Abstract
Machine learning-based tools are becoming increasingly popular in clinical practice. They offer new possibilities but are also limited in their reliability and accuracy. The present systematic review updates and discusses the existing literature regarding machine learning algorithm-based identification of cruciate ligament injury on radiographic images. PubMed was searched for articles containing machine learning algorithms related to cruciate ligament injury recognition. No additional filters or time constraints were used. All eligible studies were accessed by hand. From the 115 articles initially retrieved, 29 articles were finally included. Only one study included the posterior cruciate ligament (PCL). Deep learning algorithms in the form of convolutional neural networks (CNNs) were most frequently used. Many studies presented CNNs that identified binary decision classes of regular and torn anterior cruciate ligaments (ACLs) with a best sensitivity of 0.98, a specificity of 0.99, and an AUC ROC of 1.0. Other studies expanded the decision classes to partially torn ACLs or reconstructed ACLs, usually at the cost of sensitivity and specificity. Deep learning algorithms are excellent for identifying ACL injuries, tears, or postoperative status after reconstruction on MRI images. They are much faster but only sometimes better than the human reviewer. While the technology seems ready, barriers to ethical and legal issues and clinicians’ refusals must be overcome to some extent. It can be firmly assumed that artificial intelligence will have a future contribution in the diagnosis of cruciate ligament injuries.
1. Introduction
Anterior cruciate ligament (ACL) injuries are the most frequent ligamentous injuries of the knee joint, with a high potential for long-term disability [1,2]. For this reason, clinical and scientific interest is high among orthopaedic and trauma surgeons, radiologists, and health economists. While orthopaedic and trauma surgeons focus on delivering excellent surgical outcomes for their patients, radiologists aim to provide prompt and accurate interpretations of the imaging studies needed to support the diagnosis. Health economists constantly try to optimise patient treatment costs as healthcare systems are already exposed to a high burden. The incidence of an ACL rupture is reported to be 1 in 3500 humans per year in the USA, which makes it one of the most common knee injuries [3]. While clinical examination with, e.g., Lachman’s test is a powerful tool for diagnosis, MR imaging of the knee joint is still considered the standard of care in preoperatively diagnosing ACL ruptures with superior sensitivity and specificity [4,5]. Posterior cruciate ligament (PCL) injuries can result in significant functional impairment if left untreated. However, their incidence is reported to be considerably lower than that of anterior cruciate ligament (ACL) injuries [6]. In younger patients, complete rupture of the ACL or PCL is almost always an indication of surgery. Arthroscopic-assisted techniques allow most cases to be performed in an outpatient setting. In recent years, artificial intelligence (AI), machine learning, and its subset deep learning (DL) have proved their value in medical diagnostics and therapy by reducing workload and cost in healthcare systems worldwide [7]. A machine can imitate human intelligence, e.g., to solve complex problems based on logic or decision-making trees. DL algorithms can reliably distinguish pathological aspects from physiological findings on radiographic images of different organs [8] (Figure 1). In addition, DL methods are used to make predictions about clinical outcomes after various treatments, which helps to influence the physicians’ choices beneficially [9]. The application of artificial intelligence (AI) has increasingly extended into orthopaedic surgery, particularly in assessing ACL and PCL injuries. Numerous studies have been published using deep learning (DL) algorithms to detect ACL injuries in radiographic images. This systematic review aims to update and critically examine the current literature on using machine learning algorithms for identifying ACL and PCL injuries in radiographic imaging.
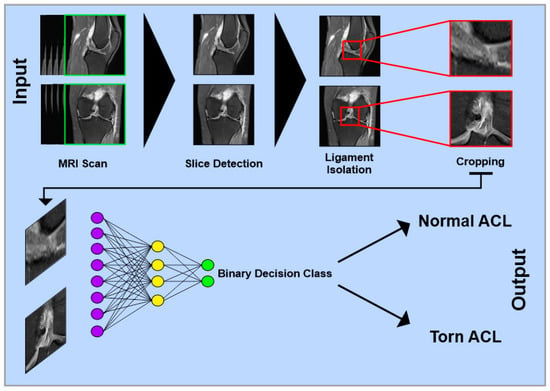
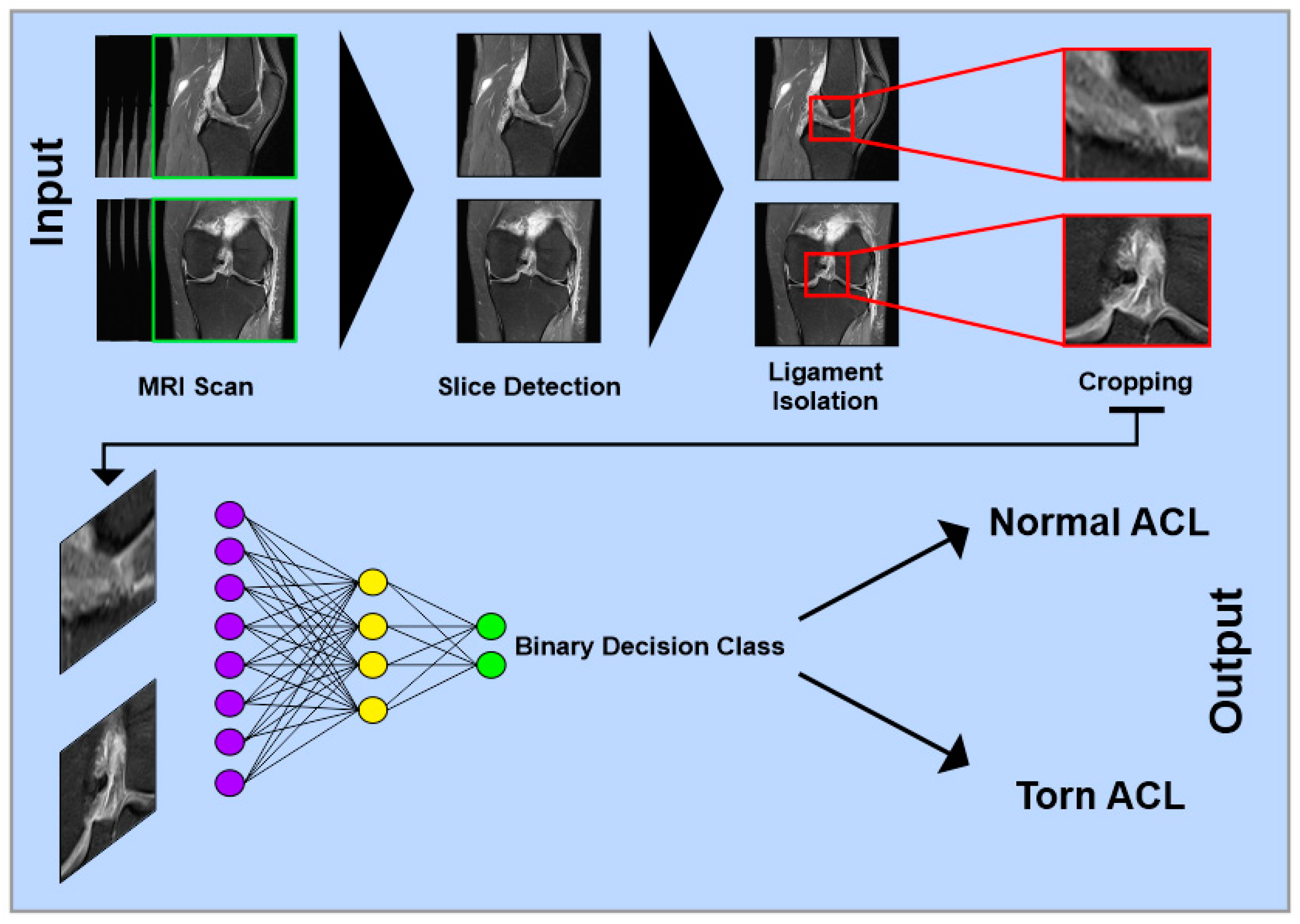
Figure 1.
