Abstract
Brain stroke is the leading cause of death and disability globally; hence, early identification and prediction are critical for better patient outcomes. Traditional diagnostic procedures, such as manually interpreting clinical images, are time consuming and error prone. This research investigates the use of hybrid deep learning models, such as recurrent neural networks (RNNs), long short-term memory (LSTM), and convolutional neural networks (CNNs), to improve stroke prediction accuracy. The current study compared the performance of these individual models with the developed hybrid model on the brain stroke dataset. By merging these models, we reached an overall accuracy of 96% in identifying stroke risk as low, medium, or high. This categorization may offer healthcare practitioners actionable insights by assisting them and allowing them to make better decisions. This technique represents a substantial improvement in stroke prediction and preventive healthcare practices. The model’s performance can further be tested with more complicated clinical and demographic data that will help to generalize the model for real-world clinical applications. Furthermore, combining this hybrid model with electronic health records (EHR) systems can also assist in early identification, tailored therapies, and improved stroke management, enhancing patient outcomes and lowering healthcare costs.
1. Introduction
Brain stroke, an ailment described by the fast loss of cerebrum capability because of an upset blood stream, stays one of the main sources of death and incapacity around the world. There are two essential kinds of strokes: ischemic and hemorrhagic strokes [1]. Ischemic strokes, which make up almost 87% of all strokes worldwide, happen when a blood coagulation or blockage keeps blood from arriving at the mind, denying it of oxygen and nutrients [1]. Hemorrhagic strokes, liable for 10–15% of stroke cases around the world, happen when a vein in the cerebrum breaks, causing draining and pressure that harms mind cells [1]. Also, transient ischemic assaults (TIAs), or “scaled down strokes”, are brief blockages in the cerebrum blood stream that act as advance notice finishes for future strokes [1]. Early discovery and treatment of these circumstances are essential, as postponed mediation can prompt serious mind harm, long-term disabilities, or even death [1]. In 2019, the World Health Organization (WHO) revealed that cerebrovascular sicknesses were connected to over 6.6 million passings universally, with ischemic strokes representing around 3.3 million and hemorrhagic strokes for 2.9 million [2]. The subsequent weight on medical services frameworks is tremendous, featuring the significance of ideal determination to relieve the effect.
Conventional strategies for diagnosing strokes, for example, clinical appraisals and neuroimaging methods like computed tomography (CT) and X-ray filters, have limits, including significant expenses, the requirement for specific skills, and postponements in analysis. These difficulties have driven the joining of computerized reasoning and artificial intelligence and machine learning (AIML) calculations into stroke finding and prediction [2]. Simulated intelligence, especially profound learning, has shown surprising potential in handling enormous datasets, recognizing designs, and giving precise stroke analysis. Convolutional neural networks (CNNs) have been especially successful in dissecting CT and X-ray pictures, essentially lessening finding times without compromising accuracy [2]. Given their significant effect on infection weight, handicap, and mortality, strokes are a significant worldwide general wellbeing concern. Artificial intelligence (AI) is changing stroke conclusions by empowering quicker, more exact recognizable proof through better examination of clinical pictures, recordings, and patient information, permitting medical services experts to make opportune and all-round informed decisions [3,4]. Moreover, man-made intelligence-controlled prescient examination is upsetting stroke care by surveying a singular’s risk of stroke utilizing segment, clinical, and way of life information, working with early preventive actions [3].
Computer-based intelligence frameworks can convey customized risk appraisals by breaking down factors like age, pulse, glucose levels, smoking propensities, and clinical history [4]. This capacity is urgent for starting early treatment, consequently further developing patient endurance rates and personal satisfaction. Also, simulated intelligence empowered wearable gadgets that continuously screen essential signs and ready patients and medical services suppliers to potential stroke risk further improve forecast accuracy [4]. In instances of hemorrhagic strokes, which are brought about by breaks in mind veins and can be deadly if untreated, simulated intelligence-fueled apparatuses can rapidly separate ischemic and hemorrhagic strokes, empowering prompt treatment. In any case, to completely burden the advantages of simulated intelligence in stroke care, difficulties, for example, the requirement for powerful, different datasets and guaranteeing that simulated intelligence models are interpretable and morally sent, should be addressed [2,3].
Late investigations on mind stroke expectation have progressively centered around utilizing advanced computational strategies, especially AI and profound learning methods. These investigations investigate the utilization of calculations to foresee stroke risk in light of a scope of clinical and segment factors, for example, age, pulse, and smoking history [3]. Scientists have applied these strategies to freely accessible datasets, determined to further develop expectation exactness by investigating designs and distinguishing key gamble factors. Different strategies, including brain organizations and helping calculations, have been investigated to upgrade stroke detection [3]. Dimensionality reduction techniques like head part examination (PCA) are normally used to improve on the information, guaranteeing that the most important highlights are held for stroke expectation. The exhibition of these models is evaluated utilizing measurements like exactness, accuracy, and review, and results are contrasted with existing strategies with show enhancements in prescient capabilities [4]. These examinations feature the continuous progressions in the field and highlight how computational strategies are turning out to be progressively necessary for stroke finding and hazard assessment [4].
The foundation for the suggested system is provided by this work, which expands upon previously published material (related work). A thorough description of the system’s architecture emphasizes how well it can anticipate and identify strokes [5]. To guarantee precision and applicability to real-world situations, the dataset is meticulously chosen and preprocessed. In order to improve prediction performance, the suggested methodology presents a hybrid deep learning model that combines together the advantages of CNN, recurrent neural networks (RNNs) and long short-term memory (LSTM), algorithms [6]. The proposed system’s features and utility highlight how useful it is in clinical settings and provide information on risk assessment and early identification. The efficacy of the system is demonstrated in the Result part by thorough examination, opening the door for more developments in the Future Scope [6]. Summarizing the study’s contributions, the Conclusion confirms that it has the potential to revolutionize stroke prevention and diagnosis.
2. Review Methodology
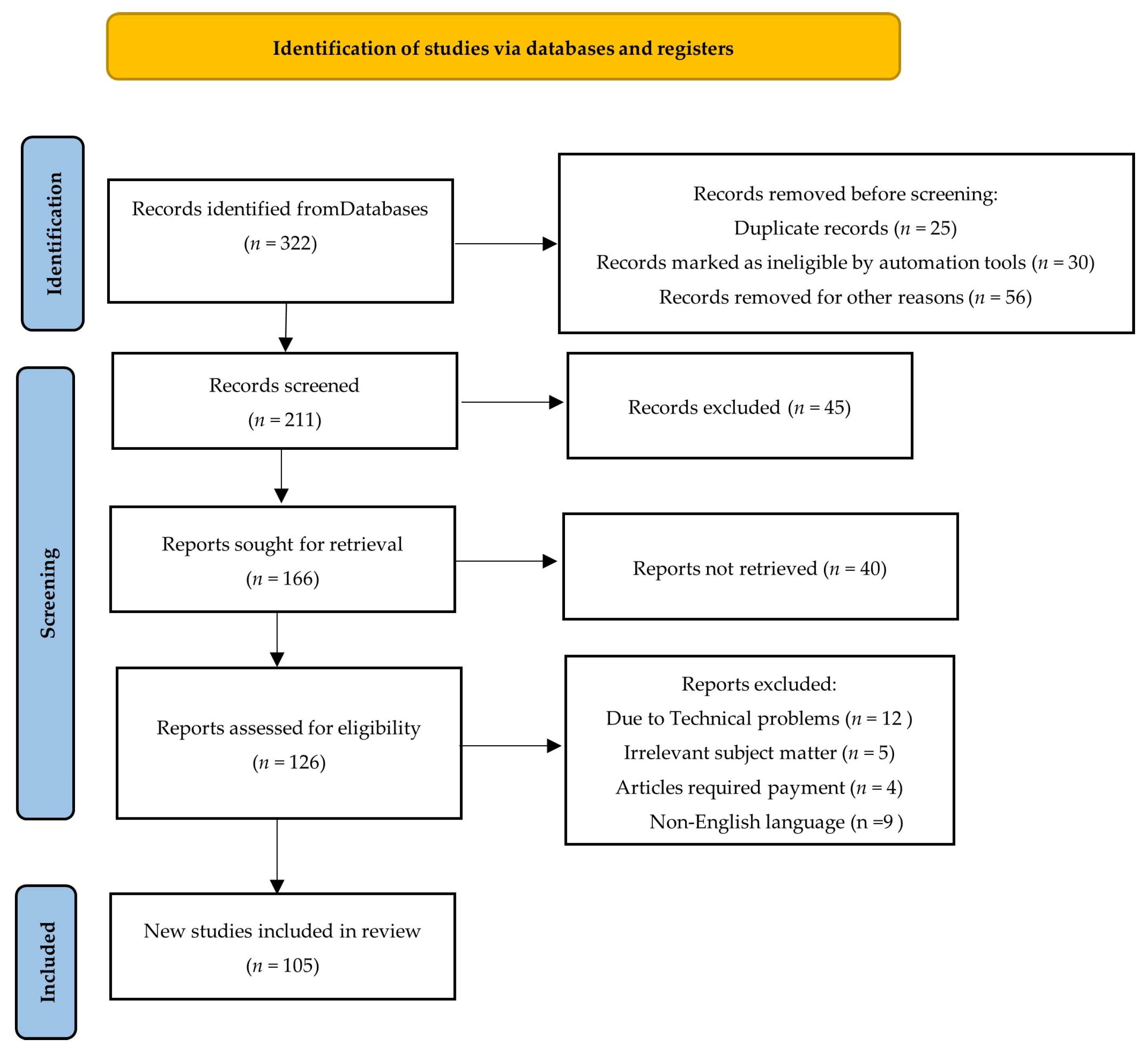
A systematic technique was used to construct the search strategy for the paper titled “A Hybrid Deep Learning Approach for Improved Detection and Prediction of Brain Stroke”. Extensive searches were conducted across significant academic databases such as PubMed, Web of Science, and Scopus to collect pertinent research papers, reviews, and reports published between 2008 and 2024. The search used specific terms like “brain stroke”, “deep learning”, “stroke prediction”, “artificial intelligence”, and “machine learning”. The inclusion criteria required that the selected literature be published in English and focus on the use of AI and deep learning techniques in stroke detection, prediction, and management. Articles were reviewed for relevance using predetermined inclusion and exclusion criteria, resulting in the inclusion of high-quality and relevant sources. The data from the selected research were thoroughly evaluated and synthesized to offer a comprehensive knowledge of how hybrid deep learning models might improve brain stroke prediction. This organized search technique enabled a thorough study of AI’s potential for improving stroke diagnosis and patient outcomes (Figure 1).
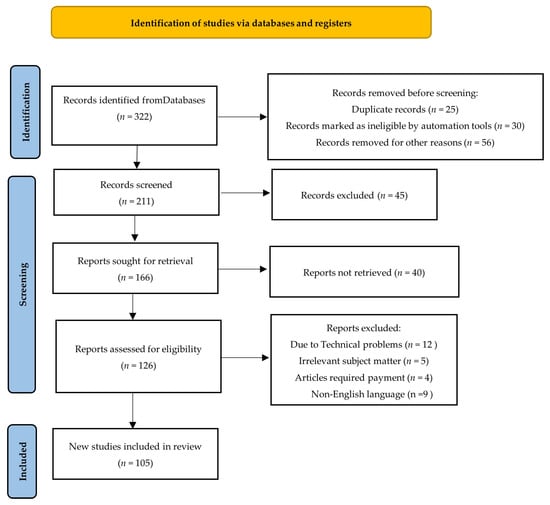
Figure 1.
PRISMA flow diagram for search strategy and selection criteria (Adapted from the preferred reporting systemic reviews and meta-analyses (PRISMA) guidelines).
3. Related Work
Recent research has greatly improved stroke risk prediction with machine learning (ML) and deep learning (DL) approaches as shown in Table 1. Rahman et al. [1] used deep neural network techniques to increase prediction accuracy and identify key risk variables. Their approach included feature selection, data preprocessing, and model training with deep neural networks and machine learning. The study found that the random forest classifier attained 99% accuracy, outperforming the 4-layer artificial neural network (ANN) (92.39%); nonetheless, this high accuracy signals probable overfitting, restricting real-world applications. Lakkshmanan et al. [2] used an adaptive new fuzzy inference system (ANFIS) for stroke prediction based on Magnetic Resonance Imaging (MRI) data to handle uncertainty and increase accuracy. Their strategy includes preparing MRI data and comparing it to other ways. The model significantly improved performance indicators such as accuracy, sensitivity, specificity, F1-score, and ROC analysis, but it was heavily dependent on parameter selection, which hampered dependability.

Table 1.
Literature survey on brain stroke detection and prediction system.
Qasim et al. [3] developed a deep neural network that uses weighted binary cross-entropy to improve stroke detection accuracy. The process included training a deep neural network (DNN) on brain imaging data and prioritizing stroke detection with a weighted loss function. The model considerably increased recall, precision, and F1-score while decreasing overall accuracy from 95.36% to 75.36%, which may influence performance when accuracy is critical. Choi et al. [4] created a real-time stroke prediction system with electroencephalogram (EEG) data and DL models, including CNN-LSTM and bidirectional LSTM. Their approach included real-time biosignal collection, feature extraction, and prediction with deep learning models. The CNN-bidirectional LSTM model obtained 94% accuracy; however, changes in data quality and noise between patients may impact real-world deployment. Gaidhani et al. [5] investigated CNN-based stroke diagnosis using medical imaging data. The approach employed deep learning, namely CNNs, to extract characteristics and categorize stroke events. The study found that LeNet achieved 96–97% accuracy for classification and 85–87% accuracy for segmentation; however, segmentation accuracy and high computational demand prevented real-time clinical applications.
