Organic Semiconducting Polymers in Photonic Devices: From Fundamental Properties to Emerging Applications
Abstract
1. Introduction
2. Fundamental Principles
3. Material Systems
4. Applications in Photonic Devices
4.1. Fabrication Approaches for Polymer Photonic Devices
4.2. Passive Components
4.3. Active Components
4.4. Emerging Applications
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Dong, H.; Hu, W. Organic Semiconductor Single Crystals for Electronics and Photonics. Adv. Mater. 2018, 30, 1801048. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; Lanzani, G. Organic Photonics for Communications. Nat. Photonics 2010, 4, 438–446. [Google Scholar] [CrossRef]
- Zuo, H.; Yu, S.; Gu, T.; Hu, J. Low Loss, Flexible Single-Mode Polymer Photonics. Opt. Express 2019, 27, 11152–11159. [Google Scholar] [CrossRef] [PubMed]
- Paquet, C.; Kumacheva, E. Nanostructured Polymers for Photonics. Mater. Today 2008, 11, 48–56. [Google Scholar] [CrossRef]
- Subbaraman, H.; Lin, X.; Ling, T.; Guo, L.J.; Chen, R.T. Towards Roll-to-Roll Manufacturing of Polymer Photonic Devices. Proc. SPIE 2014, 8991, 267–273. [Google Scholar] [CrossRef]
- Costa, J.C.; Taveira, R.J.; Lima, C.F.; Mendes, A.; Santos, L.M. Optical Band Gaps of Organic Semiconductor Materials. Opt. Mater. 2016, 58, 51–60. [Google Scholar] [CrossRef]
- Kobayashi, T.; Naito, H. Optical Properties of Materials and Their Applications; Wiley: Hoboken, NJ, USA, 2019; pp. 295–321. [Google Scholar] [CrossRef]
- Grivas, C.; Pollnau, M. Organic Solid-State Integrated Amplifiers and Lasers. Laser Photonics Rev. 2012, 6, 419–462. [Google Scholar] [CrossRef]
- Taghavi, I.; Moridsadat, M.; Tofini, A.; Raza, S.; Jaeger, N.A.; Chrostowski, L.; Shastri, B.J.; Shekhar, S. Polymer Modulators in Silicon Photonics: Review and Projections. Nanophotonics 2022, 11, 3855–3871. [Google Scholar] [CrossRef]
- Bronstein, H.; Nielsen, C.B.; Schroeder, B.C.; McCulloch, I. The Role of Chemical Design in the Performance of Organic Semiconductors. Nat. Rev. Chem. 2020, 4, 66–77. [Google Scholar] [CrossRef]
- Pathak, D.; Kumar, S.; Andotra, S.; Thomas, J.; Kaur, N.; Kumar, P.; Kumar, V. New Tailored Organic Semiconductors Thin Films for Optoelectronic Applications. Eur. Phys. J. Appl. Phys. 2021, 95, 10201. [Google Scholar] [CrossRef]
- Unge, M.; Christen, T.; Törnkvist, C. Electronic Structure of Polyethylene—Crystalline and Amorphous Phases of Pure Polyethylene and Their Interfaces. In Proceedings of the 2012 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Montreal, QC, Canada, 14–17 October 2012; pp. 525–530. [Google Scholar] [CrossRef]
- Chien, J.C. Polyacetylene: Chemistry, Physics, and Material Science; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Yang, Q.; Muntwiler, M. Charge-Transfer Excitons at Organic Semiconductor Surfaces and Interfaces. Acc. Chem. Res. 2009, 42, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Friend, R.H.; Phillips, M.; Rao, A.; Wilson, M.W.; Li, Z.; McNeill, C.R. Excitons and Charges at Organic Semiconductor Heterojunctions. Faraday Discuss. 2012, 155, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Knoester, J.; Agranovich, V.M. Frenkel and Charge-Transfer Excitons in Organic Solids. Thin Films Nanostruct. 2003, 31, 1–96. [Google Scholar] [CrossRef]
- Dimitriev, O.P. Dynamics of Excitons in Conjugated Molecules and Organic Semiconductor Systems. Chem. Rev. 2022, 122, 8487–8593. [Google Scholar] [CrossRef]
- Mikhnenko, O.V.; Blom, P.W.; Nguyen, T.Q. Exciton Diffusion in Organic Semiconductors. Energy Environ. Sci. 2015, 8, 1867–1888. [Google Scholar] [CrossRef]
- Lunt, R.R.; Benziger, J.B.; Forrest, S.R. Relationship between Crystalline Order and Exciton Diffusion Length in Molecular Organic Semiconductors. Adv. Mater. 2010, 22, 1233–1236. [Google Scholar] [CrossRef]
- Huang, M.Z.; Ching, W.Y. Calculation of Optical Excitations in Cubic Semiconductors. II. Second-Harmonic Generation. Phys. Rev. B 1993, 47, 9464. [Google Scholar] [CrossRef]
- Penner, T.L.; Motschmann, H.R.; Armstrong, N.J.; Ezenyilimba, M.C.; Williams, D.J. Efficient Phase-Matched Second-Harmonic Generation of Blue Light in an Organic Waveguide. Nature 1994, 367, 49–51. [Google Scholar] [CrossRef]
- Dalton, L.R. Organic Electro-Optics and Photonics: Molecules, Polymers and Crystals; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar] [CrossRef]
- Dalton, L.R.; Sullivan, P.A.; Bale, D.H. Electric Field Poled Organic Electro-Optic Materials: State of the Art and Future Prospects. Chem. Rev. 2010, 110, 25–55. [Google Scholar] [CrossRef]
- Sullivan, P.A.; Dalton, L.R. Theory-Inspired Development of Organic Electro-Optic Materials. Acc. Chem. Res. 2010, 43, 10–18. [Google Scholar] [CrossRef]
