Effect of Altitude on Polyphenol Content, Antioxidant Activity and Multi-Element Composition of Wildflower Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Preparation of Honey Samples
2.3. Total Phenolic Content (TPC)
2.4. Total Flavonoid Content (TFC)
2.5. ABTS
2.6. FRAP
2.7. Determination of Element Content
2.8. Analysis by ICP-MS
2.9. Statistical Analysis
3. Results and Discussions
3.1. Total Phenols, Flavonoids and Antioxidant Activity in Multiflora Honeys
3.2. Mineral Contents in Multiflora Honeys
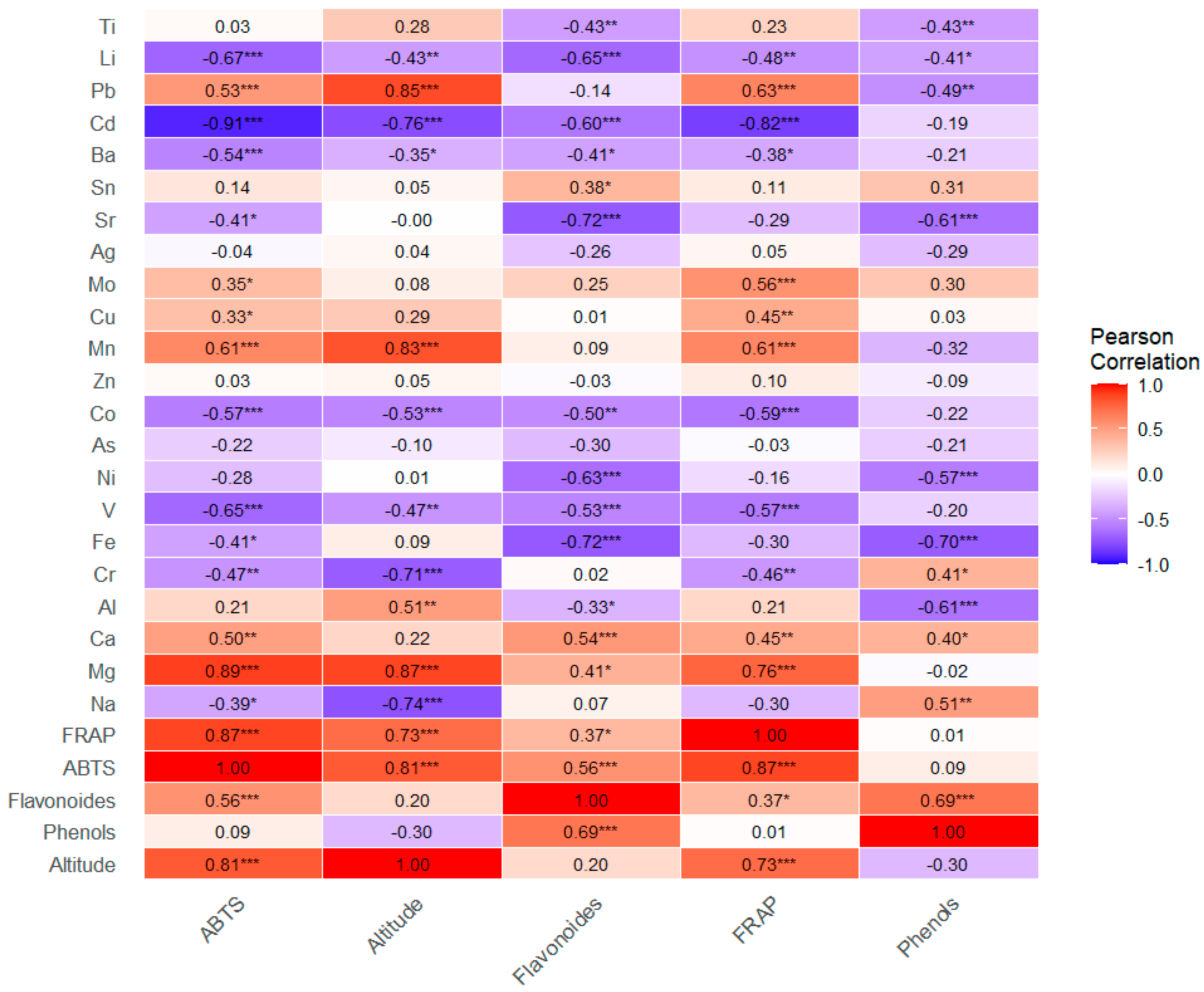
3.3. Correlation
3.4. Correlation Between Altitude and Studied Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashagrie Tafere, D. Chemical Composition and Uses of Honey: A Review. J. Food Sci. Nutr. Res. 2021, 4, 194–201. [Google Scholar] [CrossRef]
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical Properties, Minerals, Trace Elements, and Heavy Metals in Honey of Different Origins: A Comprehensive Review. Comp. Rev. Food Sci. Food Safe 2016, 15, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Haldimann, M.; Luginbühl, W.; Gallmann, P. Minerals in Honey: Environmental, Geographical and Botanical Aspects. J. Apic. Res. 2007, 46, 269–275. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tomás-Barberán, F.A.; Tornadijo, M.E.; Fresno-Baro, J.M. Bioactive Components and Antioxidant and Antibacterial Activities of Different Varieties of Honey: A Screening Prior to Clinical Application. J. Agric. Food Chem. 2019, 67, 688–698. [Google Scholar] [CrossRef]
- dos Santos Scholz, M.B.; Júnior, A.Q.; Delamuta, B.H.; Nakamura, J.M.; Baudraz, M.C.; Reis, M.O.; Kato, T.; Pedrão, M.R.; Dias, L.F.; dos Santos, D.T.R.; et al. Indication of the Geographical Origin of Honey Using Its Physicochemical Characteristics and Multivariate Analysis. J. Food Sci. Technol. 2020, 57, 1896. [Google Scholar] [CrossRef]
- Direttiva 2014/63/UE. Directive 2014/63/EU of the European Parliament and of the Council of 15 May 2014 Amending Council Directive 2001/110/EC Relating to Honey; European Union: Brussels, Belgium, 2014. [Google Scholar]
- Wu, J.; Duan, Y.; Gao, Z.; Yang, X.; Zhao, D.; Gao, J.; Han, W.; Li, G.; Wang, S. Quality Comparison of Multifloral Honeys Produced by Apis Cerana Cerana, Apis Dorsata and Lepidotrigona Flavibasis. LWT 2020, 134, 110225. [Google Scholar] [CrossRef]
