Ozone Nanobubble-Assisted Pretreatment of Lignocellulose: Enhancing Wood Liquefaction and Bio-Polyurea Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
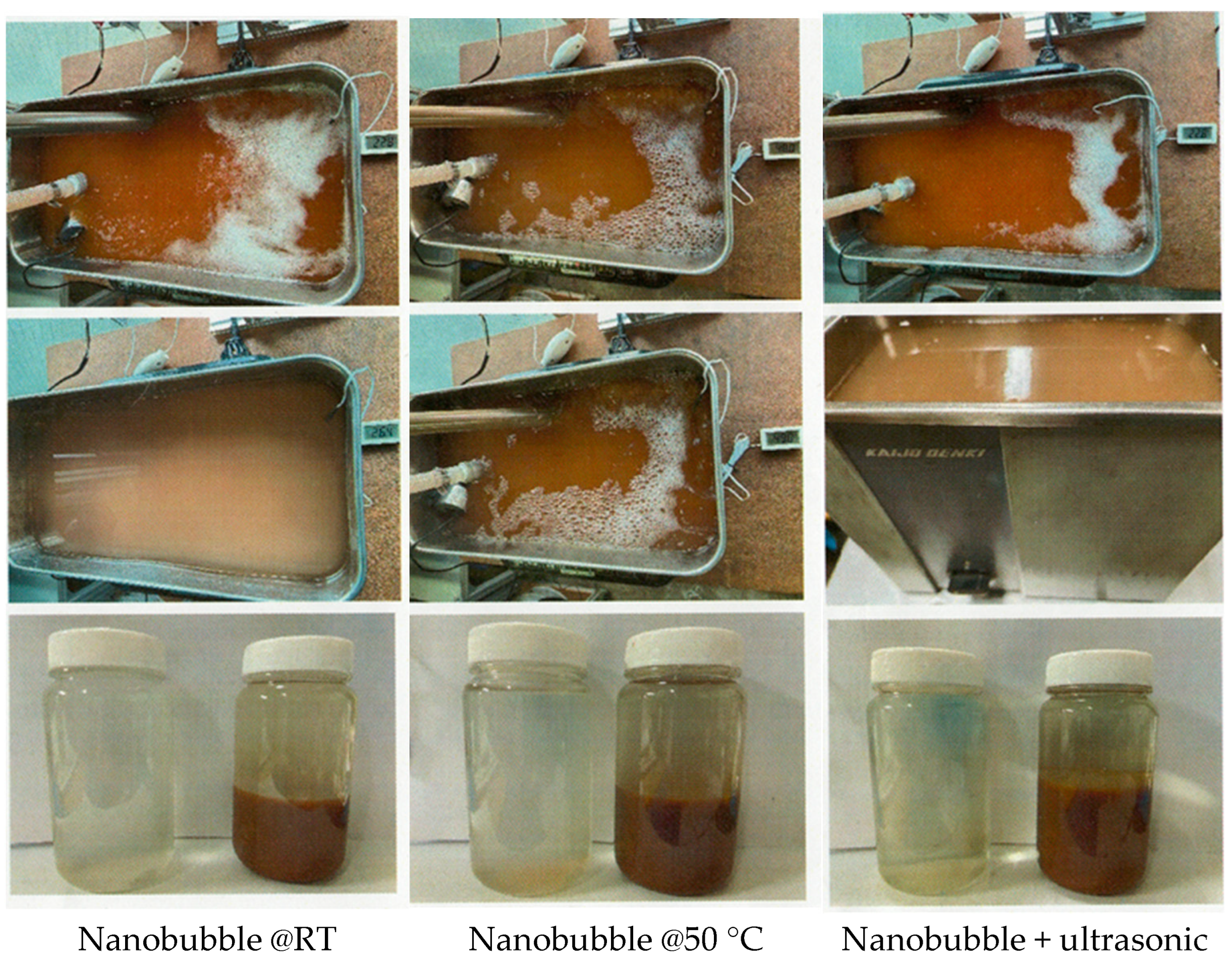
2.2. Preparation of Ozone Nanobubbled Wood Meal
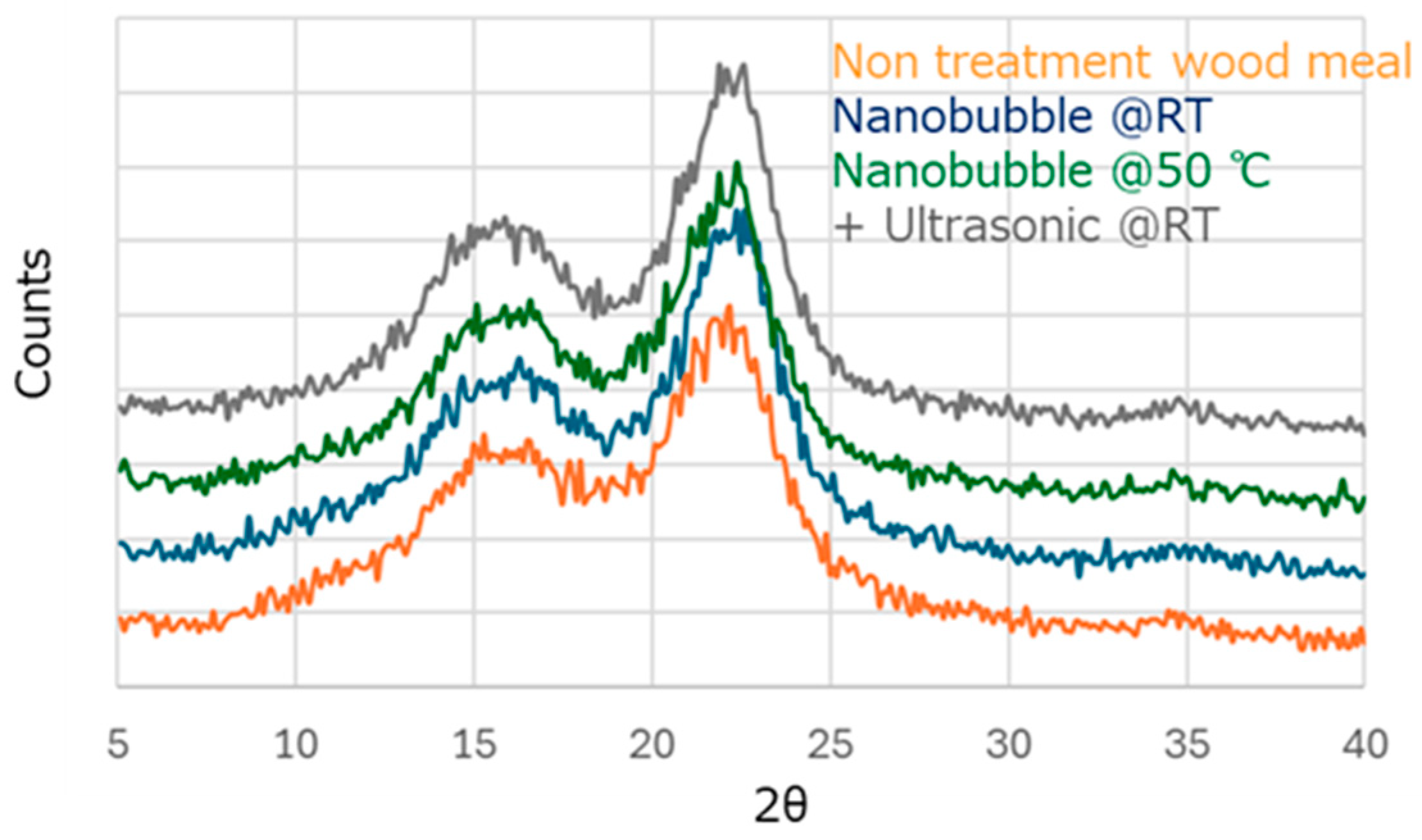
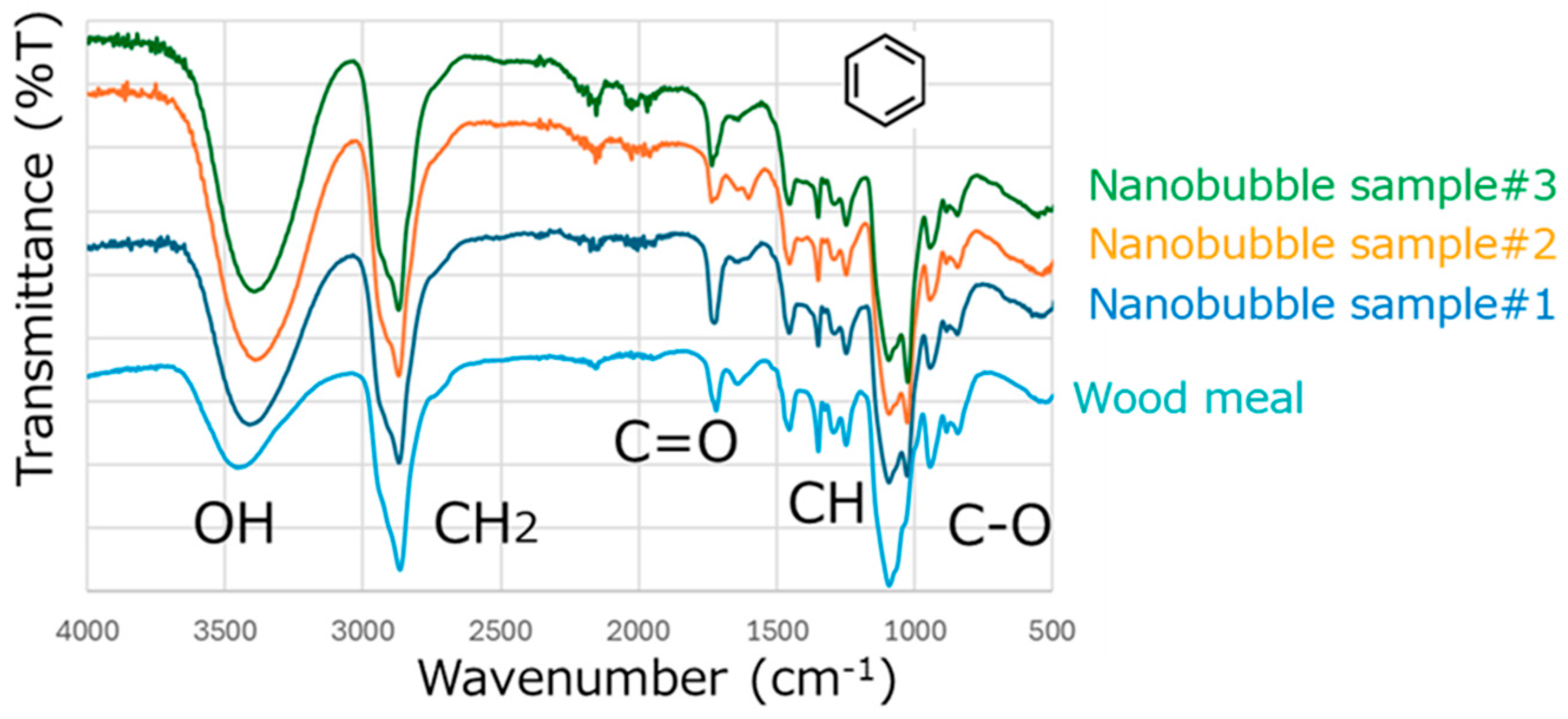
2.3. Analysis of Ozone Nanobubbled Wood Meal
 × 100
× 100
2.4. Characterization of the Liquefied Wood
2.5. Tensile Strength Test of Polyurea Films
3. Results and Discussion
3.1. Effect of Nanobubbling for Wood Meal
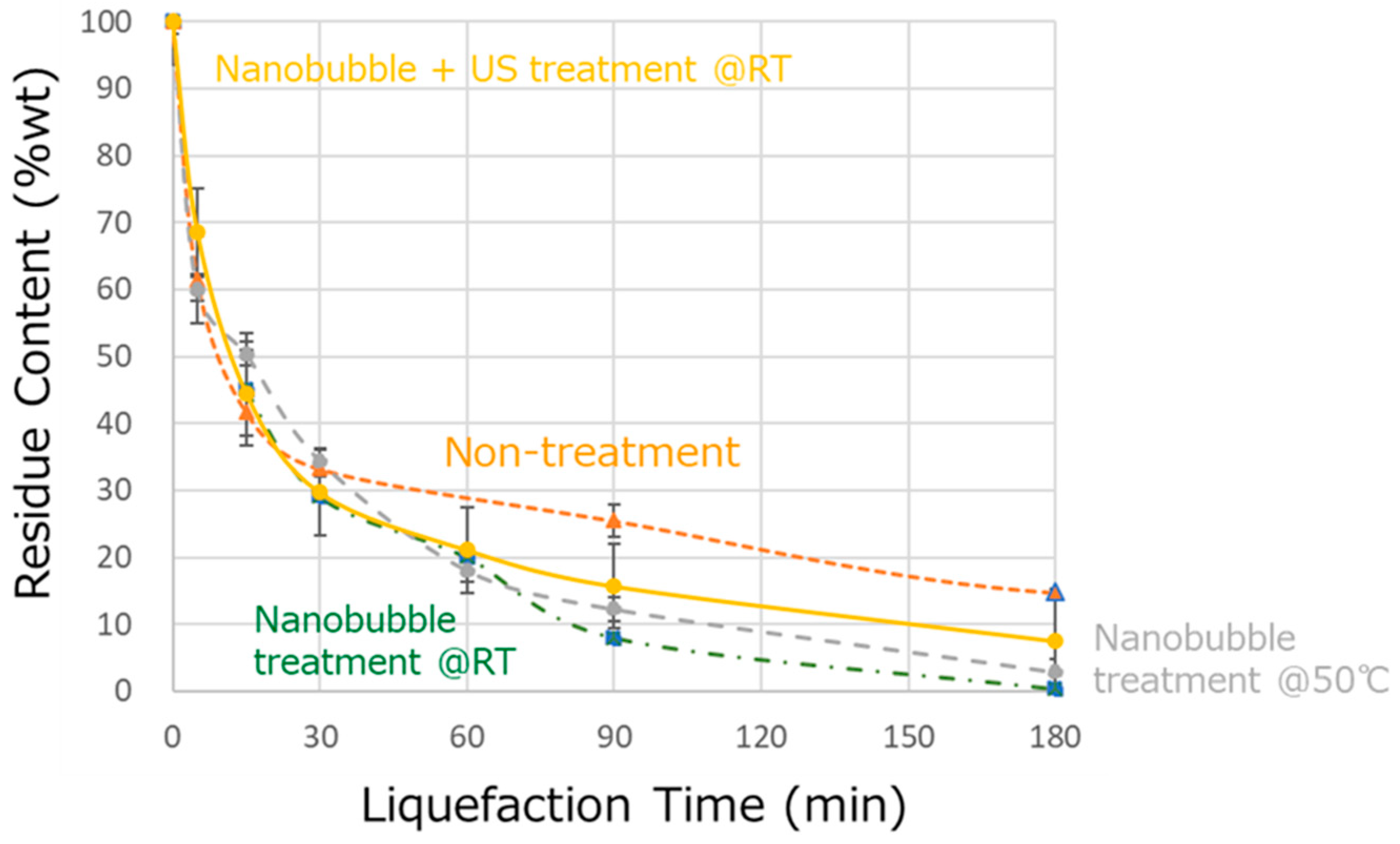
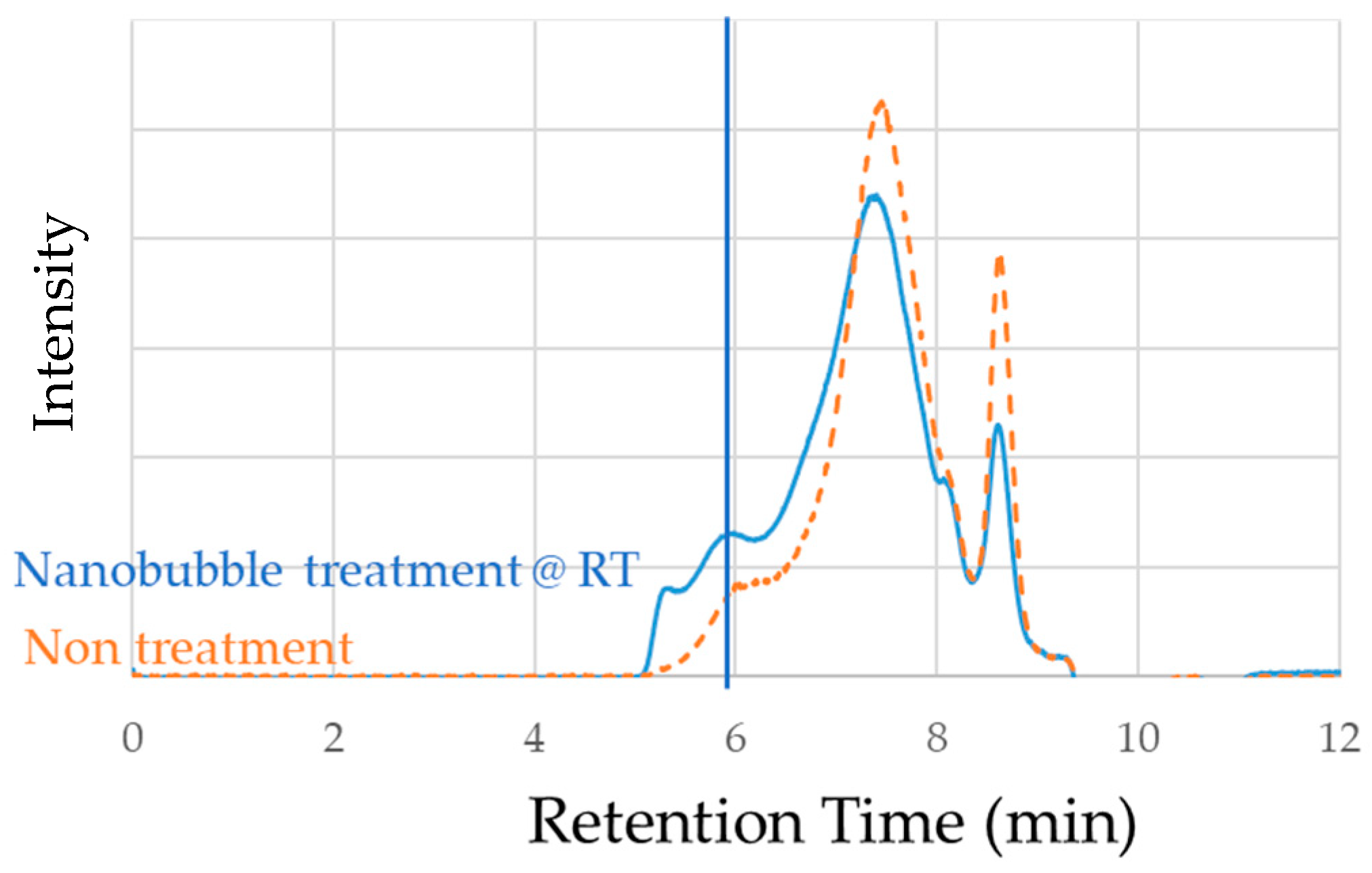
3.2. Effect of Nanobubbling on Wood Liquefaction
3.3. Effect of Nanobubbling on Bio-Polyurea Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiyohiko, T.K.A.N. Efforts toward Biomass Waste Utilization and Future Initiatives for its Widespread Adoption. Mater. Cycles Waste Manag. Res. 2024, 35, 99–106. [Google Scholar]
- Makoto, I. Utilization of Biomass Energy. Waste Manag. Res. 2002, 13, 278–287. [Google Scholar]
- Matsuda, S. Biomass Contributing to Environmental Issues. Environ. Sci. 2011, 24, 493–502. [Google Scholar]