Simplified schematic representation of a multilayer convolutional neural network for detecting anterior cruciate ligament injuries on MRI images. Single images from different views of complete MRI datasets serve as input. In the first step, relevant slices containing the ACL are identified. Next, the specific image region containing only the ACL is isolated and cropped. The processed images are then passed through multiple network layers for classification. In a binary classification model, the output can be, e.g., “Normal ACL” or “Torn ACL”.
2. Methods
2.1. Eligibility Criteria
All published studies investigating AI image-based ACL and PCL injury detection were accessed. Only articles available in English, French, Spanish and German were eligible. Original studies with evidence of I to IV, according to the Oxford Centre of Evidence-Based Medicine, were considered. Reviews, meta-analyses, preprints, letters, editorials, corrections, abstracts and posters were not considered. Animal studies and cadaveric studies were not eligible. Only studies that reported information in orthopaedic or trauma surgery, radiology, computer science, and with a health care background were eligible.
2.2. Search Strategy
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the 2020 PRISMA statement [10]. The PICO algorithm was preliminarily established as follows:
- ■
- P (problem): ACL/PCL injury;
- ■
- I (intervention): machine learning;
- ■
- C (comparison): evaluate tool;
- ■
- O (outcomes): reliability of ACL/PCL injury detection;
On 26 December 2023, the following database was accessed: PubMed. No time constraint was set for the search. Using the building block approach, the following keywords/blocks were searched using the Boolean operator “AND/OR”:
- (A)
- “Machine learning” OR “unsupervised learning” OR “supervised learning” OR “reinforcement learning” OR “unsupervised machine learning” OR “supervised machine learning” OR “deep learning”
- (B)
- “Anterior cruciate ligament” OR “Anterior Cruciate Ligament” OR “Anterior Cruciate Ligament Reconstruction” OR “Anterior Cruciate Ligament Injuries”
- (C)
- “Posterior cruciate ligament” OR “Posterior Cruciate Ligament” OR “Posterior Cruciate Ligament Reconstruction”
2.3. Selection and Data Collection
Two authors (JMW and UKH) independently performed the database search. All titles were screened by hand, and the abstract was accessed if suitable. The full text of the abstracts that matched the topic was accessed. A hand cross-reference of the bibliography of the full-text articles was also performed for inclusion. The authors debated and mutually solved disagreements. Only studies on which both investigators fully agreed were subsequently screened. A third senior author (MF) made the final decision in the case of further disagreements.
2.4. Data Items and Outcome of Interest
If a study was included, the following information was collected: names of authors and year of publication, journal, study design, country of origin, the purpose of the article, modality applied, decision classes chosen, number of datasets used for training, validation and testing, main findings (sensitivity, specificity, positive predictive value, negative predictive value, accuracy, area under the receiver operating characteristic curve (AUC ROC), dice similarity coefficient, F1-score, intersection over union). Some studies did not present precise information on the number or split ratio of MRI studies or patients used for training, validation, and testing. Although nearly all authors employed deep learning (DL) algorithms to address the task, various settings and datasets—both self-generated and pre-existing—were often used within the same study. In such cases, we report only the results from the best-performing model variation, regardless of the specific decision class. The primary objective was to evaluate the effectiveness of AI-based image analysis in detecting ACL and PCL injuries. However, the included studies lacked uniformity in defining decision classes and often differed in the number of classes used. We sought to standardise the terminology by applying unified terms whenever feasible to address this inconsistency. For example, a study might classify cases into “normal ACL” and “torn ACL”; while one study may refer to “normal ACL” as “intact ACL”, another might use the term “unruptured ACL” to describe the same condition.
3. Results
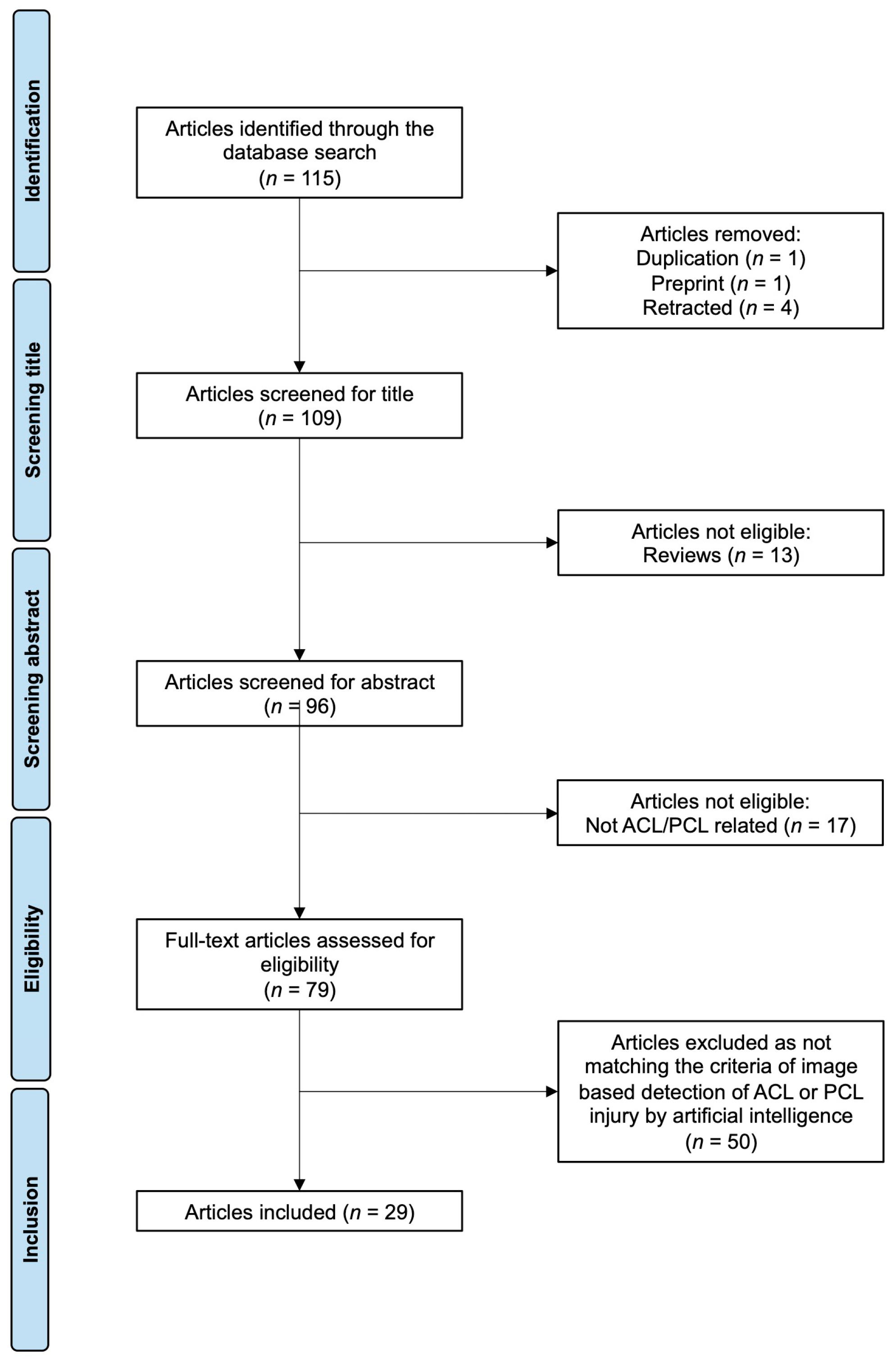
The literature search resulted in 115 articles. After screening, 29 articles fulfilled all inclusion criteria (Table 1 and Table 2, Figure 2). Of note, the search strategy did not include any restrictions on publication date. However, no relevant studies were published before 2017. Among the identified publications, 27 focused on MRI-based detection of ACL injuries, while only two examined X-ray images. Just one study addressed AI-based image recognition of PCL injuries.
