Stability and Degradation Issues of Manganese Violet Pigment in Polymeric Paints: Morphological and Chemical Changes Under SO2 and Humidity Exposure
Abstract
:1. Introduction
- To assess any changes in the morphology of the samples;
- To study and evaluate chemical changes involving the inorganic fraction;
- To investigate the degradation of the organic matrices and compare the stability of model and commercial paints.
2. Materials and Methods
2.1. Sample Preparation
2.2. Weathering Experiment
2.3. Optical Microscopy
2.4. Attenuated Total Reflection–Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.5. μ-Raman Spectroscopy
2.6. Scanning Electron Microscopy–Energy Dispersive X-Ray Analysis (SEM-EDX)
2.7. X-Ray Powder Diffraction (XRPD)
2.8. Fiber Optic Reflectance Spectroscopy (FORS)
2.9. Pyrolysis–Gas Chromatography/Mass Spectrometry (Py-GC/MS) and Thermally Assisted Hydrolysis and Methylation (THM) of Py-GC/MS (THM-Py-GC/MS)
3. Results and Discussion
3.1. Before Aging: Pigment Characterization

3.2. Before Aging: Paints Characterization
3.2.1. Oil-Based Samples
3.2.2. Alkyd-Based Samples
3.2.3. Acrylic-Based Samples
3.3. After Aging: Evaluation of Paint Samples
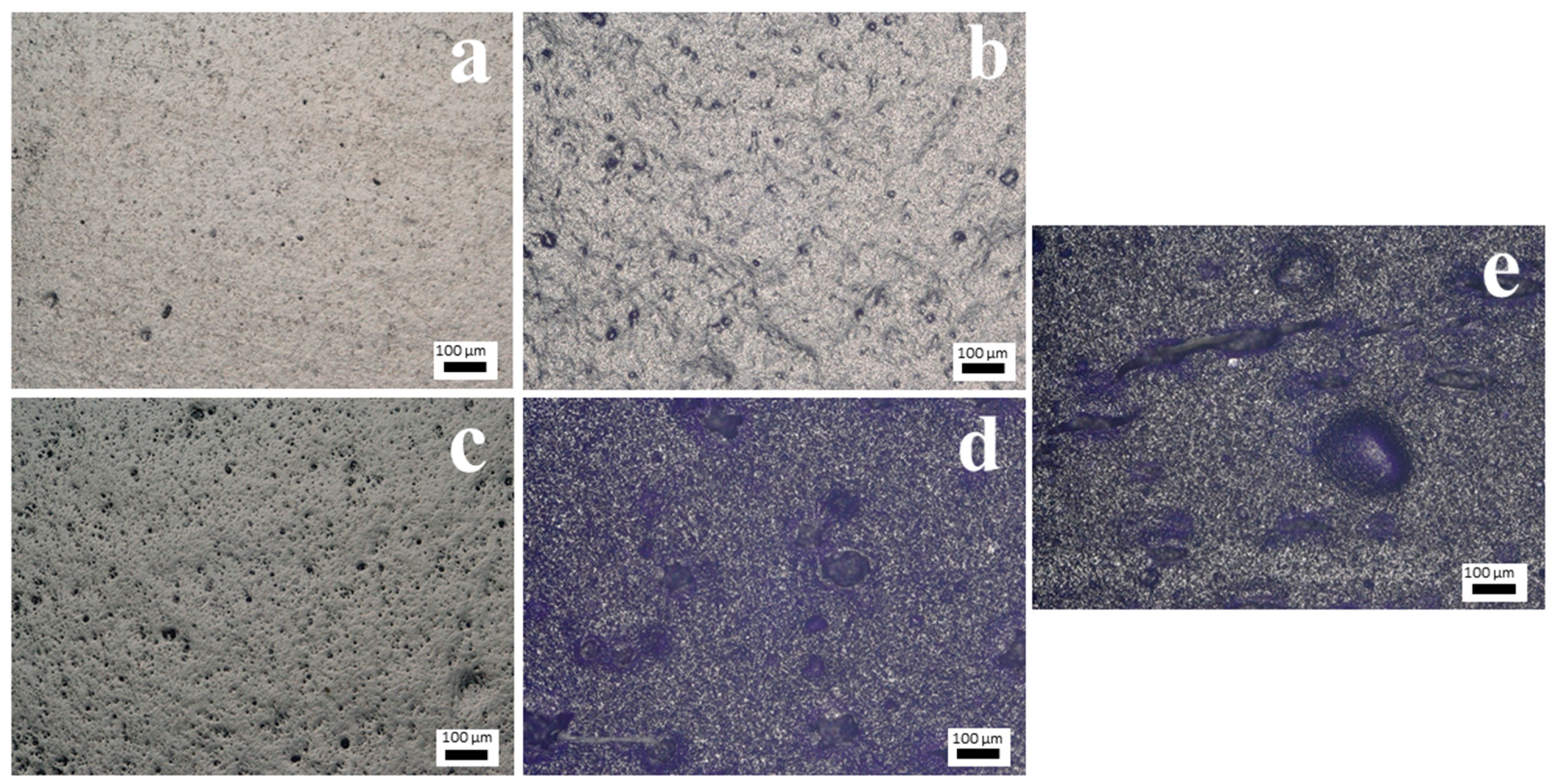
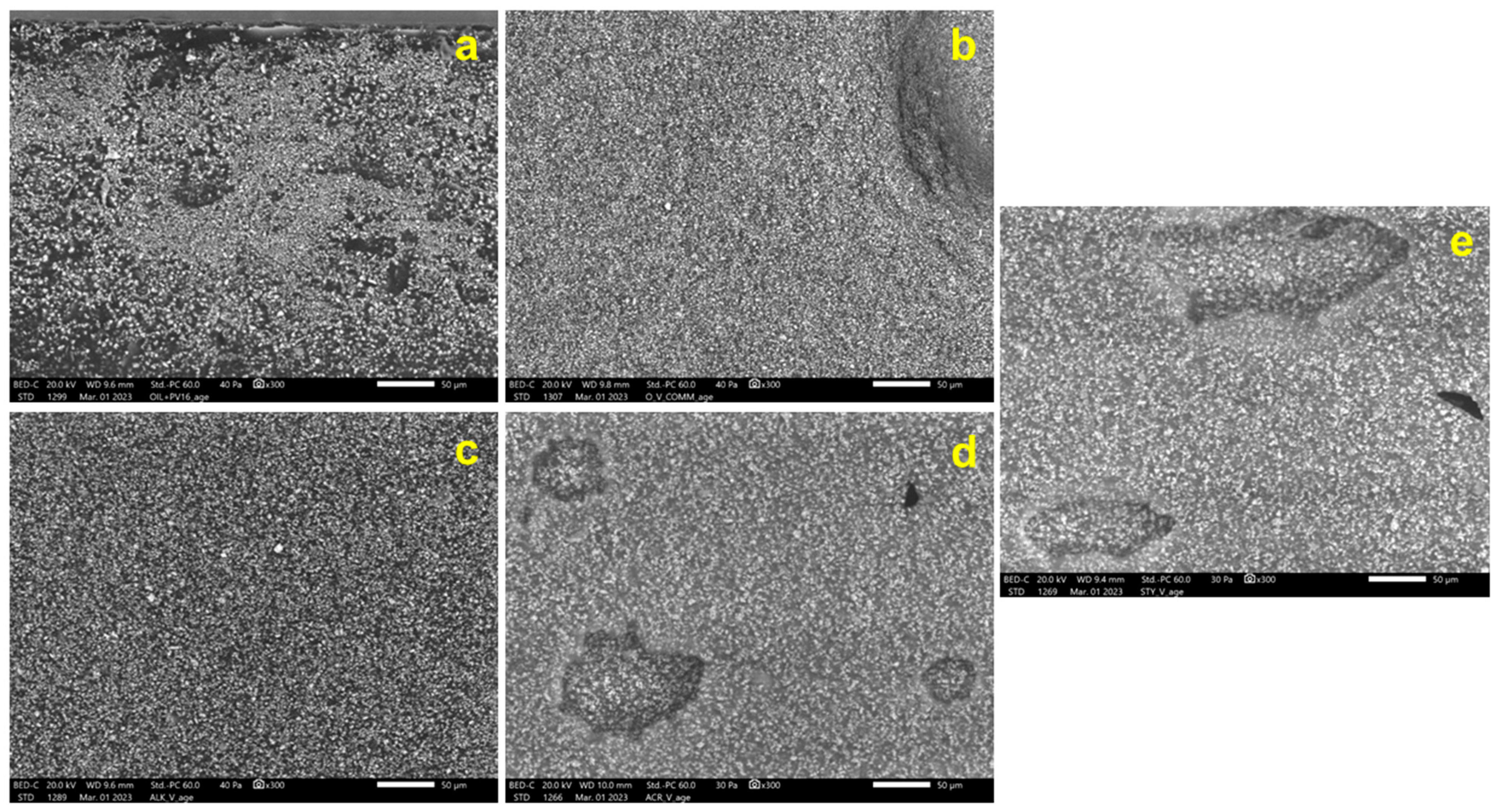
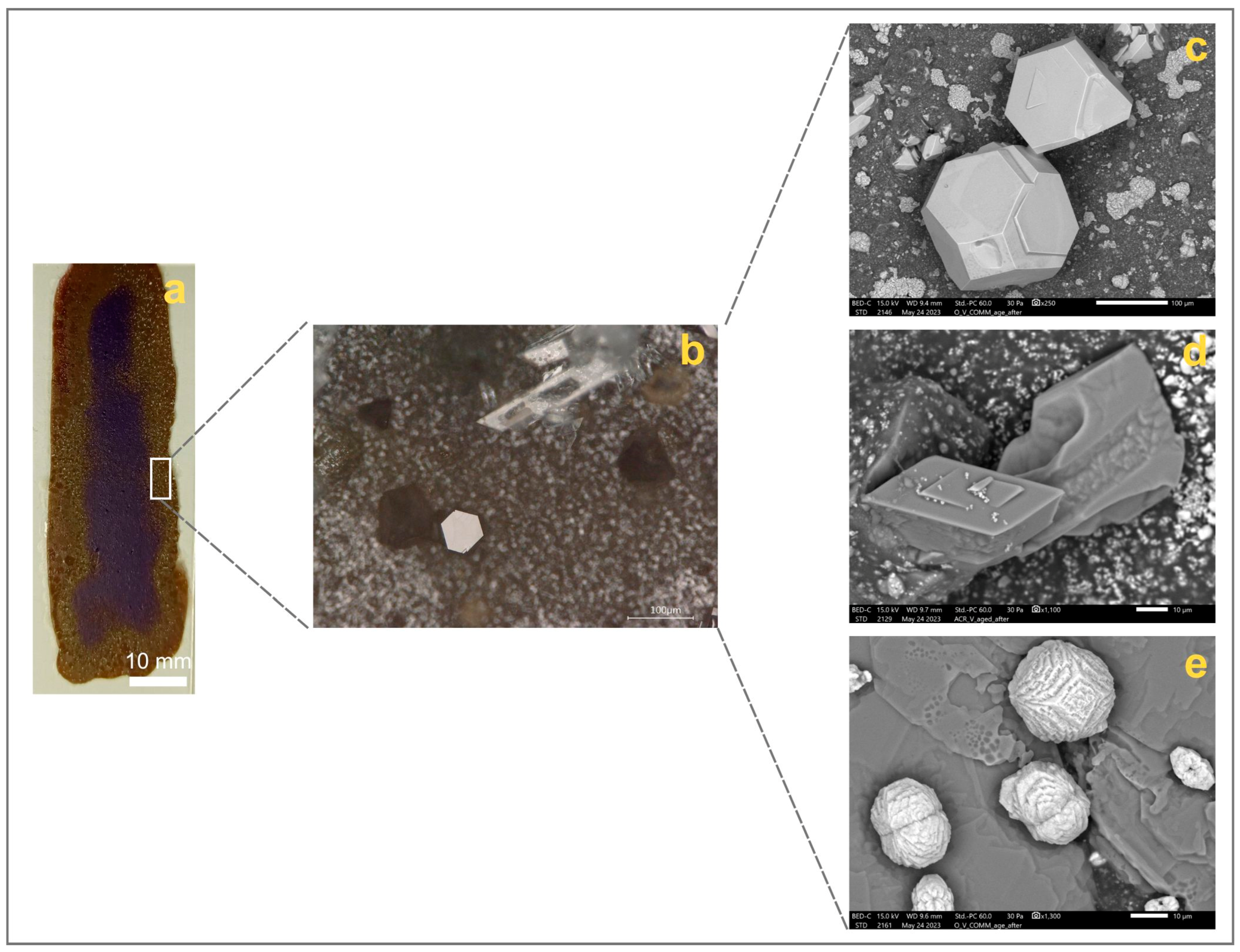