- Shiraishi, N. Liquefaction of Wood and Its Several Applications. J. Oleo Sci. 1997, 46, 1127–1236. [Google Scholar]
- Kobayashi, M.; Asano, T.; Kajiyama, M.; Tomita, B. Analysis on residue formation during wood liquefaction with polyhydric alcohol. J. Wood Sci. 2004, 50, 407–414. [Google Scholar] [CrossRef]
- Lianzhen Lin, Y.Y.; Yoshioka, M.; Shiraishi, N. Liquefaction mechanism of cellulose in the presence of phenol under acid catalysis. Carbohydr. Polym. 2004, 57, 123–129. [Google Scholar]
- Bontaş, M.G.; Diacon, A.; Călinescu, I.; Rusen, E. Lignocellulose Biomass Liquefaction: Process and Applications Development as Polyurethane Foams. Polymers 2023, 15, 563. [Google Scholar] [CrossRef]
- Yang, Y.H.W.; Zhang, W.; Zhang, D. High-Strength and Low-Cost Biobased Polyurethane Foam Composites Enhanced by Poplar Wood Powder Liquefaction. Polymers 2021, 13, 2999. [Google Scholar] [CrossRef]
- Zheng, Z. Rapid Liquefaction of Wood in Polyhydric Alcohols Under Microwave Heating and its Liquefied Products for Preparation of Rigid Polyurethane Foam. Open Mater. Sci. J. 2011, 5, 1–8. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Wang, S.; Lan, Z.; Zhang, X.; Yin, Y.; Tu, S.; Yang, S.; Ye, L. Fast liquefaction of bamboo shoot shell with liquid-phase microplasma assisted technology. Bioresour. Technol. 2016, 218, 1275–1278. [Google Scholar] [CrossRef]
