Evaluation of Achillea millefolium var. Paprika Extract with Antioxidant, Antimicrobial, and Skin Protection Potential in Topical Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Plant Extracts
2.3. Phytochemical Constituents of Achillea millefolium Extract
2.3.1. Total Polyphenol Content
2.3.2. Total Flavonoid Content
2.3.3. Total Phenolic Acid Content
2.3.4. Condensed Tannin Content
2.4. Antioxidant Activity Evaluation
2.4.1. FRAP Assay
2.4.2. DPPH Assay
2.5. Evaluation of the Antimicrobial Activity of Achillea millefolium Extract
2.5.1. Test Microorganisms
2.5.2. Minimal Inhibitory Concentration (MIC)
2.5.3. Determination of Minimum Bactericidal and Fungicidal Concentrations (MBC/MFC)
2.5.4. Evaluation of Biofilm Formation Inhibition
2.6. Cell Culture
2.7. Assessment of Cytotoxicity—Alamar Blue (AB) and Neutral Red (NR) Uptake Assays
2.8. Evaluation of Intracellular Reactive Oxygen Species (ROS) Levels in Skin Cells Without and After UVB Radiation Exposure
2.9. Assessment of Inhibition of Collagenase, Elastase, and Tyrosinase Activity
2.9.1. Determination of Anti-Collagenase and Anti-Elastase Activity
2.9.2. Determination of Anti-Tyrosinase Activity
2.10. Determination of Model Cosmetics Containing Achillea millefolium Extract
2.10.1. Preparation of the Cosmetic Cream
2.10.2. Rheological Properties of Extract-Based Cosmetic Cream
2.10.3. Determination of Sun Protection Factor (In Vitro) of Cosmetic Cream
2.11. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Constituents and Antioxidant Activity of Achillea millefolium Extract
3.2. Antimicrobial and Antibiofilm Activity of Achillea millefolium Extract
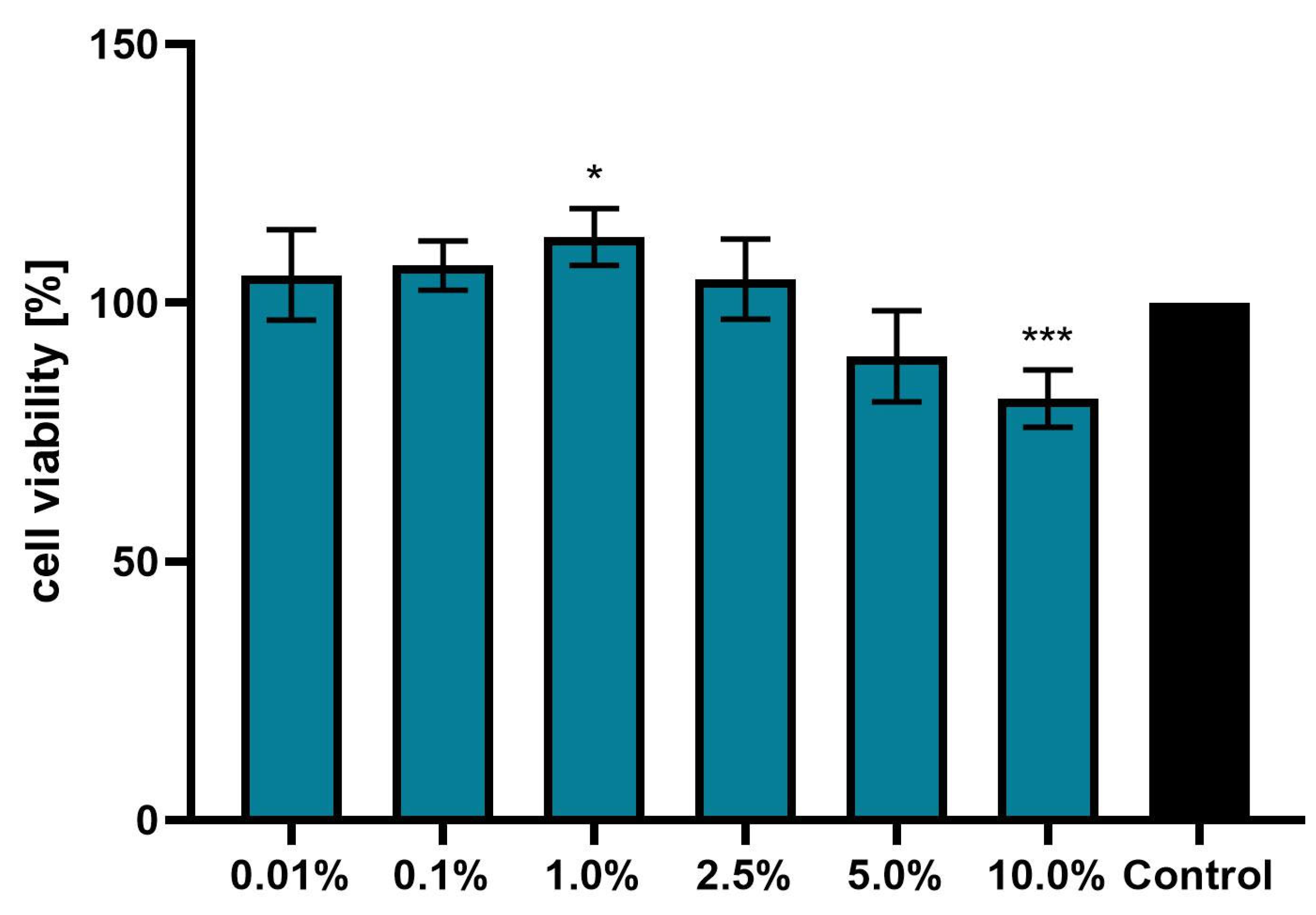
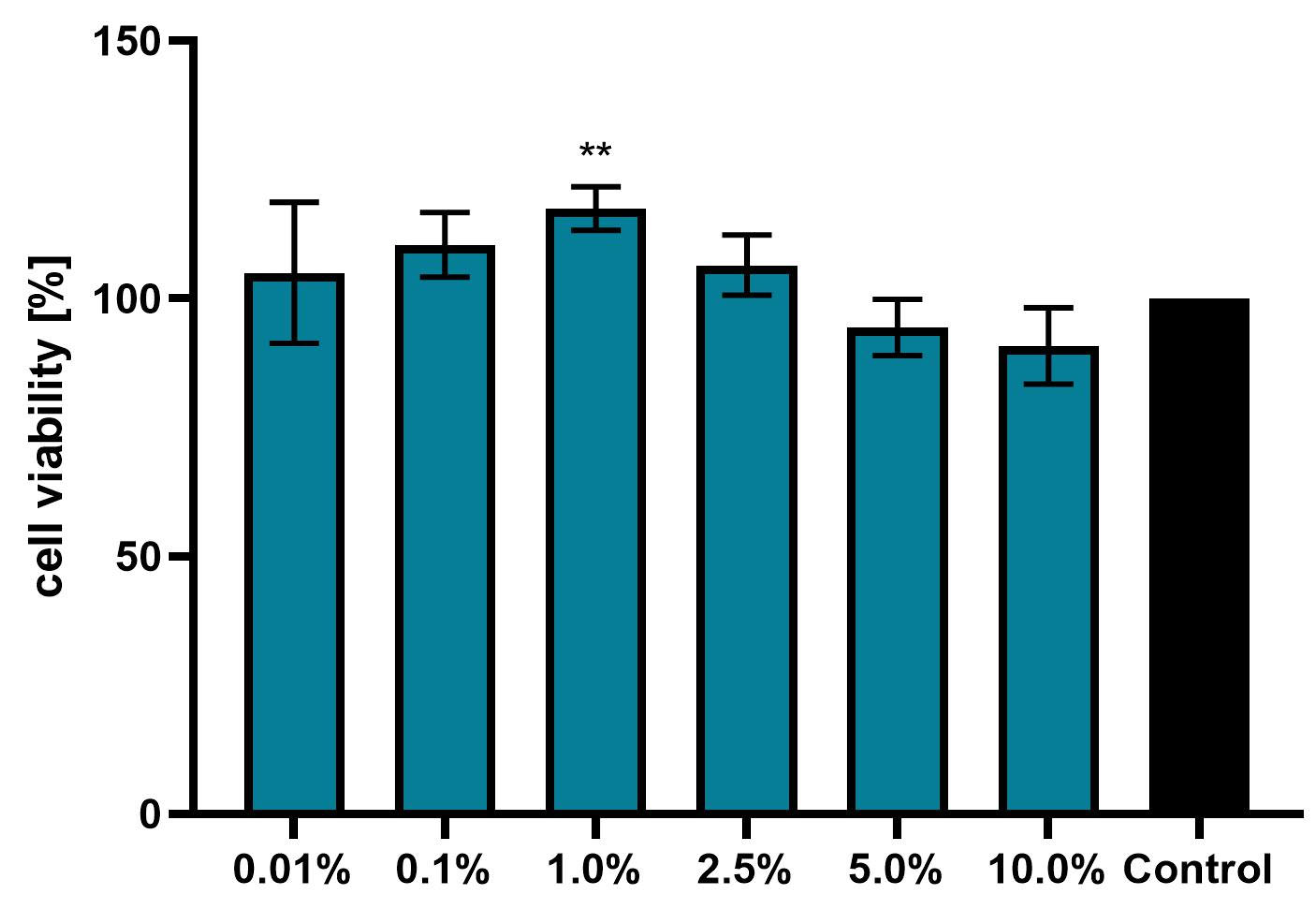
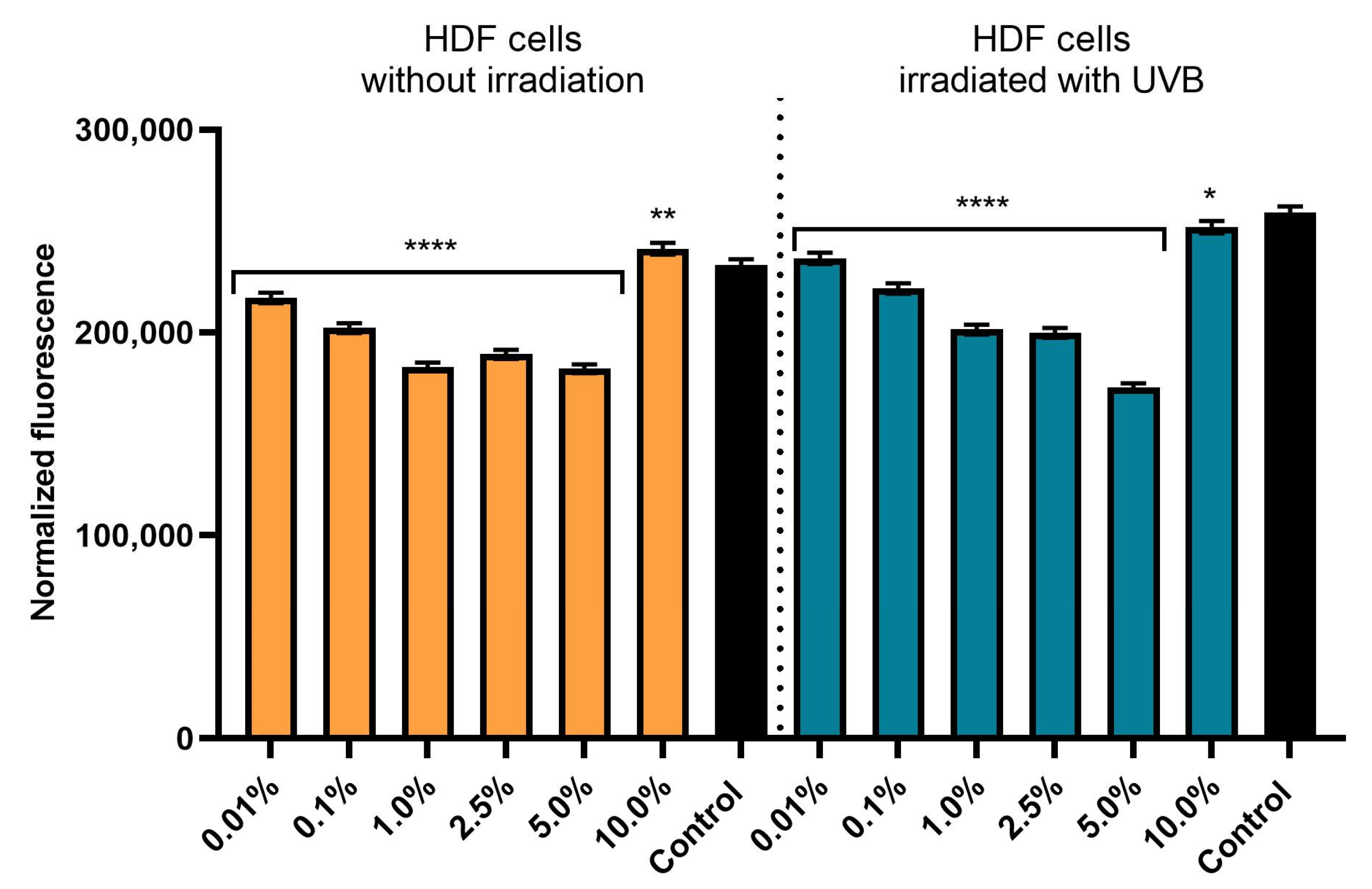
3.3. Effect of Achillea millefolium Extract on Skin Cell Viability
3.4. Anti-Ageing Activity of Achillea millefolium Extract
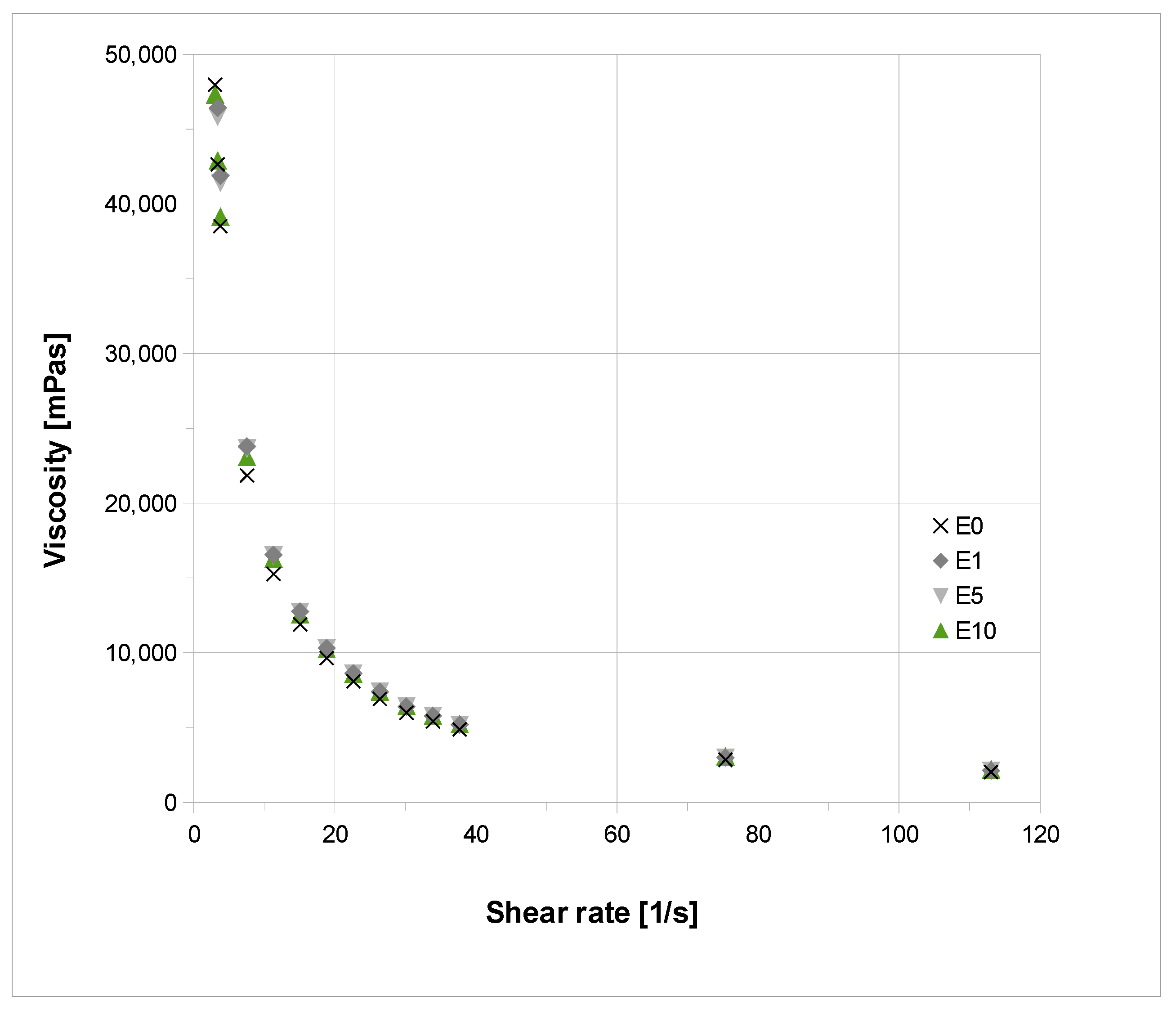
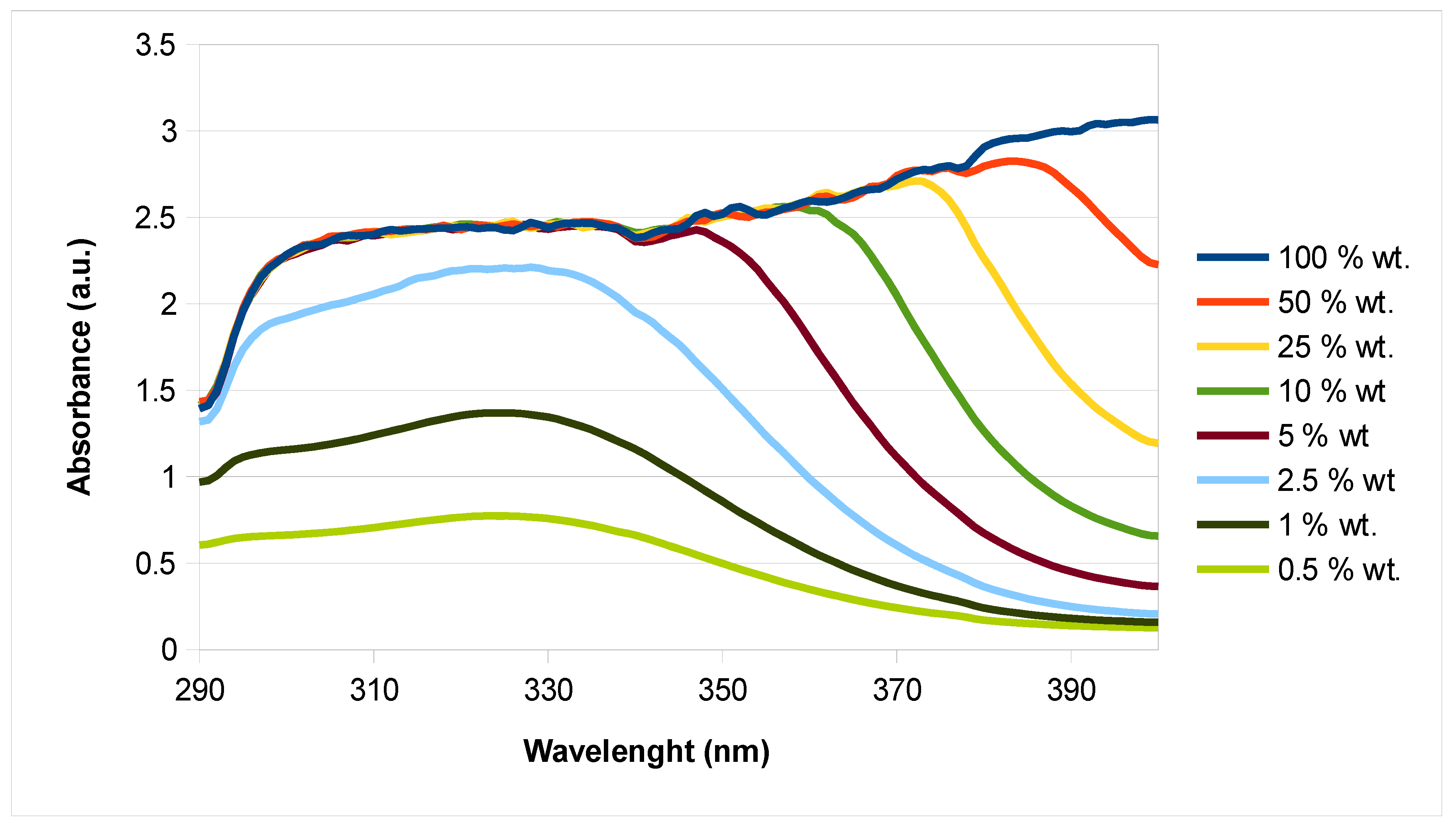