- Krausz, F.; Wintner, E.; Leising, G. Optical Third-Harmonic Generation in Polyacetylene. Phys. Rev. B 1989, 39, 3701. [Google Scholar] [CrossRef]
- Mathy, A.; Ueberhofen, K.; Schenk, R.; Gregorius, H.; Garay, R.; Müllen, K.; Bubeck, C. Third-Harmonic-Generation Spectroscopy of Poly(p-phenylenevinylene): A Comparison with Oligomers and Scaling Laws for Conjugated Polymers. Phys. Rev. B 1996, 53, 4367. [Google Scholar] [CrossRef]
- Qasymeh, M.; Cada, M.; Ponomarenko, S.A. Quadratic Electro-Optic Kerr Effect: Applications to Photonic Devices. IEEE J. Quantum Electron. 2008, 44, 740–746. [Google Scholar] [CrossRef]
- He, G.S.; Xu, G.C.; Prasad, P.N.; Reinhardt, B.A.; Bhatt, J.C.; Dillard, A.G. Two-Photon Absorption and Optical-Limiting Properties of Novel Organic Compounds. Opt. Lett. 1995, 20, 435–437. [Google Scholar] [CrossRef]
- Lin, T.C.; Chung, S.J.; Kim, K.S.; Wang, X.; He, G.S.; Swiatkiewicz, J.; Pudavar, H.E.; Prasad, P.N. Organics and Polymers with High Two-Photon Activities and Their Applications. In Polymers for Photonics Applications II; Springer: Berlin/Heidelberg, Germany, 2003; pp. 157–193. [Google Scholar] [CrossRef]
- Leclerc, M. Polyfluorenes: Twenty Years of Progress. J. Polym. Sci. Part A 2001, 39, 2867–2873. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Jenekhe, S.A. Blue Light-Emitting Diodes with Good Spectral Stability Based on Blends of Poly(9,9-dioctylfluorene): Interplay Between Morphology, Photophysics, and Device Performance. Macromolecules 2003, 36, 5285–5296. [Google Scholar] [CrossRef]
- Kabra, D.; Lu, L.P.; Song, M.H.; Snaith, H.J.; Friend, R.H. Efficient Single-Layer Polymer Light-Emitting Diodes. Adv. Mater. 2010, 22, 3194. [Google Scholar] [CrossRef]
- Mamada, M.; Komatsu, R.; Adachi, C. F8BT Oligomers for Organic Solid-State Lasers. ACS Appl. Mater. Interfaces 2020, 12, 28383–28391. [Google Scholar] [CrossRef]
- Madsen, M.; Bakke, M.R.; Gudnason, D.A.; Sandahl, A.F.; Hansen, R.A.; Knudsen, J.B.; Kodal, A.L.; Birkedal, V.; Gothelf, K.V. A Single Molecule Polyphenylene-Vinylene Photonic Wire. ACS Nano 2021, 15, 9404–9411. [Google Scholar] [CrossRef]
- Li, C.; Liu, M.; Pschirer, N.G.; Baumgarten, M.; Mullen, K. Polyphenylene-Based Materials for Organic Photovoltaics. Chem. Rev. 2010, 110, 6817–6855. [Google Scholar] [CrossRef]
- Martin, S.J.; Bradley, D.D.; Lane, P.A.; Mellor, H.; Burn, P.L. Linear and Nonlinear Optical Properties of the Conjugated Polymers PPV and MEH-PPV. Phys. Rev. B 1999, 59, 15133. [Google Scholar] [CrossRef]
- Chang, R.; Hsu, J.H.; Fann, W.S.; Liang, K.K.; Chang, C.H.; Hayashi, M.; Yu, J.; Lin, S.H.; Chang, E.C.; Chuang, K.R.; et al. Experimental and Theoretical Investigations of Absorption and Emission Spectra of the Light-Emitting Polymer MEH-PPV in Solution. Chem. Phys. Lett. 2000, 317, 142–152. [Google Scholar] [CrossRef]
- Jankus, V.; Snedden, E.W.; Bright, D.W.; Whittle, V.L.; Williams, J.A.; Monkman, A. Energy Upconversion via Triplet Fusion in Super Yellow PPV Films Doped with Palladium Tetraphenyltetrabenzoporphyrin: A Comprehensive Investigation of Exciton Dynamics. Adv. Funct. Mater. 2013, 23, 384–393. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From Fundamental Perspectives to Applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Perepichka, I.F.; Perepichka, D.F.; Meng, H.; Wudl, F. Light-Emitting Polythiophenes. Adv. Mater. 2005, 17, 2281–2305. [Google Scholar] [CrossRef]
- Tremel, K.; Ludwigs, S. P3HT Revisited—From Molecular Scale to Solar Cell Devices; Springer: Berlin, Germany, 2014; pp. 39–82. [Google Scholar] [CrossRef]
- Cook, S.; Furube, A.; Katoh, R. Analysis of the Excited States of Regioregular Polythiophene P3HT. Energy Environ. Sci. 2008, 1, 294–299. [Google Scholar] [CrossRef]
- Peters, V.N.; Faruk, M.O.; Asane, J.; Alexander, R.; Peters, D.A.; Prayakarao, S.; Rout, S.; Noginov, M.A. Effect of Strong Coupling on Photodegradation of the Semiconducting Polymer P3HT. Optica 2019, 6, 318–325. [Google Scholar] [CrossRef]
- Banerji, N.; Gagnon, E.; Morgantini, P.Y.; Valouch, S.; Mohebbi, A.R.; Seo, J.H.; Leclerc, M.; Heeger, A.J. Breaking Down the Problem: Optical Transitions, Electronic Structure, and Photoconductivity in Conjugated Polymer PCDTBT and in its Separate Building Blocks. J. Phys. Chem. C 2012, 116, 11456–11469. [Google Scholar] [CrossRef]
- Lombeck, F.; Di, D.; Yang, L.; Meraldi, L.; Athanasopoulos, S.; Credgington, D.; Sommer, M.; Friend, R.H. PCDTBT: From Polymer Photovoltaics to Light-Emitting Diodes by Side-Chain-Controlled Luminescence. Macromolecules 2016, 49, 9382–9387. [Google Scholar] [CrossRef]
