges, H.S. Producing High-Value Chemicals in Escherichia coli through Synthetic Biology and Metabolic Engineering. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2019. [Google Scholar]
- Huang, J.; Wang, J.; Liu, S. Advanced Fermentation Techniques for Lactic Acid Production from Agricultural Waste. Fermentation 2023, 9, 765. [Google Scholar] [CrossRef]
- Kaya, B.; Wijayarathna, E.K.B.; Yüceer, Y.K.; Agnihotri, S.; Taherzadeh, M.J.; Sar, T. The Use of Cheese Whey Powder in the Cultivation of Protein-Rich Filamentous Fungal Biomass for Sustainable Food Production. Front. Sustain. Food Syst. 2024, 8, 1386519. [Google Scholar] [CrossRef]
- Reardon, K.F. Practical Monitoring Technologies for Cells and Substrates in Biomanufacturing. Curr. Opin. Biotechnol. 2021, 71, 225–230. [Google Scholar] [CrossRef]
- Foncillas, R.P.; Sebastiá, M.S.; Wallberg, O.; Carlquist, M.; Gorwa-Grauslund, M.F. Assessment of the TRX2p-yEGFP Biosensor to Monitor the Redox Response of an Industrial Xylose-Fermenting Saccharomyces cerevisiae Strain during Propagation and Fermentation. J. Fungi 2023, 9, 630. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Asci, C.; Del-Rio-Ruiz, R.; Trinidad, K.; Hossain, N.I.; Kaplan, D.L.; Sonkusale, S. Multiplexed Sensing Probe for Bioreactors for Cellular Agriculture. IEEE Sens. Lett. 2023, 7, 1–4. [Google Scholar] [CrossRef]
- Kreß, S.; Schaller-Ammann, R.; Feiel, J.; Wegener, J.; Priedl, J.; Dietrich, W.; Kasper, C.; Egger, D. Innovative Platform for the Advanced Online Monitoring of Three-Dimensional Cells and Tissue Cultures. Cells 2022, 11, 412. [Google Scholar] [CrossRef]
- Pal, A.; Kant, K. Smart Sensing, Communication, and Control in Perishable Food Supply Chain. ACM Trans. Sens. Netw. TOSN 2020, 16, 1–41. [Google Scholar] [CrossRef]
- Chen, S.; Brahma, S.; Mackay, J.; Cao, C.; Aliakbarian, B. The Role of Smart Packaging System in Food Supply Chain. J. Food Sci. 2020, 85, 517–525. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Deng, Y. Latest Advances in Active Materials for Food Packaging and Their Application. Foods 2023, 12, 4055. [Google Scholar] [CrossRef]
- Bolwig, S.; Tanner, A.N.; Riemann, P.; Redlingshöfer, B.; Zhang, Y. Reducing Consumer Food Waste Using Green and Digital Technologies; UNEP DTU Partnership: Washington, DC, USA, 2021. [Google Scholar]
- Kuswandi, B. Active and Intelligent Packaging, Safety, and Quality Controls. In Fresh-Cut Fruits and Vegetables; Academic Press: New York, NY, USA, 2020; pp. 243–294. [Google Scholar]
- Mohammadian, E.; Alizadeh-Sani, M.; Jafari, S.M. Smart Monitoring of Gas/Temperature Changes within Food Packaging Based on Natural Colorants. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2885–2931. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Burak, L.C.; Sapach, A.N.; Pisarik, M.I. Intelligent Packaging For Vegetables And Fruits, Classification And Use Prospects: Scoping Review. Health Food Biotechnol. 2023, 5, 51. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Suppakul, P. Active and Intelligent Packaging: The Indication of Quality and Safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 808–831. [Google Scholar] [CrossRef]
- Kim, G.B.; Kim, W.J.; Kim, H.U.; Lee, S.Y. Machine Learning Applications in Systems Metabolic Engineering. Curr. Opin. Biotechnol. 2020, 64, 1–9. [Google Scholar] [CrossRef]
- Khaleghi, M.K.; Savizi, I.S.P.; Lewis, N.E.; Shojaosadati, S.A. Synergisms of Machine Learning and Constraint-based Modeling of Metabolism for Analysis and Optimization of Fermentation Parameters. Biotechnol. J. 2021, 16, 2100212. [Google Scholar] [CrossRef]
- Tsui, T.-H.; van Loosdrecht, M.C.; Dai, Y.; Tong, Y.W. Machine Learning and Circular Bioeconomy: Building New Resource Efficiency from Diverse Waste Streams. Bioresour. Technol. 2023, 369, 128445. [Google Scholar] [CrossRef]