Chen et al. [6] suggested a hybrid deep transfer learning system for improving stroke risk prediction. Their process includes fine-tuning a pre-trained model utilizing a hybrid approach while adhering to ethical norms and data protection protocols. The hybrid deep transfer learning-based stroke risk prediction (HDTL-SRP) approach outperformed earlier models and showed potential for 5G/B5G hospital integration; nonetheless, data privacy and heterogeneity issues may limit scalability and generalizability. Karthik et al. [7] reviewed neuroimaging and DL advances in stroke diagnosis. Their strategy included examining existing approaches, finding research gaps, and investigating the potential of deep learning in stroke detection. CNNs and fully convolutional networks (FCNs) performed well in stroke detection and segmentation but required large, annotated datasets, limiting their use.
Tan et al. [8] thoroughly assessed deep learning approaches for early ischemic stroke prediction, noting both strengths and drawbacks in current models. The process includes reviewing the literature, evaluating model efficiency, and recommending changes. The study found that the ConvNeXt Base model detected ischemic strokes with 84% accuracy using MRI scans, indicating the possibility for early intervention. Obtaining big, labeled datasets remains a significant difficulty. Cui et al. [9] studied deep learning algorithms for ischemic stroke imaging analysis. The technique entailed examining current advances in stroke imaging and measuring the performance of deep learning models. While deep learning models greatly improved ischemic stroke diagnosis and clinical outcomes, their reliance on big, high-quality datasets and difficulty generalizing across clinical contexts limited their usefulness. Table 1 provides a summary of various other literature carried out in the field of ML and DL applications to brain stroke detection and prediction, highlighting the outcomes from each work.
4. Detailed Overview of the Brain Stroke Detection and Prediction System
As far as clinical determination, the ability to appropriately figure a patient’s risk of having a stroke in light of existing clinical and segment information is very valuable [10]. Most existing stroke risk evaluation approaches utilize mathematical information from various pointers to decide the patient’s risk level and group it as high, medium, or low [11]. This segment depicts exhaustively the advances and approaches used in the improvement of the cerebrum stroke identification and expectation framework, with an accentuation on information numeration and high-level profound learning strategies [12].
The cerebrum stroke location and expectation framework utilizes state-of-the-art AI to remove qualities from a great many clinical and segment factors [13]. This strategy takes into consideration a careful assessment of an individual’s risk factors and an exact gauge of their possibilities of suffering a heart attack. Age, pulse, cholesterol levels, blood glucose levels, body mass index (BMI), and way of life propensities, for example, smoking and actual activity, are among the key factors inspected [14]. By consolidating this information, the technique partitions stroke risk into three classifications: high, medium, and low, permitting medical services specialists to tweak precaution and helpful intercessions accordingly [14].
The significant reason for the cerebrum stroke identification and expectation framework is to help clinical experts appropriately anticipate stroke risk, work on the symptomatic cycle, and work with early intercession. The framework utilizes strong, profound learning calculations, specifically brain organizations, to recognize unobtrusive examples in clinical and segment information [15]. These models are appropriate for managing nonlinear cooperations between different information types, like all-out factors (e.g., orientation and staying type) and continuous measures (e.g., glucose levels and BMI). This limit works on the framework’s capacity to find minor associations and make exact forecasts [16].
This framework’s utilization of information numeration, a procedure that in a perfect world deciphers clinical and segment sensitivity into normalized mathematical portrayals appropriate for AI calculations, is a huge development [17]. By handling and scaling information reliably, the framework ensures that straight-out and ceaseless information are blended, permitting it to find huge relationships across undeniable input factors. The careful preprocessing stage works on the model’s anticipated precision and reliability [18].
The framework’s result, which sorts risk levels as high, medium, or low, gives medical care specialists valuable data. These bits of knowledge act as the establishment for centered medicines, for example, pushing way of life changes, initiating regular checking, or recommending custom fitted prescriptions relying upon risk level [19]. Moreover, the model’s capacity to examine huge datasets makes it material to both individual patient evaluations and populace level studies [20].
The Brain Stroke Detection and Prediction System offers significant advantages, including:
- Personalized Risk Assessment: Gives customized risk profiles to every patient, empowering accurate medication strategies [21].
- Early Intervention: Medical care experts can bring down the quantity of strokes by distinguishing high-risk individuals from the get-go [21].
- Scalability: The plan upholds huge datasets, making it ideal for population-level activities and enormous scope clinical offices [22].
- Cost-Effectiveness: Early ID and intercession can impressively save medical services costs by diminishing the quantity of intense strokes and expecting expensive treatments [22].
- Continuous Learning: The profound learning viewpoint works on the framework’s exactness after some time with extra information [23].
- Integration with EHR: Constant data on patients risk levels and work on entering data for medical services experts are made conceivable via consistent cooperation with HER [24].
The brain stroke detection and prediction system is an effective and dependable technique for predicting stroke risk, allowing clinicians to make educated, proactive decisions that can save lives and improve patient outcomes.
5. Dataset Used
Healthcare predictive modeling is a strategy that uses past data to anticipate probable future health issues [24]. The work makes use of a publicly available dataset on brain stroke detection, obtained from GitHub [25]. Table 2 provides the details of the dataset and its attributes used in the current study. Several biomarkers and risk variables, such as gender, residence type, BMI, glucose level, hypertension, heart disease, smoking status, and work type as mention in Table 2. are used to develop models for individual risk stratification in stroke [26]. Table 3 provides an overview on various datasets, predictive models and their performance for stroke prediction.

Table 2.
Description of the dataset used for the proposed approaches.
Each of these factors has a significant impact on the chance of a stroke:
- Gender (Risk Factor): It may be established that gender is an important predictor of stroke. Several studies have revealed that men are more likely than women to have a stroke; however, this changes with age [26]. The integration of gender into this model enables personalized predictions based on gender-related risk indicators [27].
- Residence Type (Risk Factor): Whether an individual lives in an urban or rural area influences lifestyle aspects such as food, exercise regimen, healthcare access, and stress levels, all of which increase stroke risk. Variations in eating choices and healthcare access between urban and rural populations can influence stroke risk [28].
- Body Mass Index (BMI) (Biomarker): BMI is an important biomarker used worldwide to calculate body fat percentage. It is linked to a variety of health issues, including stroke, hypertension, diabetes, and cardiovascular disease. Individuals with a high BMI have a considerably higher risk of stroke [29,30].
- Glucose Level (Biomarker): High glucose levels are a biomarker for diabetes, which is a major risk factor for stroke. Chronic high blood sugar levels can damage blood vessels, leading to ischemic and hemorrhagic strokes [29,31].
- Hypertension (Biomarker): Hypertension (high blood pressure) is an important biomarker for stroke because it weakens blood vessels and increases the risk of clotting. Controlling blood pressure is critical for stroke prevention and risk assessment in prediction models [29,32].
- Heart Disease (Biomarker): Atrial fibrillation and coronary artery disease are biomarkers that dramatically increase the risk of stroke. These diseases can cause clot development, which, when carried to the brain, can result in a stroke. The addition of cardiac disease in prediction models improves stroke prognosis accuracy [29,33].
- Smoking Status (Risk Factor and Biomarker): Smoking is both a modifiable biomarker and a risk factor for stroke. Cigarette chemicals, notably nicotine, lead to hypertension, low oxygen levels, and vascular damage, raising stroke risk [34].
- Work Type (Risk Factor): Occupational stress, job security worries, and sedentary lifestyles all increase stroke risk. Jobs that require little physical exercise are frequently related to higher BMI and hypertension, whereas physically demanding employment may have a protective effect against stroke [35].
Integrating biomarkers and risk variables into prediction models allows healthcare providers to improve stroke risk assessment and early intervention tactics.
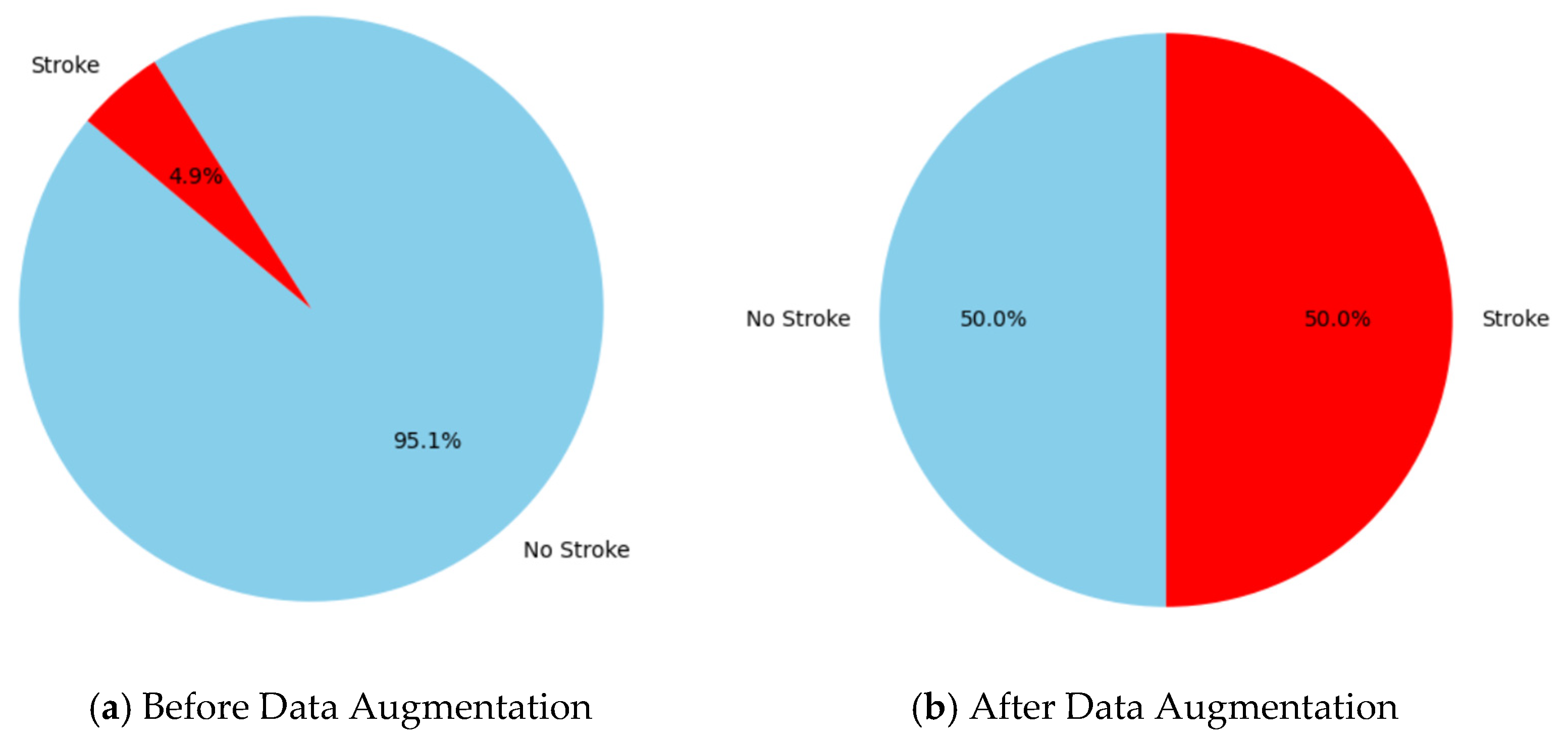
Figure 2 represents the class distribution of brain stroke cases in the dataset prior to and during data augmentation. Before augmentation (a), the dataset shows a considerable class imbalance, with 95.1% of occurrences categorized as “No Stroke” (blue) and just 4.9% as “Stroke” (red), showing the difficulties of predictive modeling in such imbalanced settings, where algorithms frequently prefer the majority class [35]. Following data augmentation (b), the class distribution is balanced, with 50% representation for both “Stroke” (red) and “No Stroke” (blue). This balanced distribution tackles the issue of class imbalance by allowing the model to learn more effectively from the minority class and enhancing its predictive performance for stroke patients, which is a vital step toward fair and accurate forecasts [36].
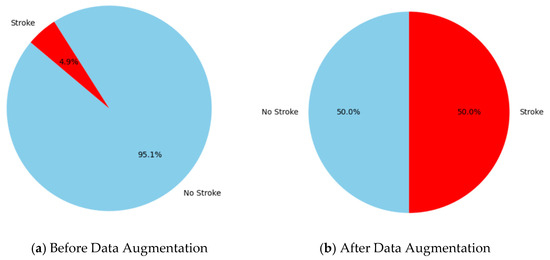
Figure 2.
The visualizing count of classes (stroke and non-stroke) (a) before data augmentation and (b) after data augmentation.
Table 3 lists the many machine learning and deep learning models that have been investigated for stroke prediction utilizing various datasets and methodologies. To improve prediction accuracy, researchers have experimented with ensemble approaches, logistic regression (LR), decision trees (DT), random forests (RF), and neural networks. To solve issues like data imbalance and feature optimization, methods including principle component analysis (PCA), feature selection, and the Synthetic Minority Over-sampling Technique (SMOTE) have been used. Although these methods increase model interpretability and accuracy, there are still significant issues with their limits, including limited datasets, unbalanced data, and the fact that certain strategies are only applicable to particular kinds of data.


Table 3.
Overview of datasets and algorithms applied in stroke prediction studies.
Table 3.