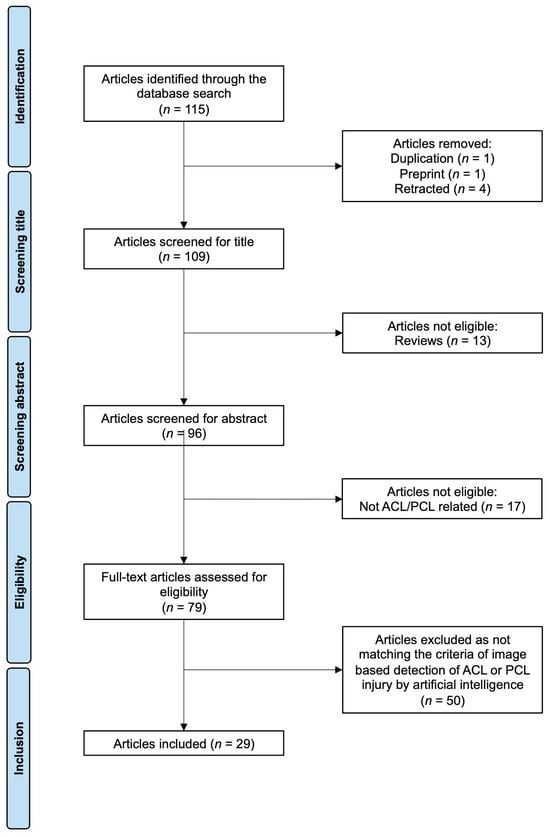
Figure 2.
PRISMA flow chart of the literature search. Abbreviations: ACL—anterior cruciate ligament; PCL—posterior cruciate ligament.
Almost all authors used artificial neural networks in the form of DL algorithms; all were level III studies. Statistical parameters used to determine the quality of the performance of the algorithms were sensitivity, specificity, positive predictive value, negative predictive value, area under the receiver operating characteristic curve (AUC ROC), dice similarity coefficient, F1-score, and intersection over union. The most frequently used statistical parameter was the sensitivity of the algorithm. The authors used datasets of very inhomogeneous size, ranging from 15 to 4086 MRI studies. Most authors used self-generated datasets, while some used pre-existing datasets such as the MRNet dataset published by the Stanford University Medical Center [11] or the dataset published by Štajduhar et al. [12].

Table 1.
A majority (15) of the included studies (29) presented machine learning algorithms that decided between two classes, e.g., “normal ACL” and “torn ACL”. In this group, sensitivity and specificity ranged between 0.87 and 0.98, and 0.86 and 0.99. Other authors used machine learning algorithms that were trained to distinguish between more than two classes, e.g., “normal ACL”, “partially torn ACL”, and “fully torn ACL”, reaching a sensitivity and specificity up to 0.98 and 0.99. Perfect sensitivity and specificity were achieved in recognising reconstructed ACLs. Nearly all the authors used the design of a convolutional neural network.
Table 1.
A majority (15) of the included studies (29) presented machine learning algorithms that decided between two classes, e.g., “normal ACL” and “torn ACL”. In this group, sensitivity and specificity ranged between 0.87 and 0.98, and 0.86 and 0.99. Other authors used machine learning algorithms that were trained to distinguish between more than two classes, e.g., “normal ACL”, “partially torn ACL”, and “fully torn ACL”, reaching a sensitivity and specificity up to 0.98 and 0.99. Perfect sensitivity and specificity were achieved in recognising reconstructed ACLs. Nearly all the authors used the design of a convolutional neural network.
| Group | Number of Studies | Best Performance | Size of the Test Dataset | Results |
|---|---|---|---|---|
| Binary: normal ACL, torn ACL | 15 | Richardson et al. [13] | 201 MRI Studies | Sensitivity of 0.98, specificity of 0.99 |
| Other than binary: e.g., normal ACL, partially torn ACL, fully torn ACL | 4 | Awan et al. [14] | 276 MRI Studies | Sensitivity of 0.98, specificity of 0.99 |
| Other than binary, including reconstructed ACLs | 2 | Namiri et al. [15] | 2248 MRI Studies | Sensitivity of 1.0, specificity of 1.0 |
| X-ray | 2 | Kim et al. [16] | 287 X-ray Studies | Sensitivity of 0.87, specificity of 0.89 |
| Image segmentation | 6 | Kulseng et al. [17] | 15 MRI Studies | Dice similarity coefficient: ACL region: 0.96, PCL region 0.97 |
Most MRI-based studies investigated the quality of AI image-based detection of ACL injury by using binary decision classes to distinguish between a normal ACL and a torn ACL in MRI images [13,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. Li et al. also included images of abnormalities other than torn ACL in the regular ACL class [32]. Of those studies with binary decision classes, the CNN used by Richardson et al. performed best with a sensitivity of 0.98, specificity of 0,99, and AUC ROC of 1.0 on a self-generated dataset using 1806 MRI studies for training and validation and 201 MRI studies for testing [13]. In other studies of that design, sensitivity, specificity, and AUC ROC ranged between 0.87 [19] and 0.98 [13], 0.86 [23] and 0.99 [13] and 0.94 [29] and 1.0 [13].
Other authors used DL algorithms, which were trained to distinguish between more than two classes, e.g., “normal ACL”, “partially torn ACL”, and “fully torn ACL” [11,12,14,15,33,34]. Among these studies, the U-Net CNN algorithm used by Awan et al. performed best with a sensitivity, specificity, and AUC ROC of 0.98, 0.99, and 0.98 on a self-generated dataset using 827 MRI studies for training and validation and 276 MRI studies for testing [34].
In works by other authors, in addition to simply the condition of the native ACL, a possible postoperative reconstructed condition was included [15,35]. One CNN designed to recognise reconstructed ACLs performed excellently with a sensitivity of 1.0 using 994 MRI studies for training and validation and 2248 MRI studies for testing [15].
Other studies took a different approach and focused on automatic image segmentation of the ACL or PCL region [17,27,36] or tear regions of the ACL [24,37,38]. For example, the DenseVNet CNN algorithm developed by Kulseng et al. achieved a dice similarity coefficient of 0.964 for ACL region segmentation and 0.973 for the PCL region, using 25 MRI studies for training and validation and 15 MRI studies for testing [17].
Two studies also worked with X-rays in ACL patients [16,39]: Kim et al. [16] developed a model to identify ACL rupture on lateral knee radiographs, outperforming non-musculoskeletal radiologists using 1146 X-ray studies for training and validation and 287 X-ray studies for testing. Lu et al. [39] developed a U-Net CNN algorithm to measure posterior tibial slope in short-leg lateral X-rays of ACL patients before and after ACL reconstruction. No significant differences were observed between manually performed posterior tibial slope measurements. The deep learning (DL) algorithm measured all 90 test images in under one minute, a substantial reduction compared to the estimated 180 min required for manual measurements by human annotators.

Table 2.
Studies investigating an artificial intelligence approach to recognise anterior cruciate ligament (ACL) or posterior cruciate ligament (PCL) pathologies. All studies had an evidence level of III, according to the Oxford Centre of Evidence-Based Medicine. All studies used a deep learning approach based on a convolutional neural network except Štajduhar et al., 2017 [12], who used a supervised learning method. Abbreviations: area under the receiver operating characteristic curve (AUC ROC), n.s. (non-specified), sensitivity (SS), specificity (SP), positive predictive value (PPV), negative predictive value (NPV), accuracy (ACC), dice similarity coefficient (DSC), F1-score (F1), intersection over union (IoU).
Table 2.