- Rohatgi, C.V.; Harada, T.; Need, E.F.; Krasowska, M.; Beattie, D.A.; Dickenson, G.D.; Smith, T.A.; Kee, T.W. Low-Bandgap Conjugated Polymer Dots for Near-Infrared Fluorescence Imaging. ACS Appl. Nano Mater. 2018, 1, 4801–4808. [Google Scholar] [CrossRef]
- Qu, Y.K.; Zheng, Q.; Fan, J.; Liao, L.S.; Jiang, Z.Q. Spiro Compounds for Organic Light-Emitting Diodes. Acc. Mater. Res. 2021, 2, 1261–1271. [Google Scholar] [CrossRef]
- Yang, S.Y.; Wang, J.; Deng, Z.; Xu, Y.; Su, X.; Zhang, L.; Yang, S.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Spiro-Materials with Aggregation-Induced Emission. Matter 2024, 7, 3390–3421. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, S.; Luo, J.; Suresh, S.; Liu, L.; Kang, S.H.; Haller, M.; Sassa, T.; Dalton, L.R.; Jen, A.Y. Highly Efficient and Thermally Stable Electro-Optical Dendrimers for Photonics. Adv. Funct. Mater. 2002, 12, 565–574. [Google Scholar] [CrossRef]
- Pitois, C.; Vestberg, R.; Rodlert, M.; Malmström, E.; Hult, A.; Lindgren, M. Fluorinated Dendritic Polymers and Dendrimers for Waveguide Applications. Opt. Mater. 2003, 21, 499–506. [Google Scholar] [CrossRef]
- Spangler, C.W.; Suo, Z.; Drobizhev, M.; Karotki, A.; Rebane, A. Organic Nanophotonics; Springer: Dordrecht, The Netherlands, 2003; pp. 139–153. [Google Scholar] [CrossRef]
- Rodlert, M.; Vestberg, R.; Malmström, E.; Persson, M.; Lindgren, M. Chiral Dendritic Polymers for Photonic Applications. Synth. Met. 2002, 127, 37–43. [Google Scholar] [CrossRef]
- Wang, L.D.; Tang, J.; Li, R.Z.; Zhang, T.; Tong, L.; Tang, J. Synthesis and Characterization of Cross-Linkable Polyurethane-Imide Electro-Optic Waveguide Polymer. Appl. Phys. A 2016, 122, 38. [Google Scholar] [CrossRef]
- Wang, L.D.; Zhang, T.; Li, R.Z.; Zhang, X.Y.; Song, Y.J. Synthesis and Characterization of Cross-Linkable Fluorinated Polyimide for Optical Waveguide. Appl. Phys. A 2015, 118, 655–664. [Google Scholar] [CrossRef]
- Song, D.P.; Li, C.; Li, W.; Watkins, J.J. Block Copolymer Nanocomposites with High Refractive Index Contrast for One-Step Photonics. ACS Nano 2016, 10, 1216–1223. [Google Scholar] [CrossRef]
- Choi, S.; Kim, W.; Shin, W.; Oh, J.; Jin, S.; Jung, Y.M.; Ryu, M.Y.; Lee, H. Effects of UV-Ozone Treatment on the Electronic Structures of F8BT and PFO Polymeric Thin Films. Curr. Appl. Phys. 2020, 20, 1359–1365. [Google Scholar] [CrossRef]
- Corcoran, N.; Ho, P.K.; Arias, A.C.; Mackenzie, J.D.; Friend, R.H.; Fichet, G.; Huck, W.T. Highly-Efficient Broadband Waveguide Outcoupling in Light-Emitting Diodes with Self-Organized Polymer Blends. Appl. Phys. Lett. 2004, 85, 2965–2967. [Google Scholar] [CrossRef]
- Morgado, J.; Moons, E.; Friend, R.H.; Cacialli, F. Optical and Morphological Investigations of Non-Homogeneity in Polyfluorene Blends. Synth. Met. 2001, 124, 63–66. [Google Scholar] [CrossRef]
- Yeh, K.M.; Chen, Y. Improved Performance of Polymer Light-Emitting Devices Based on Blend of MEH-PPV and Vinyl Copolymer with 1,3,4-Oxadiazole Chromophores. Org. Electron. 2008, 9, 45–50. [Google Scholar] [CrossRef]
- Ruderer, M.A.; Wang, C.; Schaible, E.; Hexemer, A.; Xu, T.; Müller-Buschbaum, P. Morphology and Optical Properties of P3HT:MEH-CN-PPV Blend Films. Macromolecules 2013, 46, 4491–4501. [Google Scholar] [CrossRef]
- Kuo, C.C.; Lin, C.H.; Chen, W.C. Morphology and Photophysical Properties of Light-Emitting Electrospun Nanofibers Prepared from Poly(fluorene) Derivative/PMMA Blends. Macromolecules 2007, 40, 6959–6966. [Google Scholar] [CrossRef]
- Chappell, J.; Lidzey, D.G. Phase Separation in Polyfluorene-Polymethylmethacrylate Blends Studied Using UV Near-Field Microscopy. J. Microsc. 2003, 209, 188–193. [Google Scholar] [CrossRef][Green Version]
- Chappell, J.; Lidzey, D.G.; Jukes, P.C.; Higgins, A.M.; Thompson, R.L.; O’Connor, S.; Grizzi, I.; Fletcher, R.; O’Brien, J.; Geoghegan, M.; et al. Correlating Structure with Fluorescence Emission in Phase-Separated Conjugated-Polymer Blends. Nat. Mater. 2003, 2, 616–621. [Google Scholar] [CrossRef]
- Yim, K.H.; Zheng, Z.; Friend, R.H.; Huck, W.T.; Kim, J.S. Surface-Directed Phase Separation of Conjugated Polymer Blends for Efficient Light-Emitting Diodes. Adv. Funct. Mater. 2008, 18, 2897–2904. [Google Scholar] [CrossRef]
- Shikler, R.; Chiesa, M.; Friend, R.H. Photovoltaic Performance and Morphology of Polyfluorene Blends: The Influence of Phase Separation Evolution. Macromolecules 2006, 39, 5393–5399. [Google Scholar] [CrossRef]
- Liu, C.L.; Lin, C.H.; Kuo, C.C.; Lin, S.T.; Chen, W.C. Conjugated Rod-Coil Block Copolymers: Synthesis, Morphology, Photophysical Properties, and Stimuli-Responsive Applications. Prog. Polym. Sci. 2011, 36, 603–637. [Google Scholar] [CrossRef]