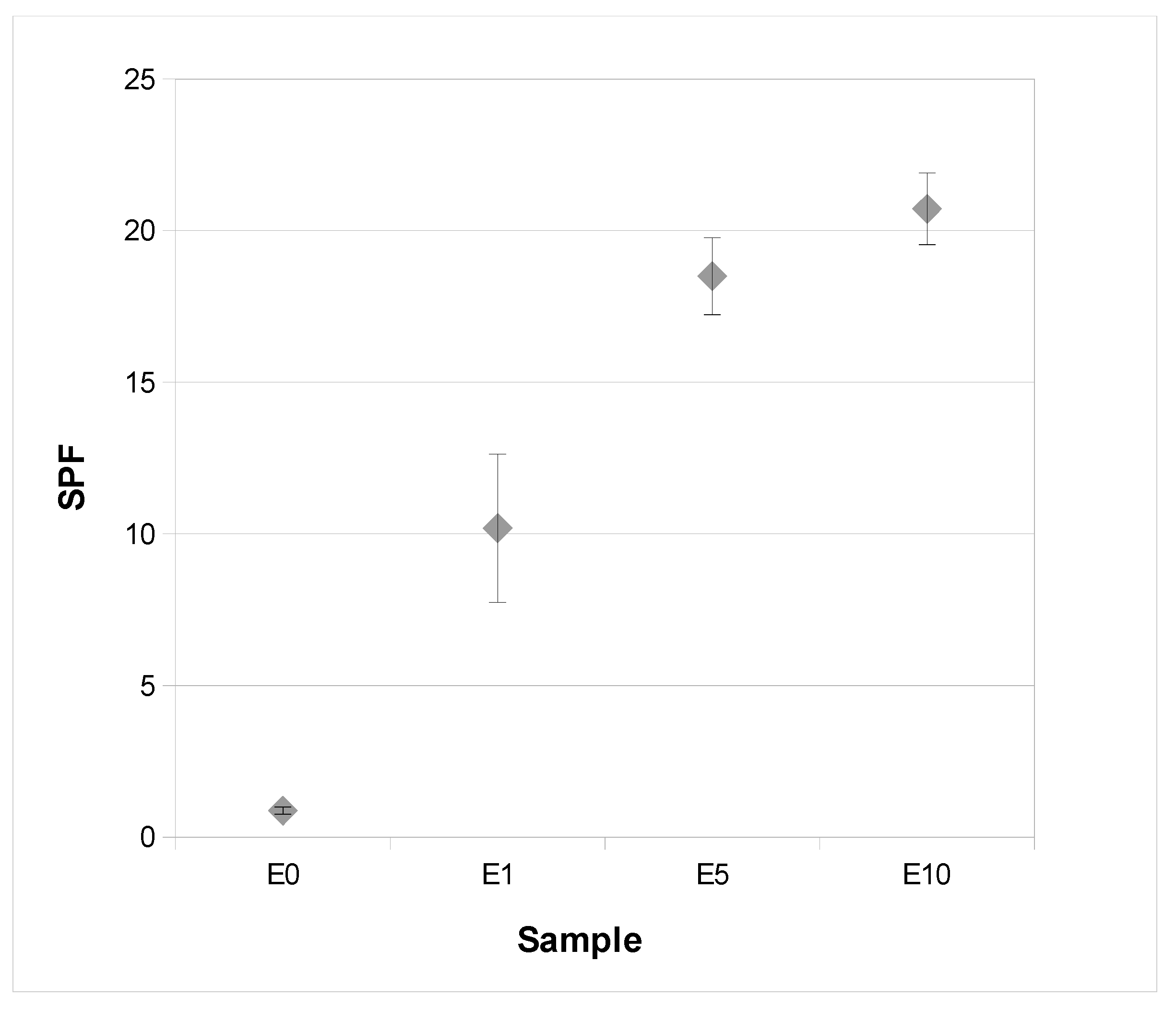
3.5. Rheological Properties and Sun Protection Factor (SPF) of an Extract-Based Cream
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EMA | European Medicines Agency |
| CosIng | Cosmetic ingredient database |
| F-C | Folin–Ciocalteu |
| TPTZ | 2,4,6-tripyridyl-s-triazine |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| UAE | Ultrasound-assisted extraction |
| SFE | Supercritical fluid extraction |
| TP | Total polyphenol |
| GAE | Gallic acid equivalents |
| TF | Total flavonoid |
| CE | Catechin equivalents |
| TPA | Total phenolic acid |
| CAE | Caffeic acid equivalents |
| CT | Condensed tannin |
| DpE | Delphinidin equivalents |
| FRAP | Ferric Reducing Antioxidant Power |
| TE | Trolox equivalent |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| MIC | Minimal Inhibitory Concentration |
| CLSI | Clinical and Laboratory Standards Institute |
| MBC | Minimum Bactericidal Concentration |