Studies investigating an artificial intelligence approach to recognise anterior cruciate ligament (ACL) or posterior cruciate ligament (PCL) pathologies. All studies had an evidence level of III, according to the Oxford Centre of Evidence-Based Medicine. All studies used a deep learning approach based on a convolutional neural network except Štajduhar et al., 2017 [12], who used a supervised learning method. Abbreviations: area under the receiver operating characteristic curve (AUC ROC), n.s. (non-specified), sensitivity (SS), specificity (SP), positive predictive value (PPV), negative predictive value (NPV), accuracy (ACC), dice similarity coefficient (DSC), F1-score (F1), intersection over union (IoU).
| Authors | Journal | Study Design | Country of Origin | Purpose of the Article | Modality | Decision Classes | Dataset | n (Training and Validation) | n (Testing) | Main Findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | SP | PPV | NPV | ACC | AUC ROC | DSC | F1 | IoU | ||||||||||
| Lu et al., 2023 [39] | Orthopaedic Journal of Sports Medicine | Retrospective cohort study | USA | To develop a deep learning algorithm for automated posterior tibial slope measurement from standard lateral knee radiographs of patients after ACL reconstruction | X-ray |
| Rochester epidemiology project: Over 500,000 medical records for residents of Olmsted County, Minnesota, neighbouring counties. Training images were balanced to maintain a 1-to-1 ratio of patient sex and a 1-to-1 ratio of the graft type utilised: allograft, bone–patellar tendon–bone autograft, and hamstring autograft. | 300 | 90 | 0.89 | ||||||||
| Li et al., 2023 [32] | Frontiers in Bioengineering and Biotechnology | Retrospective cohort study | China | To develop a deep learning algorithm for automated detection of ACL injury in MRI images | MRI |
| Own dataset (MRI-ACL, displayed results): Ningbo Hospital, China: A total number of 100 MRI scans were included. The patient characteristics, such as age and gender, were not available. MRNet dataset: Stanford University Medical Center, California, USA: A total number of 1370 MRI scans were included. The patient characteristics, such as age and gender, were not available. | 80 | 20 | 0.97 | ||||||||
| Wang et al., 2023 [18] | Arthroscopy | Retrospective comparative case series | China | To develop a deep learning algorithm for automated detection of ACL injury in MRI images | MRI |
| Own dataset: MRI scans were collected from 5 medical centers in China. The patient characteristics, such as age and gender, were not available. | 22,767 | 4086 | 0.95 | 0.95 | 0.99 | ||||||
| Awan et al., 2023 [37] | The Open Access Journal for Computer Science research | Retrospective cohort study | Malaysia | To develop a deep learning algorithm for automated localisation of the ACL tear region in MRI images | MRI |
| Dataset by Štajduhar et al. [12]: Clinical Hospital Centre Rijeka, Croatia: A total number of 969 (in this case, 917 after discarding corrupted volumes) MRI scans were included. The patient characteristics, such as age and gender, were not available. | 11,438 (images) | 3817 (images) | 0.98 | 0.98 | 0.99 | 0.98 | 0.98 | 0.95 | |||
| Dung et al., 2023 [33] | Diagnostic and Interventional Imaging | Retrospective cohort study | Vietnam | To develop a deep learning algorithm for automated segmentation and classification of ACL injury in MRI images | MRI |
| Own dataset: Hospital 1, Da Nang City, Vietnam: a total number of 297 MRI scans were included. The mean age was 36 years. Female ratio of 24%. The mean body mass index (kg/m2) was 24. | 247 | 50 | 0.80 | 0.95 | 0.92 | ||||||
| Kulseng et al., 2023 [17] | BMC Musculoskeletal Disorders | Retrospective cohort study | Norway | To develop a deep learning algorithm for automated localisation of the ACL and PCL region in MRI images | MRI |
| Own dataset: Norway: A total number of 46 MRI scans were included. The included participants were divided into independent subgroups of 20, 5, and 15 for training, validation, and test dataset, respectively. The mean ages in these subgroups were 36.7, 37.2, and 28.8 years. The ratios of men and women were 13:7, 2:3, and 2:3. | 25 | 15 | 0.96 | ||||||||
| 25 | 15 | 0.97 | |||||||||||||||
| Tran et al., 2022 [19] | European Radiology | Retrospective cohort study | France | To develop a deep learning algorithm for automated detection of ACL injury in MRI images | MRI |
| Own dataset: France: MRI scans were collected from 12 medical centers: A total number of 19,765 MRI scans were included. The mean age was 44 years. Female ratio of 48%. | 17,789 | 1976 | 0.87 | 0.91 | 0.90 | 0.94 | 0.72 | ||||
| Shin et al., 2022 [20] | Medicine | Retrospective cohort study | Republic of Korea | To develop a deep learning algorithm for automated detection of ACL injury in MRI images | MRI |
| Own dataset: Yeungnam University, Republic of Korea: A total number of 164 MRI scans were included. The mean age was 43.6 years. Female ratio of 34%. | 130 | 34 | 0.94 | ||||||||
| Joshi et al., 2022 [21] | Diagnostics | Retrospective cohort study | India | To develop a deep learning algorithm for automated detection of ACL injury in MRI images | MRI |
| MRNet dataset: Stanford University Medical Center, California, USA: A total number of 1370 MRI scans were included. The patient characteristics, such as age and gender, were not available. | 907 | 388 | 0.97 | 0.97 | 0.97 | 0.96 | |||||
| Qu et al., 2022 [38] | Frontiers in Bioengineering and Biotechnology | Retrospective cohort study | China | To develop a deep learning algorithm for automated segmentation and classification of ACL injury in MRI images | MRI |
| Own dataset: Balgrist University Hospital Zürich, Switzerland: a total number of 85 were included. The mean age was 27 years. Female ratio of 34%. | 68 | 17 | 0.86 | 0.79 | 0.80 | 0.79 | 0.83 | ||||
| Kim et al., 2022 [16] | Skeletal Radiology | Retrospective cohort study | Republic of Korea | To develop a deep learning algorithm for automated prediction of ACL injury in lateral knee radiographs | X-ray |
| Own dataset: SMG-SNU Boramae Medical Center and Konkuk University Medical Center, Republic of Korea: a total number of 1433 lateral knee radiographs were included. The mean age was 27 years. Female ratio of 30%. | 1146 | 287 | 0.87 | 0.89 | 0.88 | 0.93 | |||||
| Sridhar et al., 2022 [22] | Journal of Healthcare Engineering | Retrospective cohort study | India | To develop a deep learning algorithm for automated detection of ACL injury in MRI images | MRI |
| MRNet dataset: Stanford University Medical Center, California, USA: a total number of 1370 MRI scans were included. The patient characteristics, such as age and gender, were not available. | 959 | 411 | 0.95 | 0.96 | 0.95 | 0.95 | 0.95 | ||||
| Minamoto et al., 2022 [23] | BMC Musculoskeletal Disorders | Retrospective cohort study | Japan | To develop a deep learning algorithm for automated detection of ACL injury in MRI images and to compare the results to those of human readers | MRI |
| Own dataset: Chiba University Hospital, Japan: a total number of 200 MRI images were included. Training/validation/testing split information were not available. The patient characteristics, such as age and gender, were not available. | n.s. | n.s. | 0.91 | 0.86 | 0.87 | 0.91 | 0.89 | 0.94 | |||
| Awan et al., 2022 [24] | Sensors | Retrospective cohort study | Malaysia | To develop a deep learning algorithm for automated segmentation of ACL tears in MRI images | MRI |
| Dataset by Štajduhar et al. [12]: Clinical Hospital Centre Rijeka, Croatia: a total number of 969 MRI scans were included. The patient characteristics, such as age and gender, were not available | 11,451 | 3817 | 0.97 | 0.97 | 0.98 | 0.97 | 0.97 | 0.94 | |||
| Flannery et al., 2022 [35] | Journal of Orthopaedic Research | Retrospective cohort study | USA | To develop a deep learning algorithm for automated segmentation of repaired and reconstructed ACLs in MRI images | MRI |
| Own datasets (BEAR I, BEAR II): Boston Children’s Hospital, Massachusetts, USA: a total number of 358 MRI scans were included. BEAR I: The mean age was 24 years. Female ratio of 60%. BEAR II: The mean age was 19 years. Female ratio of 58%. | 4380 | 380 | 0.82 | 0.79 | 0.80 | ||||||
| 2200 | 200 | 0.80 | 0.78 | 0.78 | |||||||||||||
| Awan et al., 2021 [34] | Journal of Personalized Medicine | Retrospective cohort study | Malaysia | To develop a deep learning algorithm for automated detection of ACL injury in MRI images | MRI |