- Yassar, A.; Miozzo, L.; Gironda, R.; Horowitz, G. Rod-Coil and All-Conjugated Block Copolymers for Photovoltaic Applications. Prog. Polym. Sci. 2013, 38, 791–844. [Google Scholar] [CrossRef]
- de Cuendias, A.; Hiorns, R.C.; Cloutet, E.; Vignau, L.; Cramail, H. Conjugated Rod-Coil Block Copolymers and Optoelectronic Applications. Polym. Int. 2010, 59, 1452–1476. [Google Scholar] [CrossRef]
- Dogariu, A.; Gupta, R.; Heeger, A.J.; Wang, H. Time-Resolved Förster Energy Transfer in Polymer Blends. Synth. Met. 1999, 100, 95–100. [Google Scholar] [CrossRef]
- Feron, K.; Belcher, W.J.; Fell, C.J.; Dastoor, P.C. Organic Solar Cells: Understanding the Role of Förster Resonance Energy Transfer. Int. J. Mol. Sci. 2012, 13, 17019–17047. [Google Scholar] [CrossRef]
- Al-Bati, S.; Jumali, M.H.; Ibtehaj, K.; Al-Asbahi, B.A.; Yap, C.C. Förster Energy Transfer Mechanism and Color Tunability in Three Binary Conjugated Polymer Blends. Opt. Mater. 2021, 116, 111085. [Google Scholar] [CrossRef]
- Tran, T.T.; Nguyen, H.T.; Sharma, A.; Cho, Y.B.; Kumar, M.; Yun, J.H. Expanding the Spectral Responsivity of Photodetectors via the Integration of CdSe/ZnS Quantum Dots and MEH-PPV Polymer Composite. Polymers 2024, 16, 2371. [Google Scholar] [CrossRef]
- Hemdana, I.; Mahdouani, M.; Bourguiga, R. Investigation of the Optical Properties of CdSe/ZnS Core/Shell Quantum Dot Embedded in an MEH-PPV Polymer Matrix. Transp. Theory Stat. Phys. 2013, 42, 381–398. [Google Scholar] [CrossRef]
- Im, S.H.; Chang, J.A.; Kim, S.W.; Kim, S.W.; Seok, S.I. Near-Infrared Photodetection Based on PbS Colloidal Quantum Dots/Organic Hole Conductor. Org. Electron. 2010, 11, 696–699. [Google Scholar] [CrossRef]
- Cardozo, O.; Farooq, S.; Farias, P.M.; Fraidenraich, N.; Stingl, A.; Araujo, R.E. Zinc Oxide Nanodiffusers to Enhance P3HT:PCBM Organic Solar Cells Performance. J. Mater. Sci. Mater. Electron. 2022, 33, 3225–3236. [Google Scholar] [CrossRef]
- Khurana, K.; Jaggi, N. Localized Surface Plasmonic Properties of Au and Ag Nanoparticles for Sensors: A Review. Plasmonics 2021, 16, 981–999. [Google Scholar] [CrossRef]
- Chen, S.H.; Huang, C.L.; Yu, C.F.; Wu, G.F.; Kuan, Y.C.; Cheng, B.H.; Li, Y.R. Efficacy Improvement in Polymer LEDs via Silver-Nanoparticle Doping in the Emissive Layer. Opt. Lett. 2017, 42, 3411–3414. [Google Scholar] [CrossRef] [PubMed]
- Krivenkov, V.; Samokhvalov, P.; Nabiev, I.; Rakovich, Y.P. Synergy of Excitation Enhancement and the Purcell Effect for Strong Photoluminescence Enhancement in a Thin-Film Hybrid Structure Based on Quantum Dots and Plasmon Nanoparticles. J. Phys. Chem. Lett. 2020, 11, 8018–8025. [Google Scholar] [CrossRef] [PubMed]
- Signoretto, M.; Zink-Lorre, N.; Martinez-Pastor, J.P.; Font-Sanchis, E.; Chirvony, V.S.; Sastre-Santos, A.; Fernández-Lázaro, F.; Suárez, I. Purcell-Enhancement of the Radiative PL Decay in Perylenediimides by Coupling with Silver Nanoparticles into Waveguide Modes. Appl. Phys. Lett. 2017, 111, 081102. [Google Scholar] [CrossRef]
- Wenger, W.N.; Bates, F.S.; Aydil, E.S. Functionalization of Cadmium Selenide Quantum Dots with Poly(ethylene glycol): Ligand Exchange, Surface Coverage, and Dispersion Stability. Langmuir 2017, 33, 8239–8245. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, B.; Jeon, S.; Han, C.J.; Hong, S.K. Thermal Curing Property of Silicone Encapsulant Containing Quantum Dot Surrounded by Various Types of Ligands. Bull. Korean Chem. Soc. 2013, 34, 3787–3789. [Google Scholar] [CrossRef][Green Version]
- Ahangaran, F.; Navarchian, A.H. Recent Advances in Chemical Surface Modification of Metal Oxide Nanoparticles with Silane Coupling Agents: A Review. Adv. Colloid Interface Sci. 2020, 286, 102298. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. A Review of Current Coupling Agents for Modification of Metal Oxide Nanoparticles. Prog. Org. Coat. 2015, 86, 194–207. [Google Scholar] [CrossRef]
- Yin, C.; Tai, X.; Li, X.; Tan, J.; Lee, C.S.; Sun, P.; Fan, Q.; Huang, W. Side Chain Engineering of Semiconducting Polymers for Improved NIR-II Fluorescence Imaging and Photothermal Therapy. Chem. Eng. J. 2022, 428, 132098. [Google Scholar] [CrossRef]
- Pawle, R.H.; Agarwal, A.; Malveira, S.; Smith, Z.C.; Thomas III, S.W. Bandgap Engineering of Conjugated Materials with Nonconjugated Side Chains. Macromolecules 2014, 47, 2250–2256. [Google Scholar] [CrossRef]
- Wu, W.; Inbasekaran, M.; Hudack, M.; Welsh, D.; Yu, W.; Cheng, Y.; Wang, C.; Kram, S.; Tacey, M.; Bernius, M.; et al. Recent Development of Polyfluorene-Based RGB Materials for Light Emitting Diodes. Microelectron. J. 2004, 35, 343–348. [Google Scholar] [CrossRef]