| MFC | Minimum Fungicidal Concentration |
| AB | Alamar Blue |
| NR | Neutral Red |
| ROS | Reactive Oxygen Species |
| CF | Correction factor |
| EE | Erythemogenic effect |
| ABTS | 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid) |
| ORAC | Oxygen radical absorbance capacity |
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Lakshmi, T.; Goetha, R.V.; Roy, A.; Kumar, A. Yarrow (Achillea millefolium Linn.) a herbal medicinal plant with broad therapeutic use—A review. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 136–141. [Google Scholar]
- Vitalini, S.; Beretta, G.; Iriti, M.; Orsenigo, S.; Basilico, N.; Dall’Acqua, S.; Iorizzi, M.; Fico, G. Phenolic compounds from Achillea millefolium L. and their bioactivity. Acta Biochim. Pol. 2011, 58, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Far, B.F.; Behzad, G.; Khalili, H. Achillea millefolium: Mechanism of action, pharmacokinetic, clinical drug-drug interactions and tolerability. Heliyon 2023, 9, e22841. [Google Scholar] [CrossRef]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, Phytochemistry and Pharmacological Properties of Achillea millefolium L.: A Review. Phytother. Res. 2017, 8, 1140–1161. [Google Scholar] [CrossRef]
- Akram, M. Minireview on Achillea millefolium Linn. J. Membr. Biol. 2013, 9, 661–663. [Google Scholar] [CrossRef]
- Borrelli, F.; Romano, B.; Fasolino, I.; Tagliatatela-Scafati, O.; Aprea, G.; Capasso, R.; Coppola-Bottazzi, E.; Izzo, A.A. Prokinetic effect of a standardized yarrow (Achillea millefolium) extract and its constituent choline: Studies in the mouse and human stomach. Neurogastroenterol. Motil. 2012, 24, 164–171. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Achillea millefolium as Used in Cosmetics. Int. J. Toxicol. 2016, 35, 5–15. [Google Scholar] [CrossRef]
- Cosmetic Ingredient Database (CosIng). Available online: https://ec.europa.eu/growth/tools-databases/cosing/ (accessed on 3 January 2025).