| Dataset by Štajduhar et al. [12]: Clinical Hospital Centre Rijeka, Croatia: a total number of 969 MRI scans were included. The patient characteristics, such as age and gender, were not available | 827 | 276 | 0.98 | 0.99 | 0.98 | 0.99 | 0.98 | 0.98 | |||
| Li et al., 2021 [25] | Journal of Healthcare Engineering | Retrospective cohort study | China | To develop a deep learning algorithm for automated detection of ACL injury in MRI images | MRI |
| Own dataset: Peking University Shenzhen Hospital, China: a total number of 30 MRI scans were included. The mean age was 38 years. Female ratio of 30%. Training/validation/testing split information were not available. | n.s. | n.s. | 0.97 | 0.91 | 0.92 | 0.97 | |||||
| Jeon et al., 2021 [26] | Journal of Biomedical and Health Information | Retrospective cohort study | USA | To develop a deep learning algorithm for automated detection of ACL injury in MRI images | MRI |
| MRNet dataset: Stanford University Medical Center, California, USA: a total number of 1370 MRI scans were included. The patient characteristics, such as age and gender, were not available. Chiba Dataset: two institutions in Chiba, Japan: a total number of 1177 MRI scans were included. The patient characteristics, such as age and gender, were not available. Training/validation/testing split information were not available. | n.s. | n.s. | 0.93 | 0.98 | 0.98 | ||||||
| Flannery et al., 2021 [36] | Journal of Orthopaedic Research | Retrospective cohort study | USA | To develop a deep learning algorithm for automated segmentation of the ACL in MRI images | MRI |
| Own datasets (BEAR I, BEAR II): Boston Children’s Hospital, Massachusetts, USA: a total number of 358 MRI scans were included. BEAR I: the mean age was 24 years. Female ratio of 60%. BEAR II: the mean age was 19 years. Female ratio of 58%. | 217 | 29 | 0.85 | 0.82 | 0.84 | ||||||
| Awan et al., 2021 [14] | Diagnostics | Retrospective cohort study | Malaysia | To develop a deep learning algorithm for automated detection of ACL injury in MRI images | MRI |
| Dataset by Štajduhar et al. [12]: Clinical Hospital Centre Rijeka, Croatia: a total number of 969 MRI scans were included. The patient characteristics, such as age and gender, were not available | 2387 | 950 | 0.92 | 0.95 | 0.92 | 0.92 | 0.98 | 0.92 | |||
| Astuto et al., 2021 [27] | Radiology Artificial Intelligence | Retrospective cohort study | USA | To develop a deep learning algorithm for automated segmentation and classification of ACL injury in MRI images | MRI |
| Own dataset: USA: a total number of 1252 MRI scans were included for the ACL group. For the whole dataset of 1435 MRI scans the mean age was 43 years. Female ratio of 52%. The mean body mass index (kg/m2) was 24. | 1002 | 250 | 0.89 | ||||||||
| 1002 | 250 | 0.88 | 0.89 | 0.90 | |||||||||||||
| Zhang et al., 2020 [28] | Journal of Magnetic Resonance Imaging | Retrospective cohort study | China | To develop a deep learning algorithm for automated detection of ACL injury in MRI images | MRI |
| Own dataset: Third Affiliated Hospital of Southern Medical University Guangzhou, China: a total number of 408 MRI scans were included. The mean age was 50 years. Female ratio of 40%. | 366 | 42 | 0.98 | 0.94 | 0.94 | 0.98 | 0.96 | 0.96 | |||
| Germann et al., 2020 [29] | Investigative Radiology | Retrospective cohort study | Switzerland | To develop a deep learning algorithm for automated detection of ACL injury in MRI images and to compare the results to those of human readers | MRI |
| Own dataset: Switzerland: a total number of 5802 MRI scans were included for the initial model. For testing of the final model the mean age was 34 years. Female ratio of 45%. | 5302 | 500 | 0.96 | 0.93 | 0.94 | ||||||
| Namiri et al., 2020 [15] | Radiology Artificial Intelligence | Retrospective cohort study | USA | To develop a deep learning algorithm for automated detection of ACL injury and reconstructed ACLs in MRI images and to compare the results to those of human readers | MRI |
| Own dataset: University of California, USA, Mayo Clinic Rochester, USA, and Hospital for Special Surgery New York, USA. A total number of 1243 MRI scans were included. The mean age was 47 years. Female ratio of 54%. | 994 | 248 | 1.0 | 1.0 | 0.92 | ||||||
| Chang et al., 2019 [30] | Journal of Digital Imaging | Retrospective cohort study | USA | To develop a deep learning algorithm for automated detection of ACL injury in MRI images | MRI |
| Unknown institutional database: USA: a total number of 260 MRI scans were included. Subjects between ages 18 and 40 were included. Gender characteristics were not available | 200 | 60 | 1.0 | 0.93 | 0.94 | 1.0 | 0.97 | ||||
| Liu et al., 2019 [31] | Radiology Artificial Intelligence | Retrospective cohort study | USA | To develop a deep learning algorithm for automated detection of ACL injury in MRI images and to compare the results to those of human readers | MRI |
| Own dataset: Department of Radiology, University of Wisconsin School of Medicine and Public Health, USA and Department of Radiology, Boston University School of Medicine, USA: a total number of 350 MRI scans were included. Normal ACL group: the mean age was 39 years. Female ratio of 42%. Torn ACL group: the mean age was 28 years. Female ratio of 44%. | 250 | 100 | 0.96 | 0.96 | 0.98 | ||||||
| Richardson et al., 2019 [13] | Current Problems in Diagnostic Radiology | Retrospective cohort study | USA | To develop a deep learning algorithm for automated detection of ACL injury in MRI images | MRI |
| Own dataset: Department of Radiology, University of Washington, USA: a total number of 2007 MRI scans were included. Normal ACL group: the mean age was 44 years for women and 42 years for men. Female ratio of 52%. Torn ACL group: the mean age was 34 years for women and 34 years for men. Female ratio of 51%. | 1806 | 201 | 0.98 | 0.99 | 0.98 | 0.99 | 1.0 | ||||
| Bien et al., 2018 [11] | PLOS Medicine | Retrospective cohort study | USA | To develop a deep learning algorithm for automated detection of ACL injury in MRI images and to compare the results to those of human readers | MRI |
| MRNet dataset: Stanford University Medical Center, California, USA: a total number of 1370 MRI scans were included. The patient characteristics, such as age and gender, were not available. | 1250 | 120 | 0.88 | 0.71 | 0.85 | 0.94 | |||||
| Štajduhar et al., 2017 [12] | Computer Methods and Programs in Biomedicine | Retrospective cohort study | Croatia | To develop supervised learning algorithms for automated detection of ACL injury in MRI images | MRI |
| Own dataset: Clinical Hospital Centre Rijeka, Croatia: a total number of 969 MRI scans were included. The patient characteristics, such as age and gender, were not available | n.s. | n.s. | 0.94 | ||||||||
4. Discussion
The use of machine learning algorithms in managing cruciate ligament injuries is rapidly increasing. Notably, 36 of the 115 studies identified in our search were published in 2023 alone, with a marked rise in publication volume beginning in 2019. The retrieved articles appeared in journals spanning orthopaedic and trauma surgery, radiology, computer science, and health sciences, highlighting the interdisciplinary nature—and growing relevance—of this emerging field.
Many studies have focused on using DL algorithms to detect cruciate ligament injuries in MRI scans. CNNs, a subset of DL, were employed by nearly all researchers addressing this task. CNNs are widely regarded as the most suitable tool for this purpose due to their ability to extract complex, abstract features from large datasets—specifically, the identification of injured cruciate ligaments within individual MR images from extensive MRI datasets [40,41].
This leads to efficient complex information processing and enables precise decisions and predictions to be made. In line with this observation, DL and CNNs are already the standard in other complex fields such as social media, chatbots, and self-driving cars [42,43]. A disadvantage of CNNs is that they require large datasets and high computational power, making training expensive and difficult for smaller datasets. They act as black-box models with limited interpretability and often struggle to capture global context in images. Last but not least, small input changes can significantly alter predictions [44].