- Barve, K.A.; Raut, S.S.; Mishra, A.V.; Patil, V.R. Synthesis and Studies of Blue Light Emitting Polymers Containing Triphenylamine-Substituted Fluorene and Diphenylanthracene Moiety. J. Appl. Polym. Sci. 2011, 122, 3483–3492. [Google Scholar] [CrossRef]
- Amrutha, S.R.; Jayakannan, M. Structure Control of π-Conjugated Polymers for Enhanced Solid-State Luminescence: Synthesis and Liquid Crystalline and Photophysical Properties of New Bulky Poly(p-phenylenevinylene)s and Oligo(phenylenevinylene)s Bearing Tricyclodecane Pendants. Macromolecules 2007, 40, 2380–2391. [Google Scholar] [CrossRef]
- Shi, D.; Qin, X.; Li, Y.; He, Y.; Zhong, C.; Pan, J.; Dong, H.; Xu, W.; Li, T.; Hu, W.; et al. Spiro-OMeTAD Single Crystals: Remarkably Enhanced Charge-Carrier Transport via Mesoscale Ordering. Sci. Adv. 2016, 2, e1501491. [Google Scholar] [CrossRef] [PubMed]
- Ziolek, R.M.; Santana-Bonilla, A.; López-Ríos de Castro, R.; Kühn, R.; Green, M.; Lorenz, C.D. Conformational Heterogeneity and Interchain Percolation Revealed in an Amorphous Conjugated Polymer. ACS Nano 2022, 16, 14432–14442. [Google Scholar] [CrossRef]
- Cui, C.; Sun, Y.; Zhang, Z.G.; Zhang, M.; Zhang, J.; Li, Y. Effect of Branched Side Chains on the Physicochemical and Photovoltaic Properties of Poly(3-hexylthiophene) Isomers. Macromol. Chem. Phys. 2012, 213, 2267–2274. [Google Scholar] [CrossRef]
- Dang, M.T.; Wantz, G.; Bejbouji, H.; Urien, M.; Dautel, O.J.; Vignau, L.; Hirsch, L. Polymeric Solar Cells Based on P3HT:PCBM: Role of the Casting Solvent. Sol. Energy Mater. Sol. Cells 2011, 95, 3408–3418. [Google Scholar] [CrossRef]
- Zhang, Q.; Chi, L.; Hai, G.; Fang, Y.; Li, X.; Xia, R.; Huang, W.; Gu, E. An Easy Approach to Control β-Phase Formation in PFO Films for Optimized Emission Properties. Molecules 2017, 22, 315. [Google Scholar] [CrossRef]
- Lim, G.E.; Ha, Y.E.; Jo, M.Y.; Park, J.; Kang, Y.C.; Kim, J.H. Nonconjugated Anionic Polyelectrolyte as an Interfacial Layer for the Organic Optoelectronic Devices. ACS Appl. Mater. Interfaces 2013, 5, 6508–6513. [Google Scholar] [CrossRef]
- Tekoglu, S.; Petzoldt, M.; Stolz, S.; Bunz, U.H.; Lemmer, U.; Hamburger, M.; Hernandez-Sosa, G. Emissive Polyelectrolytes as Interlayer for Color Tuning and Electron Injection in Solution-Processed Light-Emitting Devices. ACS Appl. Mater. Interfaces 2016, 8, 7320–7325. [Google Scholar] [CrossRef]
- Pozas, R.; Mihi, A.; Ocaña, M.; Míguezm, H. Building Nanocrystalline Planar Defects within Self-Assembled Photonic Crystals by Spin-Coating. Adv. Mater. 2006, 18, 1183–1187. [Google Scholar] [CrossRef]
- Alamán, J.; Alicante, R.; Peña, J.I.; Sánchez-Somolinos, C. Inkjet printing of functional materials for optical and photonic applications. Materials 2016, 9, 910. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, Ł.; Shahbaz, M.; Butt, M.A.; Tyszkiewicz, C.; Karasiński, P.; Kaźmierczak, A.; Piramidowicz, R. Low-cost integrated photonic platform developed via a sol-gel dip-coating method: A brief review. Sens. Transducers 2022, 259, 82–92. [Google Scholar]
- Zhang, C.; Subbaraman, H.; Li, Q.; Pan, Z.; Ok, J.G.; Ling, T.; Chung, C.J.; Zhang, X.; Lin, X.; Chen, R.T.; et al. Printed photonic elements: Nanoimprinting and beyond. J. Mater. Chem. C 2016, 4, 5133–5153. [Google Scholar] [CrossRef]
- Habermehl, A.; Brenner, P.; Huber, R.; Mertens, A.; Winkler, F.; Hahn, L.; Guttmann, M.; Eschenbaum, C.; Lemmer, U. Roll-to-Roll Hot Embossing of 1D and 2D Photonic Nanostructures. Adv. Eng. Mater. 2019, 21, 1900110. [Google Scholar] [CrossRef]
- Jakabovič, J.; Kováč, J.; Weis, M.; Haško, D.; Srnánek, R.; Valent, P.; Resel, R. Preparation and properties of thin parylene layers as the gate dielectrics for organic field effect transistors. Microelectron. J. 2009, 40, 595–597. [Google Scholar] [CrossRef]
- Ooka, Y.; Tetsumoto, T.; Daud, N.A.; Tanabe, T. Ultrasmall in-plane photonic crystal demultiplexers fabricated with photolithography. Opt. Express 2017, 25, 1521–1528. [Google Scholar] [CrossRef]
- Goodwin, M.J.; Harteveld, C.A.; de Boer, M.J.; Vos, W.L. Deep reactive ion etching of cylindrical nanopores in silicon for photonic crystals. Nanotechnology 2023, 34, 225301. [Google Scholar] [CrossRef]
- Li, Y.; Fullager, D.B.; Park, S.; Childers, D.; Fesperman, R.; Boreman, G.; Hofmann, T. High-contrast infrared polymer photonic crystals fabricated by direct laser writing. Opt. Lett. 2018, 43, 4711–4714. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Zhang, X.; Wu, H.; Shen, J.; Chen, R.; Xiong, Y.; Li, J.; Guo, S. Progress on the layer-by-layer assembly of multilayered polymer composites: Strategy, structural control and applications. Prog. Polym. Sci. 2019, 89, 76–107. [Google Scholar] [CrossRef]