- Larsen, P.; Ahmed, M. Evaluation of Antioxidant Potential of Honey Drops and Honey Lozenges. Food Chem. Adv. 2022, 1, 100013. [Google Scholar] [CrossRef]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and Its Nutritional and Anti-Inflammatory Value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef]
- Almasaudi, S. The Antibacterial Activities of Honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Erejuwa, O.O. Chapter 31—Honey: Profile and Features: Applications to Diabetes. In Bioactive Food as Dietary Interventions for Diabetes, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 461–494. ISBN 978-0-12-813822-9. [Google Scholar]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Suleiman, M.H.A.; ALaerjani, W.M.A.; Mohammed, M.E.A. Influence of Altitudinal Variation on the Total Phenolic and Flavonoid Content of Acacia and Ziziphus Honey. Int. J. Food Prop. 2020, 23, 2077–2086. [Google Scholar] [CrossRef]
- Thakur, M.; Gupta, N.; Sharma, H.K.; Devi, S. Physicochemical Characteristics and Mineral Status of Honey from Different Agro-Climatic Zones of Himachal Pradesh, India. BFJ 2021, 123, 3789–3804. [Google Scholar] [CrossRef]
- Al, M.L.; Daniel, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-Chemical and Bioactive Properties of Different Floral Origin Honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Gasparrini, M.; Forbes-Hernández, T.Y.; Mazzoni, L.; Giampieri, F. The Composition and Biological Activity of Honey: A Focus on Manuka Honey. Foods 2014, 3, 420–432. [Google Scholar] [CrossRef]
- Özkök, A.; Keskin, M.; Tanuğur Samancı, A.E.; Yorulmaz Önder, E.; Takma, Ç. Determination of Antioxidant Activity and Phenolic Compounds for Basic Standardization of Turkish Propolis. Appl. Biol. Chem. 2021, 64, 37. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A.; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as Well as Their Radical Scavenging Activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ahmed, T.; Sun, X.; Udenigwe, C.C. Role of Structural Properties of Bioactive Peptides in Their Stability during Simulated Gastrointestinal Digestion: A Systematic Review. Trends Food Sci. Technol. 2022, 120, 265–273. [Google Scholar] [CrossRef]
- Pichichero, E.; Canuti, L.; Canini, A. Characterisation of the Phenolic and Flavonoid Fractions and Antioxidant Power of Italian Honeys of Different Botanical Origin. J. Sci. Food Agric. 2009, 89, 609–616. [Google Scholar] [CrossRef]