- Zhuang, X.; Liu, J.; Wang, C.; Zhang, Q.; Ma, L. Microwave-assisted hydrothermal liquefaction for biomass valorization: Insights into the fuel properties of biocrude and its liquefaction mechanism. Fuel 2022, 317, 123462. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.; Gu, Z.; Fang, D.; Hong, Y.; Cheng, L.; Li, Z. Ultrasonic pretreatment improves the high-temperature liquefaction of corn starch at high concentrations. Starch–Stärke 2016, 69, 1600002. [Google Scholar] [CrossRef]
- Lu, Z.; Wu, Z.; Fan, L.; Zhang, H.; Liao, Y.; Zheng, D.; Wang, S. Rapid and solvent-saving liquefaction of woody biomass using microwave-ultrasonic assisted technology. Bioresour. Technol. 2016, 199, 423–426. [Google Scholar] [CrossRef]
- Brand, S.; Susanti, R.F.; Kim, S.K.; Lee, H.-S.; Kim, J.; Sang, B.-I. Supercritical ethanol as an enhanced medium for lignocellulosic biomass liquefaction: Influence of physical process parameters. Energy 2013, 59, 173–182. [Google Scholar] [CrossRef]
- Gu, X.; Fu, X.; Chen, S. Hydrothermal liquefaction conversion of lignocelluloses with enhanced fungal pretreatment. Ind. Crops Prod. 2021, 162, 113268. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.; Mei, D.; Zhou, R.; Jiang, C.; Zhang, X.; Fang, Z.; Ostrikov, K.K. Liquid discharge plasma for fast biomass liquefaction at mild conditions: The effects of homogeneous catalysts. Front. Chem. Sci. Eng. 2020, 14, 763–771. [Google Scholar] [CrossRef]
- Fernandes, A.; Cruz-Lopes, L.; Esteves, B.; Evtuguin, D.V. Microwaves and Ultrasound as Emerging Techniques for Lignocellulosic Materials. Materials 2023, 16, 7351. [Google Scholar] [CrossRef]
- Lee, J.I.; Huh, H.S.; Park, J.Y.; Han, J.G.; Kim, J.M. Coarsening behavior of bulk nanobubbles in water. Sci. Rep. 2021, 11, 19173. [Google Scholar] [CrossRef]
- Lee, J.I.; Yim, B.S.; Kim, J.M. Effect of dissolved-gas concentration on bulk nanobubbles generation using ultrasonication. Sci. Rep. 2020, 10, 18816. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, J.M. Generation and Stability of Bulk Nanobubbles. Langmuir 2017, 33, 3818–3823. [Google Scholar] [CrossRef]
- Seridou, P.; Kalogerakis, N. Disinfection applications of ozone micro- and nanobubbles. Environ. Sci. Nano 2021, 8, 3493–3510. [Google Scholar] [CrossRef]