Overview of datasets and algorithms applied in stroke prediction studies.
| Ref. | Dataset | Algorithm Used | Technique | Advantages | Limitations |
|---|---|---|---|---|---|
| [37,38] | 5110 instances, 12,249:4861 split | LR, SGD, DT, AdaBoost, Gaussian, QDA, MLP, K-neighbors, GBC, XGB | Working with different ML models, imbalanced data | Exploring data on different ML models | Imbalanced data |
| [39,40] | 5110 instances, 12,249:4861 split | LR, DT, RF, Voting classifier | SMOTE for balanced data | Better performance with upscaled data | Upscaled data may not be accurate for real-time |
| [41,42] | 250:67,897 instances | DT, NB, neural network | Demographic data | Importance of demographic data on stroke prediction | No risk factors considered |
| [43,44] | 29,072 instances, 12,548:28,524 split | DT, RF, neural networks | Feature correlation analysis, stepwise analysis, PCA | Get optimum set of features | Limited features in dataset |
| [45,46] | 2439 instances, 1556:1075 split | RLR, SVM, RF | ROS, RUS, SMOTE | Improve predictive performance with imbalanced data | Small dataset |
| [47,48] | 43,400 instances, 12,548:28,524 split | Two-class decision jungle, two-class boosted decision tree | Chi-squared based feature selection | Extracts most important features from original dataset | Applicable only for categorical data |
| [1,49] | 3522 instances, 3820:43,561 split | Deep neural network, RF, LR | Deep neural network | Multiple layers can represent complex outcomes in stroke patients | Small dataset |
| [50,51] | 29,072 instances, 12,548:28,524 split | DT, RF, neural networks | PCA | Identifying the impact of risk factors on stroke prediction | Random downsampling technique |
6. Proposed Methodology
The proposed methodology employs a hybrid DL model integrating RNNs, LSTMs, and CNNs to enhance brain stroke prediction [52,53]. This technique detects temporal connections in sequential health data as well as spatial links between static health measures. RNNs assess sequential trends, LSTMs deal with long-term dependencies, and CNNs extract features from structured numerical data [54,55]. The following subsections describe each model component’s involvement in the overall system process.
6.1. Recurrent Neural Networks (RNNs)
RNNs are powerful in learning consecutive connections inside a dataset. These organizations cycle input highlights by consolidating criticism circles, which permit the maintenance of data from past strides in a succession and catch fleeting conditions between data of interest [56]. For stroke expectation, RNNs are particularly helpful while breaking down time-reliant or consecutive information, for example, variances in glucose levels, BMI patterns, or other powerful wellbeing markers [57]. Despite the fact that RNNs succeed at demonstrating such examples, they might battle with long-term conditions and may require variations like LSTMs to further develop execution and stability [58].
By examining sequential health data to identify temporal relationships in patient records, such as changes in blood pressure or heart rate over time, RNNs are used to predict brain strokes as illustrated in Figure 3 [59]. By using its recurrent layers, the RNN examines this time-series input, taking into account both recent data and historical context at each stage as shown in Figure 3. This allows the model to comprehend how prior health patterns influence the risk of stroke [51,60]. RNNs are appropriate over applications involving context-aware evaluation of sequential medical data because they can accurately forecast the chance of stroke through acquiring these temporal patterns [52,61].
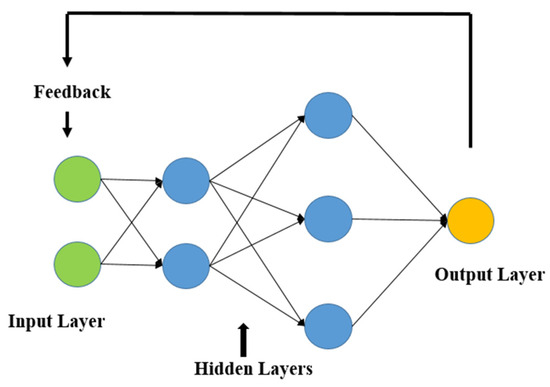
Figure 3.
Basic recurrent neural network architecture.
6.2. Long Short-Term Memory Networks (LSTMs)
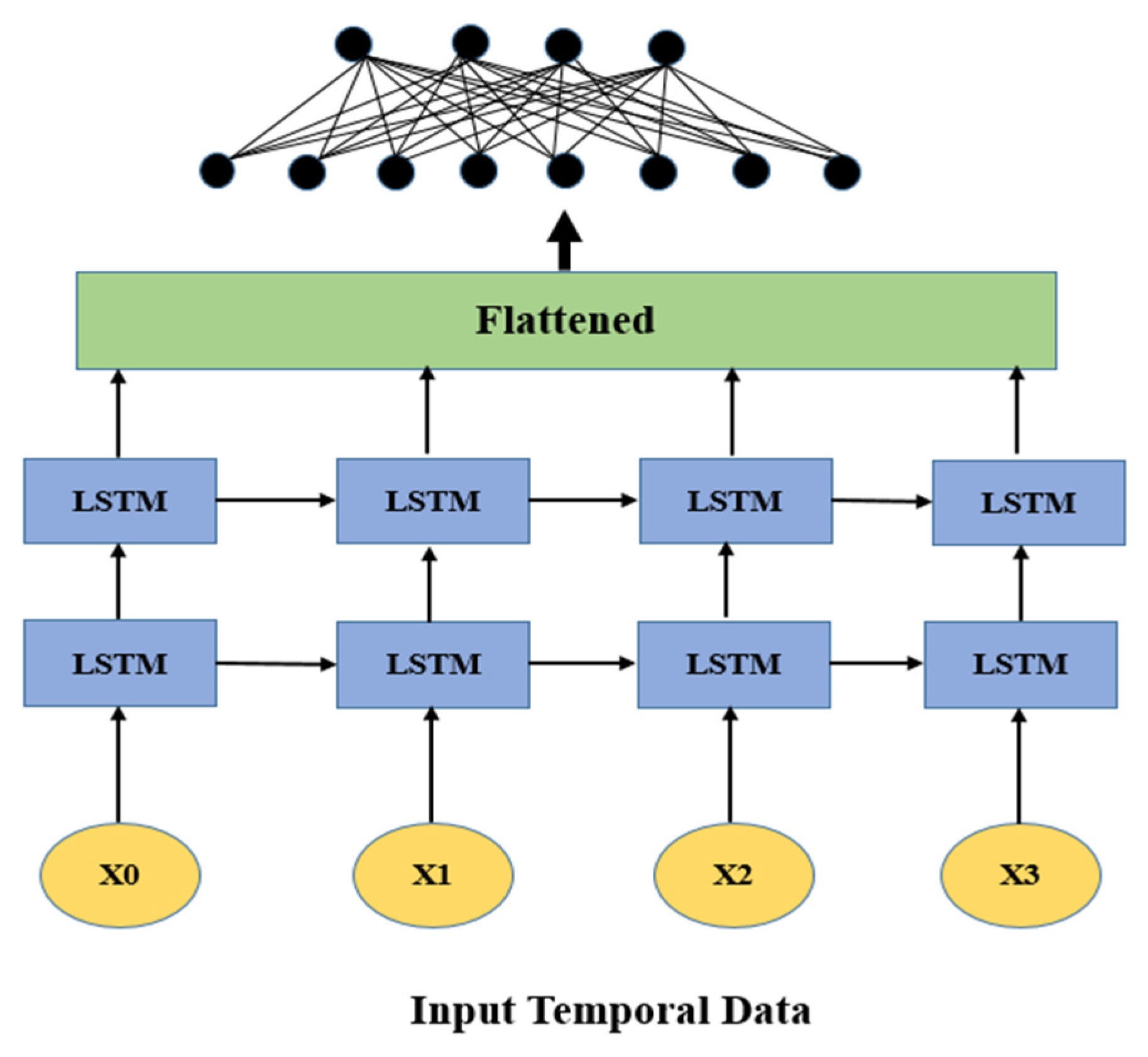
LSTMs are a specific kind of RNN that are especially proficient at taking care of consecutive information and gaining from long-range conditions [62]. This trademark is important in the clinical field, where patient information is in many cases transient, for example, changes in glucose levels, BMI after some time, or varieties in pulse [63]. LSTMs are fit for handling these time-series inputs successfully, catching the movement and worldly elements that might show an expanded gamble of stroke [64]. They are intended to hold data over lengthy successions, making them appropriate for displaying advancing ailments and distinguishing patterns that may not be apparent in static datasets [65].
Because LSTM networks can model long-term dependencies and assess time-series health data, including patterns in blood pressure or heart rate, they are very successful for both detecting and predicting brain strokes [66]. They may choose to store and discard information because of their unique cell structure, which includes forget, input, and output gates as shown in Figure 4. This ensures that important temporal patterns that affect stroke risk are recorded [67]. Sequential input data are processed by LSTMs using memory cells, and the output layer predicts the low, medium, and high-risk levels for stroke [67,68]. LSTMs provide accurate and context-aware predictions by learning these temporal patterns as sown in Figure 4, which makes them perfect for risk assessment and early detection [68,69].
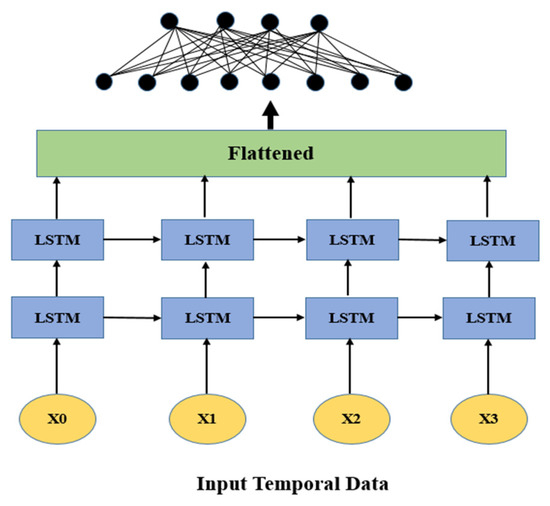
Figure 4.
Basic long short-term memory (LSTM) architecture.
6.3. Convolutional Neural Networks (CNNs)
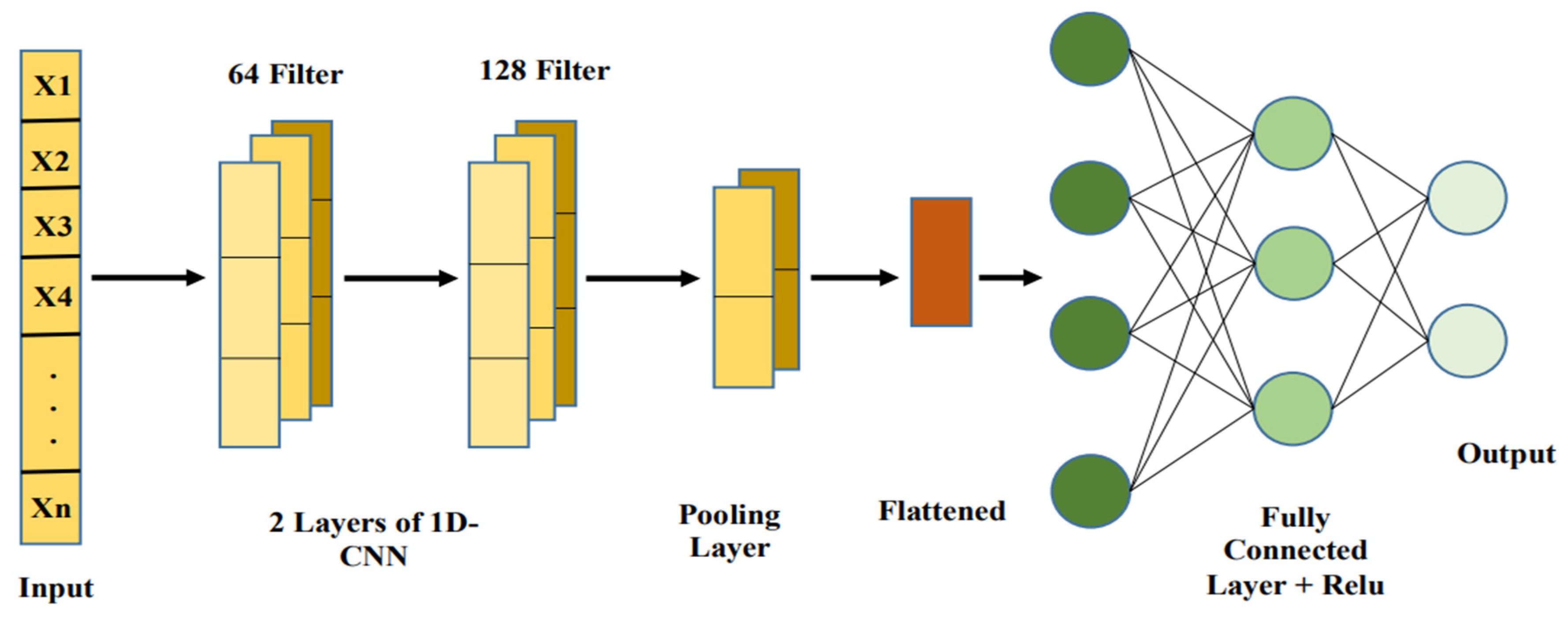
While CNNs are generally connected with picture information, they can be adjusted to work with organized numeric information sources, like patient information, by utilizing 1D convolutional layers [60]. CNNs are magnificent at distinguishing neighborhood examples and conditions inside information, which makes them ideal for recognizing connections between various wellbeing boundaries that may not be promptly clear in crude numeric form [70]. With regards to stroke forecast, CNNs can be utilized to distinguish unpretentious relationships, for example, those between glucose level variances and BMI, in any event, when the information is loud or fragmented. By gaining from these cooperations, CNNs assist with working on the model’s capacity to identify complex, non-straight examples in understanding wellbeing metrics [71].
By treating numerical data as a structured input in grid-like forms, CNNs can also be used for brain stroke diagnosis and prediction [72]. A 2D matrix is used to arrange numerical characteristics, including age, blood pressure, BMI, glucose levels, and lifestyle variables as shown in Figure 5. CNN layers use convolutional filters to identify patterns and spatial correlations among these features [73]. In order to prevent overfitting and preserve important patterns, pooling layers minimize the dimensionality. The retrieved characteristics are processed by fully connected layers to categorize results, including risk level prediction [74]. This method provides precise and accurate predictions by utilizing CNNs’ feature extraction ability for structured numerical data [75].
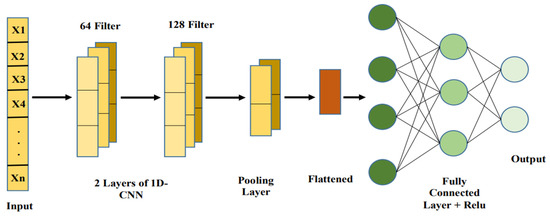
Figure 5.