- Habryka, C.; Socha, R.; Juszczak, L. Effect of Bee Pollen Addition on the Polyphenol Content, Antioxidant Activity, and Quality Parameters of Honey. Antioxidants 2021, 10, 810. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Gruyaert, S.; Poliwoda, A.; Kafarski, P. Chemical Profiling of Polyfloral Belgian Honey: Ellagic Acid and Pinocembrin as Antioxidants and Chemical Markers. J. Chem. 2017, 2017, 5393158. [Google Scholar] [CrossRef]
- Nascimento, K.S.D.; Gasparotto Sattler, J.A.; Lauer Macedo, L.F.; Serna González, C.V.; Pereira De Melo, I.L.; Da Silva Araújo, E.; Granato, D.; Sattler, A.; De Almeida-Muradian, L.B. Phenolic Compounds, Antioxidant Capacity and Physicochemical Properties of Brazilian Apis Mellifera Honeys. LWT 2018, 91, 85–94. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the Phenolic Content, Antioxidant Activity and Colour of Slovenian Honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Ghorab, A.; Rodríguez-Flores, M.S.; Nakib, R.; Escuredo, O.; Haderbache, L.; Bekdouche, F.; Seijo, M.C. Sensorial, Melissopalynological and Physico-Chemical Characteristics of Honey from Babors Kabylia’s Region (Algeria). Foods 2021, 10, 225. [Google Scholar] [CrossRef]
- Kivima, E.; Tanilas, K.; Martverk, K.; Rosenvald, S.; Timberg, L.; Laos, K. The Composition, Physicochemical Properties, Antioxidant Activity, and Sensory Properties of Estonian Honeys. Foods 2021, 10, 511. [Google Scholar] [CrossRef]
- Gośliński, M.; Nowak, D.; Kłębukowska, L. Antioxidant Properties and Antimicrobial Activity of Manuka Honey versus Polish Honeys. J. Food Sci. Technol. 2020, 57, 1269–1277. [Google Scholar] [CrossRef]
- Cabrera, M.C.; Perez, M.; Gallez, L.; Andrada, A.; Balbarrey, G. Colour, Antioxidant Capacity, Phenolic and Flavonoid Content of Honey from the Humid Chaco Region, Argentina. Phyton 2017, 86, 124–130. [Google Scholar] [CrossRef]
- Mahnot, N.K.; Saikia, S.; Mahanta, C.L. Quality Characterization and Effect of Sonication Time on Bioactive Properties of Honey from North East India. J. Food Sci. Technol. 2019, 56, 724–736. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, N.; He, L.; Peng, G.; Xue, X.; Wu, L.; Cao, W. Antioxidant and Hepatoprotective Effects of A. Cerana Honey against Acute Alcohol-Induced Liver Damage in Mice. Food Res. Int. 2017, 101, 35–44. [Google Scholar] [CrossRef]
- Đogo Mračević, S.; Krstić, M.; Lolić, A.; Ražić, S. Comparative Study of the Chemical Composition and Biological Potential of Honey from Different Regions of Serbia. Microchem. J. 2020, 152, 104420. [Google Scholar] [CrossRef]
