First Insights into Macromolecular Components Analyses of an Insect Meal Using Hyperspectral Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Moisture and Total Fat Content Determination
2.3. HSI Data Acquisition
2.4. Spectra Processing
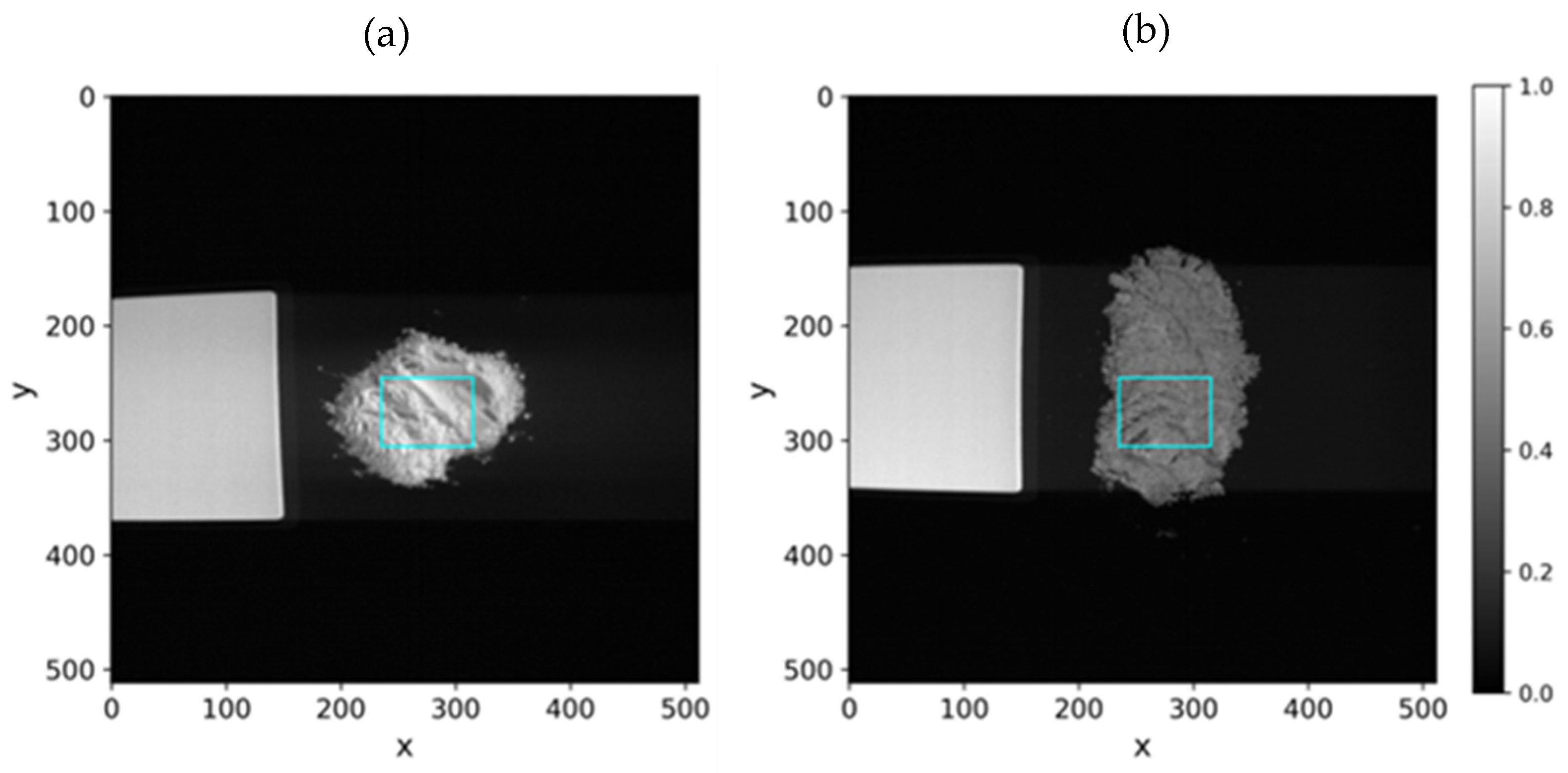
- Normalization of the bands to allow pixel intensity comparison between different hypercubes and definition of a Region Of Interest (ROI) within the sample (Figure 5);
- Computation of the mean spectra of the pixels inside the ROI, for noise reduction and increase representativeness (Figure 6).