Basic CNN architecture.
6.4. Hybrid Deep Learning Model
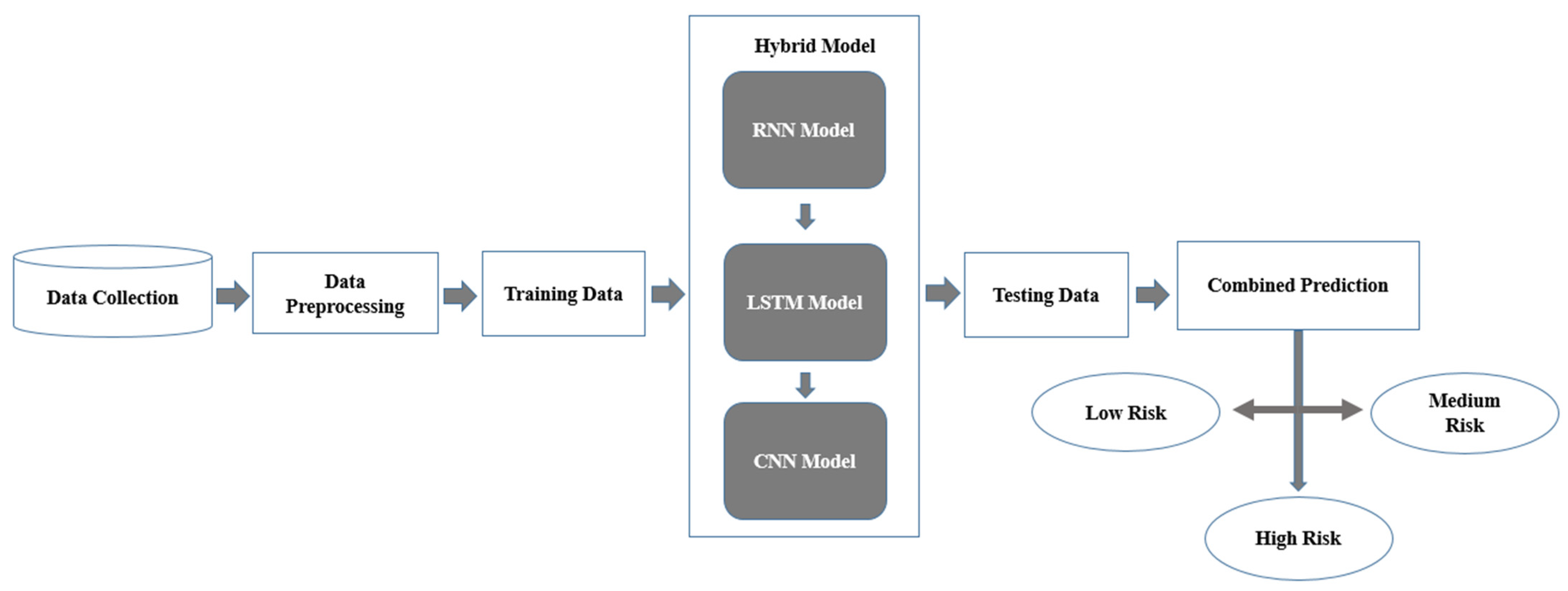
Profound learning has shown to be an amazing asset in clinical conclusions by empowering the extraction of highlights from complex datasets, including patient narratives, wellbeing markers, and physiological signs, without the requirement for express programming [76,77]. Nonetheless, independent profound learning models, even profound semantic organizations, frequently battle with summed-up issues and boisterous information, which are normal in clinical datasets. To defeat these difficulties, half-breed profound learning models have been created by consolidating different structures to improve prescient capabilities as shown in Figure 6 [78,79]. In mind stroke forecast and discovery, especially utilizing numeric datasets, crossover models can coordinate CNNs, recurrent neural networks (RNNs), and long transient memory organizations (LSTMs) to deal with patient boundaries, for example, orientation, glucose level, BMI, work type, hypertension, coronary illness, and smoking status [80,81]. Figure 6 provides the workflow that has been adopted in current study for application of hybrid DL model for brain stroke prediction.
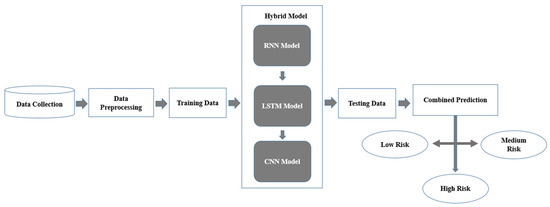
Figure 6.
Flowchart of the hybrid deep learning model.
- Data Collection: The method begins with gathering relevant information from various sources, including electronic health records (EHRs), clinical datasets, and shrewd wellbeing checking gadgets [82]. This information contains both static components, for example, BMI, glucose levels, hypertension status, and segment data, and dynamic elements, which record fleeting changes in wellbeing markers, for example, day-to-day glucose level swings or circulatory strain patterns over the long run [83]. Thorough and enhanced information gathering guarantees that the model gets a general image of patient gamble and wellbeing factors [84].
- Data Pre-processing: The technique begins with gathering relevant information from different sources, including EHRs, clinical datasets, and savvy well-being-observing gadgets [85]. This information contains both static components, for example, BMI, glucose levels, hypertension status, and segment data, and dynamic highlights, which record transient changes in wellbeing pointers, for example, everyday glucose level swings or pulse patterns over the long term [86]. Exhaustive and enhanced information gathering guarantees that the model gets a general image of patient gamble and wellbeing factors [87].
- Training Data: Following the preprocessing stage, the information is isolated into two datasets: preparing and testing. A preparation set has been used to prepare the cross-breed model, permitting it to distinguish examples and relationships in the information [83,86]. The testing dataset, which was held back all through preparing, is subsequently utilized for evaluating the model’s exhibition on already concealed information, guaranteeing that it sums up really and maintains a strategic distance from overfitting [88]. This division is basic to making a reliable and strong forecast framework.
- Hybrid Model: The crossover model proposes the qualities of three particular brain network plans to deal with both static and dynamic elements effectively:RNN Component: This module dissects consecutive information, catching examples and fleeting connections in unique qualities like glucose levels and circulatory strain variances across time [89,90].LSTM Component: In light of the RNN, LSTMs succeed at grasping conditions that go the distance over the long run, permitting the model to understand connections that range over extensive spans, for example, what relentless hypertension might mean for stroke risk [90].CNN Component: This module centers around static boundaries like BMI, glucose levels, and hypertension, removing itemized designs and deciding connections between them [91]. The CNN’s ability to catch complex component communications further develops general forecast precision.These components work together to guarantee that the model can fully process and incorporate both static and temporal elements of the input.
- Testing Data: Features like gender, residence type, BMI, glucose levels, hypertension, heart disease, smoking status, and work type are among the testing data used by the stroke detection and prediction system [92]. It is preprocessed using encoding and normalization to guarantee consistency with the training data. Using metrics like accuracy, precision, recall, and F1-score, performance is assessed by comparing the predictions for stroke risk levels (low, medium, and high) made from the processed data with the actual values [73].
- Combined Prediction: The results of the RNN, LSTM, and CNN parts are converged to make a solitary expectation. This convergence of results permits the framework to consider each model’s particular assets transient investigation from RNN and LSTM, and complex static element extraction from CNN [90,93]. The joint forecast gives a reasonable and intensive assessment of the patient’s gamble level; it is substantial and exact to ensure that the discoveries.
- Risk Classification: The combined prediction classifies individuals into one of three stroke risk levels [80]:
- −
- Low Risk: Patients with few indications of stroke risk who may essentially require occasional assessment and solid way of life direction.
- −
- Medium Risk: Patients with moderate gamble factors require more forceful measures, for example, way of life changes and ordinary wellbeing checks.
- −
- High risk: patients have impressive gamble signs and require fast clinical consideration, as well as possibly progressed demonstrative or safeguard intercessions.
This categorization enables healthcare practitioners to customize therapies to individual risk levels, encouraging early detection and effective resource allocation.
- Performance Metrics
Five measurable factors were used in this review to assess the presentation and helpfulness of the classifiers: exactness, accuracy, review/awareness (review and responsiveness are similar in parallel order), F1-score, and AUC bend [81]. Coming up next are the meanings of the measurable boundaries.
where,
- TP = true positive,
- FN = false negative,
- FP = false positive,
- TN = true negative.
7. Result
The brain stroke expectation framework separates individuals into okay, medium gamble, and high gamble classes in light of clinical and segment factors, for example, age, orientation, hypertension, coronary illness, smoking status, BMI, glucose levels, work type, and habitation. These boundaries are examined utilizing a blend of LSTM, CNN, and RNN profound learning models to distinguish complex connections and examples in the information.
The technique permits medical care specialists to make preventive moves by recognizing high-risk patients right off the bat. The technique makes patients with high gamble aware of the requirement for early mediation, for example, way of life change projects or clinical treatments, to bring down the gamble of a stroke occurrence. Those in the okay gathering could profit from progressing, observing, and helping to keep up with their wellbeing through way of life changes like regular workouts and adjusted eating. People with medium gamble might require month-to-month subsequent meet-ups to guarantee that any new changes in their wellbeing pointers are seen and taken care of right away. Utilizing this delineated methodology, the framework works on individualized medical care, not just expanding the effectiveness of stroke preventive endeavors but also prompting better long-term wellbeing results. It adds to bringing down medical care costs by empowering centered activities that forestall strokes before they happen. Moreover, the framework’s adaptability and association with EHR ensure that it stays current with every patient’s developing wellbeing profile, so helping the precision and reliability of future conjectures.
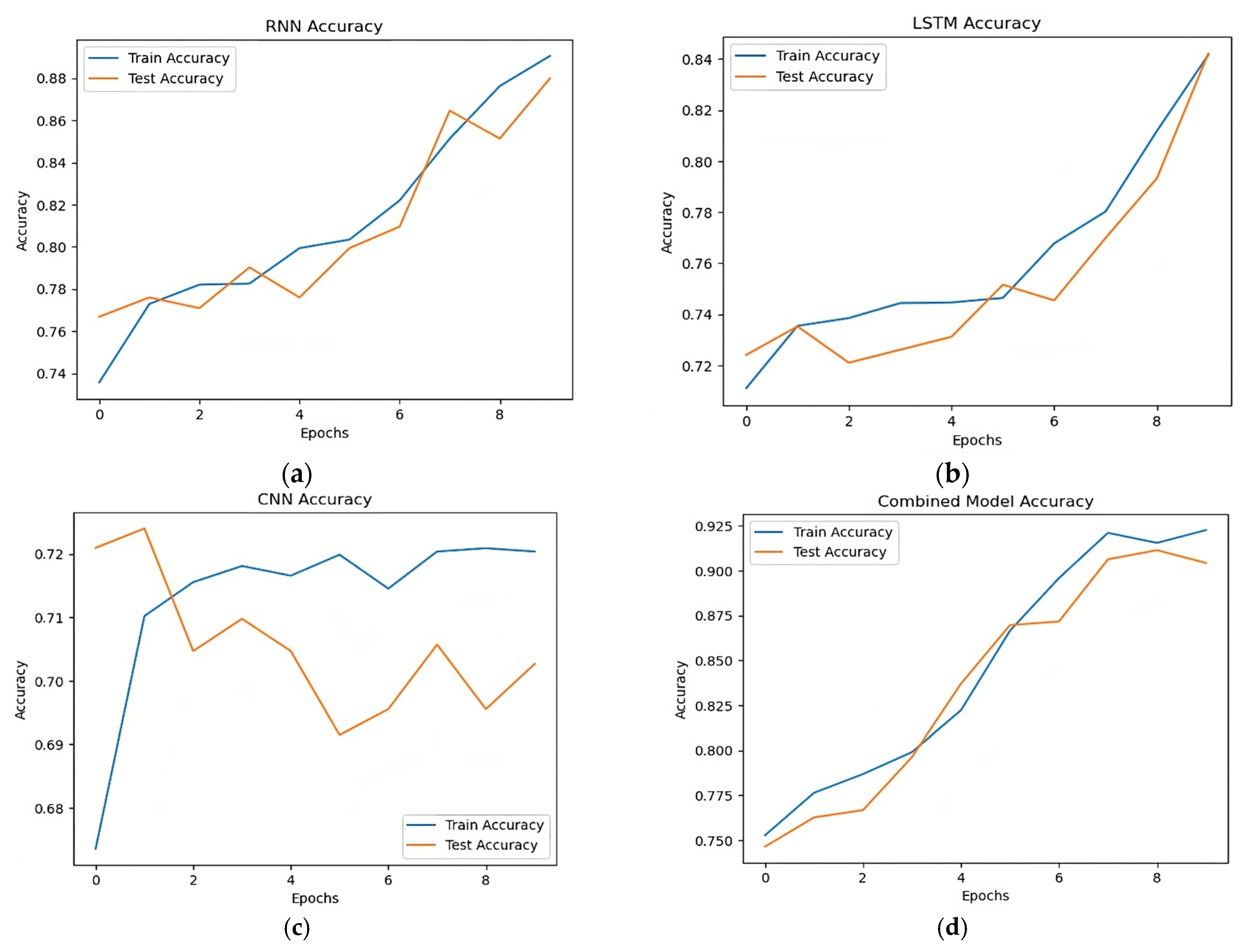
Table 4 and Figure 7 demonstrate that the models’ performance was comparatively poorer before data augmentation. With an accuracy of 0.89 for the RNN model and 0.84 for the LSTM model, both models demonstrated their capacity to identify long-term dependencies. While getting a significant recall of 0.91, the CNN model had the lowest accuracy of 0.70. The hybrid model, which included many architectures, demonstrated its efficacy in stroke prediction by achieving the best accuracy of 0.92. These findings, however, imply that more advancements in data augmentation methods might be employed to increase model performance.

Table 4.
Evaluation metrics of deep learning models and hybrid approach.