- Kumar, I.; Rawat, J.; Mohd, N.; Husain, S. Opportunities of Artificial Intelligence and Machine Learning in the Food Industry. J. Food Qual. 2021, 2021, 4535567. [Google Scholar] [CrossRef]
- Wu, D.; Xu, Y.; Xu, F.; Shao, M.; Huang, M. Machine Learning Algorithms for In-Line Monitoring during Yeast Fermentations Based on Raman Spectroscopy. Vib. Spectrosc. 2024, 132, 103672. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S. Low-Cost Methods to Assess Beer Quality Using Artificial Intelligence Involving Robotics, an Electronic Nose, and Machine Learning. Fermentation 2020, 6, 104. [Google Scholar] [CrossRef]
- Galimberti, A.; Bruno, A.; Agostinetto, G.; Casiraghi, M.; Guzzetti, L.; Labra, M. Fermented Food Products in the Era of Globalization: Tradition Meets Biotechnology Innovations. Curr. Opin. Biotechnol. 2021, 70, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Ahire, J.J.; Taneja, N.K. Advancing Microbial Food Safety and Hazard Analysis through Predictive Mathematical Modeling. Microbe 2024, 2, 100049. [Google Scholar] [CrossRef]
- Medina, V.Y. Predictive Microbiology and Machine Learning by Optimization Productive Process: Metanalysis. Acta Sci. Microbiol. 2023, 6, 54–66. [Google Scholar] [CrossRef]
- Karanth, S.; Tanui, C.K.; Meng, J.; Pradhan, A.K. Exploring the Predictive Capability of Advanced Machine Learning in Identifying Severe Disease Phenotype in Salmonella enterica. Food Res. Int. 2022, 151, 110817. [Google Scholar] [CrossRef]
- Huang, L.; Jia, Z.; Hwang, C.-A. Growth and No-Growth Boundary of Listeria monocytogenes in Beef–A Logistic Modeling. Food Res. Int. 2022, 152, 110919. [Google Scholar] [CrossRef]
- Yesodha, K.R.K.; Jagadeesan, A.; Logeshwaran, J. IoT Applications in Modern Supply Chains: Enhancing Efficiency and Product Quality. In Proceedings of the IEEE 2nd International Conference on Industrial Electronics: Developments & Applications (ICIDeA), Imphal, India, 29–30 September 2023; pp. 366–371. [Google Scholar]
- Taiwo, O.R.; Onyeaka, H.; Oladipo, E.K.; Oloke, J.K.; Chukwugozie, D.C. Advancements in Predictive Microbiology: Integrating New Technologies for Efficient Food Safety Models. Int. J. Microbiol. 2024, 2024, 6612162. [Google Scholar] [CrossRef]
- O’Brien, A.; Zhang, H.; Allwood, D.M.; Rawsthorne, A. From Data to Draught: Modelling and Predicting Mixed-Culture Beer Fermentation Dynamics Using Autoregressive Recurrent Neural Networks. Modelling 2024, 5, 201–222. [Google Scholar] [CrossRef]
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2020, 10, 69. [Google Scholar] [CrossRef]
- Pennone, V.; Cobo-Díaz, J.F.; Prieto, M.; Alvarez-Ordóñez, A. Application of Genomics and Metagenomics to Improve Food Safety Based on an Enhanced Characterisation of Antimicrobial Resistance. Curr. Opin. Food Sci. 2022, 43, 183–188. [Google Scholar] [CrossRef]
- Fytsilis, V.D.; Urlings, M.J.; van Schooten, F.-J.; de Boer, A.; Vrolijk, M.F. Toxicological Risks of Dairy Proteins Produced through Cellular Agriculture: Current State of Knowledge, Challenges and Future Perspectives. Future Foods 2024, 10, 100412. [Google Scholar] [CrossRef]
- Puvaca, N.; Vapa, B. Implementation of Food Safety Policy in the European Union-Guidance, Variety, and Resolution of Challenges. Law Theory Pr. 2024, 41, 18. [Google Scholar] [CrossRef]
- Mukherjee, A.; Gómez-Sala, B.; O’Connor, E.M.; Kenny, J.G.; Cotter, P.D. Global Regulatory Frameworks for Fermented Foods: A Review. Front. Nutr. 2022, 9, 902642. [Google Scholar] [CrossRef] [PubMed]