3.3.1. Morphological Observations
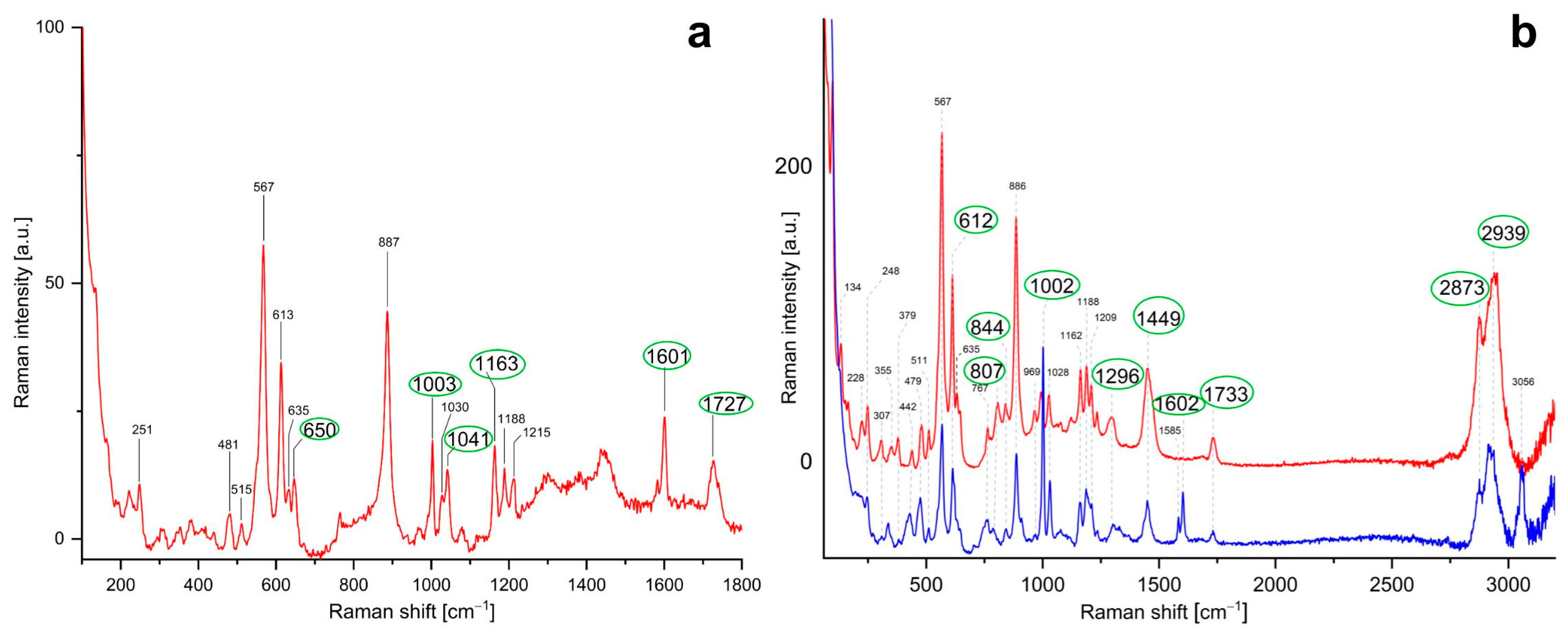
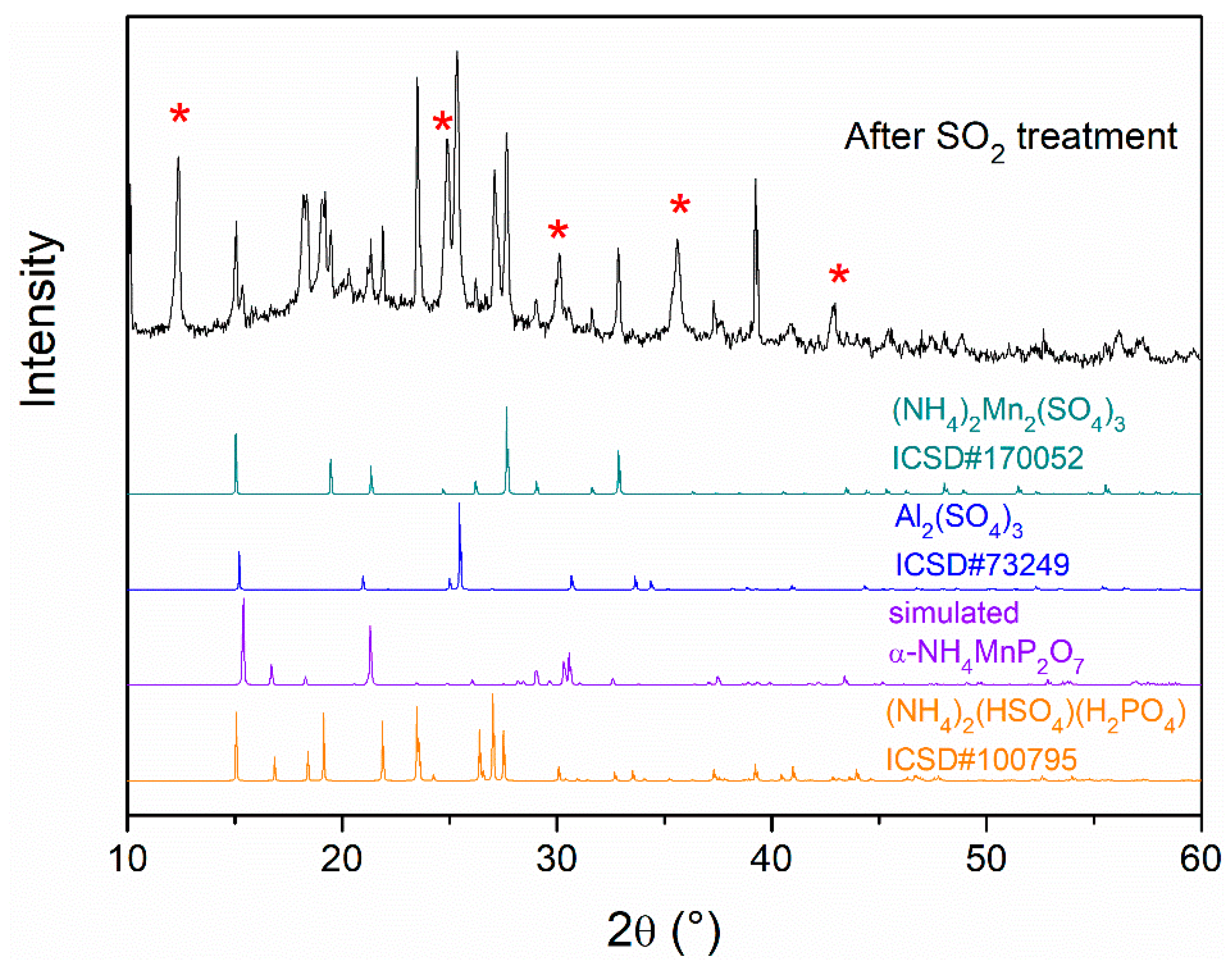
3.3.2. Inorganic Fraction
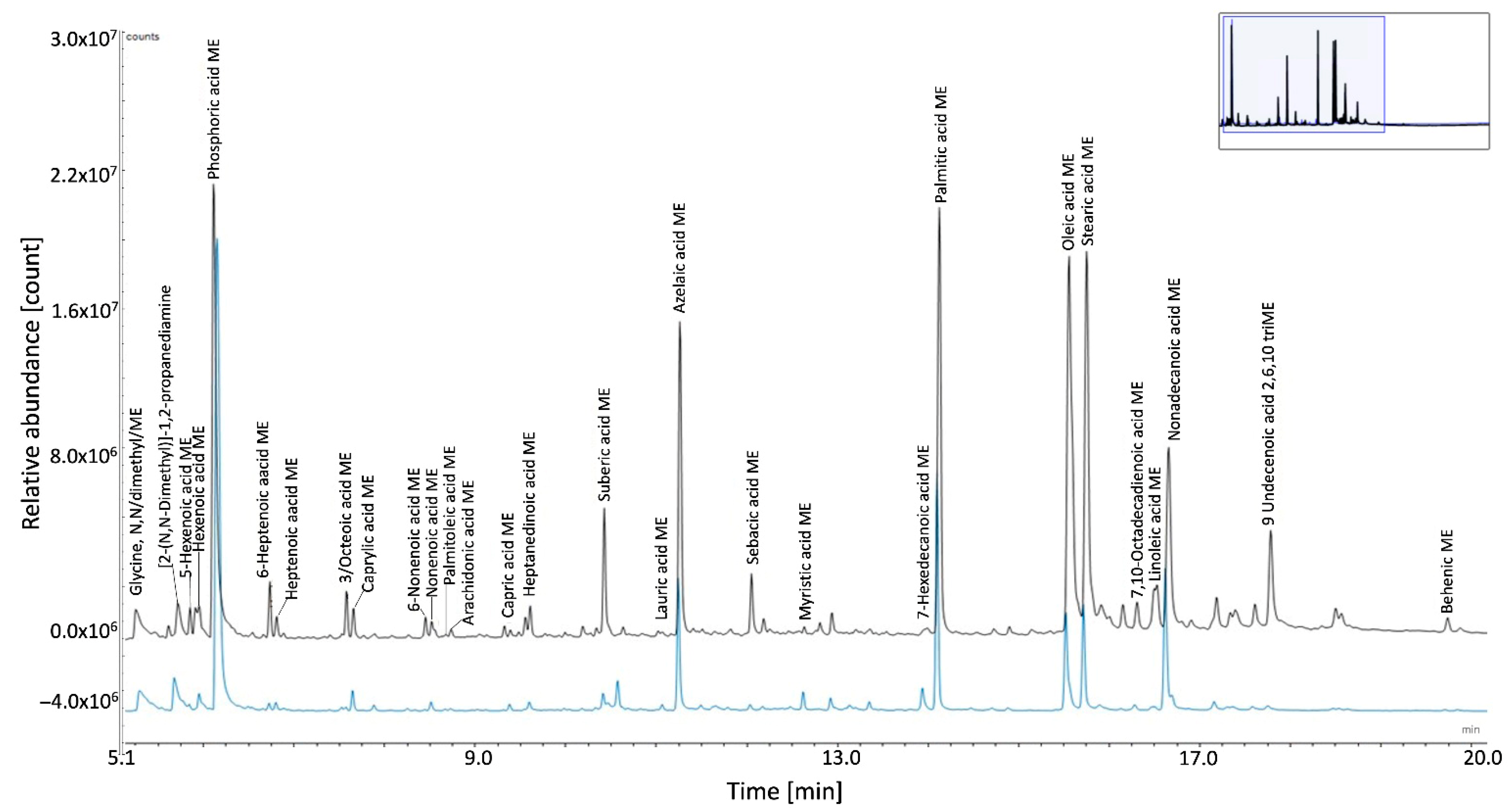
3.3.3. Organic Fraction
Linseed Oil
Commercial Oil
Alkyd Resin
Acrylic Emulsion
Styrene–Acrylic Emulsion
4. Final Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grzywacz, C.M. Monitoring for Gaseous Pollutants in Museum Environments; Getty Publications: Los Angeles, CA, USA, 2006. [Google Scholar]
- Motta, O.; Pironti, C.; Ricciardi, M.; Rostagno, C.; Bolzacchini, E.; Ferrero, L.; Cucciniello, R.; Proto, A. Leonardo da Vinci’s “Last Supper”: A case study to evaluate the influence of visitors on the Museum preservation systems. Environ. Sci. Pollut. Res. Int. 2022, 29, 29391–29398. [Google Scholar] [CrossRef]
- Franey, J.P.; Graedel, T.E. Corrosive Effects of Mixtures of Pollutants. J. Air Pollut. Control Assoc. 1985, 35, 644–648. [Google Scholar] [CrossRef]
- Kreislova, K.; Knotkova, D.; Geiplova, H. Atmospheric corrosion of historical industrial structures. Corros. Conserv. Cult. Herit. Met. Artefacts 2013, 65, 311–343. [Google Scholar] [CrossRef]
- European Environment Agency. Air Pollution Fact Sheet 2013; European Environment Agency: Copenhagen, Denmark, 2013.