- Yasuda, K. Characteristics of Ultrafine Bubbles (Bulk Nanobubbles) and Their Application to Particle-Related Technology. KONA Powder Part. J. 2024, 41, 183–196. [Google Scholar] [CrossRef]
- Foudas, A.W.; Kosheleva, R.I.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C.; Kyzas, G.Z. Fundamentals and applications of nanobubbles: A review. Chem. Eng. Res. Des. 2023, 189, 64–86. [Google Scholar] [CrossRef]
- Jia, J.; Zhu, Z.; Chen, H.; Pan, H.; Jiang, L.; Su, W.-H.; Chen, Q.; Tang, Y.; Pan, J.; Yu, K. Full life circle of micro-nano bubbles: Generation, characterization and applications. Chem. Eng. J. 2023, 471, 144621. [Google Scholar] [CrossRef]
- Sugisawa, M.; Suda, D.; Arakawa, S. Prevention of postoperative infection after implant placement using ozon nano-bubble water Antibacterial effects against Streptococcus mutans. J. Bio-Integlation 2016, 6, 57–62. [Google Scholar]
- Lee, J.I.; Kim, J.-M. Influence of temperature on bulk nanobubble generation by ultrasonication. Colloid Interface Sci. Commun. 2022, 49, 100639. [Google Scholar] [CrossRef]
- Han, Z.; Kurokawa, H.; Matsui, H.; He, C.; Wang, K.; Wei, Y.; Dodbiba, G.; Otsuki, A.; Fujita, T. Stability and Free Radical Production for CO2 and H2 in Air Nanobubbles in Ethanol Aqueous Solution. Nanomaterials 2022, 12, 237. [Google Scholar] [CrossRef]