CNNs can be further divided into subfamilies like U-Net, ResNet and Inception. ResNet uses residual connections to train very deep networks effectively but requires high computational power. It works well for general tasks but may be unnecessary for simpler problems [45]. Inception captures features at multiple scales, making it highly effective for object detection, although its implementation is complex. As a result, it reduces computational costs but incurs a longer inference time [46]. U-Net performs well with small datasets but is not ideal for classification. It is great for image segmentation and preserving fine details, but needs a lot of memory and is prone to overfitting [47]. Choosing the right model always depends on the task.
Larger datasets improve a model’s capacity to learn meaningful patterns and generalise more effectively to new data. In contrast, smaller datasets often result in overfitting, where the model memorises specific training examples instead of identifying the underlying features. Techniques such as dropout, data augmentation, and transfer learning can improve generalisation when data are limited. However, extremely large datasets can be costly and may not yield significant performance gains. A diverse and well-balanced dataset is more valuable than sheer volume alone. Ensuring high-quality, varied data are essential for building robust models that perform well in real-world scenarios [40,48].
The application of AI in the diagnosis and treatment of cruciate ligament injuries has not yet been fully integrated into routine clinical practice and remains largely experimental. Orthopaedic and trauma surgeons still use their clinical examination as the foundation for their decision-making regarding diagnostics and therapy, something that was thought never likely to be changed. Specific clinical tests of the ACL provide good sensitivity and specificity of 0.83 and 0.85 (95% CI, 0.77–0.88) for the anterior drawer test and 0.81 and 0.85 (95% CI, 0.73–0.87) for the Lachman’s test, as shown in a meta-analysis by Sokal et al. [49]. The sensitivity and specificity of detecting cruciate ligament injuries increase when additionally using MR imaging: In a recent meta-analysis, Li et al. reported a combined sensitivity of 0.87 (95% CI, 0.84–0.90) and a specificity of 0.90 (95% CI, 0.88–0.92) [50]. The best DL CNN reviewed in this study was presented by Richardson et al. [13], which outperformed these values with a sensitivity of 0.98 and a specificity of 0.99 when recognising ACL tears on fat-saturated weighted MR images. Additionally, other models using binary decision classes (normal ACL, torn ACL) have outperformed combined clinical and MRI assessments by physicians in terms of sensitivity and specificity. However, the reality is far more complex than simple binary decision-making. When expanding the model to include other dimensions, sensitivity and specificity lowered in a relevant way. For example, the three-category model from Bien et al. [11] had a sensitivity significantly lower than that of general radiologists, while the specificity was not considerably higher. Germann et al. [29] also reached a specificity with their CNN that was significantly lower than the control group, while sensitivity was not significantly different. These findings are consistent with those of other studies [23,31]. While DL-based detection of ACL injuries is not inherently superior to human analysis, its performance is highly context-dependent.
Physical examination, MRI, or arthroscopy allow multiple and more complex questions to be answered simultaneously. In contrast, a machine learning algorithm must be extensively programmed and trained to decide between various classes. When conditions can be standardised, however, performance seems to remain relatively high in more complex decisions, as shown by Awan et al. [34], who presented a CNN that differentiated between a standard ACL, a partially torn ACL, and a fully torn ACL with a sensitivity of 0.98 and a specificity of 0.99. Namiri et al. [15] even presented an algorithm that identified reconstructed ACLs on MRI images with a sensitivity and specificity of 1.0. This outstanding result might be due to the bundled, cylindric structure of commonly used autografts, which significantly differs from the physiological, more trapezoid, and flat shape of the ACL. Additional tasks are relatively easy for machine learning algorithms. Setting up complex machine learning algorithms might be time- and cost-intensive. Still, it must be remembered that a physician who reaches top statistical values for detecting ACL injuries in physical examinations or MRI images also needs several years of training.
With the rising burden on the health care systems because of, e.g., demographic changes, effective use of the workforce becomes more critical. Bien et al. [11] demonstrated that their DL model identified ACL tears at least ninety times faster than the clinical experts. While the human expert needed more than three hours to review 120 exams, the DL model completed the task in just two minutes. Lu et al. [39] also highlighted that their DL tool for automated radiographic determination of posterior tibial slope in patients with ACL injuries only needed less than one minute to obtain angle measurements from ninety images.
This raises an important question: Can healthcare systems afford to overlook the support of artificial intelligence? From a cost-benefit perspective, a machine-learning algorithm for detecting ACL tears may initially appear superior. However, as with many AI applications, ethical considerations must also be carefully addressed. A machine learning algorithm will always be faster than a human reviewer, making it significantly cheaper [51]. While practitioners represent ongoing expenses for healthcare systems, a machine learning tool is a one-time investment. Although advanced deep learning algorithms can cost over USD 1,000,000—significantly surpassing the average annual salary of a practitioner—they can perform tasks up to ninety times faster, making them cost-effective even in the short term [39]. Ultimately, the true measure of cost-effectiveness lies in the benefit to the patient. If AI underperforms compared to a human reviewer regarding diagnostic quality, cost becomes a secondary concern in a healthcare system prioritising patient outcomes. However, once AI consistently outperforms humans in diagnostic accuracy—such as detecting ligamentous injuries on MRI scans—the cost-benefit debate will no longer be a point of contention. In such cases, the machine will surpass the practitioner in specific tasks, though only within defined domains. For instance, clinical examinations are unlikely to be replaced by AI shortly, as they still require human judgment and practical considerations.
Given the reliable and fast classification of cruciate ligament injuries raises the question of why machine learning algorithms have yet to find their way into clinical practice. Implementing new techniques in clinical medicine always takes time; on average, it takes 17 years [52]. Remember that the first study dealing with AI-based detection of ACL injuries was published in 2017 by Štajduhar et al. [12], which gives an idea of the expected progress. In addition, there might be a group of clinicians interested in not promoting these techniques for fear that their workforce might get replaced at some point in the future. This fear seems, however, unwarranted. From what we can tell today, AI will more likely be used to support, e.g., radiologists instead of replacing them [53]. There is also an ongoing ethical legal debate about the usage of AI in medicine because, at this point, physicians are legally responsible for their actions, regardless of the influence of machine learning tools in the decision-making process [54]. This could also raise potential barriers to clinical implementation. If, in the future, machine learning algorithms and their developers become liable for outcomes, this is a critical issue that needs to be addressed. There are already commercially available DL tools developed by tech giants for different purposes in the digital world, which could be used as a blueprint for tools that can detect cruciate ligaments on radiographic images. It is believed, however, that these companies would decline any responsibility for such use in the medical field. For now, the available algorithms used on the given topic are most commonly based on open-source CNNs that computer scientists and clinicians set up in a more experimental setting, which excludes liability for good reasons. This is why, in our opinion, the concept of AI assistance in the sense of a second opinion would be the next realistic milestone.
5. Conclusions
Deep learning (DL) algorithms demonstrate excellent performance in identifying ACL injuries, tears, and postoperative status following reconstruction on MRI images. While these algorithms are significantly faster, they are not necessarily superior to human reviewers. However, although the technology appears ready, ethical, and legal, and clinician scepticism present barriers that must be addressed; it is reasonable to expect that AI will play an increasingly important role in diagnosing cruciate ligament injuries. In the context of ACL injuries, machine learning tools are also anticipated to contribute to other clinical aspects, such as graft selection, functional outcomes, and cost management.
Author Contributions
J.M.W.: literature search, study selection and data extraction, writing; U.K.H. conception and design, drafting (original and revision); M.P. and M.D.: supervision, revision; F.M.: literature search, risk of bias assessment; M.F.: conception, supervision and writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
AI—artificial intelligence; DL—deep learning; CNN—convolutional neural network; ACL—anterior cruciate ligament; PCL—posterior cruciate ligament.