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant Activities and Total Phenolics of Different Types of Honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Aljadi, A.M.; Kamaruddin, M.Y. Evaluation of the Phenolic Contents and Antioxidant Capacities of Two Malaysian Floral Honeys. Food Chem. 2004, 85, 513–518. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic Compounds and Their Antioxidant Activity in Plants Growing under Heavy Metal Stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability; Soto-Hernndez, M., Palma-Tenango, M., Garcia-Mateos, M.D.R., Eds.; InTech: Sydney, Austrilia, 2017. [Google Scholar]
- Gutiérrez-Grijalva, E.; Marcela, G.; Santos-Zea, L. Editorial: Trends in the Design of Functional Foods for Human Health. Front. Nutr. 2024, 11, 1393366. [Google Scholar] [CrossRef]
- Derouich, M.; Bouhlali, E.D.T.; Bammou, M.; Hmidani, A.; Sellam, K.; Alem, C. Bioactive Compounds and Antioxidant, Antiperoxidative, and Antihemolytic Properties Investigation of Three Apiaceae Species Grown in the Southeast of Morocco. Scientifica 2020, 2020, 3971041. [Google Scholar] [CrossRef]
- Grassi, G.; Capasso, G.; Gambacorta, E.; Perna, A.M. Chemical and Functional Characterization of Propolis Collected from Different Areas of South Italy. Foods 2023, 12, 3481. [Google Scholar] [CrossRef]
- Neupane, B.; Malla, K.; Kaundinnyayana, A.; Poudel, P.; Thapa, R.; Shrestha, S. Antioxidant Properties of Honey from Different Altitudes of Nepal Himalayas. Pol. J. Food Nutr. Sci. 2015, 65, 87–91. [Google Scholar] [CrossRef]
- Shantal Rodríguez Flores, M.; Escuredo, O.; Carmen Seijo, M. Assessment of Physicochemical and Antioxidant Characteristics of Quercus Pyrenaica Honeydew Honeys. Food Chem. 2015, 166, 101–106. [Google Scholar] [CrossRef]
- Mouhoubi-Tafinine, Z.; Ouchemoukh, S.; Tamendjari, A. Antioxydant Activity of Some Algerian Honey and Propolis. Ind. Crops Prod. 2016, 88, 85–90. [Google Scholar] [CrossRef]
- Rashed, M.N.; Soltan, M.E. Major and Trace Elements in Different Types of Egyptian Mono-Floral and Non-Floral Bee Honeys. J. Food Compos. Anal. 2004, 17, 725–735. [Google Scholar] [CrossRef]