- Pozo-Antonio, J.S.; Rivas, T.; Dionísio, A.; Barral, D.; Cardell, C. Effect of a SO2 Rich Atmosphere on Tempera Paint Mock-Ups. Part 1: Accelerated Aging of Smalt and Lapis Lazuli-Based Paints. Minerals 2020, 10, 427. [Google Scholar] [CrossRef]
- Yue, Y.; Cheng, J.; Lee, K.S.; Stocker, R.; He, X.; Yao, M.; Wang, J. Effects of relative humidity on heterogeneous reaction of SO2 with CaCO3 particles and formation of CaSO4·2H2O crystal as secondary aerosol. Atmos. Environ. 2022, 268, 118776. [Google Scholar] [CrossRef]
- Chianese, E.; Riccio, A.; Dura, I.; Trifuoggi, M.; Iovino, P.; Capasso, S.; Barone, G. Measurements for indoor air quality assessment at the Capodimonte Museum in Naples (Italy). Int. J. Environ. Res. 2012, 6, 509–518. [Google Scholar]
- Faulkner, E.B.; Hollenberg, J.C. Coloring the Cosmetic World: Using Pigments in Decorative Cosmetic Formulations, 2nd ed.; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Rajadurai, R.S.; Lee, J.-H.; Choi, E.; Kang, J.-W. MnNH4P2O7-Based Coating for High Temperature Assessment on the Surfaces of Cement Composites. Coatings 2020, 10, 396. [Google Scholar] [CrossRef]
- Finlay, V. The Brilliant History of Color in Art, 1st ed.; J. Paul Getty Museum: Los Angeles, CA, USA, 2014. [Google Scholar]
- Kirby, J.; Stonor, K.; Roy, A.; Burnstock, A.; Grout, R.; White, R. Seurat’s Painting Practice: Theory, Development and Technology. In National Gallery Technical Bulletin; The National Gallery: London, UK, 2003; pp. 4–37. Available online: https://www.nationalgallery.org.uk/research/research-resources/technical-bulletin/seurats-painting-practice-theory-development-and-technology (accessed on 31 October 2024).
- Haddad, A.; Pastorelli, G.; Ortiz Miranda, A.S.; Ludvigsen, L.; Centeno, S.A.; Duvernois, I.; Hoover, C.; Duffy, M.; Aviram, A.; Zycherman, L. Exploring the private universe of Henri Matisse in The Red Studio. Herit. Sci. 2022, 10, 168. [Google Scholar] [CrossRef]
- Calza, C.; Pedreira, A.; Lopes, R.T. Analysis of paintings from the nineteenth century Brazilian painter Rodolfo Amoedo using EDXRF portable system. X-Ray Spectrom. 2009, 38, 327–332. [Google Scholar] [CrossRef]
- Roldán, C.; Ferrero, J.; Juanes, D.; Murcia, S.; Ripollés, V. Joaquin Sorolla’s pigment characterisation of the paintings “Vision of Spain” by means of EDXRF portable system. X-Ray Spectrom. 2011, 40, 289–296. [Google Scholar] [CrossRef]
- Anselmi, C.; Vagnini, M.; Cartechini, L.; Grazia, C.; Vivani, R.; Romani, A.; Rosi, F.; Sgamellotti, A.; Miliani, C. Molecular and structural characterization of some violet phosphate pigments for their non-invasive identification in modern paintings. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 439–444. [Google Scholar] [CrossRef]
- Sirois, P.J.; Stewart, C.; Helwig, K.; Moffatt, E.; Legate, K.M. A Technical Study of the Materials and Methods Used by David B. Milne in his Oil Paintings. J. Can. Assoc. Conserv. 2007, 32, 17–33. [Google Scholar]
- Rosi, F.; Grazia, C.; Fontana, R.; Gabrieli, F.; Pensabene Buemi, L.; Pampaloni, E.; Romani, A.; Stringari, C.; Miliani, C. Disclosing Jackson Pollock’s palette in Alchemy (1947) by non-invasive spectroscopies. Herit. Sci. 2016, 4, 18. [Google Scholar] [CrossRef]
- Defeyt, C.; Mazurek, J.; Zebala, A.; Burchett-Lere, D. Insight into Sam Francis’ painting techniques through the analytical study of twenty-eight artworks made between 1946 and 1992. Appl. Phys. A Mater. Sci. Process. 2016, 122, 991. [Google Scholar] [CrossRef]
- Sabatini, F. Ageing and Fading of Organic Pigments in Art: A Multi-Analytical Study Based on Mass Spectrometric Techniques. Ph.D. Thesis, University of Pisa, Pisa, Italy, 2018. [Google Scholar]
- La Nasa, J.; Doherty, B.; Rosi, F.; Braccini, C.; Broers, F.T.H.; Degano, I.; Matinero, J.M.; Miliani, C.; Modugno, F.; Sabatini, F.; et al. An integrated analytical study of crayons from the original art materials collection of the MUNCH museum in Oslo. Sci. Rep. 2021, 11, 7152. [Google Scholar] [CrossRef]
- Pagnin, L.; Calvini, R.; Wiesinger, R.; Schreiner, M. SO2− and NOx− initiated atmospheric degradation of polymeric films: Morphological and chemical changes, influence of relative humidity and inorganic pigments. Microchem. J. 2021, 164, 106087. [Google Scholar] [CrossRef]
- Pagnin, L.; Wiesinger, R.; Koyun, A.N.; Schreiner, M. The effect of pollutant gases on surfactant migration in acrylic emulsion films: A comparative study and preliminary evaluation of surface cleaning. Polymers 2021, 13, 1941. [Google Scholar] [CrossRef]
- Pagnin, L.; Calvini, R.; Sterflinger, K.; Izzo, F.C. Data Fusion Approach to Simultaneously Evaluate the Degradation Process Caused by Ozone and Humidity on Modern Paint Materials. Polymers 2022, 14, 1787. [Google Scholar] [CrossRef] [PubMed]
- Pagnin, L.; Zendri, E.; Izzo, F.C. How Can Ozone and Relative Humidity Affect Artists’ Alkyd Paints? A FT-IR and Py-GC/MS Systematic Study. Polymers 2022, 14, 1831. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Mennig, M. (Eds.) Sol-Gel Technologies for Glass Producers and Users; Springer: Boston, MA, USA, 2004. [Google Scholar] [CrossRef]