- Pospori, A.; Marques, C.A.; Sagias, G.; Lamela-Rivera, H.; Webb, D.J. Novel thermal annealing methodology for permanent tuning polymer optical fiber Bragg gratings to longer wavelengths. Opt. Express 2018, 26, 1411–1421. [Google Scholar] [CrossRef]
- Guo, T.; Wang, Y.; Qiao, Y.; Yuan, X.; Zhao, Y.; Ren, L. Thermal property of photonic crystals (PCs) prepared by solvent annealing self-assembly of bottlebrush PS-b-PtBA. Polymer. 2020, 194, 122389. [Google Scholar] [CrossRef]
- Signoretto, M.; Zink-Lorre, N.; Suarez, I.; Font-Sanchis, E.; Sastre-Santos, A.; Chirvony, V.S.; Fernandez-Lazaro, F.; Martinez-Pastor, J.P. Efficient Optical Amplification in a Sandwich-Type Active-Passive Polymer Waveguide Containing Perylenediimides. ACS Photonics 2017, 4, 114–120. [Google Scholar] [CrossRef]
- Jiang, L.; Gerhardt, K.P.; Myer, B.; Zohar, Y.; Pau, S. Evanescent-Wave Spectroscopy Using an SU-8 Waveguide for Rapid Quantitative Detection of Biomolecules. J. Microelectromech. Syst. 2008, 17, 1495–1500. [Google Scholar] [CrossRef]
- Sharma, N.; Petri, C.; Jonas, U.; Bach, M.; Tovar, G.; Mrkvová, K.; Vala, M.; Homola, J.; Knoll, W.; Dostálek, J. Molecularly Imprinted Polymer Waveguides for Direct Optical Detection of Low-Molecular-Weight Analytes. Macromol. Chem. Phys. 2014, 215, 2295–2304. [Google Scholar] [CrossRef]
- Jena, S.; Tokas, R.B.; Thakur, S.; Udupa, D.V. Tunable Mirrors and Filters in 1D Photonic Crystals Containing Polymers. Phys. E 2019, 114, 113627. [Google Scholar] [CrossRef]
- Li, Z.; Olah, A.; Baer, E. Micro-and Nano-Layered Processing of New Polymeric Systems. Prog. Polym. Sci. 2020, 102, 101210. [Google Scholar] [CrossRef]
- Bunning, T.J.; Natarajan, L.V.; Tondiglia, V.P.; Sutherland, R.L. Holographic Polymer-Dispersed Liquid Crystals (H-PDLCs). Annu. Rev. Mater. Sci. 2000, 30, 83–115. [Google Scholar] [CrossRef]
- Liu, Y.J.; Sun, X.W. Holographic Polymer-Dispersed Liquid Crystals: Materials, Formation, and Applications. Adv. OptoElectron. 2008, 2008, 684349. [Google Scholar] [CrossRef]
- Zito, G.; Pissadakis, S. Holographic Polymer-Dispersed Liquid Crystal Bragg Grating Integrated Inside a Solid Core Photonic Crystal Fiber. Opt. Lett. 2013, 38, 3253–3256. [Google Scholar] [CrossRef]
- Rossi, R.; Martella, D.; Parmeggiani, C.; Hirschmann, M.; Fuoco, T. Photoswitches in Order: One-Pot Synthesis of Azobenzene Main-Chain and Segmented Copolymers. ACS Appl. Polym. Mater. 2024, 6, 1563–1572. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Doerk, G.S. Thin Film Block Copolymer Self-Assembly for Nanophotonics. Nanotechnology 2022, 33, 292001. [Google Scholar] [CrossRef]
- Kim, K.H.; Huh, Y.; Song, I.; Ryu, D.Y.; Son, J.G.; Bang, J. Recent Advances in Block Copolymer Self-Assembly: From the Lowest to the Highest. J. Polym. Sci. 2024, 62, 679–692. [Google Scholar] [CrossRef]
- Jiang, H.; Zhu, Y.; Chen, C.; Shen, J.; Bao, H.; Peng, L.; Yang, X.; Li, C. Photonic Crystal pH and Metal Cation Sensors Based on Poly(vinyl alcohol) Hydrogel. New J. Chem. 2012, 36, 1051–1056. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Li, L.; Wang, J.; Dong, X.; Meng, Z.; Xue, M. Real-Time and High-Sensitive Colorimetric Sensor Based on Photonic Crystal for Amniotic Fluid Identification. Microchem. J. 2024, 202, 110691. [Google Scholar] [CrossRef]
- Zhang, W.; Froyen, A.A.; Schenning, A.P.; Zhou, G.; Debije, M.G.; de Haan, L.T. Temperature-Responsive Photonic Devices Based on Cholesteric Liquid Crystals. Adv. Photonics Res. 2021, 2, 2100016. [Google Scholar] [CrossRef]
- Hussain, S.; Zourob, M. Solid-State Cholesteric Liquid Crystals as an Emerging Platform for the Development of Optical Photonic Sensors. Small 2024, 20, 2304590. [Google Scholar] [CrossRef]
- Nagai, H.; Urayama, K. Thermal Response of Cholesteric Liquid Crystal Elastomers. Phys. Rev. E 2015, 92, 022501. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Jiang, S.A.; Lin, J.D.; Lee, C.R. Bio-Inspired Design of Active Photo-Mechano-Chemically Dual-Responsive Photonic Film Based on Cholesteric Liquid Crystal Elastomers. J. Mater. Chem. C 2020, 8, 5517–5524. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Lee, S.Y.; Chin, M.K.; Kassim, N.M.; Mohammad, A.B. Multimode Interference Optical Splitter Based on Photodefinable Benzocyclobutene (BCB 4024-40) Polymer. Opt. Eng. 2007, 46, 013401. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jang, K.; Yoon, Y.J.; Kim, Y.J. A Novel Relative Humidity Sensor Based on Microwave Resonators and a Customized Polymeric Film. Sens. Actuators B 2006, 117, 315–322. [Google Scholar] [CrossRef]
- Roth, G.L.; Hessler, S.; Kefer, S.; Girschikofsky, M.; Esen, C.; Hellmann, R. Femtosecond Laser Inscription of Waveguides and Bragg Gratings in Transparent Cyclic Olefin Copolymers. Opt. Express 2020, 28, 18077–18084. [Google Scholar] [CrossRef] [PubMed]