- Schmidlová, S.; Javůrková, Z.; Tremlová, B.; Hernik, J.; Prus, B.; Marcinčák, S.; Marcinčáková, D.; Štarha, P.; Čížková, H.; Kružík, V.; et al. Exploring the Influence of Soil Types on the Mineral Profile of Honey: Implications for Geographical Origin Prediction. Foods 2024, 13, 2006. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Lo Turco, V.; Potortì, A.G.; Bua, G.D.; Fede, M.R.; Dugo, G. Geographical Discrimination of Italian Honey by Multi-Element Analysis with a Chemometric Approach. J. Food Compos. Anal. 2015, 44, 25–35. [Google Scholar] [CrossRef]
- Pisani, A.; Protano, G.; Riccobono, F. Minor and Trace Elements in Different Honey Types Produced in Siena County (Italy). Food Chem. 2008, 107, 1553–1560. [Google Scholar] [CrossRef]
- Perna, A.M.; Grassi, G.; Gambacorta, E.; Simonetti, A. Minerals Content in Basilicata Region (Southern Italy) Honeys from Areas with Different Anthropic Impact. Int. J. Food Sci. Technol. 2021, 56, 4465–4472. [Google Scholar] [CrossRef]
- Oroian, M.; Prisacaru, A.; Hretcanu, E.C.; Stroe, S.-G.; Leahu, A.; Buculei, A. Heavy Metals Profile in Honey as a Potential Indicator of Botanical and Geographical Origin. Int. J. Food Prop. 2016, 19, 1825–1836. [Google Scholar] [CrossRef]
- Tutun, H.; Kahraman, H.A.; Aluc, Y.; Avci, T.; Ekici, H. Investigation of Some Metals in Honey Samples from West Mediterranean Region of Turkey. Vet. Res. Forum 2019, 10, 181–186. [Google Scholar] [CrossRef]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. Metals in Honeys from Different Areas of Southern Italy. Bull. Environ. Contam. Toxicol. 2014, 92, 253–258. [Google Scholar] [CrossRef]
- Altundag, H.; Bina, E.; Altıntıg, E. The Levels of Trace Elements in Honey and Molasses Samples That Were Determined by ICP-OES After Microwave Digestion Method. Biol. Trace Elem. Res. 2016, 170, 508–514. [Google Scholar] [CrossRef]
- Yücel, Y.; Sultanoğlu, P. Characterization of Honeys from Hatay Region by Their Physicochemical Properties Combined with Chemometrics. Food Biosci. 2013, 1, 16–25. [Google Scholar] [CrossRef]
- Perna, A.; Simonetti, A.; Intaglietta, I.; Sofo, A.; Gambacorta, E. Metal Content of Southern Italy Honey of Different Botanical Origins and Its Correlation with Polyphenol Content and Antioxidant Activity. Int. J. Food Sci. Tech. 2012, 47, 1909–1917. [Google Scholar] [CrossRef]