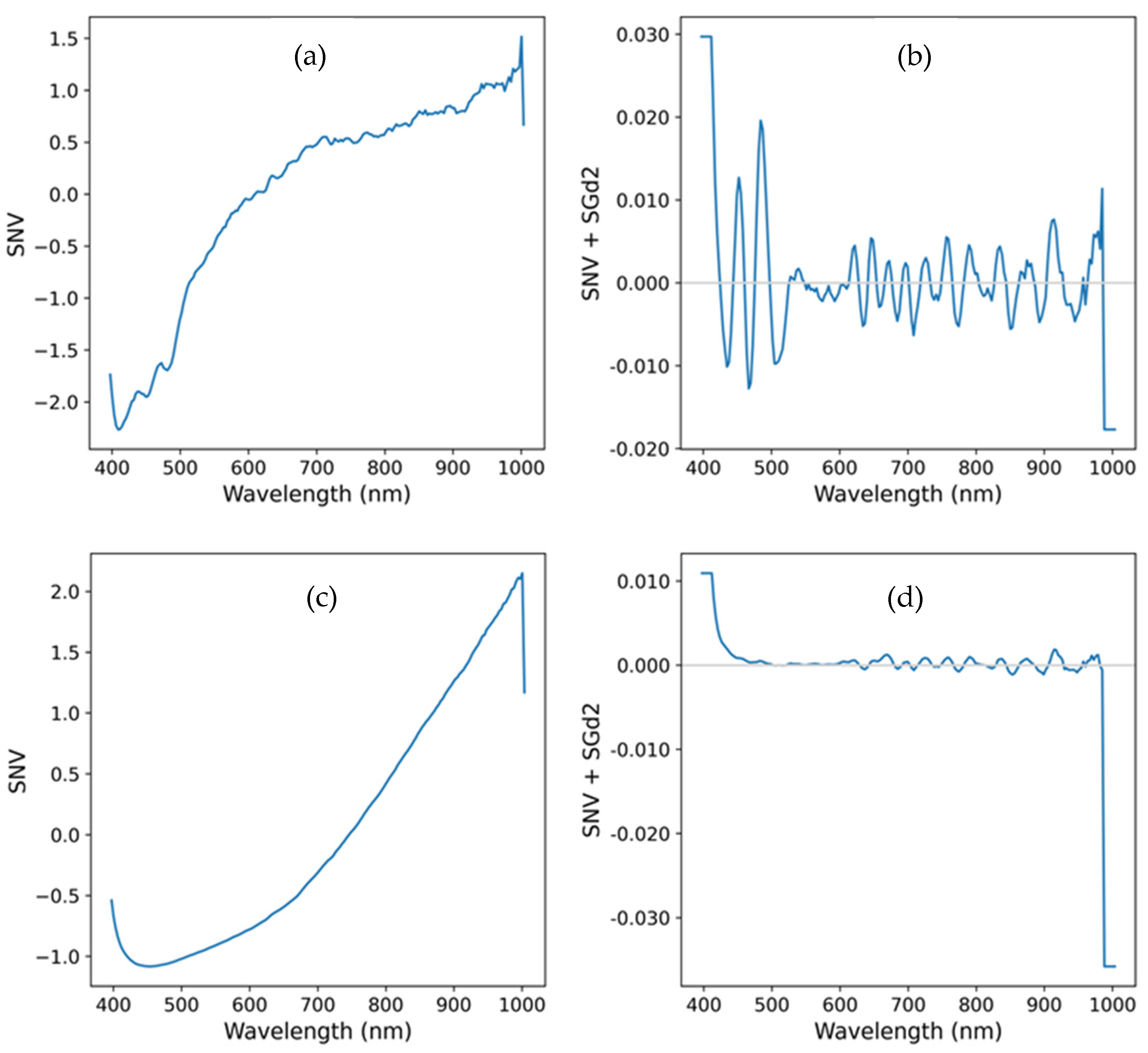
- Mean spectrum pre-processing (Figure 7):
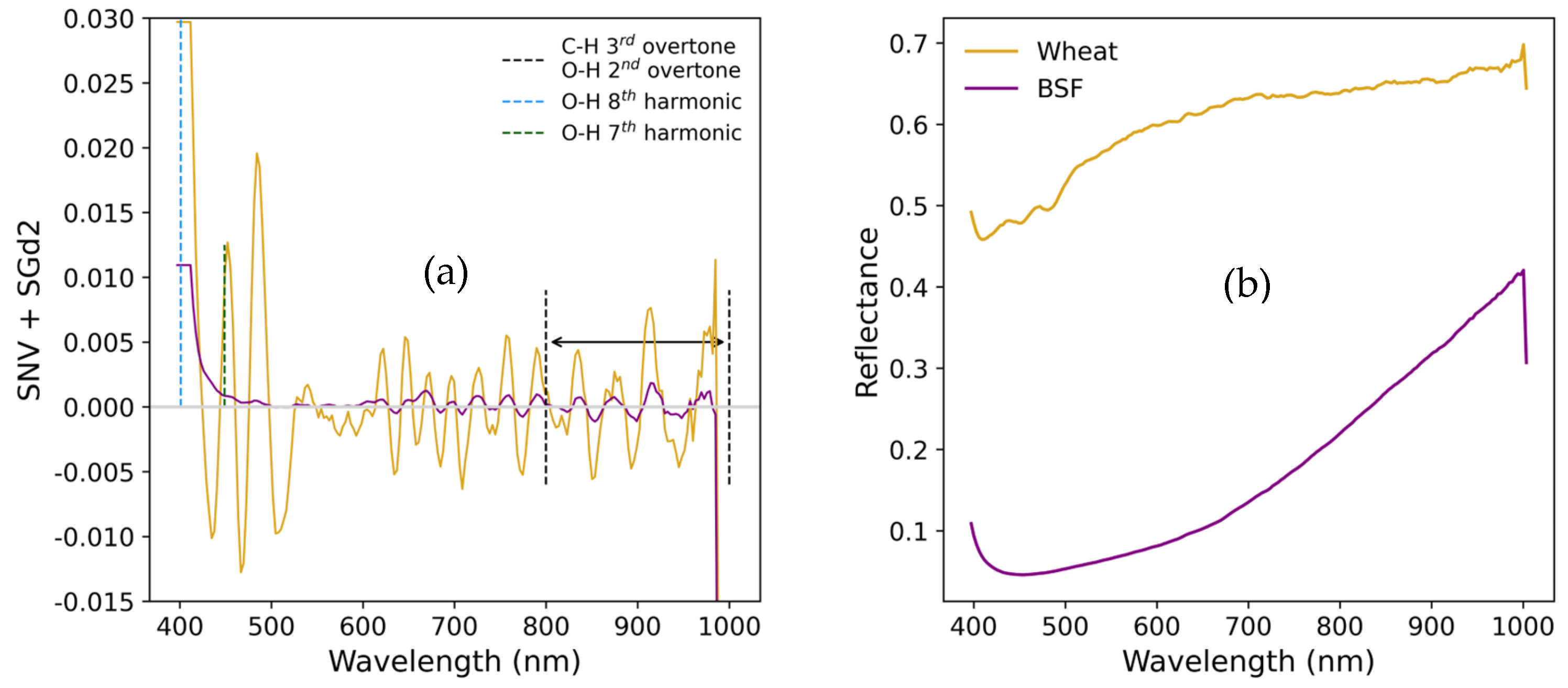
- Computation of the Standard Normal Variate to reduce variability introduced by particle size, surface scattering effects, and correct baseline shifts [23];
- Determination of quantitative similarity metrics for the SNV-transformed spectra of each sample: Spectral Angle Mapper (SAM) [24], and Pearson Coefficient of Correlation;
- Application of the Savitzky-Golay filter with a 7-points window and a 2nd order polynomial, for noise reduction, and 2nd derivative computation (SGd2) to highlight spectral features, namely, absorption peaks not visible in the raw spectrum [25].