- Koerdt, M.; Vollertsen, F. Fabrication of an Integrated Optical Mach-Zehnder Interferometer Based on Refractive Index Modification of Polymethylmethacrylate by Krypton Fluoride Excimer Laser Radiation. Appl. Surf. Sci. 2011, 257, 5237–5240. [Google Scholar] [CrossRef]
- Li, H.; Ma, S.; Ding, M.; Zhang, L.; Zuo, L.; Xie, F.; Meng, W.; Zhang, S.; Cao, L.; Ren, F.; et al. New Class of Optical Blood Glucose Sensors Based on a PMMA Mach-Zehnder Interferometer. ACS Photonics 2024, 11, 1684–1692. [Google Scholar] [CrossRef]
- Koynov, K.; Goutev, N.; Fitrilawati, F.; Bahtiar, A.; Best, A.; Bubeck, C.; Hörhold, H.H. Nonlinear Prism Coupling of Waveguides of the Conjugated Polymer MEH-PPV and Their Figures of Merit for All-Optical Switching. J. Opt. Soc. Am. B 2002, 19, 895–901. [Google Scholar] [CrossRef]
- Almarashi, J.Q.; Gadallah, A.S.; Ellabban, M.A.; Mohamed, A.A. Improvement of MEH-PPV Performance for Laser and Optoelectronic Applications. Opt. Mater. 2024, 150, 115240. [Google Scholar] [CrossRef]
- Burunkova, Y.E.; Denisyuk, I.Y.; Aref’eva, N.N.; Litvin, A.P.; Minozhenko, O.A. Polymeric Electrooptic Composite Based on Disperse Red and its Derivatives for Use in Photonics. J. Opt. Technol. 2010, 77, 643–648. [Google Scholar] [CrossRef]
- Nahata, A.; Shan, J.; Yardley, J.T.; Wu, C. Electro-Optic Determination of the Nonlinear-Optical Properties of a Covalently Functionalized Disperse Red 1 Copolymer. J. Opt. Soc. Am. B 1993, 10, 1553–1564. [Google Scholar] [CrossRef]
- Ko, Y.; Kim, M.; Noh, C.; Kim, M.J.; Lee, K.; Kim, J.; Kim, S.D.; Jee, S.S. Organic-Inorganic Hybrid Electro-Optic Material with Disperse Red 1 Chromophore Fabricated by Flow Chemistry. J. Flow Chem. 2022, 12, 79–90. [Google Scholar] [CrossRef]
- Ohmori, Y.; Kajii, H.; Kaneko, M.; Yoshino, K.; Ozaki, M.; Fujii, A.; Hikita, M.; Takenaka, H.; Taneda, T. Realization of Polymeric Optical Integrated Devices Utilizing Organic Light-Emitting Diodes and Photodetectors Fabricated on a Polymeric Waveguide. IEEE J. Sel. Top. Quantum Electron. 2004, 10, 70–78. [Google Scholar] [CrossRef]
- Ramuz, M.; Bürgi, L.; Stanley, R.; Winnewisser, C. Coupling Light from an Organic Light Emitting Diode (OLED) into a Single-Mode Waveguide: Toward Monolithically Integrated Optical Sensors. J. Appl. Phys. 2009, 105, 084508. [Google Scholar] [CrossRef]
- Ratcliff, E.L.; Veneman, P.A.; Simmonds, A.; Zacher, B.; Huebner, D.; Saavedra, S.S.; Armstrong, N.R. A Planar, Chip-Based, Dual-Beam Refractometer Using an Integrated Organic Light-Emitting Diode (OLED) Light Source and Organic Photovoltaic (OPV) Detectors. Anal. Chem. 2010, 82, 2734–2742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jin, T.; Ye, X.; Geng, D.; Chen, W.; Hu, W. Organic Field Effect Transistor-Based Photonic Synapses: Materials, Devices, and Applications. Adv. Funct. Mater. 2021, 31, 2106151. [Google Scholar] [CrossRef]
- Zhao, P.; Ji, R.; Lao, J.; Xu, W.; Jiang, C.; Luo, C.; Lin, H.; Peng, H.; Duan, C.G. Two-Terminal Organic Optoelectronic Synapse Based on Poly(3-hexylthiophene) for Neuromorphic Computing. Org. Electron. 2022, 100, 106390. [Google Scholar] [CrossRef]
- He, L.; Yang, Z.; Wang, Z.; Leydecker, T.; Orgiu, E. Organic Multilevel (Opto)Electronic Memories Towards Neuromorphic Applications. Nanoscale 2023, 15, 11434–11456. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, S.T.; Sonar, P.; Roy, V.A. Nonvolatile Multilevel Data Storage Memory Device from Controlled Ambipolar Charge Trapping Mechanism. Sci. Rep. 2013, 3, 2319. [Google Scholar] [CrossRef]
- Hou, L.; Adhikari, S.; Tian, Y.; Scheblykin, I.G.; Orrit, M. Absorption and Quantum Yield of Single Conjugated Polymer Poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene](MEH-PPV) Molecules. Nano Lett. 2017, 17, 1575–1581. [Google Scholar] [CrossRef]
- Squeo, B.M.; Gregoriou, V.G.; Avgeropoulos, A.; Baysec, S.; Allard, S.; Scherf, U.; Chochos, C.L. BODIPY-Based Polymeric Dyes as Emerging Horizon Materials for Biological Sensing and Organic Electronic Applications. Prog. Polym. Sci. 2017, 71, 26–52. [Google Scholar] [CrossRef]
- Nagai, A.; Kokado, K.; Miyake, J.; Chujo, Y. Quantum Yield and Morphology Control of BODIPY-Based Supramolecular Self-Assembly with a Chiral Polymer Inhibitor. Polym. J. 2010, 42, 37–42. [Google Scholar] [CrossRef][Green Version]
- Lee, N.; Kim, R.; Kim, J.Y.; Ko, J.B.; Park, S.H.; Kim, S.O.; Brongersma, M.L.; Shin, J. Self-Assembled Nano-Lotus Pod Metasurface for Light Trapping. ACS Photonics 2021, 8, 1616–1622. [Google Scholar] [CrossRef]
- Guo, M.; Qu, Z.; Min, F.; Li, Z.; Qiao, Y.; Song, Y. Advanced Unconventional Techniques for Sub-100 nm Nanopatterning. InfoMat 2022, 4, e12323. [Google Scholar] [CrossRef]