- Quinto, M.; Miedico, O.; Spadaccino, G.; Paglia, G.; Mangiacotti, M.; Li, D.; Centonze, D.; Chiaravalle, A.E. Characterization, Chemometric Evaluation, and Human Health-Related Aspects of Essential and Toxic Elements in Italian Honey Samples by Inductively Coupled Plasma Mass Spectrometry. Environ. Sci. Pollut. Res. 2016, 23, 25374–25384. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.E.; Canepari, S.; Finoia, M.G.; Mele, G.; Astolfi, M.L. Characterization of Italian Multifloral Honeys on the Basis of Their Mineral Content and Some Typical Quality Parameters. J. Food Compos. Anal. 2018, 74, 102–113. [Google Scholar] [CrossRef]
- Halagarda, M.; Groth, S.; Popek, S.; Rohn, S.; Pedan, V. Antioxidant Activity and Phenolic Profile of Selected Organic and Conventional Honeys from Poland. Antioxidants 2020, 9, 44. [Google Scholar] [CrossRef]
- Ibrahimi, H.; Hajdari, A. Phenolic and Flavonoid Content, and Antioxidant Activity of Honey from Kosovo. J. Apic. Res. 2020, 59, 452–457. [Google Scholar] [CrossRef]
- Shamsudin, S.; Selamat, J.; Sanny, M.; Abd Razak, S.-B.; Jambari, N.N.; Mian, Z.; Khatib, A. Influence of Origins and Bee Species on Physicochemical, Antioxidant Properties and Botanical Discrimination of Stingless Bee Honey. Int. J. Food Prop. 2019, 22, 239–264. [Google Scholar] [CrossRef]
- Khiati, B. Evaluation of Physicochemical and Antioxidant Properties of Raw Honey from Algeria. J. Microb. Biochem. Technol. 2014, 4, 6. [Google Scholar] [CrossRef]
- Idris, Y.M.A.; Mariod, A.A.; Hamad, S.I. Physicochemical Properties, Phenolic Contents and Antioxidant Activity of Sudanese Honey. Int. J. Food Prop. 2011, 14, 450–458. [Google Scholar] [CrossRef]
- Hailu, D.; Belay, A. Melissopalynology and Antioxidant Properties Used to Differentiate Schefflera Abyssinica and Polyfloral Honey. PLoS ONE 2020, 15, e0240868. [Google Scholar] [CrossRef]
- Mokaya, H.O.; Bargul, J.L.; Irungu, J.W.; Lattorff, H.M.G. Bioactive Constituents, in Vitro Radical Scavenging and Antibacterial Activities of Selected Apis Mellifera Honey from Kenya. Int. J. Food Sci. Technol. 2020, 55, 1246–1254. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Al-Belushi, S.; Al-Amri, A.; Al-Hadhrami, A.; Al-Rusheidi, M.; Al-Alawi, A. Quality Evaluation of Omani Honey. Food Chem. 2018, 262, 162–167. [Google Scholar] [CrossRef]
- Marić, A.; Jovanov, P.; Sakač, M.; Novaković, A.; Hadnađev, M.; Pezo, L.; Mandić, A.; Milićević, N.; Đurović, A.; Gadžurić, S. A Comprehensive Study of Parameters Correlated with Honey Health Benefits. RSC Adv. 2021, 11, 12434–12441. [Google Scholar] [CrossRef] [PubMed]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P.; Sergiel, I.; Stecka, H. Determination and Fractionation of Metals in Honey. Crit. Rev. Anal. Chem. 2009, 39, 276–288. [Google Scholar] [CrossRef]