- European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/search?search_api_fulltext=achillea&f%5B0%5D=ema_search_entity_is_document%3ADocument (accessed on 4 January 2025).
- Agbor, G.A.; Vinson, J.A.; Donnelly, P.E. Folin-Ciocalteau Reagent for Polyphenolic Assay. Int. J. Food Sci. Nutr. Diet. 2014, 3, 147–156. [Google Scholar] [CrossRef]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321. [Google Scholar] [CrossRef]
- Jain, R.; Rao, B.; Tare, A.B. Comparative analysis of the spectrophotometry based total phenolic acid estimation methods. J. Anal. Chem. 2017, 72, 972–976. [Google Scholar] [CrossRef]
- Tlili, H.; Hanen, N.; Ben Arfa, A.; Neffati, M.; Boubakri, A.; Buonocore, D.; Dossena, M.; Verri, M.; Doria, E. Biochemical profile and in vitro biological activities of extracts from seven folk medicinal plants growing wild in southern Tunisia. PLoS ONE 2019, 14, e0213049. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition, CLSI Document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. 2005, 22, 1B.1.1–1B.1.18. [Google Scholar] [CrossRef]
- Terzić, J.; Stanković, M.; Stefanović, O. Extracts of Achillea millefolium L. inhibited biofilms and biofilm-related virulence factors of pathogenic bacteria isolated from wounds. Microb. Pathog. 2025, 199, 107219. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Zagórska-Dziok, M.; Mokrzyńska, A.; Nizioł-Łukaszewska, Z.; Szczepanek, D.; Sowa, I.; Wójciak, M. Comparison of Anti-Inflammatory and Antibacterial Properties of Raphanus sativus L. Leaf and Root Kombucha-Fermented Extracts. Int. J. Mol. Sci. 2024, 25, 5622. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Mokrzyńska, A.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Sowa, I.; Feldo, M.; Wójciak, M. Assessment of the Antioxidant and Photoprotective Properties of Cornus mas L. Extracts on HDF, HaCaT and A375 Cells Exposed to UVA Radiation. Int. J. Mol. Sci. 2024, 25, 10993. [Google Scholar] [CrossRef]
- Krochmal-Marczak, B.; Zagórska-Janiszewska, M.; Michalak, M.; Kiełtyka-Dadasiewicz, A. Comparative assessment of phenolic content, cellular antioxidant, antityrosinase and protective activities on skin cells of extracts from three sweet potato (Ipomoea batatas (L.) Lam.) cultivars. J. King Saud Univ. Sci. 2021, 33, 6. [Google Scholar] [CrossRef]
- Mansur, J.D.S.; Breder, M.N.R.; Mansur, M.C.D.A.; Azulay, R.D. Determinaçäo do fator de proteçäo solar por espectrofotometria. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef]
- Villalva, M.; Santoyo, S.; Salas-Pérez, L.; Nieves Siles-Sánchez, M.; Rodríguez García-Risco, M.; Fornari, T.; Reglero, G.; Jaime, L. Sustainable Extraction Techniques for Obtaining Antioxidant and Anti-Inflammatory Compounds from the Lamiaceae and Asteraceae Species. Foods 2021, 10, 2067. [Google Scholar] [CrossRef] [PubMed]
- Abu Safe, F.A.; Badr, A.N.; Shehata, M.G.; El-Sayyad, G.S. Antimicrobial and anti-aflatoxigenic activities of nanoemulsions based on Achillea millefolium and Crocus sativus flower extracts as green promising agents for food preservatives. BMC Microbiol. 2023, 23, 289. [Google Scholar] [CrossRef] [PubMed]
- Barbinta-Patrascu, M.-E.; Nichita, C.; Enculescu, M.; Maraloiu, V.-A.; Bacalum, M.; Ungureanu, C.; Negrila, C.C.; Zgura, I. Bioactive Hybrids Containing Artificial Cell Membranes and Phyto-Gold–Silver Chloride Bio-Nanoparticles. Int. J. Mol. Sci. 2024, 25, 11929. [Google Scholar] [CrossRef] [PubMed]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from plants protect against skin photoaging. Oxidative Med. Cell. Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef]
- Benetis, R.; Radušienė, J.; Jakštas, V.; Janulis, V.; Malinauskas, F. Development of an RP-HPLC Method for the Analysis of Phenolic Compounds in Achillea millefolium L. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 596–610. [Google Scholar] [CrossRef]