- Waché, Y.; Do, T.-L.; Do, T.-B.-H.; Do, T.-Y.; Haure, M.; Ho, P.-H.; Kumar Anal, A.; Le, V.-V.-M.; Li, W.-J.; Licandro, H. Prospects for Food Fermentation in South-East Asia, Topics from the Tropical Fermentation and Biotechnology Network at the End of the AsiFood Erasmus+Project. Front. Microbiol. 2018, 9, 2278. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Yoneoka, D.; Ishizuka, A.; Adachi, M.; Hayabuchi, H.; Nishimura, T.; Takemi, Y.; Uneyama, H.; Nakamura, H.; Lwin, K.S. Modelling of Salt Intake Reduction by Incorporation of Umami Substances into Japanese Foods: A Cross-Sectional Study. BMC Public Health 2023, 23, 516. [Google Scholar] [CrossRef]
- Farag, M.A.; Zain, A.E.; Hariri, M.L.; el Aaasar, R.; Khalifa, I.; Elmetwally, F. Potential Food Safety Hazards in Fermented and Salted Fish in Egypt (Feseekh, Renga, Moloha) as Case Studies and Controlling Their Manufacture Using HACCP System. J. Food Saf. 2022, 42, e12973. [Google Scholar] [CrossRef]
- Uzoigwe, D.O.; Kongolo, D. Integration of Hazard Analysis and Critical Control Points HACCP with Maintenance Practices: Enhancing Food Safety in the Food and Beverage Industry; A Review. Int. J. Latest Technol. Eng. Manag. Appl. Sci. 2024, 13, 88–101. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. Persistence of Microbiological Hazards in Food and Feed Production and Processing Environments. EFSA J. 2024, 22, e8521. [Google Scholar] [CrossRef]
- Heo, S.; Kim, T.; Na, H.-E.; Lee, G.; Park, J.-H.; Park, H.-J.; Jeong, D.-W. Safety Assessment Systems for Microbial Starters Derived from Fermented Foods. J. Microbiol. Biotechnol. 2022, 32, 1219. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Ong, H.C.; Yong, A.M.H.; Fattori, V.; Mukherjee, K. Addressing the Safety of New Food Sources and Production Systems. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13341. [Google Scholar] [CrossRef]
- Lee, J.-G.; Lee, Y.; Kim, C.S.; Han, S.B. Codex Alimentarius Commission on Ensuring Food Safety and Promoting Fair Trade: Harmonization of Standards between Korea and Codex. Food Sci. Biotechnol. 2021, 30, 1151–1170. [Google Scholar] [CrossRef]
- Wui, P.; Kim, K.; Seo, G. The Influence of Codex Guidelines on International Trade: An Analysis Focused on Kimchi. Int. J. Asian Soc. Sci. 2023, 13, 282–292. [Google Scholar] [CrossRef]
- Verbruggen, P.; Havinga, T. Transnational Business Governance Interactions in Food Safety Regulation: Exploring the Promises and Risks of Enrolment. In Transnational Business Governance Interactions; Edward Elgar Publishing: Cheltenham, UK, 2019; pp. 28–51. ISBN 1-78811-473-6. [Google Scholar]
- Eruaga, M.A. Enhancing Global Food Safety Standards through International Collaboration and Policy Harmonization. Int. J. Sch. Res. Multidiscip. Stud. 2024, 4, 20–32. [Google Scholar]
- World Health Organization (WHO). Global Strategy for Food Safety 2022–2030: Towards Stronger Food Safety Systems and Global Cooperation; World Health Organization: Geneva, Switzerland, 2022; ISBN 92-4-005768-4. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niyigaba, T.; Küçükgöz, K.; Kołożyn-Krajewska, D.; Królikowski, T.; Trząskowska, M. Advances in Fermentation Technology: A Focus on Health and Safety. Appl. Sci. 2025, 15, 3001. https://doi.org/10.3390/app15063001
Niyigaba T, Küçükgöz K, Kołożyn-Krajewska D, Królikowski T, Trząskowska M. Advances in Fermentation Technology: A Focus on Health and Safety. Applied Sciences. 2025; 15(6):3001. https://doi.org/10.3390/app15063001
Chicago/Turabian StyleNiyigaba, Theoneste, Kübra Küçükgöz, Danuta Kołożyn-Krajewska, Tomasz Królikowski, and Monika Trząskowska. 2025. "Advances in Fermentation Technology: A Focus on Health and Safety" Applied Sciences 15, no. 6: 3001. https://doi.org/10.3390/app15063001
APA StyleNiyigaba, T., Küçükgöz, K., Kołożyn-Krajewska, D., Królikowski, T., & Trząskowska, M. (2025). Advances in Fermentation Technology: A Focus on Health and Safety. Applied Sciences, 15(6), 3001. https://doi.org/10.3390/app15063001