- Moridani, M.Y.; Pourahmad, J.; Bui, H.; Siraki, A.; O’Brien, P.J. Dietary Flavonoid Iron Complexes as Cytoprotective Superoxide Radical Scavengers. Free. Radic. Biol. Med. 2003, 34, 243–253. [Google Scholar] [CrossRef]
- Chaoui, A.; Mazhoudi, S.; Ghorbal, M.H.; El Ferjani, E. Cadmium and Zinc Induction of Lipid Peroxidation and Effects on Antioxidant Enzyme Activities in Bean (Phaseolus vulgaris L.). Plant Sci. 1997, 127, 139–147. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Shakoori, Z.; Salaseh, E.; Mehrabian, A.R.; Tehrani, D.M.; Dardashti, N.F.; Salmanpour, F. The Amount of Antioxidants in Honey Has a Strong Relationship with the Plants Selected by Honey Bees. Sci. Rep. 2024, 14, 351. [Google Scholar] [CrossRef]
- Teron, G.; Bordoloi, R.; Paul, A.; Singha, L.B.; Tripathi, O.P. Effect of Altitude on Soil Physico-Chemical Properties and Microbial Biomass Carbon in the Eaglenest Wildlife Sanctuary of Arunachal Pradesh. Geol. Ecol. Landsc. 2024, 1–19. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary Plant Polyphenols: Effects of Food Processing on Their Content and Bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef]
- Nemzer, B.V.; Al-Taher, F.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Health-Improving Effects of Polyphenols on the Human Intestinal Microbiota: A Review. Int. J. Mol. Sci. 2025, 26, 1335. [Google Scholar] [CrossRef]
- Tew, N.E.; Memmott, J.; Vaughan, I.P.; Bird, S.; Stone, G.N.; Potts, S.G.; Baldock, K.C.R. Quantifying Nectar Production by Flowering Plants in Urban and Rural Landscapes. J. Ecol. 2021, 109, 1747–1757. [Google Scholar] [CrossRef]
- Cardoso, F.C.G.; Marques, R.; Botosso, P.C.; Marques, M.C.M. Stem Growth and Phenology of Two Tropical Trees in Contrasting Soil Conditions. Plant Soil. 2012, 354, 269–281. [Google Scholar] [CrossRef]
- Loix, C.; Huybrechts, M.; Vangronsveld, J.; Gielen, M.; Keunen, E.; Cuypers, A. Reciprocal Interactions between Cadmium-Induced Cell Wall Responses and Oxidative Stress in Plants. Front. Plant Sci. 2017, 8, 1867. [Google Scholar] [CrossRef] [PubMed]
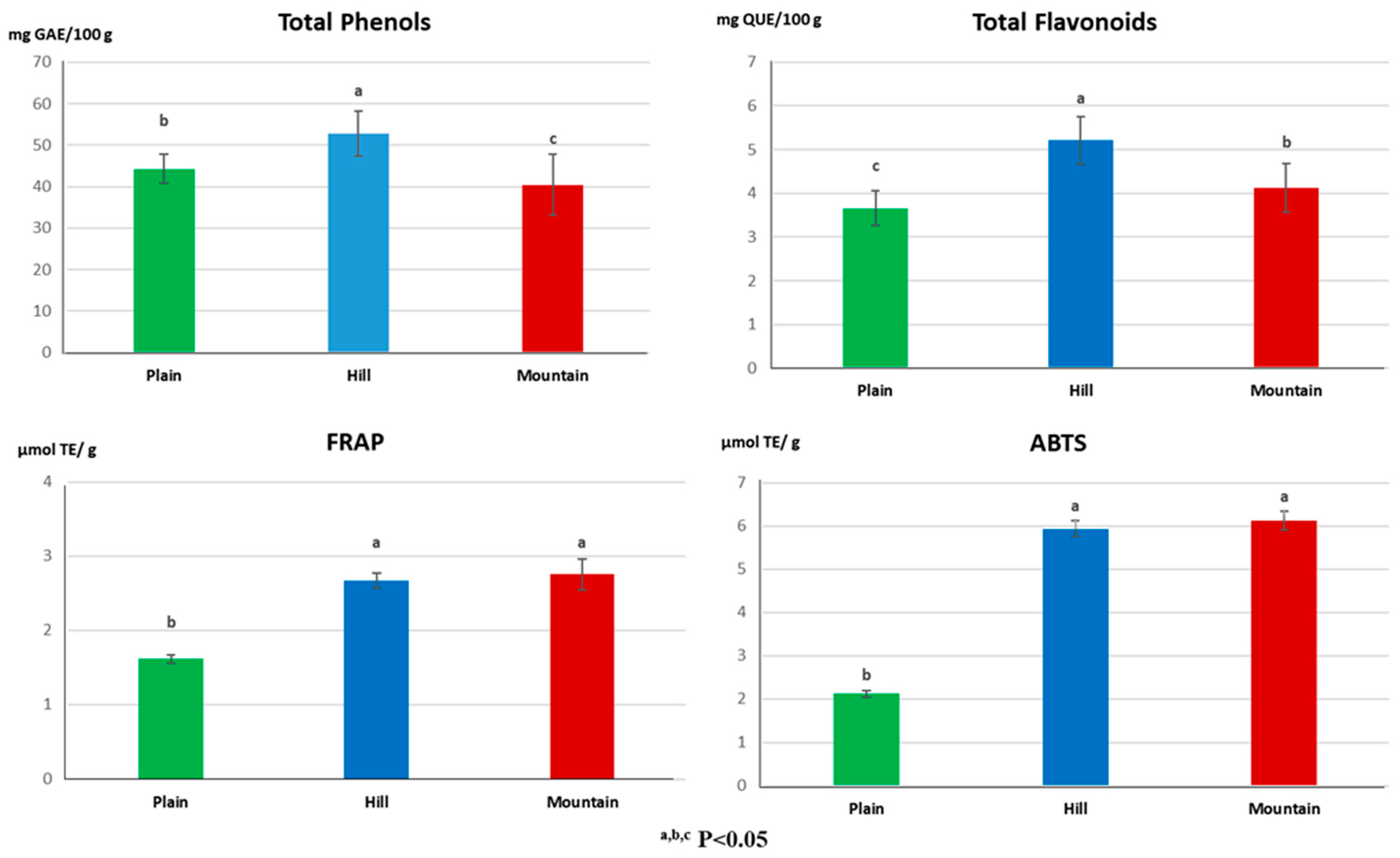
| Elements | Plain | Hill | Mountain | |||
|---|---|---|---|---|---|---|
| µ | SD | µ | SD | µ | SD | |
| mg kg−1 | ||||||
| Ca | 4.44 b | 0.54 | 5.39 a | 0.66 | 4.87 ab | 0.47 |
| Mg | 13.73 c | 1.51 | 20.74 b | 1.83 | 23.34 a | 1.86 |