- Jadhav, A.J.; Barigou, M. Bulk Nanobubbles or Not Nanobubbles: That is the Question. Langmuir 2020, 36, 1699–1708. [Google Scholar] [CrossRef]
- Maie, N.; Anzai, S.; Tokai, K.; Kakino, W.; Taruya, H.; Ninomiya, H. Using oxygen/ozone nanobubbles for in situ oxidation of dissolved hydrogen sulfide at a residential tunnel-construction site. J. Environ. Manag. 2022, 302 Pt B, 114068. [Google Scholar] [CrossRef]
- Ulatowski, K.; Cecuga, A.; Sobieszuk, P. The Pursuit of Energy Reduction in Generation of Stable Nanobubbles. Processes 2023, 11, 2739. [Google Scholar] [CrossRef]
- Aikawa, A.; Kioka, A.; Nakagawa, M.; Anzai, S. Nanobubbles as corrosion inhibitor in acidic geothermal fluid. Geothermics 2021, 89, 101962. [Google Scholar] [CrossRef]
- Dhungana, P.; Bhandari, B. Development of a continuous membrane nanobubble generation method applicable in liquid food processing. Int. J. Food Sci. Technol. 2021, 56, 4268–4277. [Google Scholar] [CrossRef]
- Anzaikantetsu. 2025. Available online: https://anzaimcs.com/main/nanobubble.html (accessed on 28 February 2025).