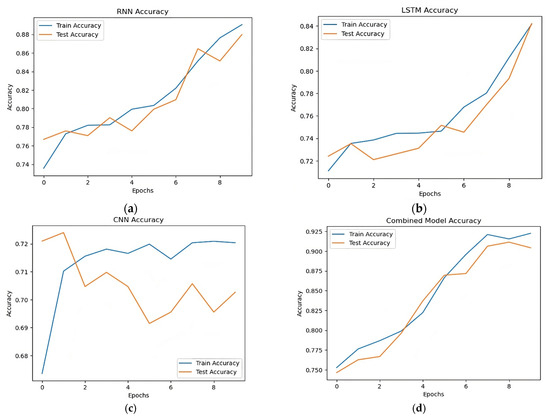
Figure 7.
Accuracy comparison RNN, LSTM, CNN, and combined models. (a) Accuracy of RNN model, (b) accuracy of LSTM model, (c) accuracy of CNN model, (d) accuracy of hybrid model.
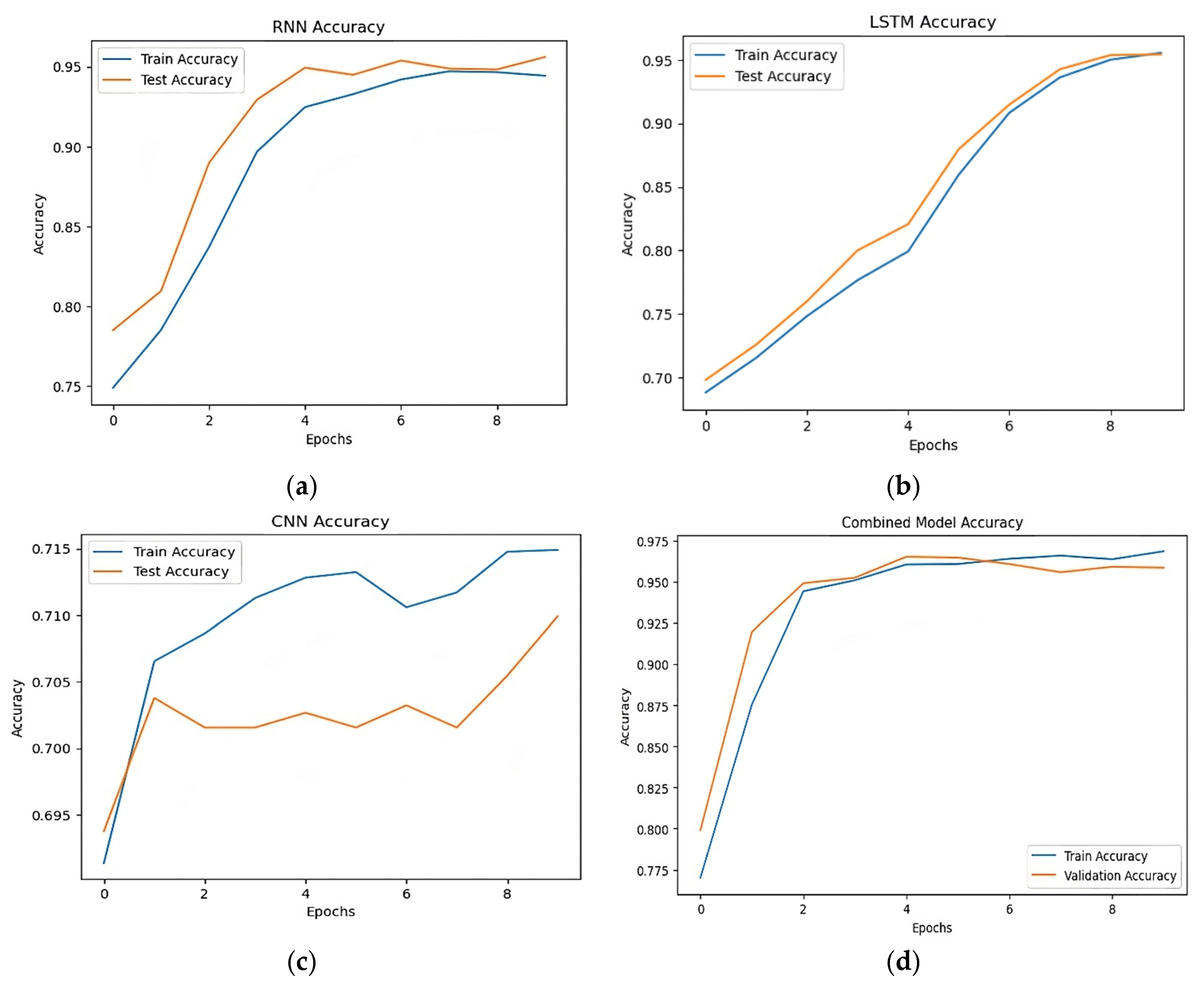
Table 5 demonstrate how successful the suggested hybrid technique is when comparing the post-augmentation accuracy of various models. The accuracy scores of the RNN, LSTM, and CNN models were 0.94, 0.95, and 0.70, respectively. The precision and recall of the LSTM model were marginally higher. With an accuracy of 0.96, the hybrid model which combines many architectures performed better than any of the separate models. Figure 8 shows the accuracy trends over epochs show that the hybrid model continuously output forms standalone models in terms of accuracy and stability, hence enhancing its resilience in stroke prediction.

Table 5.
Evaluation metrics of deep learning models and hybrid approach after data augmentation.
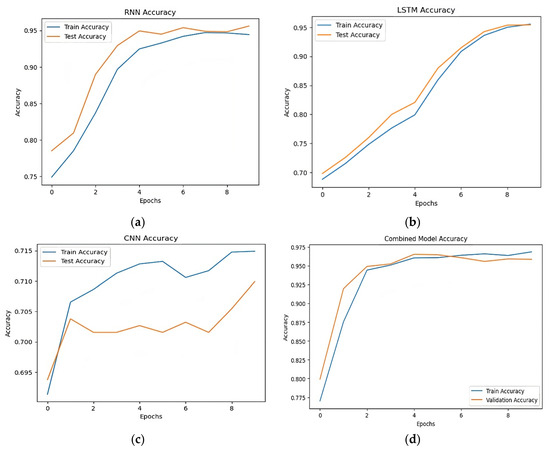
Figure 8.
Post-augmentation accuracy comparison. (a) Accuracy of RNN model, (b) accuracy of LSTM model, (c) accuracy of CNN model, (d) accuracy of hybrid model.
As Table 6 illustrates, the model’s stroke risk prediction findings reveal how different health parameters influence varying risk levels. In Case 1, a 35-year-old man without a history of heart disease, hypertension, or smoking is predicted by the model to have a low risk of stroke. The model identifies the risk as medium in Case 2, when the person is older (56 years), has hypertension, and has slightly raised blood sugar levels. A 59-year-old man with heart disease, high blood sugar, hypertension, and a history of smoking is classified as high risk in Case 3. These outcomes demonstrate the model’s capacity to evaluate several factors and accurately forecast the risk of stroke from patient data.

Table 6.
Predicted Stroke Risk Levels Based on Health Parameters.
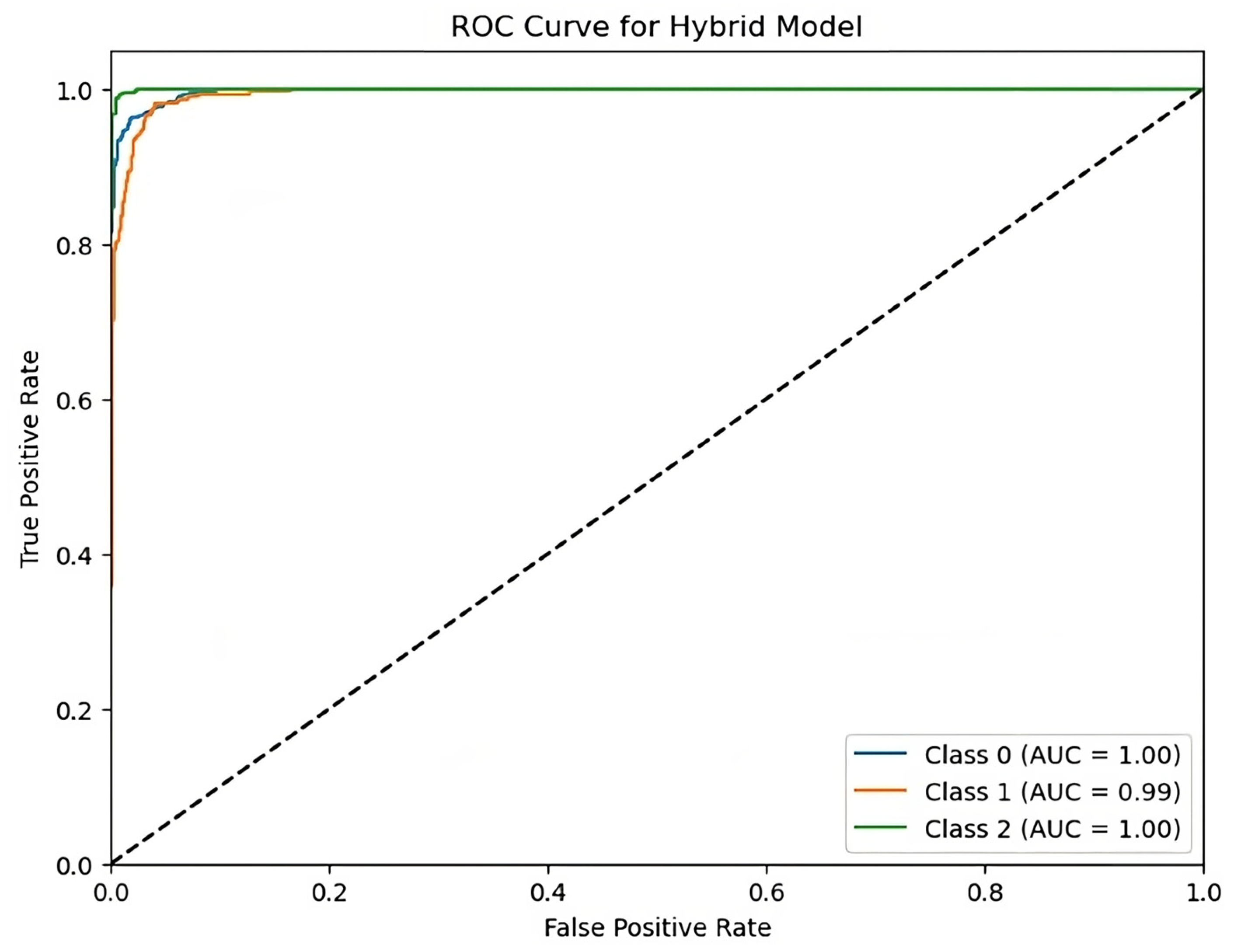
Figure 9 shows the ROC curve measures the performance of a hybrid model in a multi-class classification test. The X-axis indicates the false positive rate (FPR), while the Y-axis reflects the true positive rate as depicted in Figure 9. Each curve represents a class (class 0, class 1, class 2), with the following AUC (area under curve) scores: class 0 = 1.00, class 1 = 0.99, and class 2 = 1.00. The curves’ proximity to the top-left corner and high AUC values suggest good model performance and discriminatory abilities across all classes.
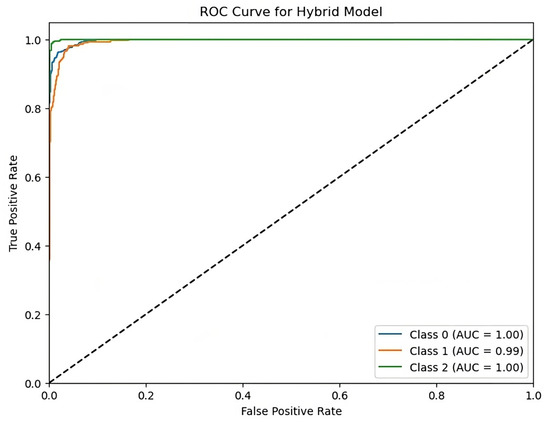
Figure 9.
ROC curve for hybrid model with AUC scores.
The hybrid model’s results demonstrate outstanding accuracy in predicting stroke risk levels, at 92%. The model has well-balanced accuracy, recall, and F1-scores, surpassing individual RNN, LSTM, and CNN models. The ROC curve demonstrates the hybrid model’s capacity to accurately distinguish across risk levels, with AUC scores near to one for all classes. These findings support the model’s robustness and promise for improving stroke risk prediction and early intervention options.
8. Features of the Proposed System and Its Utility
The recommended Brain Stroke Discovery and Forecast Framework has three significant advantages:
Personalized Risk Assessment: The system utilized is complex, since it coordinates different boundaries like segment data, wellbeing markers, and way of life qualities to give customized stroke risk appraisals [94]. This granularity empowers medical services specialists to make individualized treatment regimens for every patient, considering exceptional gamble factors, for example, BMI, glucose levels, and hypertension [95]. Customized assessments likewise work on quiet contribution by giving ideas that are custom-made to their particular wellbeing profiles, bringing about better adherence to protection measures and treatment programs [96].
Early Intervention: By recognizing stroke risk at low, medium, and undeniable levels, the model takes into account brief intercession, especially for people at medium or high gamble. Early location empowers the execution of precautionary intercessions, for example, way of life changes, prescriptions, or clinical medicines, which emphatically lessens the gamble or seriousness of a stroke [97]. People delegated high-risk, for instance, may be focused on for thorough reconnaissance and early treatment intercessions, hence diminishing long-term outcomes [97].
Scalability: The framework’s ability to take care of and assess tremendous datasets makes it proper for an assortment of medical services settings. It could be carried out in significant medical clinics with enormous patient datasets and extended to country regions with negligible assets [93]. Likewise, the model’s engineering advances multi-provincial reconciliation, considering relative examination and facilitated medical care programs across fluctuated populations [98].