| Na | 52.48 a | 5.42 | 52.60 a | 6.33 | 36.25 b | 4.57 |
| µg kg−1 | ||||||
| Ag | 0.37 a | 0.04 | 0.35 a | 0.05 | 0.37 a | 0.04 |
| Al | 1384.81 b | 155.16 | 1217.43 b | 118.91 | 1673.32 a | 238.04 |
| As | 0.91 a | 0.15 | 0.83 a | 0.14 | 0.87 a | 0.16 |
| Ba | 171.38 a | 19.3 | 134.49 b | 23.59 | 146.35 b | 24.34 |
| Cd | 0.91 a | 0.12 | 0.38 b | 0.07 | 0.40 b | 0.05 |
| Co | 4.58 a | 0.66 | 3.32 b | 0.74 | 3.40 b | 0.58 |
| Cr | 16.77 a | 1.81 | 15.68 a | 2.15 | 12.63 b | 1.24 |
| Cu | 248.21 a | 28.98 | 267.90 a | 47.95 | 280.53 a | 55.27 |
| Fe | 1615.14 a | 239.69 | 617.31 b | 94.15 | 1637.43 a | 301.41 |
| Li | 10.78 a | 0.94 | 7.97 b | 0.85 | 8.91 b | 1.5 |
| Mn | 254.89 c | 30.18 | 318.72 b | 45.63 | 454.42 a | 83.7 |
| Mo | 4.25 b | 0.46 | 5.31 a | 0.88 | 4.52 ab | 0.96 |
| Ni | 34.58 a | 4.47 | 26.35 b | 4.60 | 33.90 a | 2.46 |
| Pb | 7.51 b | 0.72 | 8.38 b | 1.26 | 12.92 a | 1.64 |
| Sn | 29.07 a | 3.16 | 32.45 a | 5.05 | 29.86 a | 3.53 |
| Sr | 124.27 a | 14.34 | 79.71 b | 10.62 | 120.06 a | 15.24 |
| Ti | 133.38 ab | 16.03 | 118.75 b | 18.53 | 146.86 a | 23.99 |
| V | 2.33 a | 0.21 | 1.67 b | 0.28 | 1.85 b | 0.31 |
| Zn | 975.27 a | 114.49 | 971.84 a | 157.34 | 990.28 a | 147.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassi, G.; Capasso, G.; Cillo, A.; Miedico, O.; Pompa, C.; Nardelli, V.; Perna, A.M. Effect of Altitude on Polyphenol Content, Antioxidant Activity and Multi-Element Composition of Wildflower Honey. Appl. Sci. 2025, 15, 3255. https://doi.org/10.3390/app15063255
Grassi G, Capasso G, Cillo A, Miedico O, Pompa C, Nardelli V, Perna AM. Effect of Altitude on Polyphenol Content, Antioxidant Activity and Multi-Element Composition of Wildflower Honey. Applied Sciences. 2025; 15(6):3255. https://doi.org/10.3390/app15063255
Chicago/Turabian StyleGrassi, Giulia, Giambattista Capasso, Alessandra Cillo, Oto Miedico, Ciro Pompa, Valeria Nardelli, and Anna Maria Perna. 2025. "Effect of Altitude on Polyphenol Content, Antioxidant Activity and Multi-Element Composition of Wildflower Honey" Applied Sciences 15, no. 6: 3255. https://doi.org/10.3390/app15063255
APA StyleGrassi, G., Capasso, G., Cillo, A., Miedico, O., Pompa, C., Nardelli, V., & Perna, A. M. (2025). Effect of Altitude on Polyphenol Content, Antioxidant Activity and Multi-Element Composition of Wildflower Honey. Applied Sciences, 15(6), 3255. https://doi.org/10.3390/app15063255