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniso, E.; Tulli, F.; Cardinaletti, G.; Cerri, R.; Tibaldi, E. Molecular Approach for Insect Detection in Feed and Food: The Case of Gryllodes Sigillatus. Eur. Food Res. Technol. 2020, 246, 2373–2381. [Google Scholar] [CrossRef]
- COMMISSION REGULATION (EU) 2017/893 of 24 May 2017-Amending Annexes I and IV to Regu-518 Lation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation 519 (EU). Available online: https://eur-lex.europa.eu/eli/reg/2017/893/oj/eng (accessed on 1 January 2025).
- Tan, X.; Yang, H.S.; Wang, M.; Yi, Z.F.; Ji, F.J.; Li, J.Z.; Yin, Y.L. Amino Acid Digestibility in Housefly and Black Soldier Fly Prepupae by Growing Pigs. Anim. Feed Sci. Technol. 2020, 263, 114446. [Google Scholar] [CrossRef]
- Star, L.; Arsiwalla, T.; Molist, F.; Leushuis, R.; Dalim, M.; Paul, A. Gradual Provision of Live Black Soldier Fly (Hermetia illucens) Larvae to Older Laying Hens: Effect on Production Performance, Egg Quality, Feather Condition and Behavior. Animals 2020, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Li, J.; Tomerlin, J.K.; Xiao, X.; ur Rehman, K.; Cai, M.; Zheng, L.; YU, Z. Black Soldier Fly: A New Vista for Livestock and Poultry Manure Management. J. Integr. Agric. 2021, 20, 1167–1179. [Google Scholar] [CrossRef]
- Parodi, A.; Gerrits, W.J.J.; Van Loon, J.J.A.; De Boer, I.J.M.; Aarnink, A.J.A.; Van Zanten, H.H.E. Black Soldier Fly Reared on Pig Manure: Bioconversion Efficiencies, Nutrients in the Residual Material, Greenhouse Gas and Ammonia Emissions. Waste Manag. 2021, 126, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Rajan, D.K.; Muralisankar, T.; Ganesan, A.R.; Sathishkumar, P.; Revathi, N. Use of Black Soldier Fly (Hermetia illucens L.) Larvae Meal in Aquafeeds for a Sustainable Aquaculture Industry: A Review of Past and Future Needs. Aquaculture 2022, 553, 738095. [Google Scholar] [CrossRef]
- Alamu, E.; Maziya-Dixon, B.; Zum Felde, T.; Kulakow, P.; Parkes, E. Application of near Infrared Reflectance Spectroscopy in Screening of Fresh Cassava (Manihot Esculenta Crantz) Storage Roots for Provitamin A Carotenoids. In Proceedings of the 18th International Conference on Near Infrared Spectroscopy, Gold Cost, Australia, 15–20 September 2019; IM Publications Open LLP: Chichester, UK, 2019; pp. 91–97. [Google Scholar]
- Adebayo, S.E.; Hashim, N.; Abdan, K.; Hanafi, M. Application and Potential of Backscattering Imaging Techniques in Agricultural and Food Processing—A Review. J. Food Eng. 2016, 169, 155–164. [Google Scholar] [CrossRef]