Cost-Effective: Mechanized risk forecasts limit dependence on extensive analytic testing, subsequently streamlining medical care spending [99]. The methodology empowers powerful asset use by zeroing specifically on medicines for high-risk individuals. This cost-adequacy is particularly valuable for underfunded medical services foundations since it considers broad protection care while keeping up with quality [100].
Continuous Learning: The model has a continuous training system, which permits it to adjust and work on as new information is given [41]. This adaptability ensures that the framework stays fruitful in powerful medical care settings in what personas and hazard factors could modify over the long term [101]. By gaining from an assortment of datasets, the model might detect recent fads and work on its gauges’ exactness.
Integration with EHR: The consistent connection point with EHRs brings about worked-on information passage and result methods. This network not only considers constant updates on understanding gamble levels but also assists with holding a total wellbeing history [102]. By incorporating EHR information, the framework gives steady and precise figures, permitting medical care suppliers to quickly pursue instructed decisions [102]. It likewise takes into consideration longitudinal examinations, which inspect patient outcomes over the long term and assist with further developing medical services systems.
9. Challenges in the Hybrid Deep Learning Model for Brain Stroke Detection and Prediction
The challenges of the hybrid DL model for brain stroke detection and prediction come from the complexity of medical data, model tuning, and real-world application. Addressing these problems is critical for increasing model performance and providing accurate stroke prediction in medical applications.
9.1. Data Imbalance
Stroke datasets frequently suffer from class imbalance, with non-stroke cases greatly outnumbering stroke cases. This might cause the model to be biased toward the majority class, resulting in low sensitivity in recognizing stroke patients. Oversampling, undersampling, and synthetic data synthesis (such as SMOTE) are necessary to address this problem. However, artificially balancing the data may create noise or impair the model’s ability to generalize to real-world situations. Furthermore, class imbalance affects performance parameters like precision, recall, and F1-score, necessitating a thorough study in addition to accuracy.
9.2. Limited Availability of Medical Datasets
High-quality medical datasets are difficult to get due to privacy concerns and rigorous rules on patient data exchange. Many hospitals and institutes have restrictions on making patient information publicly available, which results in a scarcity of varied and large-scale datasets for training DL models. This constraint reduces the capacity to create strong and generalizable models. Furthermore, publicly accessible stroke datasets may be obsolete, missing values, or lacking essential properties, rendering them unsuitable for real-world clinical applications. Collaborations with medical institutions, as well as the usage of federated learning methodologies, may help to solve this difficulty.
9.3. Hyperparameter Tuning Complexity
Training a hybrid model entails adjusting a variety of hyperparameters, including learning rates, batch sizes, dropout rates, and network design. Fine-tuning these parameters necessitates substantial testing and domain knowledge, making the model creation process time consuming. Furthermore, deep learning models may require distinct hyperparameter setups depending on the dataset, making transferability problematic. Automated hyperparameter optimization techniques, such as Bayesian optimization or evolutionary algorithms, can aid, but they incur additional computing expenses.
9.4. Overfitting on Training Data
Overfitting happens when a model performs extraordinarily well on training data but suffers in untested real-world scenarios. This might be caused by excessive model complexity, inadequate training samples, or noise in the dataset. Overfitting produces incorrect predictions in clinical contexts, limiting the model’s utility. Regularization techniques like dropout, weight decay, and data augmentation can assist in minimizing overfitting, but they need precise parameter tweaking. Furthermore, cross-validation procedures must be used to confirm that the model generalizes effectively across diverse patient groups.
Addressing these challenges is crucial for increasing the model’s dependability, assuring accurate stroke prediction, and enabling practical use in clinical settings. Using sophisticated data-balancing approaches, optimizing model hyperparameters, and utilizing joint research efforts can dramatically improve predictive performance. A well-optimized hybrid deep learning model has the potential to enable early and precise stroke diagnosis, resulting in enhanced patient care and medical decision-making.
10. Future Scope
The brain stroke prediction framework, which combines a hybrid DL model with metaheuristic optimization, has the potential to greatly enhance healthcare by allowing for early identification and individualized intervention. Future developments may focus on incorporating continuous monitoring via wearable devices such as smartwatches and fitness trackers, allowing for real-time gathering of vital health parameters such as blood pressure, heart rate, and glucose levels. This live data might be incorporated into the prediction model, allowing for quick risk assessments and proactive preventative actions, especially for high-risk people with a history of transient ischemic attacks (TIAs) or early stroke symptoms.
To improve practical applicability, the model’s real-time effectiveness in clinical settings must be rigorously tested. To ensure dependability in various clinical situations, deployment in real-world hospital settings necessitates rigorous validation across a broad patient population. Addressing patient demographic variability, such as age, gender, ethnicity, and lifestyle characteristics, will be critical for maintaining accuracy and avoiding biases in predictions. Ensuring that the model adapts to a wide range of patient characteristics would enhance its use as a generalizable and egalitarian diagnostic tool. Furthermore, the system should be tuned to discriminate between distinct stroke subtypes, such as ischemic and hemorrhagic strokes, for more accurate risk prediction and treatment planning.
The use of explainable AI (XAI) approaches will be crucial in increasing the framework’s transparency, allowing healthcare practitioners to evaluate forecasts and comprehend underlying risk factors for individual patients. This will increase trust, improve clinical decision-making, and facilitate regulatory approval. Furthermore, more study into hybrid metaheuristic optimization methodologies could increase the model’s efficiency by lowering computing complexity while retaining high prediction accuracy, especially in resource-constrained environments. Integrating EHRs with AI-driven decision support systems can improve stroke risk assessment, allowing neurologists to make better-informed and faster judgments.
Ethical considerations must also be addressed because AI-powered healthcare systems handle very sensitive medical information. Ensuring fairness, reducing biases in prediction models, and eliminating inadvertent discrimination in stroke risk assessment will be critical. Furthermore, patient permission, data ownership, and responsibility in AI-generated judgments must be explicitly stated in order to comply with ethical medical standards. Strict compliance with data security requirements such as Health Insurance Portability and Accountability Act (HIPAA), General Data Protection Regulation (GDPR), and Health Level Seven International Fast Healthcare Interoperability Resources (HL7 FHIR) will be required to ensure patient privacy while allowing for wider implementation. Expanding the framework’s capabilities to improve stroke recurrence prediction and post-stroke rehabilitation recommendations might result in a holistic stroke prevention strategy. Integrating this technology into telemedicine infrastructures can improve remote consultations, especially in underprivileged areas, by expanding access to powerful predictive analytics.
Finally, scalable solutions that use cloud-based platforms and international healthcare data partnerships may enable population-level research, eventually enhancing public health outcomes. By encouraging global collaboration in predicting stroke healthcare, the framework has the potential to pave the way for precision neurology, AI-assisted early stroke therapies, and individualized treatment options globally. With these advances, this approach is poised to transform stroke prevention and management in modern healthcare.
11. Conclusions
The brain stroke prediction framework, which employs hybrid DL methods (LSTM, CNN, and RNN), offers a promising approach to the early identification and prediction of stroke risk. Using these complicated neural network designs, the framework can assess numerical data from clinical and demographic characteristics and categorize stroke risk as low, medium, or high. This hybrid technique combines each model’s capabilities, with LSTM excelling in time-series prediction, CNN effectively recognizing spatial patterns, and RNN competent at capturing sequential correlations in data. The framework’s ability to accurately assess and identify risk levels demonstrates its potential to increase diagnostic precision and allow for prompt actions. Deep learning models are more resilient in handling complicated correlations between input variables, giving them an edge over classic stroke prediction algorithms.
The combination (hybrid) model has an accuracy of 92%, with 90% precision, 91% recall, and a 90% F1-score. This high degree of performance demonstrates the hybrid approach’s usefulness in making accurate stroke risk forecasts. Furthermore, the framework’s scalability and integration features enable it to be used in a variety of healthcare contexts, including individual patient evaluations and population-level health monitoring. With the increasing availability of real-time monitoring via wearable devices and connection with EHR, this technique has the potential to improve stroke prediction and prevention. Its emphasis on individualized healthcare means that therapies are adapted to each patient’s unique risk profile, leading to a more proactive approach to stroke therapy. As AI and deep learning technologies advance, the framework’s capabilities will expand, paving the path for complete, data-driven solutions that improve patient outcomes, lower healthcare costs, and help in the overall battle against stroke.
However, there are several obstacles and restrictions to consider. The model’s accuracy and dependability are determined by the quality and amount of accessible data, and data biases may have an impact on the model’s predictions. Furthermore, the intricate structure of deep learning models necessitates large computational resources, making real-time applications hard in resource-constrained environments. Furthermore, the models’ interpretability remains a worry, as healthcare practitioners may struggle to grasp the logic behind the model’s predictions, therefore reducing confidence and acceptance in clinical settings.
Future research might focus on enhancing model explainability and combining various datasets to increase generalizability across populations. The use of other data sources, such as genetic information and lifestyle variables, may improve stroke risk prediction. Furthermore, investigating hybrid models that integrate deep learning with other machine learning approaches may enhance performance and resilience. The continuing improvement of wearable devices and IoT (Internet of Things) technology opens up the possibility of real-time stroke monitoring and prevention, enhancing the framework’s potential to transform stroke management in the medical industry.
Author Contributions
Conceptualization, G.T., R.R. and C.P.; methodology, G.T. and R.R.; validation, G.T., R.R., C.P. and P.V.; writing—original draft preparation, G.T., R.R. and C.P.; writing—review and editing, G.T., R.R. and P.V.; visualization, G.T. and R.R.; supervision, P.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data presented in the study are openly available online on https://www.kaggle.com/datasets/fedesoriano/stroke-prediction-dataset (accessed on 1 November 2024).
Acknowledgments
The authors would like to thank the owners of Brain Stroke Healthcare dataset for providing the dataset free of cost for education and research purpose.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Notations | Abbreviations |
| RNN | Recurrent Neural Network |
| LSTM | Long Short Term Memory |
| LR | Logistics regression |
| CNN | Cconvolutional neural networks |
| EHR | Electronic Health Record |
| DT | Decision tree classifier |
| TIAs | transient ischemic assaults |
| WHO | World Health Organization |
| CT | Computer Tomography |
| AIML | artificial intelligence and machine learning |
| AI | Artificial intelligence |
| RF | Random forest |
| NB | Naive Bayes |
| RNN | Recurrent neural networks |
| SVM | Support vector machine |
| ANFIS | Adaptive new fuzzy inference system |
| MRI | Magnetic Resonance Imaging |
| SMOTE | Synthetic minority oversampling technique |
| PCA | Principal component analysis |
| ANN | Artificial neural network |
| DNN | Deep neural network |
| EEG | Electroencephalogram |
| HDTL-SRP | Hybrid deep transfer learning-based stroke risk prediction |
| FCN | Fully convolutional networks |
| BMI | Body mass index |
| SGD | Stochastic gradient descent |
| AdaBoost | AdaBoot classifier |
| QDA | Quadratic discriminant analysis |
| MLP | Multilayer perceptron classifier |
| GBC | Gradient boosting classifier |
| XGB | XGBoost classifier |
| RLR | Regularized logistic regression |
| ROS | Random oversampling |
| RUS | Random undersampling |
References
- Rahman, S.; Hasan, M.; Sarkar, A.K. Prediction of brain stroke using machine learning algorithms and deep neural network techniques. Eur. J. Electr. Eng. Comput. Sci. 2023, 7, 23–30. [Google Scholar] [CrossRef]
- Lakkshmanan, A.; Priyanka, N.; Anand, D.B.; Nagaraju, K.; Naidu, U.G. A novel machine learning-based stroke prediction system using magnetic resonance imaging and adaptive new fuzzy inference system. Int. J. Intell. Syst. Appl. Eng. 2024, 12, 576–585. [Google Scholar]
- Qasim, A.N.; Alani, S.; Mahmood, S.N.; Mohammed, S.S.; Aziz, D.A.; Ata, K.I.M. Enhancing Brain Stroke Detection: A Novel Deep Neural Network with Weighted Binary Cross Entropy Training. Rev. D’Intelligence Artif. 2024, 38, 777. [Google Scholar] [CrossRef]
- Choi, Y.A.; Park, S.J.; Jun, J.A.; Pyo, C.S.; Cho, K.H.; Lee, H.S.; Yu, J.H. Deep learning-based stroke disease prediction system using real-time bio signals. Sensors 2021, 21, 4269. [Google Scholar] [CrossRef]
- Gaidhani, B.R.; Rajamenakshi, R.R.; Sonavane, S. Brain stroke detection using convolutional neural network and deep learning models. In Proceedings of the 2019 2nd International Conference on Intelligent Communication and Computational Techniques (ICCT), Jaipur, India, 28–29 September 2019; IEEE: New York, NY, USA; pp. 242–249. [Google Scholar]
- Chen, J.; Chen, Y.; Li, J.; Wang, J.; Lin, Z.; Nandi, A.K. Stroke risk prediction with hybrid deep transfer learning framework. IEEE J. Biomed. Health Inform. 2021, 26, 411–422. [Google Scholar] [CrossRef]
- Karthik, R.; Menaka, R.; Johnson, A.; Anand, S. Neuroimaging and deep learning for brain stroke detection-A review of recent advancements and future prospects. Comput. Methods Programs Biomed. 2020, 197, 105728. [Google Scholar] [CrossRef]
- Tan, K.; Marvell, Y.A.; Gunawan, A.A.S. Early ischemic stroke detection using deep learning: A systematic literature review. In Proceedings of the 2023 International Seminar on Application for Technology of Information and Communication (iSemantic), Semarang, Indonesia, 16–17 September 2023; IEEE: New York, NY, USA; pp. 7–11. [Google Scholar]
- Cui, L.; Fan, Z.; Yang, Y.; Liu, R.; Wang, D.; Feng, Y.; Lu, J.; Fan, Y. Deep learning in ischemic stroke imaging analysis: A comprehensive review. BioMed Res. Int. 2022, 2022, 2456550. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and treatment of stroke: Present status and future perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Abedi, V.; Khan, A.; Chaudhary, D.; Misra, D.; Avula, V.; Mathrawala, D.; Kraus, C.; Marshall, K.A.; Chaudhary, N.; Li, X.; et al. Using artificial intelligence for improving stroke diagnosis in emergency departments: A practical framework. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420938962. [Google Scholar] [CrossRef]
- Yalçın, S.; Vural, H. Brain stroke classification and segmentation using encoder-decoder based deep convolutional neural networks. Comput. Biol. Med. 2022, 149, 105941. [Google Scholar] [CrossRef]
- Liu, L.; Kurgan, L.; Wu, F.X.; Wang, J. Attention convolutional neural network for accurate segmentation and quantification of lesions in ischemic stroke disease. Med. Image Anal. 2020, 65, 101791. [Google Scholar] [CrossRef]
- Kumar, M.A.; Abirami, N.; Prasad, M.G.; Mohankumar, M. Stroke disease prediction based on ECG signals using deep learning techniques. In Proceedings of the 2022 International Conference on Computational Intelligence and Sustainable Engineering Solutions (CISES), Greater Noida, India, 20–21 May 2022; IEEE: New York, NY, USA; pp. 453–458. [Google Scholar]
- Alsubaie, M.G.; Luo, S.; Shaukat, K. Alzheimer’s Disease Detection Using Deep Learning on Neuroimaging: A Systematic Review. Mach. Learn. Knowl. Extr. 2024, 6, 464–505. [Google Scholar] [CrossRef]
- Lameesa, A.; Hoque, M.; Alam, M.S.B.; Ahmed, S.F.; Gandomi, A.H. Role of metaheuristic algorithms in healthcare: A comprehensive investigation across clinical diagnosis, medical imaging, operations management, and public health. J. Comput. Des. Eng. 2024, 11, 223–247. [Google Scholar] [CrossRef]
- Rajwar, K.; Deep, K.; Das, S. An exhaustive review of the metaheuristic algorithms for search and optimization: Taxonomy, applications, and open challenges. Artif. Intell. Rev. 2023, 56, 13187–13257. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.H.; Wu, Y.H.; Wu, T.W.; Chu, H.Y.; Shih, M.H. Stroke Prediction Using Deep Learning and Transfer Learning Approaches. IEEE Access 2024, 12, 130091–130104. [Google Scholar] [CrossRef]
- Hairani, H.; Widiyaningtyas, T.; Prasetya, D.D. Feature Selection and Hybrid Sampling with Machine Learning Methods for Health Data Classification. Rev. D’Intelligence Artif. 2024, 38, 1255–1261. [Google Scholar] [CrossRef]
- Liu, X.; Song, L.; Liu, S.; Zhang, Y. A review of deep-learning-based medical image segmentation methods. Sustainability 2021, 13, 1224. [Google Scholar] [CrossRef]
- Asadi, F.; Rahimi, M.; Daeechini, A.H.; Paghe, A. The most efficient machine learning algorithms in stroke prediction: A systematic review. Health Sci. Rep. 2024, 7, e70062. [Google Scholar] [CrossRef]
- Shichkina, Y.; Irishina, Y.; Stanevich, E.; de Jesus Plasencia Salgueiro, A. Application of genetic algorithms for the selection of neural network architecture in the monitoring system for patients with Parkinson’s disease. Appl. Sci. 2021, 11, 5470. [Google Scholar] [CrossRef]
- Kaifi, R. A review of recent advances in brain tumor diagnosis based on AI-based classification. Diagnostics 2023, 13, 3007. [Google Scholar] [CrossRef]
- Yao, A.D.; Cheng, D.L.; Pan, I.; Kitamura, F. Deep learning in neuroradiology: A systematic review of current algorithms and approaches for the new wave of imaging technology. Radiol. Artif. Intell. 2020, 2, e190026. [Google Scholar] [CrossRef]
- Stroke Prediction Dataset. Available online: https://www.kaggle.com/datasets/fedesoriano/stroke-prediction-dataset (accessed on 15 October 2024).