- Lyu, T.; Wu, Y.; Zhang, Y.; Fan, W.; Wu, S.; Mortimer, R.J.G.; Pan, G. Nanobubble aeration enhanced wastewater treatment and bioenergy generation in constructed wetlands coupled with microbial fuel cells. Sci. Total Environ. 2023, 895, 165131. [Google Scholar] [CrossRef] [PubMed]
- Sueishi, N.; Ohshima, T.; Oikawa, T.; Takemura, H.; Kasai, M.; Kitano, K.; Maeda, N.; Nakamura, Y. Plaque-removal effect of ultrafine bubble water: Oral application in patients undergoing orthodontic treatment. Dent. Mater. J. 2021, 40, 272–278. [Google Scholar] [CrossRef]
- Takahashi, M. Micro/nano Bubble; Fundamental Properties and Application in Surface Cleaning. J. Oleo Sci. 2017, 17, 413–419. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, K.; Cen, C.; Wu, X.; Mao, R.; Zheng, Y. Role of bulk nanobubbles in removing organic pollutants in wastewater treatment. AMB Express 2021, 11, 96. [Google Scholar] [CrossRef]
- Zhu, Y.; Lyu, T.; Li, D.; Zhang, Z.; Guo, J.; Li, X.; Xiong, W.; Dong, R.; Wang, S. Process mechanisms of nanobubble technology enhanced hydrolytic acidification of anaerobic digestion of lignocellulosic biomass. Chem. Eng. J. 2024, 480, 147956. [Google Scholar] [CrossRef]
- Masuda, G.; Enyoh, C.E.; Wang, W.; Suzuki, M.; Honda, Y.; Wang, Q. Synthesis and Characterization of Bio-Based Polyurea Derived from Liquefied Wood of Wooden Musical Instrument Offcuts. Macromol 2024, 4, 739–752. [Google Scholar] [CrossRef]
- Masuda, G.; Akuta, S.; Wang, W.; Suzuki, M.; Honda, Y.; Wang, Q. Study on Fast Liquefaction and Characterization of Produced Polyurethane Foam Materials from Moso Bamboo. Materials 2024, 17, 3751. [Google Scholar] [CrossRef]
- Masuda, G.; Nagao, A.; Wang, W.; Wang, Q. Evaluation of Polyurethane Foam Derived from the Liquefied Driftwood Approaching for Untapped Biomass. Processes 2023, 11, 2929. [Google Scholar] [CrossRef]
- Kobayashi, M.; Asano, T.; Kajiyama, M.; Tomita, B. Effect of ozone treatment of wood on its liquefaction. J. Wood Sci. 2005, 51, 348–356. [Google Scholar] [CrossRef]
- Ertaş, M.; Fidan, M.S.; Alma, H.M. Preparation and characterization of biodegradable rigid polyurethane foams from the liquefied eucalyptus and pine woods. Wood Res. 2014, 59, 97–108. [Google Scholar]
- ISO 527-3:2018; Plastics—Determination of Tensile Properties Part 3: Test Conditions for Films and Sheets. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/70307.html (accessed on 28 February 2025).