- Villalva, M.; Silvan, J.M.; Alarcón-Cavero, T.; Villanueva-Bermejo, D.; Jaime, L.; Santoyo, S.; Martinez-Rodriguez, A.J. Antioxidant, Anti-Inflammatory, and Antibacterial Properties of an Achillea millefolium L. Extract and Its Fractions Obtained by Supercritical Anti-Solvent Fractionation against Helicobacter pylori. Antioxidants 2022, 11, 1849. [Google Scholar] [CrossRef]
- Ghazi, S. Do the polyphenolic compounds from natural products can protect the skin from ultraviolet rays? Res. Chem. 2022, 4, 100428. [Google Scholar] [CrossRef]
- D’Orazio, N.; Gammone, M.A.; Gemello, E.; De Girolamo, M.; Ficoneri, C.; Riccioni, G. Skin protection against UV rays: A review of in vivo and in vitro studies. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Farjadmand, F.; Karimpour-Razkenari, E.; Nabavi, S.M.; Ardekani, M.R.S.; Saeedi, M. Plant Polyphenols: Natural and Potent UV-Protective Agents for the Prevention and Treatment of Skin Disorders. Mini. Rev. Med. Chem. 2021, 21, 576–585. [Google Scholar] [CrossRef]
- Rajashekar, C.B. Dual Role of Plant Phenolic Compounds as Antioxidants and Prooxidants. Am. J. Plant Sci. 2023, 14, 15–28. [Google Scholar] [CrossRef]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Kandaswami, C.; Lee, L.T.; Lee, P.P.; Hwang, J.J.; Ke, F.C.; Huang, Y.T.; Lee, M.T. The antitumor activities of flavonoids. In Vivo 2005, 19, 895–909. [Google Scholar]
- Farhan, M.; Rozvi, A. Understanding the Prooxidant Action of Plant Polyphenols in the Cellular Microenvironment of Malignant Cells: Role of Copper and Therapeutic Implications. Front. Pharmacol. 2022, 13, 929853. [Google Scholar] [CrossRef]
- Isaac, V.L.B.; Chiari-Andréo, B.G.; Marto, J.M.; Moraes, J.D.D.; Leone, B.A.; Corrêa, M.A.; Ribeiro, H.M. Rheology as a tool to predict the release of alpha-lipoic acid from emulsions used for the prevention of skin aging. BioMed. Res. Int. 2015, 1, 818656. [Google Scholar] [CrossRef]
- Kwak, M.S.; Ahn, H.J.; Song, K.W. Rheological investigation of body cream and body lotion in actual application conditions. Korea-Aust. Rheol. J. 2015, 27, 241–251. [Google Scholar] [CrossRef]
- Fuentes, J.L.; Pedraza Barrera, C.A.; Villamizar Mantilla, D.A.; Flórez González, S.J.; Sierra, L.J.; Ocazionez, R.E.; Stashenko, E.E. Flower Extracts from Ornamental Plants as Sources of Sunscreen Ingredients: Determination by In Vitro Methods of Photoprtective Efficacy, Antigenotoxicity and Safety. Molecules 2022, 27, 5525. [Google Scholar] [CrossRef]
- Liyan, L.; Chong, L.; Huang, T.; Ma, Y.; Li, Y.; Ding, H. Natural products and extracts from plants as natural UV filters for sunscreens: A review. Anim. Models Exp. Med. 2023, 6, 183–195. [Google Scholar] [CrossRef]
- Lionetti, N.; Rigano, L. The New Sunscreens among Formulation Strategy, Stability Issues, Changing Norms, Safety and Efficacy Evaluations. Cosmetics 2017, 4, 15. [Google Scholar] [CrossRef]
- Mansur, M.C.P.; Guimarães Leitãoa, S.; Cerqueira-Coutinhoa, C.; Vermelhob, A.B.; Silvac, R.S.; Octávio, A.F.; Presgravec Leitãod, A.A.C.; Leitãoe, G.G.; Ricci-Júniora, E.; Santosaa Faculdade, E.P. In vitro and in vivo evaluation of efficacy and safety of photoprotective formulations containing antioxidant extracts. Rev. Bras. Farmacog. 2016, 26, 251–258. [Google Scholar] [CrossRef]
- Misra, B.B.; Dey, S. Comparative phytochemical analysis and antibacterial efficacy of in vitro and in vivo extracts from East Indian sandalwood tree (Santalum album L.). Lett. Appl. Microbiol. 2012, 55, 476–486. [Google Scholar] [CrossRef]
- Ng, S.Y.; Eh Suk, V.R.; Gew, L.T. Plant polyphenols as green sunscreen ingredients: A systematic review. J. Cosmet. Dermatol. 2022, 21, 5409–5444. [Google Scholar] [CrossRef]
- Santha, S.; Dwivedi, C. Anticancer effects of sandalwood (Santalum album). Anticancer Res. 2015, 35, 3137–3145. [Google Scholar]
- Santhra Krishnan, P.; Salian, A.; Dutta, S.; Mandal, S. A roadmap to UV-protective natural resources: Classification, characteristics, and applications. Mater. Chem. Front. 2021, 5, 7696–7723. [Google Scholar] [CrossRef]