- Adesokan, M.; Alamu, E.O.; Otegbayo, B.; Maziya-Dixon, B. A Review of the Use of Near-Infrared Hyperspectral Imaging (NIR-HSI) Techniques for the Non-Destructive Quality Assessment of Root and Tuber Crops. Appl. Sci. 2023, 13, 5226. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Huang, D. A Review of Imaging Techniques for Plant Phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef]
- Rady, A.; Guyer, D.; Lu, R. Evaluation of Sugar Content of Potatoes Using Hyperspectral Imaging. Food Bioproc. Technol. 2015, 8, 995–1010. [Google Scholar] [CrossRef]
- Amjad, W.; Crichton, S.O.J.; Munir, A.; Hensel, O.; Sturm, B. Hyperspectral Imaging for the Determination of Potato Slice Moisture Content and Chromaticity during the Convective Hot Air Drying Process. Biosyst. Eng. 2018, 166, 170–183. [Google Scholar] [CrossRef]
- Nguyen Do Trong, N.; Erkinbaev, C.; Nicolaï, B.; Saeys, W.; Tsuta, M.; De Baerdemaeker, J. Spatially Resolved Spectroscopy for Nondestructive Quality Measurements of Braeburn Apples Cultivated in Sub-Fertilization Condition; Kondo, N., Ed.; SPIE: Bellingham, WA, USA, 2013; p. 88810L. [Google Scholar]
- Cruz-Tirado, J.P.; Amigo, J.M.; Barbin, D.F. Determination of Protein Content in Single Black Fly Soldier (Hermetia illucens L.) Larvae by near Infrared Hyperspectral Imaging (NIR-HSI) and Chemometrics. Food Control 2023, 143, 109266. [Google Scholar] [CrossRef]
- Osborne, B.G. Near-Infrared Spectroscopy in Food Analysis. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Zhou, M.; Long, T.; Zhao, Z.; Chen, J.; Wu, Q.; Wang, Y.; Zou, Z. Honey Quality Detection Based on Near-Infrared Spectroscopy. Food Sci. Technol. 2023, 43, e98822. [Google Scholar] [CrossRef]
- Zou, Z.; Chen, J.; Wang, L.; Wu, W.; Yu, T.; Wang, Y.; Zhao, Y.; Huang, P.; Liu, B.; Zhou, M.; et al. Nondestructive Detection of Peanuts Mildew Based on Hyperspectral Image Technology and Machine Learning Algorithm. Food Sci. Technol. 2022, 42, e71322. [Google Scholar] [CrossRef]
- Zou, Z.; Long, T.; Chen, J.; Wang, L.; Wu, X.; Zou, B.; Xu, L. Rapid Identification of Adulterated Safflower Seed Oil by Use of Hyperspectral Spectroscopy. Spectrosc. Lett. 2021, 54, 675–684. [Google Scholar] [CrossRef]
- Specim, Spectral Imaging Datacube, Specim IQ and Spectral Imaging. Available online: https://www.specim.com/downloads/iq/manual/software/iq/topics/data-cube.html (accessed on 6 March 2025).
- Specim. Specim IQ Check Calibration. Specim IQ User Manual. Available online: https://www.specim.com/downloads/iq/manual/spectral-cameras/IQ/topics/checking-the-calibration.html (accessed on 6 March 2025).
- NV5. ENVI Image Files. Docs Center. Available online: https://www.nv5geospatialsoftware.com/docs/enviimagefiles.html (accessed on 6 March 2025).