References
- Nicolini, A.P.; De Carvalho, R.T.; Matsuda, M.M.; Filho, J.S.; Cohen, M. Common injuries in athletes’ knee: Experience of a specialized center. Acta Ortop. Bras. 2014, 22, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Hewett, T.E.; Myer, G.D.; Ford, K.R.; Paterno, M.V.; Quatman, C.E. Mechanisms, prediction, and prevention of ACL injuries: Cut risk with three sharpened and validated tools. J. Orthop. Res. 2016, 34, 1843–1855. [Google Scholar] [CrossRef]
- Kaeding, C.C.; Léger-St-Jean, B.; Magnussen, R.A. Epidemiology and Diagnosis of Anterior Cruciate Ligament Injuries. Clin. Sports Med. 2017, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Inoue, Y.; Masuda, Y.; Tian, H.; Jung, H.; Tanaka, R. Diagnostic Accuracy of Physical Examination Tests for Suspected Acute Anterior Cruciate Ligament Injury: A Systematic Review and Meta-Analysis. Int. J. Sports Phys. Ther. 2022, 17, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhou, Y.; Chang, J.; Hu, J.; Liu, H.; Wang, S.; Si, D.; Yuan, Y.; Li, H. The accuracy of MRI in the diagnosis of anterior cruciate ligament injury. Ann. Transl. Med. 2020, 8, 1657. [Google Scholar] [CrossRef]
- Sanders, T.L.; Pareek, A.; Barrett, I.J.; Kremers, H.M.; Bryan, A.J.; Stuart, M.J.; Levy, B.A.; Krych, A.J. Incidence and long-term follow-up of isolated posterior cruciate ligament tears. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3017–3023. [Google Scholar] [CrossRef]
- Bohr, A.; Memarzadeh, K. Chapter 2—The rise of artificial intelligence in healthcare applications. In Artificial Intelligence in Healthcare; Academic Press: Cambridge, MA, USA, 2020; pp. 25–60. [Google Scholar]
- Oren, O.; Gersh, B.J.; Bhatt, D.L. Artificial intelligence in medical imaging: Switching from radiographic pathological data to clinically meaningful endpoints. Lancet Digit. Health 2020, 2, e486–e488. [Google Scholar] [CrossRef]
- Shamout, F.; Zhu, T.; Clifton, D.A. Machine Learning for Clinical Outcome Prediction. IEEE Rev. Biomed. Eng. 2021, 14, 116–126. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Bien, N.; Rajpurkar, P.; Ball, R.L.; Irvin, J.; Park, A.; Jones, E.; Bereket, M.; Patel, B.N.; Yeom, K.W.; Shpanskaya, K.; et al. Deep-learning-assisted diagnosis for knee magnetic resonance imaging: Development and retrospective validation of MRNet. PLoS Med. 2018, 15, e1002699. [Google Scholar] [CrossRef]
- Štajduhar, I.; Mamula, M.; Miletić, D.; Uenal, G. Semi-automated detection of anterior cruciate ligament injury from MRI. Comput. Methods Programs Biomed. 2017, 140, 151–164. [Google Scholar] [CrossRef]
- Richardson, M.L. MR Protocol Optimization with Deep Learning: A Proof of Concept. Curr. Probl. Diagn. Radiol. 2021, 50, 168–174. [Google Scholar] [CrossRef]
- Javed Awan, M.; Mohd Rahim, M.S.; Salim, N.; Mohammed, M.A.; Garcia-Zapirain, B.; Abdulkareem, K.H. Efficient Detection of Knee Anterior Cruciate Ligament from Magnetic Resonance Imaging Using Deep Learning Approach. Diagnostics 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Namiri, N.K.; Flament, I.; Astuto, B.; Shah, R.; Tibrewala, R.; Caliva, F.; Link, T.M.; Pedoia, V.; Majumdar, S. Deep Learning for Hierarchical Severity Staging of Anterior Cruciate Ligament Injuries from MRI. Radiol. Artif. Intell. 2020, 2, e190207. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Chai, J.W.; Kang, J.H.; Lee, J.H.; Kim, H.J.; Seo, J.; Choi, J.W. Ensemble deep learning model for predicting anterior cruciate ligament tear from lateral knee radiograph. Skelet. Radiol. 2022, 51, 2269–2279. [Google Scholar] [CrossRef]
- Kulseng, C.P.S.; Nainamalai, V.; Grøvik, E.; Geitung, J.-T.; Årøen, A.; Gjesdal, K.-I. Automatic segmentation of human knee anatomy by a convolutional neural network applying a 3D MRI protocol. BMC Musculoskelet. Disord. 2023, 24, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Y.; Liu, S.-G.; Ding, J.; Sun, A.-L.; Jiang, D.; Jiang, J.; Zhao, J.-Z.; Chen, D.-S.; Ji, G.; Li, N.; et al. A Deep Learning Model Enhances Clinicians’ Diagnostic Accuracy to More Than 96% for Anterior Cruciate Ligament Ruptures on Magnetic Resonance Imaging. Arthrosc. J. Arthrosc. Relat. Surg. 2024, 40, 1197–1205. [Google Scholar] [CrossRef]
- Tran, A.; Lassalle, L.; Zille, P.; Guillin, R.; Pluot, E.; Adam, C.; Charachon, M.; Brat, H.; Wallaert, M.; D’assignies, G.; et al. Deep learning to detect anterior cruciate ligament tear on knee MRI: Multi-continental external validation. Eur. Radiol. 2022, 32, 8394–8403. [Google Scholar] [CrossRef]
- Shin, H.; Choi, G.S.; Chang, M.C. Development of convolutional neural network model for diagnosing tear of anterior cruciate ligament using only one knee magnetic resonance image. Medicine 2022, 101, e31510. [Google Scholar] [CrossRef]
- Joshi, K.; Suganthi, K. Anterior Cruciate Ligament Tear Detection Based on Deep Convolutional Neural Network. Diagnostics 2022, 12, 2314. [Google Scholar] [CrossRef]
- Sridhar, S.; Amutharaj, J.; Valsalan, P.; Arthi, B.; Ramkumar, S.; Mathupriya, S.; Rajendran, T.; Waji, Y.A. A Torn ACL Mapping in Knee MRI Images Using Deep Convolution Neural Network with Inception-v3. J. Health Eng. 2022, 2022, 7872500. [Google Scholar] [CrossRef]
- Minamoto, Y.; Akagi, R.; Maki, S.; Shiko, Y.; Tozawa, R.; Kimura, S.; Yamaguchi, S.; Kawasaki, Y.; Ohtori, S.; Sasho, T. Automated detection of anterior cruciate ligament tears using a deep convolutional neural network. BMC Musculoskelet. Disord. 2022, 23, 577. [Google Scholar] [CrossRef] [PubMed]
- Awan, M.J.; Rahim, M.S.M.; Salim, N.; Rehman, A.; Garcia-Zapirain, B. Automated Knee MR Images Segmentation of Anterior Cruciate Ligament Tears. Sensors 2022, 22, 1552. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ren, S.; Zhou, R.; Jiang, X.; You, T.; Li, C.; Zhang, W. Deep Learning-Based Magnetic Resonance Imaging Image Features for Diagnosis of Anterior Cruciate Ligament Injury. J. Health Eng. 2021, 2021, 4076175. [Google Scholar] [CrossRef]
- Jeon, Y.S.; Yoshino, K.; Hagiwara, S.; Watanabe, A.; Quek, S.T.; Yoshioka, H.; Feng, M. Interpretable and Lightweight 3-D Deep Learning Model for Automated ACL Diagnosis. IEEE J. Biomed. Health Inform. 2021, 25, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Astuto, B.; Flament, I.; Namiri, N.K.; Shah, R.; Bharadwaj, U.; Link, T.M.; Bucknor, M.D.; Pedoia, V.; Majumdar, S. Automatic Deep Learning–assisted Detection and Grading of Abnormalities in Knee MRI Studies. Radiol. Artif. Intell. 2021, 3, e200165. [Google Scholar] [CrossRef]