- Yuan, Y.; Hou, W.; Qin, W.; Wu, C. Recent Advances in Semiconducting Polymer Dots as Optical Probes for Biosensing. Biomater. Sci. 2021, 9, 328–346. [Google Scholar] [CrossRef]
- Shen, Q.; Song, G.; Lin, H.; Bai, H.; Huang, Y.; Lv, F.; Wang, S. Sensing, Imaging, and Therapeutic Strategies Endowing by Conjugate Polymers for Precision Medicine. Adv. Mater. 2024, 36, 2310032. [Google Scholar] [CrossRef]


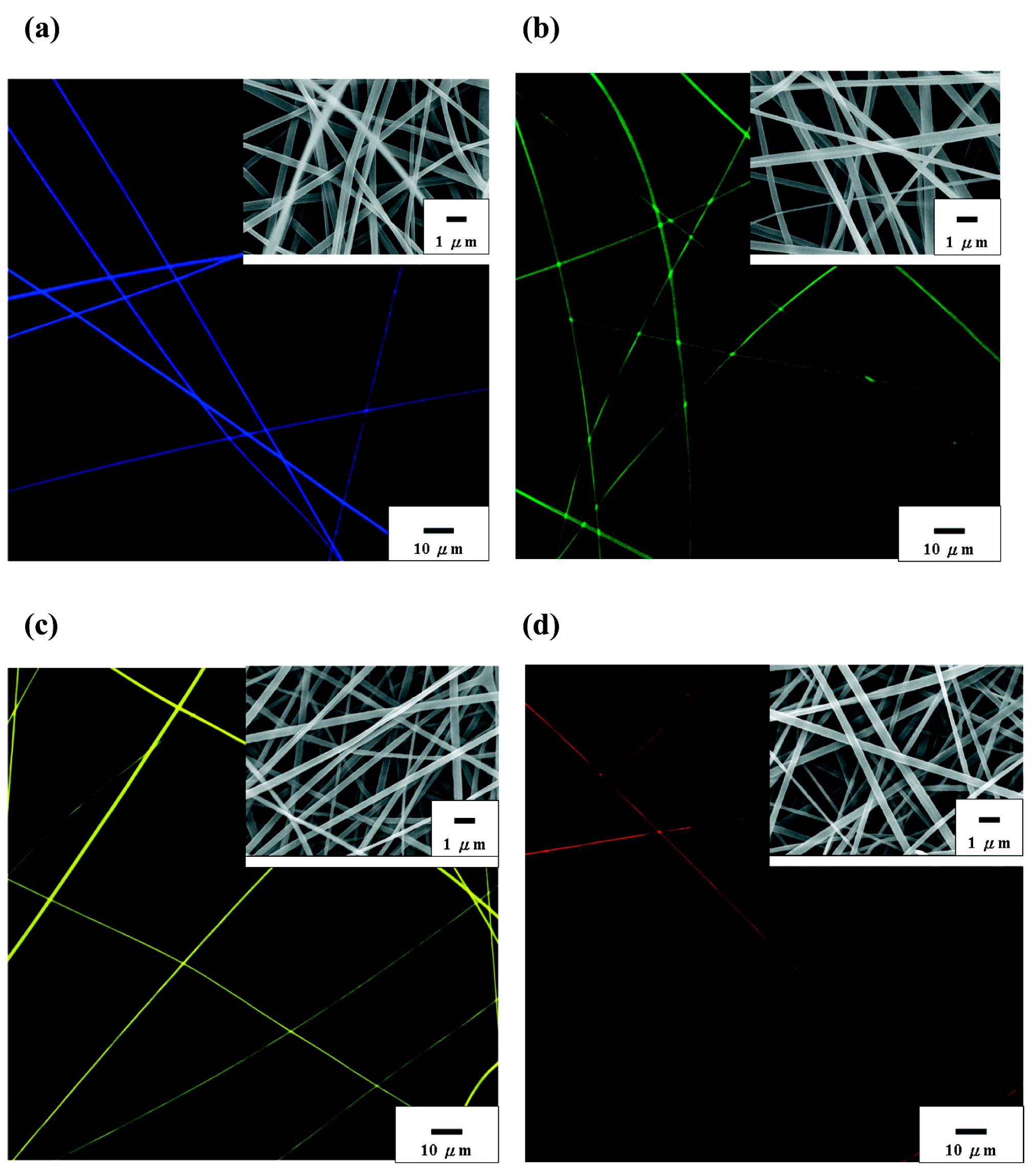
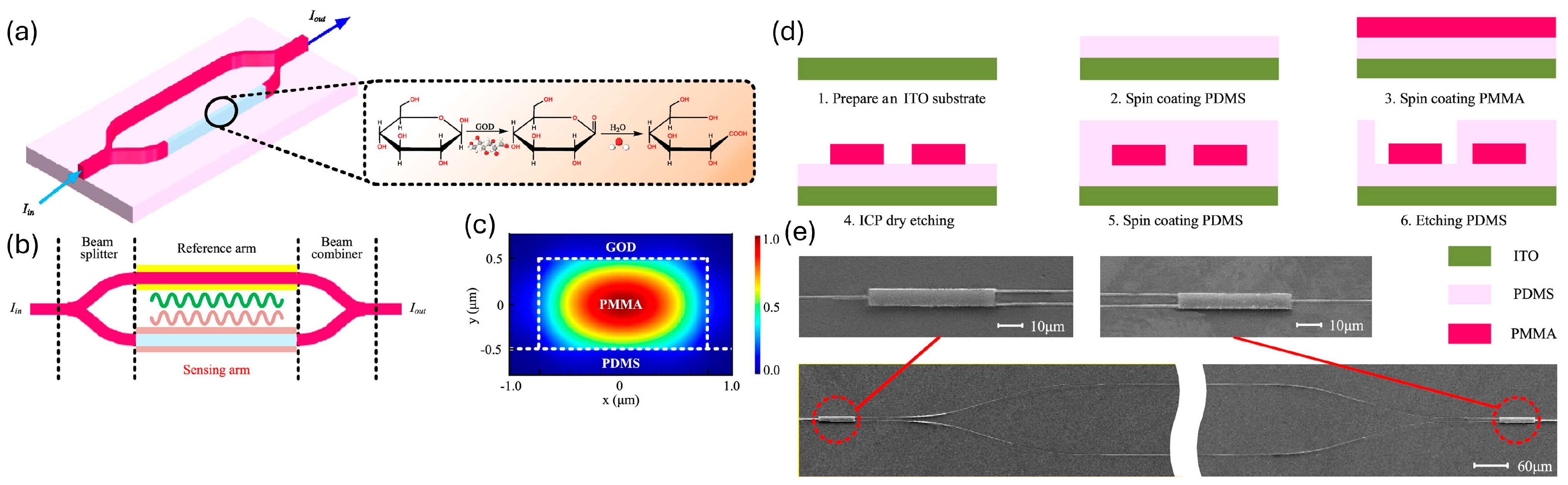
| Fabrication Method | Category | Key Features | Resolution | Application | Compatible Materials |
|---|---|---|---|---|---|
| Spin Coating | Solvent-based | Uniform thin films, precise thickness control | 10 nm (thickness) | Waveguide cores, thin film layers | PMMA, SU-8, polyfluorenes |
| Inkjet Printing | Solvent-based | Direct patterning, maskless, multi-material | 20–50 μm | Multicomponent devices, displays | Low-viscosity polymer solutions |
| Solution Casting | Solvent-based | Simple process, variable thickness | >1 μm | Large-area films, flexible devices | Most soluble polymers |
| Thermal Nanoimprint | Solvent-free | High-resolution, pattern transfer | <100 nm | Photonic crystals, gratings | Thermoplastic polymers |
| Hot Embossing | Solvent-free | Large-area patterning, cost-effective | >1 μm | Waveguides, microfluidic integration | Thermoplastic polymers |
| Polymer Vapor Deposition | Solvent-free | Conformal coatings, pinhole-free | 10 nm (thickness) | Waveguide claddings, barriers | Parylene, some conjugated polymers |
| Photolithography | Patterning | Parallel processing, industry-standard | 0.5–2 μm | Complex waveguide networks | Photosensitive polymers (SU-8) |
| Direct Laser Writing | Patterning | Maskless, 3D capabilities | 100 nm–1 μm | Photonic crystals, complex structures | Photo-cross-linkable polymers |
| Reactive Ion Etching | Patterning | High anisotropy, vertical sidewalls | 100 nm–1 μm | High-contrast waveguides | Most solid polymers |
| Layer-by-Layer Assembly | Multi-material | Precise thickness, multi-functionality | 1–10 nm (layer) | Multilayer photonic structures | Polyelectrolytes, functional polymers |
| Thermal Annealing | Post-processing | Enhanced crystallinity, reduced defects | N/A | Improved optical transmission | Semi-crystalline polymers |
| Solvent Vapor Annealing | Post-processing | Controlled reorganization | N/A | Enhanced interchain interactions | Conjugated polymers |
| Device Type | Category | Material Family | Example Material | Application | Ref. |
|---|---|---|---|---|---|
| Waveguides | Passive | Polyfluorenes | PFO | Light guiding, sensing | [62] |
| Modified PMMA | Doped PMMA | Temperature sensing | [109] | ||
| SU-8 | Functionalized SU-8 | Bio-sensing | [110] | ||
| Filters | Passive | PS/PMMA | Multilayer stacks | Wavelength selection | [112] |
| Azobenzene polymers | DR1-PMMA | Photo-switchable filtering | [133] | ||
| H-PDLCs | LC-polymer composites | Tunable reflection | [114] | ||
| Photonic Crystals | Passive | Block copolymers | PS-b-PMMA | Structural color | [118] |
| Hydrogels | Poly(acrylic acid) | pH sensing | [120] | ||
| Liquid crystals | Cholesteric polymers | Strain sensing | [125] | ||
| Light-emitting devices | Active | Polyfluorenes | F8BT | Light sources | [33] |
| PPV derivatives | MEH-PPV | Light sources | [131] | ||
| Photodetectors | Active | Polythiophenes | P3HT | Light sensing | [41] |
| Low-bandgap polymers | PCDTBT | NIR detection | [45] | ||
| Optical amplifiers | Active | PPV derivatives | MEH-PPV | Signal amplification | [132] |
| Polyfluorenes | F8BT | Optical gain | [57] | ||
| Modulators/Switches | Active | Electro-optic polymers | DR1-PMMA | Signal modulation | [133] |
| Thermo-optic polymers | PFO derivatives | Optical switching | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weis, M. Organic Semiconducting Polymers in Photonic Devices: From Fundamental Properties to Emerging Applications. Appl. Sci. 2025, 15, 4028. https://doi.org/10.3390/app15074028
Weis M. Organic Semiconducting Polymers in Photonic Devices: From Fundamental Properties to Emerging Applications. Applied Sciences. 2025; 15(7):4028. https://doi.org/10.3390/app15074028
Chicago/Turabian StyleWeis, Martin. 2025. "Organic Semiconducting Polymers in Photonic Devices: From Fundamental Properties to Emerging Applications" Applied Sciences 15, no. 7: 4028. https://doi.org/10.3390/app15074028
APA StyleWeis, M. (2025). Organic Semiconducting Polymers in Photonic Devices: From Fundamental Properties to Emerging Applications. Applied Sciences, 15(7), 4028. https://doi.org/10.3390/app15074028