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and De-Trending of Near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Kruse, F.A.; Lefkoff, A.B.; Boardman, J.W.; Heidebrecht, K.B.; Shapiro, A.T.; Barloon, P.J.; Goetz, A.F.H. The Spectral Image Processing System (SIPS)—Interactive Visualization and Analysis of Imaging Spectrometer Data. Remote Sens. Environ. 1993, 44, 145–163. [Google Scholar] [CrossRef]
- Abraham, S.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Sindhu, M. Physicochemical Properties and Nutritional Evaluation of Wheat and Water Chestnut Composite Flour. Int. J. Chem. Stud. 2020, 8, 1914–1919. [Google Scholar] [CrossRef]
- Sujitha, J.; Muneer, M.R.S.; Mahendran, T.; Kiruthiga, B. Influence of Storage Temperature on the Quality Parameters of Wheat Flour during Short Term Storage. Sabaragamuwa Univ. J. 2018, 16, 53–57. [Google Scholar] [CrossRef]
- David, O.; Arthur, E.; Kwadwo, S.O.; Badu, E.; Sakyi, P. Proximate Composition and Some Functional Properties of Soft Wheat Flour. Int. J. Innov. Res. Sci. Eng. Technol. 2015, 4, 753–758. [Google Scholar]
- Lu, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; Thongpea, S.; Paengkoum, S.; Purba, R.A.P.; et al. Nutritional Composition of Black Soldier Fly Larvae (Hermetia illucens L.) and Its Potential Uses as Alternative Protein Sources in Animal Diets: A Review. Insects 2022, 13, 831. [Google Scholar] [CrossRef]
- Tognocchi, M.; Abenaim, L.; Adamaki-Sotiraki, C.; Athanassiou, G.C.; Rumbos, I.C.; Mele, M.; Conti, B.; Conte, G. Effect of Different Diet Composition on the Fat Profile of Two Different Black Soldier Fly Larvae Populations. Animal 2024, 18, 101205. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, Y.; Fresch, M.; Ott, D.; Brockmeyer, J.; Scherber, C. Diet Composition and Social Environment Determine Food Consumption, Phenotype and Fecundity in an Omnivorous Insect. R. Soc. Open Sci. 2020, 7, 200100. [Google Scholar] [CrossRef] [PubMed]
- Elwert, C.; Ivonne, K.; Peter, K. A Novel Protein Source: Maggot Meal of the Black Soldier Fly (Hermetia illucens) in Broiler Feed. Tag. Schweine Geflügelernährung 2010, 11, 140–142. [Google Scholar]
- Pope, R.M.; Fry, E.S. Absorption Spectrum (380–700 Nm) of Pure Water II Integrating Cavity Measurements. Appl. Opt. 1997, 36, 8710. [Google Scholar] [CrossRef]





| Parameters | BSF Meal (%) | Wheat Flour (%) |
|---|---|---|
| Moisture | 7.20 ± 0.07 | 3.10 ± 0.13 |
| Crude fat | 28.40 ± 0.21 | 1.00 ± 0.07 |
| Other compounds 1 | 64.40 ± 0.18 | 95.90 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.M.O.d.; Fidalgo, L.G.; Inácio, R.S.; Fantatto, R.; Carvalho, M.J.; Murta, D.; Pereira, N.S.A. First Insights into Macromolecular Components Analyses of an Insect Meal Using Hyperspectral Imaging. Appl. Sci. 2025, 15, 3822. https://doi.org/10.3390/app15073822
Silva FMOd, Fidalgo LG, Inácio RS, Fantatto R, Carvalho MJ, Murta D, Pereira NSA. First Insights into Macromolecular Components Analyses of an Insect Meal Using Hyperspectral Imaging. Applied Sciences. 2025; 15(7):3822. https://doi.org/10.3390/app15073822
Chicago/Turabian StyleSilva, Flávia Matias Oliveira da, Liliana G. Fidalgo, Rita S. Inácio, Rafaela Fantatto, Maria J. Carvalho, Daniel Murta, and Nuno S. A. Pereira. 2025. "First Insights into Macromolecular Components Analyses of an Insect Meal Using Hyperspectral Imaging" Applied Sciences 15, no. 7: 3822. https://doi.org/10.3390/app15073822
APA StyleSilva, F. M. O. d., Fidalgo, L. G., Inácio, R. S., Fantatto, R., Carvalho, M. J., Murta, D., & Pereira, N. S. A. (2025). First Insights into Macromolecular Components Analyses of an Insect Meal Using Hyperspectral Imaging. Applied Sciences, 15(7), 3822. https://doi.org/10.3390/app15073822