- Zhang, L.; Li, M.; Zhou, Y.; Lu, G.; Zhou, Q. Deep Learning Approach for Anterior Cruciate Ligament Lesion Detection: Evaluation of Diagnostic Performance Using Arthroscopy as the Reference Standard. J. Magn. Reson. Imaging 2020, 52, 1745–1752. [Google Scholar] [CrossRef]
- Germann, C.; Marbach, G.; Civardi, F.; Fucentese, S.F.; Fritz, J.; Sutter, R.; Pfirrmann, C.W.; Fritz, B. Deep convolutional neural network–based diagnosis of anterior cruciate ligament tears: Performance comparison of homogenous versus heterogeneous knee MRI cohorts with different pulse sequence protocols and 1.5-T and 3-T magnetic field strengths. Investig. Radiol. 2020, 55, 499–506. [Google Scholar] [CrossRef]
- Chang, P.D.; Wong, T.T.; Rasiej, M.J. Deep Learning for Detection of Complete Anterior Cruciate Ligament Tear. J. Digit. Imaging 2019, 32, 980–986. [Google Scholar] [CrossRef]
- Liu, F.; Guan, B.; Zhou, Z.; Samsonov, A.; Rosas, H.; Lian, K.; Sharma, R.; Kanarek, A.; Kim, J.; Guermazi, A.; et al. Fully Automated Diagnosis of Anterior Cruciate Ligament Tears on Knee MR Images by Using Deep Learning. Radiol. Artif. Intell. 2019, 1, 180091. [Google Scholar] [CrossRef]
- Li, F.; Zhai, P.; Yang, C.; Feng, G.; Yang, J.; Yuan, Y. Automated diagnosis of anterior cruciate ligament via a weighted multi-view network. Front. Bioeng. Biotechnol. 2023, 11, 1268543. [Google Scholar] [CrossRef] [PubMed]
- Dung, N.T.; Thuan, N.H.; Van Dung, T.; Van Nho, L.; Tri, N.M.; Vy, V.P.T.; Hoang, L.N.; Phat, N.T.; Chuong, D.A.; Dang, L.H. End-to-end deep learning model for segmentation and severity staging of anterior cruciate ligament injuries from MRI. Diagn. Interv. Imaging 2023, 104, 133–141. [Google Scholar] [CrossRef]
- Awan, M.J.; Rahim, M.S.M.; Salim, N.; Rehman, A.; Nobanee, H.; Shabir, H. Improved Deep Convolutional Neural Network to Classify Osteoarthritis from Anterior Cruciate Ligament Tear Using Magnetic Resonance Imaging. J. Pers. Med. 2021, 11, 1163. [Google Scholar] [CrossRef]
- Flannery, S.W.; Kiapour, A.M.; Edgar, D.J.; Murray, M.M.; Beveridge, J.E.; Fleming, B.C. A transfer learning approach for automatic segmentation of the surgically treated anterior cruciate ligament. J. Orthop. Res. 2021, 40, 277–284. [Google Scholar] [CrossRef]
- Flannery, S.W.; Kiapour, A.M.; Edgar, D.J.; Murray, M.M.; Fleming, B.C. Automated magnetic resonance image segmentation of the anterior cruciate ligament. J. Orthop. Res. 2021, 39, 831–840. [Google Scholar] [CrossRef]
- Awan, M.J.; Rahim, M.S.M.; Salim, N.; Nobanee, H.; Asif, A.A.; Attiq, M.O. MGACA-Net: A novel deep learning based multi-scale guided attention and context aggregation for localization of knee anterior cruciate ligament tears region in MRI images. PeerJ Comput. Sci. 2023, 9, e1483. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Yang, H.; Wang, C.; Wang, C.; Ying, M.; Chen, Z.; Yang, K.; Zhang, J.; Li, K.; Dimitriou, D.; et al. A deep learning approach for anterior cruciate ligament rupture localization on knee MR images. Front. Bioeng. Biotechnol. 2022, 10, 1024527. [Google Scholar] [CrossRef]
- Lu, Y.; Pareek, A.; Yang, L.; Rouzrokh, P.; Khosravi, B.; Okoroha, K.R.; Krych, A.J.; Camp, C.L. Deep Learning Artificial Intelligence Tool for Automated Radiographic Determination of Posterior Tibial Slope in Patients With ACL Injury. Orthop. J. Sports Med. 2023, 11, 23259671231215820. [Google Scholar] [CrossRef] [PubMed]
- Sarker, I.H. Deep Learning: A Comprehensive Overview on Techniques, Taxonomy, Applications and Research Directions. SN Comput. Sci. 2021, 2, 420. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Sarker, I.H. Machine Learning: Algorithms, Real-World Applications and Research Directions. SN Comput. Sci. 2021, 2, 160. [Google Scholar] [CrossRef]
- Fayyad, J.; Jaradat, M.A.; Gruyer, D.; Najjaran, H. Deep Learning Sensor Fusion for Autonomous Vehicle Perception and Localization: A Review. Sensors 2020, 20, 4220. [Google Scholar] [CrossRef] [PubMed]
- Alzubaidi, L.; Zhang, J.; Humaidi, A.J.; Al-Dujaili, A.; Duan, Y.; Al-Shamma, O.; Santamaría, J.; Fadhel, M.A.; Al-Amidie, M.; Farhan, L. Review of deep learning: Concepts, CNN architectures, challenges, applications, future directions. J. Big Data 2021, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Fu, Y.-L.; Zhu, D. ResNet and its application to medical image processing: Research progress and challenges. Comput. Methods Programs Biomed. 2023, 240, 107660. [Google Scholar] [CrossRef] [PubMed]
- Szegedy, C.; Liu, W.; Jia, Y.; Sermanet, P.; Reed, S.; Anguelov, D.; Erhan, D.; Vanhoucke, V.; Rabinovich, A. Going deeper with convolutions. In Proceedings of the 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Boston, MA, USA, 7–12 June 2015. [Google Scholar] [CrossRef]
- Lu, H.; She, Y.; Tie, J.; Xu, S. Half-UNet: A Simplified U-Net Architecture for Medical Image Segmentation. Front. Neurosci. 2022, 16, 911679. [Google Scholar] [CrossRef]
- Bailly, A.; Blanc, C.; Francis, É.; Guillotin, T.; Jamal, F.; Wakim, B.; Roy, P. Effects of dataset size and interactions on the prediction performance of logistic regression and deep learning models. Comput. Methods Programs Biomed. 2022, 213, 106504. [Google Scholar] [CrossRef]
- Sokal, P.A.; Norris, R.; Maddox, T.W.; Oldershaw, R.A. The diagnostic accuracy of clinical tests for anterior cruciate ligament tears are comparable but the Lachman test has been previously overestimated: A systematic review and meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 3287–3303. [Google Scholar] [CrossRef]
- Li, K.; Du, J.; Huang, L.-X.; Ni, L.; Liu, T.; Yang, H.-L. The diagnostic accuracy of magnetic resonance imaging for anterior cruciate ligament injury in comparison to arthroscopy: A meta-analysis. Sci. Rep. 2017, 7, 7583. [Google Scholar] [CrossRef]
- Khanna, N.N.; Maindarkar, M.A.; Viswanathan, V.; Fernandes, J.F.E.; Paul, S.; Bhagawati, M.; Ahluwalia, P.; Ruzsa, Z.; Sharma, A.; Kolluri, R.; et al. Economics of Artificial Intelligence in Healthcare: Diagnosis vs. Treatment. Healthcare 2022, 10, 2493. [Google Scholar] [CrossRef]
- Morris, Z.S.; Wooding, S.; Grant, J. The answer is 17 years, what is the question: Understanding time lags in translational research. J. R. Soc. Med. 2011, 104, 510–520. [Google Scholar] [CrossRef]
- Langlotz, C.P. Will artificial intelligence replace radiologists? Radiol. Artif. Intell. 2019, 1, e190058. [Google Scholar] [CrossRef] [PubMed]
- Gerke, S.; Minssen, T.; Cohen, G. Chapter 12—Ethical and legal challenges of artificial intelligence-driven healthcare. In Artificial Intelligence in Healthcare; Bohr, A., Memarzadeh, K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 295–336. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).