- Camuffo, D.; Brimblecombe, P.; Van Grieken, R.; Busse, H.-J.; Sturaro, G.; Valentino, A.; Bernardi, A.; Blades, N.; Shooter, D.; De Bock, L.; et al. Indoor air quality at the Correr Museum, Venice, Italy. Sci. Total Environ. 1999, 236, 135–152. [Google Scholar] [CrossRef]
- Krupińska, B.; Van Grieken, R.; De Wael, K. Air quality monitoring in a museum for preventive conservation: Results of a three-year study in the Plantin-Moretus Museum in Antwerp, Belgium. Microchem. J. 2013, 110, 350–360. [Google Scholar] [CrossRef]
- Izzo, F.C.; Ferriani, B.; den Berg, K.J.V.; Van Keulen, H.; Zendri, E. 20th century artists’ oil paints: The case of the Olii by Lucio Fontana. J. Cult. Herit. 2014, 15, 557–563. [Google Scholar] [CrossRef]
- Pintus, V.; Baragona, A.J.; Cappa, F.; Haiml, C.; Hierl, C.; Sterflinger, K.; Schreiner, M. Multi-Analytical Investigations of Andy Warhol’s “Orange Car Crash”: Polymeric Materials in Modern Paints. Polymers 2022, 14, 633. [Google Scholar] [CrossRef]
- Nguyen, D.K.; Bach, Q.-V.; Lee, J.-H.; Kim, I.-T. Synthesis and Irreversible Thermochromic Sensor Applications of Manganese Violet. Materials 2018, 11, 1693. [Google Scholar] [CrossRef] [PubMed]
- Brouzi, K.; Ennaciri, A.; Harcharras, M.; Capitelli, F. Structure and vibrational spectra of a new trihydrate diphosphate, MnNH4NaP2O7·3H2O. J. Raman Spectrosc. 2004, 35, 41–46. [Google Scholar] [CrossRef]
- Bih, H.; Saadoune, I.; Bih, L.; Mansori, M.; ToufiK, H.; Fuess, H.; Ehrenberg, H. Synthesis, Rietveld refinements, Infrared and Raman spectroscopy studies of the sodium diphosphate NaCryFe1−yP2O7 (0 ≤ y ≤ 1). J. Mol. Struct. 2016, 1103, 103–109. [Google Scholar] [CrossRef]
- Narayanan, P.S. Raman spectrum of ammonium di-hydrogen phosphate. Proc. Indian Acad. Sci. (Math. Sci.) 1948, 28, 469. [Google Scholar] [CrossRef]
- Chapman, A.C.; Thirlwell, L.E. Spectra of phosphorus compounds-The infra-red spectra of orthophosphates. Spectrochim. Acta 1964, 20, 937–947. [Google Scholar] [CrossRef]
- Derrick, M.R.; Landry, J.M.; Stulik, D.C. Infrared Spectroscopy in Conservation Science; The Getty Conservation Institute: Los Angeles, CA, USA, 1999. [Google Scholar]
- Tironi, A.; Trezza, M.; Irassar, E.; Scian, A.N. Thermal Treatment of Kaolin: Effect on the Pozzolanic Activity. Procedia Mater. Sci. 2012, 1, 343–350. [Google Scholar] [CrossRef]
- Johnston, C.; Sposito, G.; Birge, R. Raman Spectroscopic Study of Kaolinite in Aqueous Suspension. Clays Clay Miner. 1985, 33, 483–489. [Google Scholar] [CrossRef]
- Begum, Y.; Wright, A.J. Relating highly distorted Jahn–Teller MnO6 to colouration in manganese violet pigments. J. Mater. Chem. 2012, 22, 21110–21116. [Google Scholar] [CrossRef]
- Nicholas Walsh, V.C. Pigment Compendium: A Dictionary and Optical Microscopy of Historical Pigments; Taylor & Francis Ltd.: Oxfordshire, UK, 2008. [Google Scholar]
- Wicks, Z.W., Jr. Alkyd Resins. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Learner, T. A review of synthetic binding media in twentieth-century paints. Conservator 2000, 24, 96–103. [Google Scholar] [CrossRef]
- Nakayama, Y. Polymer blend systems for water-borne paints. Prog. Org. Coat. 1998, 33, 108–116. [Google Scholar] [CrossRef]
- Androver Gracia, I. Applicazioni Della Spettrofotometria IR allo Studio dei Beni Culturali; Il Prato: Saonara, Italy, 2001. [Google Scholar]
- Silverstein, R.M.; Kiemle, D.J.; Webster, F.X.; Bryce, D.L.; Zanardi, F. Identificazione Spettrometrica di Composti Organici, 3rd ed.; CEA: Rozzano, Italy, 2016. [Google Scholar]
- Burgio, L.; Clark, R.J.H. Library of FT-Raman spectra of pigments, minerals, pigment media and varnishes, and supplement to existing library of Raman spectra of pigments with visible excitation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 1491–1521. [Google Scholar] [CrossRef]
- Carlesi, S.; Becucci, M.; Ricci, M. Vibrational Spectroscopies and Chemometry for Nondestructive Identification and Differentiation of Painting Binders. J. Chem. 2017, 2017, 3475659. [Google Scholar] [CrossRef]
- Izzo, F.C. 20th Century Artists’ Oil Paints: A Chemical-Physical Survey. Ph.D. Thesis, Università Ca’ Foscari Venezia, Venezia, Italy, 2010. Available online: http://dspace.unive.it/handle/10579/1100 (accessed on 31 October 2024).
- Colombini, M.P.; Modugno, F. Organic Mass Spectrometry in Art and Archaeology; Wiley: Chichester, UK, 2009. [Google Scholar]
- Tammekivi, E.; Vahur, S.; Vilbaste, M.; Leito, I. Quantitative GC-MS Analysis of Artificially Aged Paints with Variable Pigment and Linseed Oil Ratios. Molecules 2021, 26, 2218. [Google Scholar] [CrossRef]
- Wiesinger, R.; Pagnin, L.; Anghelone, M.; Moretto, L.M.; Orsega, E.F.; Schreiner, M. Pigment and Binder Concentrations in Modern Paint Samples Determined by IR and Raman Spectroscopy. Angew. Chem. 2018, 57, 7401–7407. [Google Scholar] [CrossRef]
- Learner, T.J.S. Analysis of Modern Paints; The Getty Conservation Institute: Los Angeles, CA, USA, 2004. [Google Scholar]
- Ormsby, B.; Learner, T.; Schilling, M.; Druzik, J.; Khanjian, H.; Carson, D.; Foster, G.; Sloan, M. The Effects of Surface Cleaning on Acrylic Emulsion Paintings: A Preliminary Investigation. Tate Pap. 2012, 6, 1–4. Available online: https://www.tate.org.uk/research/tate-papers/06/effects-of-surface-cleaning-on-acrylic-emulsion-painting-preliminary-investigation (accessed on 31 October 2024).