- Reeves, M.J.; Bushnell, C.D.; Howard, G.; Gargano, J.W.; Duncan, P.W.; Lynch, G.; Khatiwoda, A.; Lisabeth, L. Sex differences in stroke: Epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008, 7, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Girijala, R.L.; Sohrabji, F.; Bush, R.L. Sex differences in stroke: Review of current knowledge and evidence. Vasc. Med. 2017, 22, 135–145. [Google Scholar] [CrossRef]
- Hammond, G.; Luke, A.A.; Elson, L.; Towfighi, A.; Joynt Maddox, K.E. Urban-rural inequities in acute stroke care and in-hospital mortality. Stroke 2020, 51, 2131–2138. [Google Scholar] [CrossRef]
- Driga, M.P.; Catalin, B.; Olaru, D.G.; Slowik, A.; Plesnila, N.; Hermann, D.M.; Popa-Wagner, A. The need for new biomarkers to assist with stroke prevention and prediction of post-stroke therapy based on plasma-derived extracellular vesicles. Biomedicines 2021, 9, 1226. [Google Scholar] [CrossRef]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke risk factors, genetics, and prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef]
- Grelli, K.N.; Gindville, M.C.; Walker, C.H.; Jordan, L.C. Association of blood pressure, blood glucose, and temperature with neurological outcome after childhood stroke. JAMA Neurol. 2016, 73, 829–835. [Google Scholar] [CrossRef]
- Nielsen, A.; Hansen, M.B.; Tietze, A.; Mouridsen, K. Prediction of tissue outcome and assessment of treatment effect in acute ischemic stroke using deep learning. Stroke 2018, 49, 1394–1401. [Google Scholar] [CrossRef]
- Tusher, A.N.; Sadik, M.S.; Islam, M.T. Early brain stroke prediction using machine learning. In Proceedings of the 2022 11th International Conference on System Modeling & Advancement in Research Trends (SMART), Moradabad, India, 16–17 December 2022; IEEE: New York, NY, USA; pp. 1280–1284. [Google Scholar]
- Bahaddad, A.A.; Ragab, M.; Ashary, E.B.; Khalil, E.M. Metaheuristics with Deep Learning-Enabled Parkinson’s Disease Diagnosis and Classification Model. J. Healthc. Eng. 2022, 2022, 9276579. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Kiral-Kornek, I.; Harrer, S. ChronoNet: A deep recurrent neural network for abnormal EEG identification. In Proceedings of the Artificial Intelligence in Medicine: 17th Conference on Artificial Intelligence in Medicine, AIME 2019, Poznan, Poland, 26–29 June 2019; Proceedings 17. Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 47–56. [Google Scholar]
- Sheela Lavanya, J.M.; Subbulakshmi, P. Unveiling the potential of machine learning approaches in predicting the emergence of stroke at its onset: A predicting framework. Sci. Rep. 2024, 14, 20053. [Google Scholar]
- Emon, M.U.; Keya, M.S.; Meghla, T.I.; Rahman, M.M.; Al Mamun, M.S.; Kaiser, M.S. Performance analysis of machine learning approaches in stroke prediction. In Proceedings of the 2020 4th International Conference on Electronics, Communication and Aerospace Technology (ICECA), Coimbatore, India, 5–7 November 2020; IEEE: New York, NY, USA; pp. 1464–1469. [Google Scholar]
- Tazin, T.; Alam, M.N.; Dola, N.N.; Bari, M.S.; Bourouis, S.; Monirujjaman Khan, M. Stroke disease detection and prediction using robust learning approaches. J. Healthc. Eng. 2021, 2021, 7633381. [Google Scholar] [CrossRef]
- Kansadub, T.; Thammaboosadee, S.; Kiattisin, S.; Jalayondeja, C. Stroke risk prediction model based on demographic data. In Proceedings of the 2015 8th Biomedical Engineering International Conference (BMEiCON), Pattaya, Thailand, 25–27 November 2015; IEEE: New York, NY, USA; pp. 1–3. [Google Scholar]
- Anitescu, C.; Atroshchenko, E.; Alajlan, N.; Rabczuk, T. Artificial neural network methods for the solution of second order boundary value problems. Comput. Mater. Contin. 2019, 59, 345–359. [Google Scholar] [CrossRef]
- Nwosu, C.S.; Dev, S.; Bhardwaj, P.; Veeravalli, B.; John, D. Predicting stroke from electronic health records. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; IEEE: New York, NY, USA; pp. 5704–5707. [Google Scholar]
- Dev, S.; Wang, H.; Nwosu, C.S.; Jain, N.; Veeravalli, B.; John, D. A predictive analytics approach for stroke prediction using machine learning and neural networks. Healthc. Anal. 2022, 2, 100032. [Google Scholar] [CrossRef]
- Wu, Y.; Fang, Y. Stroke prediction with machine learning methods among older Chinese. Int. J. Environ. Res. Public Health 2020, 17, 1828. [Google Scholar] [CrossRef]
- Guo, H.; Zhuang, X.; Rabczuk, T. A deep collocation method for the bending analysis of Kirchhoff plate. arXiv 2021, arXiv:2102.02617. [Google Scholar] [CrossRef]
- Arshad, F.M.; Sharmila, K. Advances in Machine Learning for Stroke Prediction: A Comprehensive Review. In Proceedings of the 2024 8th International Conference on I-SMAC (IoT in Social, Mobile, Analytics and Cloud) (I-SMAC), Kirtipur, Nepal, 3–5 October 2024; IEEE: New York, NY, USA; pp. 1798–1802. [Google Scholar]
- Chantamit-O-Pas, P.; Goyal, M. Prediction of stroke using deep learning model. In Proceedings of the Neural Information Processing: 24th International Conference, ICONIP 2017, Guangzhou, China, 14–18 November 2017; Proceedings, Part V 24. Springer International Publishing: Berlin/Heidelberg, Germany; pp. 774–781. [Google Scholar]
- Hossain, M.M.; Ahmed, M.M.; Nafi, A.A.N.; Islam, M.R.; Ali, M.S.; Haque, J.; Miah, M.S.; Rahman, M.M.; Islam, M.K. A novel hybrid ViT-LSTM model with explainable AI for brain stroke detection and classification in CT images: A case study of Rajshahi region. Comput. Biol. Med. 2025, 186, 109711. [Google Scholar] [CrossRef] [PubMed]
- Bathla, P.; Kumar, R. A hybrid system to predict brain stroke using a combined feature selection and classifier. Intell. Med. 2024, 4, 75–82. [Google Scholar] [CrossRef]
- Alawneh, J.A.; Jones, P.S.; Mikkelsen, I.K.; Cho, T.H.; Siemonsen, S.; Mouridsen, K.; Ribe, L.; Morris, R.S.; Hjort, N.; Antoun, N.; et al. Infarction of ‘non-core–non-penumbral’tissue after stroke: Multivariate modelling of clinical impact. Brain 2011, 134, 1765–1776. [Google Scholar] [CrossRef]
- Dhillon, S.; Bansal, C.; Sidhu, B. Machine learning based approach using XGboost for heart stroke prediction. In Proceedings of the International Conference on Emerging Technologies: AI, IoT, and CPS for Science & Technology Applications, Chandigarh, India, 6–7 September 2021; pp. 1–6. [Google Scholar]
- Widianto, M.H.; Putra, V.H.C.; Kenardi, M.P. Interpretation and Performance Machine Learning Model for Detection of Brain Stroke Based on Classifier. In Proceedings of the 2023 6th International Conference on Information and Communications Technology (ICOIACT), Yogyakarta, Indonesia, 10 November 2023; IEEE: New York, NY, USA; pp. 183–188. [Google Scholar]
- Chin, C.L.; Lin, B.J.; Wu, G.R.; Weng, T.C.; Yang, C.S.; Su, R.C.; Pan, Y.J. An automated early ischemic stroke detection system using CNN deep learning algorithm. In Proceedings of the 2017 IEEE 8th International Conference on Awareness Science and Technology (iCAST), Taichung, Taiwan, 8–10 November 2017; IEEE: New York, NY, USA; pp. 368–372. [Google Scholar]
- Biswas, N.; Uddin, K.M.M.; Rikta, S.T.; Dey, S.K. A comparative analysis of machine learning classifiers for stroke prediction: A predictive analytics approach. Healthc. Anal. 2022, 2, 100116. [Google Scholar] [CrossRef]
- Khosla, A.; Cao, Y.; Lin, C.C.Y.; Chiu, H.K.; Hu, J.; Lee, H. An integrated machine learning approach to stroke prediction. In Proceedings of the 16th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, Washington, DC, USA, 25–28 July 2010; pp. 183–192. [Google Scholar]
- Hung, C.Y.; Lin, C.H.; Lan, T.H.; Peng, G.S.; Lee, C.C. Development of an intelligent decision support system for ischemic stroke risk assessment in a population-based electronic health record database. PLoS ONE 2019, 14, e0213007. [Google Scholar] [CrossRef]
- Chandra, J.; Tjoaquinn, C. Predicting Stroke Diagnosis Using Hyperparameter Tuning. In Proceedings of the 2024 7th International Conference of Computer and Informatics Engineering (IC2IE), Bali, Indonesia, 12–13 September 2024; IEEE: New York, NY, USA; pp. 1–7. [Google Scholar]
- Singh, M.S.; Choudhary, P. Stroke prediction using artificial intelligence. In Proceedings of the 2017 8th Annual Industrial Automation and Electromechanical Engineering Conference (IEMECON), Bangkok, Thailand, 16–18 August 2017; IEEE: New York, NY, USA; pp. 158–161. [Google Scholar]
- Rao, M.G.; Reddy, K.H.K.; Pawar, S.; Kotian, K.R.; Hegde, D.P. Employing Machine Learning in A Multimodal Approach for Brain Stroke Modelling and Identification. In Proceedings of the 2023 IEEE International Conference on Distributed Computing, VLSI, Electrical Circuits and Robotics (DISCOVER), Mangalore, India,, 13–14 October 2023; IEEE: New York, NY, USA; pp. 219–224. [Google Scholar]
- Engedal, T.S.; Hjort, N.; Hougaard, K.D.; Simonsen, C.Z.; Andersen, G.; Mikkelsen, I.K.; Boldsen, J.K.; Eskildsen, S.F.; Hansen, M.B.; Angleys, H.; et al. Transit time homogenization in ischemic stroke–A novel biomarker of penumbral microvascular failure? J. Cereb. Blood Flow. Metab. 2018, 38, 2006–2020. [Google Scholar] [CrossRef] [PubMed]
- Sailasya, G.; Kumari, G.L.A. Analyzing the performance of stroke prediction using ML classification algorithms. Int. J. Adv. Comput. Sci. Appl. 2021, 12. [Google Scholar] [CrossRef]
- Chadaga, K.; Sampathila, N.; Prabhu, S.; Chadaga, R. Multiple explainable approaches to predict the risk of stroke using artificial intelligence. Information 2023, 14, 435. [Google Scholar] [CrossRef]
- Islam, M.R.; Rahman, M.S.; Paul, R.R.; Hosen, M.F.; Khairunnahar, M.; Nayeem, M.J. Intensify Stroke Prediction Model Associated with Hypertensive State. In Proceedings of the 2024 3rd International Conference on Advancement in Electrical and Electronic Engineering (ICAEEE), Gazipur, Bangladesh, 25–27 April 2024; IEEE: New York, NY, USA; pp. 1–5. [Google Scholar]
- Ahmed, F.U.; Mahi, F.H.; Suha, S.A.; Islam, M.N. Development of an Intelligent System for Brain Stroke Prediction using Ensemble Feature Selection and Machine Learning Technique. In Proceedings of the 2023 26th International Conference on Computer and Information Technology (ICCIT), Cox’s Bazar, Bangladesh, 13–15 December 2023; IEEE: New York, NY, USA; pp. 1–6. [Google Scholar]