- Mohammad Tehrani, A.S. Effect of Chain Length Distribution on Mechanical Behavior of Polymeric Networks. Eur. Polym. J. 2016, 87, 136–146. [Google Scholar] [CrossRef]



| Equipment | RIGAKU Ultima Ⅲ |
| Condituons | Range: 2θ = 5–40° (Reflection Method) Method: 2θ/θ Step: 0.02° Scan speed: 2°/min (2θ) |
| Calculation | DIFFRAC EVA 3.2 (Bruker AXS) |
| GPC Measurement | Conditions |
|---|---|
| Mobile phase | THF |
| Flow rate | 1 mL/min |
| Dilute sample concentration | 0.4 g/L |
| THF column temp. | 40 °C |
| Sample loop volume | 100 μL |
| Wood Meal | C (%) | H (%) | N (%) | Extractive (%) | Holocellulose (%) |
|---|---|---|---|---|---|
| Untreated | 46.6 (0.1) | 5.7 (0.1) | 0.1 (0.1) | 6.4 | 77.4 |
| Nanobubble @RT | 46.1 (1.4) | 5.6 (0.2) | 0.1 (0.1) | 4.6 | 70.9 |
| Nanobubble @50 °C | 45.1 (1.4) | 5.5 (0.3) | 0.2 (0.1) | 4.7 | 69.7 |
| +Ultrasonic @RT | 44.8 (0.4) | 5.7 (0.1) | 0.1 (0.1) | 2.0 | 72.3 |
| Wood Meal | Klason Lignin (%) | α-Cellulose (%) | Hemicellulose (%) |
|---|---|---|---|
| Untreated | 23.8 | 57.1 | 42.8 |
| Nanobubble @RT | 23.8 | 64.1 | 35.9 |
| Nanobubble @50 °C | 23.8 | 65.1 | 34.9 |
| +Ultrasonic @RT | 20.7 | 61.5 | 38.4 |
| XRD Sample | Crystallinity (%) | Amorphous (%) |
|---|---|---|
| Untreated | 37.7 | 62.3 |
| Nanobubble @RT | 43.4 | 56.6 |
| Nanobubble @50 °C | 40.3 | 59.7 |
| +Ultrasonic @RT | 49.7 | 50.3 |
| Liquefied Products | OH Number (KOH/mg) | Residue (wt%) | Viscosity (cP) |
|---|---|---|---|
| Untreated | 341 | 14.7 | 684 |
| Nanobubble @RT | 387 | 0.4 | 246 |
| Nanobubble @50 °C | 557 | 2.9 | 232 |
| +Ultrasonic @RT | 581 | 7.5 | 198 |
| Polyurea Film | OH Number (KOH/mg) | Stress (MPa) | Strain (%) |
|---|---|---|---|
| Non treatment | 341 | 43.6 (3.2) | 4.3 (0.6) |
| Nanobubble @RT | 387 | 40.9 (4.8) | 5.8 (1.8) |
| Nanobubble @50 °C | 557 | 46.7 (10.3) | 5.9 (1.3) |
| +Ultrasonic @RT | 581 | 42.8 (6.0) | 6.1 (0.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuda, G.; Enyoh, C.E.; Wang, W.; Anzai, S.; Wang, Q. Ozone Nanobubble-Assisted Pretreatment of Lignocellulose: Enhancing Wood Liquefaction and Bio-Polyurea Development. Appl. Sci. 2025, 15, 4618. https://doi.org/10.3390/app15094618
Masuda G, Enyoh CE, Wang W, Anzai S, Wang Q. Ozone Nanobubble-Assisted Pretreatment of Lignocellulose: Enhancing Wood Liquefaction and Bio-Polyurea Development. Applied Sciences. 2025; 15(9):4618. https://doi.org/10.3390/app15094618
Chicago/Turabian StyleMasuda, Go, Christian Ebere Enyoh, Weiqian Wang, Satoshi Anzai, and Qingyue Wang. 2025. "Ozone Nanobubble-Assisted Pretreatment of Lignocellulose: Enhancing Wood Liquefaction and Bio-Polyurea Development" Applied Sciences 15, no. 9: 4618. https://doi.org/10.3390/app15094618
APA StyleMasuda, G., Enyoh, C. E., Wang, W., Anzai, S., & Wang, Q. (2025). Ozone Nanobubble-Assisted Pretreatment of Lignocellulose: Enhancing Wood Liquefaction and Bio-Polyurea Development. Applied Sciences, 15(9), 4618. https://doi.org/10.3390/app15094618