- Singh, K. Determination of Phenolics and Flavonoids in Ethanolic Extract of Rosa centifolia Using UV-Visible Spectroscopy. NVEO J. 2024, 11, 1–6. [Google Scholar] [CrossRef]
- Colipa. International sun protection factor test method. In The European Cosmetic; Toiletry and Perfumery Association: London, UK, 2006. [Google Scholar]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Kukula-Koch, W. Achillea Species as Sources of Active Phytochemicals for Dermatological and Cosmetic Applications. Oxidative Med. Cell. Longev. 2021, 2021, 6643827. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Czop, M.; Sakipova, Z.; Głowniak, K.; Kukula-Koch, W. Achillea millefolium L. and Achillea biebersteinii Afan. Hydroglycolic Extracts–Bioactive Ingredients for Cosmetic Use. Molecules 2020, 25, 3368. [Google Scholar] [CrossRef]






| Ingredients (INCI) | Composition [%w/w] | |||
|---|---|---|---|---|
| E0 | E1 | E5 | E10 | |
| Oil phase | ||||
| Capric/Caprylic Triglycerides | 8.0 | 8.0 | 8.0 | 8.0 |
| Cetearyl Alcohol | 7.0 | 7.0 | 7.0 | 7.0 |
| Ceteareth-20 | 3.0 | 3.0 | 3.0 | 3.0 |
| Glyceryl Stearate | 2.0 | 2.0 | 2.0 | 2.0 |
| Butyrospermum Parkii Butter | 1.0 | 1.0 | 1.0 | 1.0 |
| Isopropyl Palmitate | 1.0 | 1.0 | 1.0 | 1.0 |
| Aqueous phase | ||||
| Glycerin | 2.0 | 2.0 | 2.0 | 2.0 |
| Sodium Benzoate | 0.5 | 0.5 | 0.5 | 0.5 |
| Potassium Sorbate | 0.5 | 0.5 | 0.5 | 0.5 |
| A. millefolium extract | - | 1.0 | 5.0 | 10.0 |
| Aqua | up to 100 | up to 100 | up to 100 | up to 100 |
| Extract | TP (mg GAE/mL ± SD) | TF (mg CE/mL ± SD) | TPA (mg CAE/mL ± SD) | CT (mg DpE/mL ± SD) | DPPH (%) | FRAP (mmol/L ± SD) |
|---|---|---|---|---|---|---|
| UAE | 50.06 ± 0.09 a | 14.58 ± 0.09 a | 3.31 ± 0.04 a | 4.93 ± 0.06 a | 70.00 ± 0.14 a | 1.53 ± 0.03 a |
| SFE | 2.44 ± 0.02 b | 0.041 ± 0.00 b | 0.91 ± 0.04 b | 0.79 ± 0.03 b | 20.04 ± 0.44 b | 0.77 ± 0.04 b |
| MBC/MFC | MIC | Test Microorganism |
|---|---|---|
| 16 | 2 | Candida albicans ATCC10231 |
| 8 | 8 | Streptococcus agalactiae PCM 2683 |
| >16 | >16 | Enterococcus faecalis PCM 2784 |
| 16 | 8 | Proteus mirabilis ATTC 29906 |
| 8 | 8 | Streptococcus mutans ATTC 25175 |
| 8 | 8 | Staphylococcus epidermidis ATTC 8853 |
| 16 | 8 | Streptococcus pyogenes ATTC 19615 |
| 16 | 16 | Escherichia coli UPEC PCM 176 |
| 8 | 8 | Enterococcus hirae ATCC 10541 |
| 16 | 2 | Bacillus subtilis PCM 486 |
| 8 | 8 | Staphylococcus aureus 6538P |
| 8 | 4 | Staphylococcus epidermidis PCM 2118 |
| 16 | 8 | Escherichia coli ATCC 8739 |
| 8 | 2 | Pseudomonas aeruginosa PAO1 |
| 4 | 2 | Ralstonia solanacearum Z1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalak, M.; Zagórska-Dziok, M.; Żarnowiec, P.; Bocho-Janiszewska, A.; Stryjecka, M.; Kostrzewa, D.; Dobros, N.; Paradowska, K. Evaluation of Achillea millefolium var. Paprika Extract with Antioxidant, Antimicrobial, and Skin Protection Potential in Topical Application. Appl. Sci. 2025, 15, 4631. https://doi.org/10.3390/app15094631
Michalak M, Zagórska-Dziok M, Żarnowiec P, Bocho-Janiszewska A, Stryjecka M, Kostrzewa D, Dobros N, Paradowska K. Evaluation of Achillea millefolium var. Paprika Extract with Antioxidant, Antimicrobial, and Skin Protection Potential in Topical Application. Applied Sciences. 2025; 15(9):4631. https://doi.org/10.3390/app15094631
Chicago/Turabian StyleMichalak, Monika, Martyna Zagórska-Dziok, Paulina Żarnowiec, Anita Bocho-Janiszewska, Małgorzata Stryjecka, Dorota Kostrzewa, Natalia Dobros, and Katarzyna Paradowska. 2025. "Evaluation of Achillea millefolium var. Paprika Extract with Antioxidant, Antimicrobial, and Skin Protection Potential in Topical Application" Applied Sciences 15, no. 9: 4631. https://doi.org/10.3390/app15094631
APA StyleMichalak, M., Zagórska-Dziok, M., Żarnowiec, P., Bocho-Janiszewska, A., Stryjecka, M., Kostrzewa, D., Dobros, N., & Paradowska, K. (2025). Evaluation of Achillea millefolium var. Paprika Extract with Antioxidant, Antimicrobial, and Skin Protection Potential in Topical Application. Applied Sciences, 15(9), 4631. https://doi.org/10.3390/app15094631