- Fang, G.; Huang, Z.; Wang, Z. Predicting ischemic stroke outcome using deep learning approaches. Front. Genet. 2022, 12, 827522. [Google Scholar] [CrossRef] [PubMed]
- Bala, N. Identify Brain Stroke in Severity and Symptoms in Sex Differences Using AIML. In Proceedings of the 2023 3rd International Conference on Technological Advancements in Computational Sciences (ICTACS), Tashkent, Uzbekistan, 1–3 November 2023; IEEE: New York, NY, USA; pp. 1554–1559. [Google Scholar]
- Rahman, M.M. A web-based heart disease prediction system using machine learning algorithms. Netw. Biol. 2022, 12, 64. [Google Scholar]
- Parashar, G. Brain stroke detection and prediction using machine learning approach: A cloud deployment perspective. In Proceedings of the 2023 International Conference on Circuit Power and Computing Technologies (ICCPCT), Kollam, India, 10–11 August 2023; IEEE: New York, NY, USA; pp. 1705–1714. [Google Scholar]
- Mary, M.F.; Rajeswari, M.; Amalasweena, M. Neural Network-based Prognostic Model for Cerebrovascular Accident using CT Scans. In Proceedings of the 2023 International Conference on Sustainable Computing and Data Communication Systems (ICSCDS), Erode, India, 23–25 March 2023; IEEE: New York, NY, USA; pp. 497–502. [Google Scholar]
- Payabvash, S.; Qureshi, M.H.; Khan, S.M.; Khan, M.; Majidi, S.; Pawar, S.; Qureshi, A.I. Differentiating intraparenchymal hemorrhage from contrast extravasation on post-procedural noncontrast CT scan in acute ischemic stroke patients undergoing endovascular treatment. Neuroradiology 2014, 56, 737–744. [Google Scholar] [CrossRef]
- Gurjar, R.; Sahana, H.K.; Neelambika, C.; Sathish, S.B.; Ramys, S. Stroke risk prediction using machine learning algorithms. Int. J. Sci. Res. Comput. Sci. Eng. Inf. Technol. 2022, 8, 20–25. [Google Scholar] [CrossRef]
- Sahriar, S.; Akther, S.; Mauya, J.; Amin, R.; Mia, M.S.; Ruhi, S.; Reza, M.S. Unlocking stroke prediction: Harnessing projection-based statistical feature extraction with ML algorithms. Heliyon 2024, 10, e27411. [Google Scholar] [CrossRef]
- García-Temza, L.; Risco-Martín, J.L.; Ayala, J.L.; Roselló, G.R.; Camarasaltas, J.M. Comparison of different machine learning approaches to model stroke subtype classification and risk prediction. In Proceedings of the 2019 Spring Simulation Conference (SpringSim), Tucson, AZ, USA, 29 April–2 May 2019; IEEE: New York, NY, USA; pp. 1–10. [Google Scholar]
- Pedersen, A.; Stanne, T.M.; Nilsson, S.; Klasson, S.; Rosengren, L.; Holmegaard, L.; Jood, K.; Blennow, K.; Zetterberg, H.; Jern, C. Circulating neurofilament light in ischemic stroke: Temporal profile and outcome prediction. J. Neurol. 2019, 266, 2796–2806. [Google Scholar] [CrossRef]
- Kwon, H.S.; Lee, D.; Lee, M.H.; Yu, S.; Lim, J.S.; Yu, K.H.; Oh, M.S.; Lee, J.S.; Hong, K.S.; Lee, E.J.; et al. Post-stroke cognitive impairment as an independent predictor of ischemic stroke recurrence: PICASSO sub-study. J. Neurol. 2020, 267, 688–693. [Google Scholar] [CrossRef]
- Kokkotis, C.; Giarmatzis, G.; Giannakou, E.; Moustakidis, S.; Tsatalas, T.; Tsiptsios, D.; Vadikolias, K.; Aggelousis, N. An explainable machine learning pipeline for stroke prediction on imbalanced data. Diagnostics 2022, 12, 2392. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Parsi, B.; Speier, W.; Arnold, C.; Lou, M.; Scalzo, F. LSTM network for prediction of hemorrhagic transformation in acute stroke. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Shenzhen, China, 13–17 October 2019; Springer International Publishing: Cham, Switzerland, 2019; pp. 177–185. [Google Scholar]
- Hakim, M.A.; Hasan, M.Z.; Alam, M.M.; Hasan, M.M.; Huda, M.N. An efficient modified bagging method for early prediction of brain stroke. In Proceedings of the 2019 International Conference on Computer, Communication, Chemical, Materials and Electronic Engineering (IC4ME2), Rajshahi, Bangladesh, 11–12 July 2019; IEEE: New York, NY, USA; pp. 1–4. [Google Scholar]
- Ananya, P.R.; Pachisia, V.; Ushasukhanya, S. Optimization of CNN in capsule networks for Alzheimer’s disease prediction using CT images. In Proceedings of International Conference on Deep Learning, Computing and Intelligence: ICDCI 2021; Springer Nature: Singapore, 2022; pp. 551–560. [Google Scholar]
- Anusha Bai, R.; Sangeetha, V. Anusha Bai, R.; Sangeetha, V. A comparative study on brain intracerebral hemorrhage classification using head CT scan for stroke analysis. In International Conference on Soft Computing for Security Applications; Springer Nature: Singapore, 2023; pp. 633–649. [Google Scholar]
- Zhang, Y.; Yu, M.; Tong, C.; Zhao, Y.; Han, J. CA-UNet Segmentation makes a good ischemic stroke risk prediction. Interdiscip. Sci. Comput. Life Sci. 2024, 16, 58–72. [Google Scholar] [CrossRef]
- Weng, Y.T.; Chan, H.W.; Huang, T.Y. Automatic segmentation of brain tumor from 3D MR images using SegNet, U-Net, and PSP-Net. In Proceedings of the Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries: 5th International Workshop, BrainLes 2019, Held in Conjunction with MICCAI 2019, Shenzhen, China, 17 October 2019; Revised Selected Papers, Part II 5. Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 226–233. [Google Scholar]
- Odeh, S.; Kyriacou, E.; Pattichis, C.S. Using ANFIS to Predict the Long and Short Term Stroke Risk Based on Ultrasound Carotid Imaging and Clinical data of Initially Asymptomatic Patients. J. Millimeterwave Commun. Optim. Model. 2023, 3, 27–31. [Google Scholar]
- Hwangbo, L.; Kang, Y.J.; Kwon, H.; Lee, J.I.; Cho, H.J.; Ko, J.K.; Sung, S.M.; Lee, T.H. Stacking ensemble learning model to predict 6-month mortality in ischemic stroke patients. Sci. Rep. 2022, 12, 17389. [Google Scholar] [CrossRef] [PubMed]
- Bali, B.; Garba, E.J. Neuro-fuzzy approach for prediction of neurological disorders: A systematic review. SN Comput. Sci. 2021, 2, 307. [Google Scholar] [CrossRef]
- Hanifa, S.M.; Raja, S.K. Stroke risk prediction through non-linear support vector classification models. Int. J. Adv. Res. Comput. Sci. 2010, 1, 4753. [Google Scholar]
- Jia, Q.; Zheng, H.; Zhao, X.; Wang, C.; Liu, G.; Wang, Y.; Liu, L.; Li, H.; Zhong, L.; Wang, Y. Abnormal glucose regulation in patients with acute stroke across China: Prevalence and baseline patient characteristics. Stroke 2012, 43, 650–657. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, Y.; Maidaiti, X.; Liang, B.; Wei, Y.; Sun, F. Integrating Machine Learning into Statistical Methods in Disease Risk Prediction Modeling: A Systematic Review. Health Data Sci. 2024, 4, 0165. [Google Scholar] [CrossRef]
- Melnykova, N.; Patereha, Y.; Chereshchuk, L.O.; Sala, D. Machine Learning for Predicting Stroke Occurrences Using Imbalanced Data. In Data-Centric Business and Applications: Advancements in Information and Knowledge Management; Springer Nature: Cham, Switzerland, 2024; Volume 2, pp. 281–305. [Google Scholar]
- Priya, K.; Manju, T.; Chitra, R. Predictive model of stroke disease using hybrid neuro-genetic approach. Int. J. Eng. Comput. Sci. 2013, 2, 781–788. [Google Scholar]
- Ashrafuzzaman, M.; Saha, S.; Nur, K. Prediction of stroke disease using deep CNN based approach. J. Adv. Inf. Technol. 2022, 13, 604–613. [Google Scholar] [CrossRef]
- Telu, V.S.; Padimi, V.; Ningombam, D.D. Optimizing predictions of brain stroke using machine learning. J. Neutrosophic Fuzzy Syst. (JNFS) 2022, 2, 31–43. [Google Scholar]
- Rohit, A.; Chowdary, M.U.; Ashish, G.; Anitha, V.; Sana, S. ML approach for brain stroke prediction using IST database. Int. J. Eng. Appl. Sci. Technol. 2022, 7, 72–75. [Google Scholar] [CrossRef]
- Park, E.; Chang, H.J.; Nam, H.S. Use of machine learning classifiers and sensor data to detect neurological deficit in stroke patients. J. Med. Internet Res. 2017, 19, e120. [Google Scholar] [CrossRef] [PubMed]
- Mezher, M.A. Genetic folding (GF) algorithm with minimal kernel operators to predict stroke patients. Appl. Artif. Intell. 2022, 36, 2151179. [Google Scholar] [CrossRef]
- Uchida, K.; Kouno, J.; Yoshimura, S.; Kinjo, N.; Sakakibara, F.; Araki, H.; Morimoto, T. Development of machine learning models to predict probabilities and types of stroke at prehospital stage: The Japan urgent stroke triage score using machine learning (JUST-ML). Transl. Stroke Res. 2022, 13, 370–381. [Google Scholar] [CrossRef]
- Mostafa, S.A.; Elzanfaly, D.S.; Yakoub, A.E. A machine learning ensemble classifier for prediction of brain strokes. Int. J. Adv. Comput. Sci. Appl. 2022, 13. [Google Scholar] [CrossRef]
- Shoily, T.I.; Islam, T.; Jannat, S.; Tanna, S.A.; Alif, T.M.; Ema, R.R. Detection of stroke disease using machine learning algorithms. In Proceedings of the 2019 10th International Conference on Computing, Communication and Networking Technologies (ICCCNT), Kanpur, India, 6–8 July 2019; IEEE: New York, NY, USA; pp. 1–6. [Google Scholar]
- Stier, N.; Vincent, N.; Liebeskind, D.; Scalzo, F. Deep learning of tissue fate features in acute ischemic stroke. In Proceedings of the 2015 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Washington, DC, USA, 9–12 November 2015; IEEE: New York, NY, USA; pp. 1316–1321. [Google Scholar]
- Al-Maini, M.; Maindarkar, M.; Kitas, G.D.; Khanna, N.N.; Misra, D.P.; Johri, A.M.; Mantella, L.; Agarwal, V.; Sharma, A.; Singh, I.M.; et al. Artificial intelligence-based preventive, personalized and precision medicine for cardiovascular disease/stroke risk assessment in rheumatoid arthritis patients: A narrative review. Rheumatol. Int. 2023, 43, 1965–1982. [Google Scholar] [CrossRef]
- Lee, J.; Apriliyandi, K.; Gunawan, E.; Honova, S.M.; Salim, B.V.; Anggreainy, M.S.; Parmonangan, I.H. Comparative Analysis of Neural Network Method on Stroke Prediction. In Proceedings of the 2023 10th International Conference on ICT for Smart Society (ICISS), Bandung, Indonesia, 6–7 September 2023; IEEE: New York, NY, USA; pp. 1–6. [Google Scholar]
- Mavrogiorgou, A.; Kiourtis, A.; Kleftakis, S.; Mavrogiorgos, K.; Zafeiropoulos, N.; Kyriazis, D. A catalogue of machine learning algorithms for healthcare risk predictions. Sensors 2022, 22, 8615. [Google Scholar] [CrossRef]
- Gulzar, S.; Kiani, B.H.; Akram, R.W.; Hussein, A.M.; Alamri, A. Gender-Based Differences in Stroke Types and Risk Factors Among Young Adults: A Comparative Retrospective Analysis. J. Clin. Med. 2025, 14, 663. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).