- Melchiorre Di Crescenzo, M.; Zendri, E.; Miliani, C.; Rosi, F. A preliminary FTIR-based exploration of the surfactant phase-separation process in contemporary mural paintings. E-Preserv. Sci. 2013, 10, 10–18. [Google Scholar]
- Sridevi, N.A.; Karuppasamy, K.; Balakumar, S.; Shajan, S. Structural and Ionic Conductivity Studies on Nanochitosan Incorporated Polymer Electrolytes for Rechargeable Magnesium Batteries. Chem. Sci. Trans. 2012, 1, 311–316. [Google Scholar] [CrossRef]
- de Oliveira, A.G.G.; Wiercigroch, E.; Gomes, J. de A.; Malek, K. Infrared and Raman spectroscopy of automotive paints for forensic identification of natural weathering. Analalytical Methods 2018, 10, 1203–1212. [Google Scholar] [CrossRef]
- Bauer, P.; Buettner, A. Characterization of Odorous and Potentially Harmful Substances in Artists’ Acrylic Paint. Front. Public Health 2018, 6, 350. [Google Scholar] [CrossRef] [PubMed]
- Fardi, T.; Pintus, V.; Kampasakali, E.; Pavlidou, E.; Schreiner, M.; Kyriacou, G. Analytical characterization of artist’s paint systems based on emulsion polymers and synthetic organic pigments. J. Anal. Appl. Pyrolysis 2018, 135, 231–241. [Google Scholar] [CrossRef]
- Abd El-Wahab, H.; Saleh, T.; Zayed, E.; El-Sayed, A.; Assaker, R. Synthesis and evaluation of new anti-microbial additive based on pyrazole and triazole derivative incorporated physically into polyurethane varnish. Egypt. J. Chem. 2014, 57, 27–43. [Google Scholar]
- Flamm, D.L.; Bacon, D.D.; Kinsbron, E.; English, A.T. Chemical Reaction of Sulfur Dioxide at High Humidity and Temperature: Implications for Accelerated Testing. J. Electrochem. Soc. 1981, 128, 679. [Google Scholar] [CrossRef]
- Moustafa, Y.M.; El-Egili, K. Infrared spectra of sodium phosphate glasses. J. Non-Cryst. Solids 1998, 240, 144–153. [Google Scholar] [CrossRef]
- Frost, R.L.; Scholz, R.; López, A.; Xi, Y.; Lana, C. Vibrational spectroscopy of the sulphate mineral sturmanite from Kuruman manganese deposits, South Africa. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 24–30. [Google Scholar] [CrossRef]
- Mabrouk, K.B.; Kauffmann, T.H.; Aroui, H.; Fontana, M.D. Raman study of cation effect on sulfate vibration modes in solid state and in aqueous solutions. J. Raman Spectrosc. 2013, 44, 1603–1608. [Google Scholar] [CrossRef]
- Sekar, G.; Ramakrishnan, V.; Aruldhas, G. IR and polarized Raman spectra of (NH4)2M(SO4)2 · 6H2O(M =Zn, Mn). J. Solid State Chem. 1988, 74, 424–427. [Google Scholar] [CrossRef]
- Jeong, D.I.; Kim, S.; Koo, J.S.; Lee, S.Y.; Kim, M.; Kim, K.Y.; Azad, M.O.K.; Karmakar, M.; Chu, S.; Chae, B.-J.; et al. Manganese Sulfate Nanocomposites Fabricated by Hot-Melt Extrusion for Chemodynamic Therapy of Colorectal Cancer. Pharmaceutics 2023, 15, 1831. [Google Scholar] [CrossRef]
- Qiu, G.; Gao, Z.; Yin, H.; Feng, X.; Tan, W.; Liu, F. Synthesis of MnPO4·H2O by refluxing process at atmospheric pressure. Solid State Sci. 2010, 12, 808–813. [Google Scholar] [CrossRef]
- Frost, R.L.; López, A.; Scholz, R.; Wang, L. A Raman and infrared spectroscopic study of the sulphate mineral aluminite Al2(SO4)(OH)4·7H2O. Spectrochim Acta A Mol. Biomol. Spectrosc. 2015, 148, 232–236. [Google Scholar] [CrossRef]
- Rao, B.L. Raman spectra of some crystalline nitrates and sulphates. Proc. Indian Acad. Sci. (Math. Sci.) 1941, 14, 48–55. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; J. Wiley and Sons: Chichester, UK, 2001. [Google Scholar]
- Wolfram, R.; Gert, I. Raman Spectroscopic Investigation of Speciation in MnSO4(aq). J. Solut. Cgemistry 2014, 43, 465–485. [Google Scholar] [CrossRef]
- Kück, S.; Hartung, S.; Hurling, S.; Petermann, K.; Huber, G. Emission of octahedrally coordinated Mn3+ in garnets. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54, 1741–1749. [Google Scholar] [CrossRef]
- McClure, D.S. Optical Spectra of Transition-Metal Ions in Corundum. J. Chem. Phys. 1962, 36, 2757–2779. [Google Scholar] [CrossRef]
- Kück, S.; Hartung, S.; Hurling, S.; Petermann, K.; Huber, G. Optical transitions in Mn3+ -doped garnets. Phys. Rev. B 1998, 57, 2203–2216. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Hao, Z.-M.; Ng, S.W. Langbeinite-type (NH4)2Mn2(SO4)3. Acta Cryst E 2005, 61, i82–i83. [Google Scholar] [CrossRef]
- de Viguerie, L.; Payard, P.A.; Portero, E.; Walter, P.; Cotte, M. The drying of linseed oil investigated by Fourier transform infrared spectroscopy: Historical recipes and influence of lead compounds. Prog. Org. Coat. 2016, 93, 46–60. [Google Scholar] [CrossRef]
- Duce, C.; Della Porta, V.; Tiné, M.R.; Spepi, A.; Ghezzi, L.; Colombini, M.P.; Bramanti, E. FTIR study of ageing of fast drying oil colour (FDOC) alkyd paint replicas. Spectrochim Acta A Mol Biomol Spectrosc 2014, 130, 214–221. [Google Scholar] [CrossRef]
- Izzo, F.C.; Kratter, M.; Nevin, A.; Zendri, E. A Critical Review on the Analysis of Metal Soaps in Oil Paintings. ChemistryOpen 2021, 10, 904–921. [Google Scholar] [CrossRef]
- Simendinger, W.H.; Balik, C.M. Chemical reaction kinetics of sulfur dioxide and oxygen with unsaturated drying oils. J. Coat. Technol. 1994, 66, 39–45. [Google Scholar]
- Pizzimenti, S.; Bernazzani, L.; Tinè, M.R.; Treil, V.; Duce, C.; Bonaduce, I. Oxidation and Cross-Linking in the Curing of Air-Drying Artists’ Oil Paints. ACS Appl. Polym. Mater. 2021, 3, 1912–1922. [Google Scholar] [CrossRef]
- Lazzari, M.; Chiantore, O. Drying and oxidative degradation of linseed oil. Polym. Degrad. Stab. 1999, 65, 303–313. [Google Scholar] [CrossRef]
- La Nasa, J.; Degano, I.; Modugno, F.; Colombini, M.P. Effects of acetic acid vapour on the ageing of alkyd paint layers: Multi-analytical approach for the evaluation of the degradation processes. Polym. Degrad. Stab. 2014, 105, 257–264. [Google Scholar] [CrossRef]
- Perrin, F.X.; Irigoyen, M.; Aragon, E.; Vernet, J.L. Evaluation of accelerated weathering tests for three paint systems: A comparative study of their aging behaviour. Polym. Degrad. Stab. 2001, 72, 115–124. [Google Scholar] [CrossRef]
- Pintus, V.; Wei, S.; Schreiner, M. Accelerated UV ageing studies of acrylic, alkyd, and polyvinyl acetate paints: Influence of inorganic pigments. Microchem. J. 2016, 124, 949–961. [Google Scholar] [CrossRef]
- Francisco-Vieira, L.; Benavides, R.; Da Silva, L.; Cuara-Diaz, E.; Morales-Acosta, D. Effect of sulfonating agent in the properties of styrene copolymers for PEMFC membranes. Int. J. Hydrogen Energy 2022, 47, 30303–30314. [Google Scholar] [CrossRef]
- Melo, L.; Benavides, R.; Martínez, G.; Da Silva, L.; Paula, M.M.S. Degradation reactions during sulphonation of poly(styrene-co-acrylic acid) used as membranes. Polym. Degrad. Stab. 2014, 109, 343–352. [Google Scholar] [CrossRef]
- Papliaka, Z.E.; Andrikopoulos, K.S.; Varella, E.A. Study of the stability of a series of synthetic colorants applied with styrene-acrylic copolymer, widely used in contemporary paintings, concerning the effects of accelerated ageing. J. Cult. Herit. 2010, 11, 381–391. [Google Scholar] [CrossRef]


| Pigment | Chemical Composition | Colour Index (C.I.) Number |
| Manganese violet | NH4MnP2O7 | PV16 |
| Binders | Chemical composition | Commercial name |
| Oil | Linseed stand oil | Leinöl Stand oil 450 P (Kremer Pigmente, Germany) |
| Alkyd resin | Oil-modified polyester-resin | Alkyd Medium 4 (Lukas, Germany) |
| Acrylic emulsion | p(nBA/MMA) | Plextol® D498 (Kremer Pigmente, Germany) |
| Styrene–acrylic emulsion | Styrene acrylate copolymer | Acronal® S790 (BASF, Germany) |
| Samples abbreviations | Composition | |
| Oil_V | PV16 + stand oil | |
| Oil_V_COMM | Commercial oil paint tube | |
| Alk_V | PV16 + alkyd resin | |
| Acr_V | PV16 + acrylic emulsion | |
| Sty_V | PV16 + styrene–acrylic emulsion | |
| CRY_1 | CRY_2 | CRY_3 | |
|---|---|---|---|
| Mn | 17.65% ± 0.4 | 1.04% ± 0.1 | 22.73% ± 0.8 |
| S | 15.73% ± 0.2 | 12.63% ± 0.2 | 2.01% ± 0.1 |
| P | 2.01% ± 0.1 | 14.48% ± 0.2 | 16.52% ± 0.3 |
| N | 8.37% ± 0.3 | 11.65% ± 0.5 | - |
| O | 49.69 ± 0.4 | 41.22 ± 0.5 | 45.12 ± 0.7 |
| Sample | P/S | A/P | A/Sub | O/S | D/P | %D | PE% | PhA% | BA% | |
|---|---|---|---|---|---|---|---|---|---|---|
| Oil_V | UNAGED | 0.90 | 0.65 | 3.08 | 1.24 | 0.97 | 20.90 | - | ||
| AGED | 1.05 | 0.24 | 4.27 | 0.99 | 0.31 | 9.59 | ||||
| Oil_V_COMM | UNAGED | 1.96 | 0.55 | 9.29 | 1.16 | 0.63 | 22.75 | - | ||
| AGED | 2.12 | 1.13 | 6.44 | 0.64 | 1.34 | 42.30 | ||||
| Alk_V | UNAGED | 1.48 | 0.48 | 7.17 | 2.43 | 0.58 | 14.33 | 12.01 | 16.44 | 12.05 |
| AGED | 2.34 | 0.22 | 6.82 | 1.92 | 0.25 | 10.07 | 1.90 | 29.42 | 14.62 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagnin, L.; Cardin, G.; Pintus, V.; Back, M.; Pintér, F.; Sterflinger, K.; Izzo, F.C. Stability and Degradation Issues of Manganese Violet Pigment in Polymeric Paints: Morphological and Chemical Changes Under SO2 and Humidity Exposure. Appl. Sci. 2025, 15, 4630. https://doi.org/10.3390/app15094630
Pagnin L, Cardin G, Pintus V, Back M, Pintér F, Sterflinger K, Izzo FC. Stability and Degradation Issues of Manganese Violet Pigment in Polymeric Paints: Morphological and Chemical Changes Under SO2 and Humidity Exposure. Applied Sciences. 2025; 15(9):4630. https://doi.org/10.3390/app15094630
Chicago/Turabian StylePagnin, Laura, Giulia Cardin, Valentina Pintus, Michele Back, Farkas Pintér, Katja Sterflinger, and Francesca Caterina Izzo. 2025. "Stability and Degradation Issues of Manganese Violet Pigment in Polymeric Paints: Morphological and Chemical Changes Under SO2 and Humidity Exposure" Applied Sciences 15, no. 9: 4630. https://doi.org/10.3390/app15094630
APA StylePagnin, L., Cardin, G., Pintus, V., Back, M., Pintér, F., Sterflinger, K., & Izzo, F. C. (2025). Stability and Degradation Issues of Manganese Violet Pigment in Polymeric Paints: Morphological and Chemical Changes Under SO2 and Humidity Exposure. Applied Sciences, 15(9), 4630. https://doi.org/10.3390/app15094630








