Bioremediation of Persistent Organic Pollutant—Oxybenzone with Pleurotus djamor
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Mushroom Material
2.3. Preparation of Pleurotus djamor Mycelial Cultures
2.4. Experimental Mycelial Cultures of Pleurotus djamor
2.5. Sample Preparation for Analysis
2.6. HPLC Analysis
2.7. UPLC Analysis
2.8. Statistical Analysis
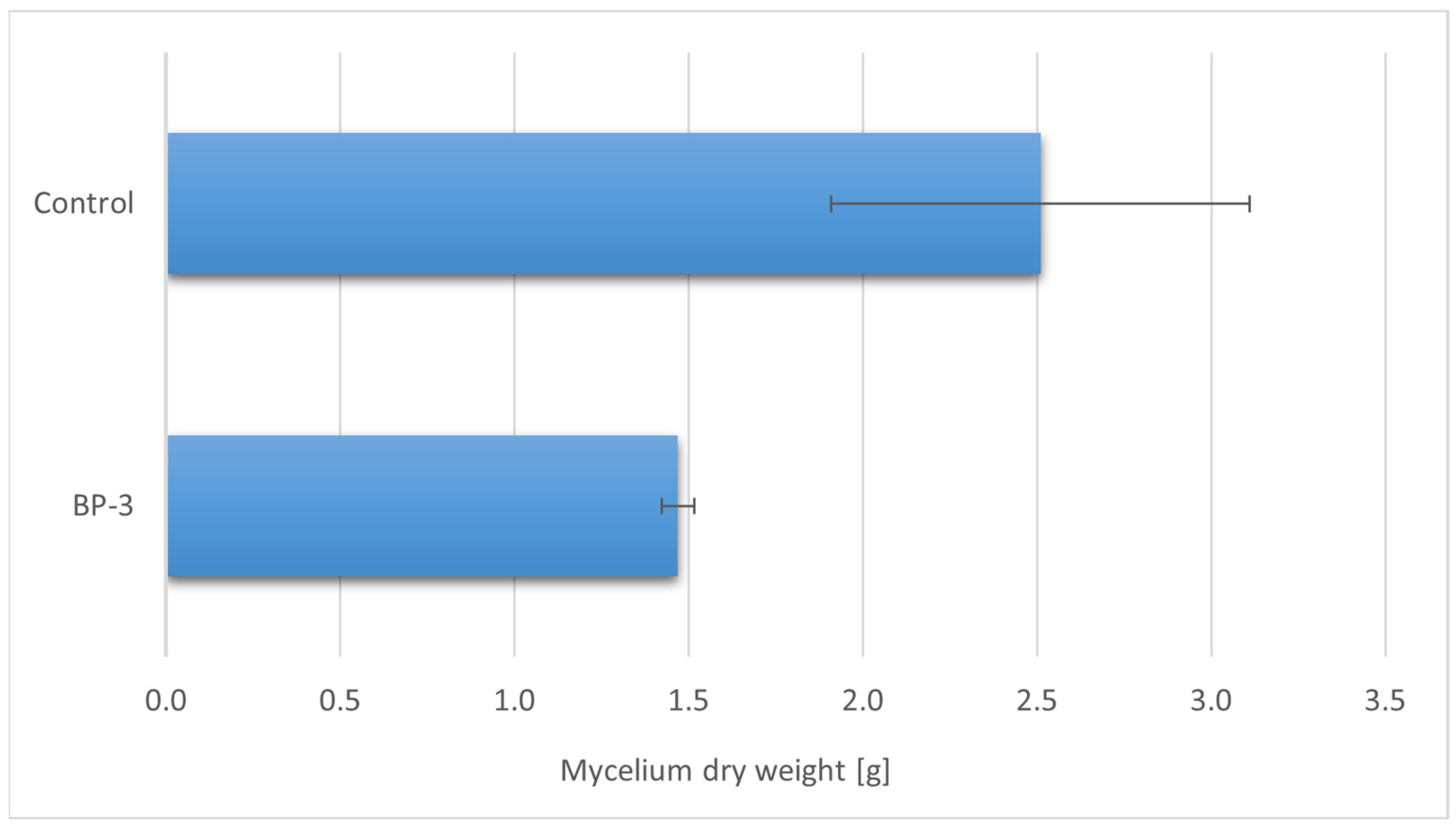
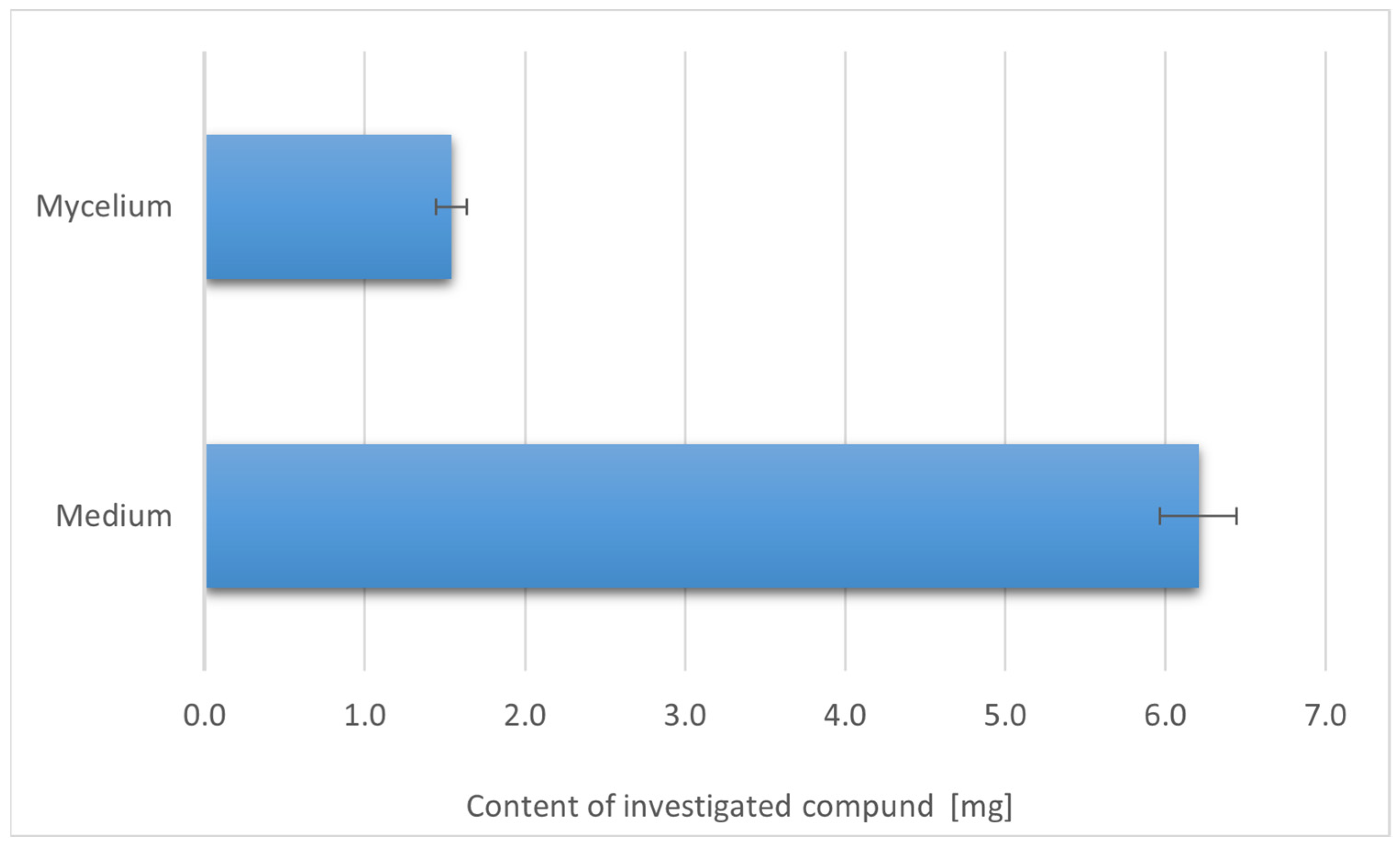
3. Results
3.1. Profile of Organic Compounds with Health Benefits
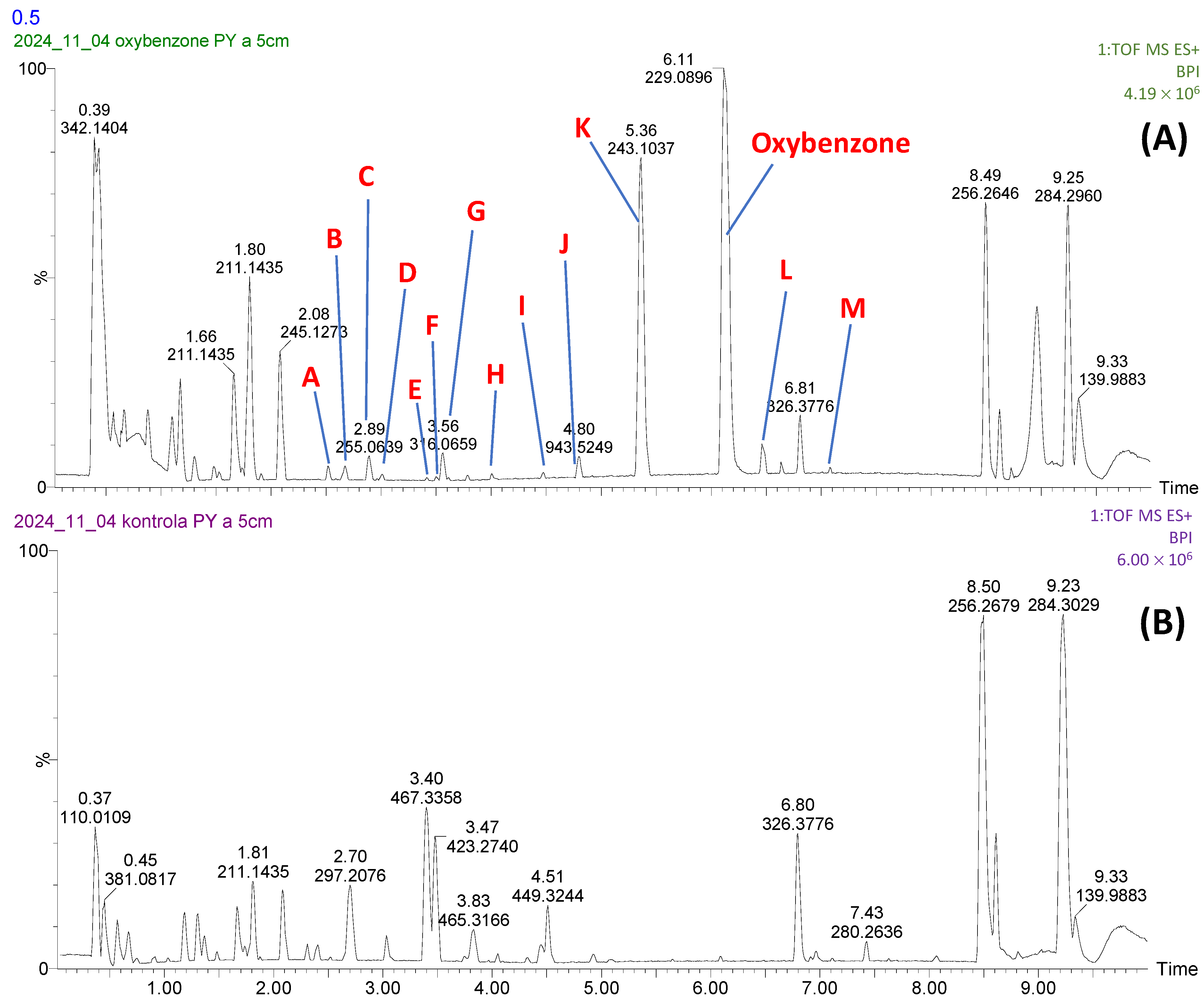
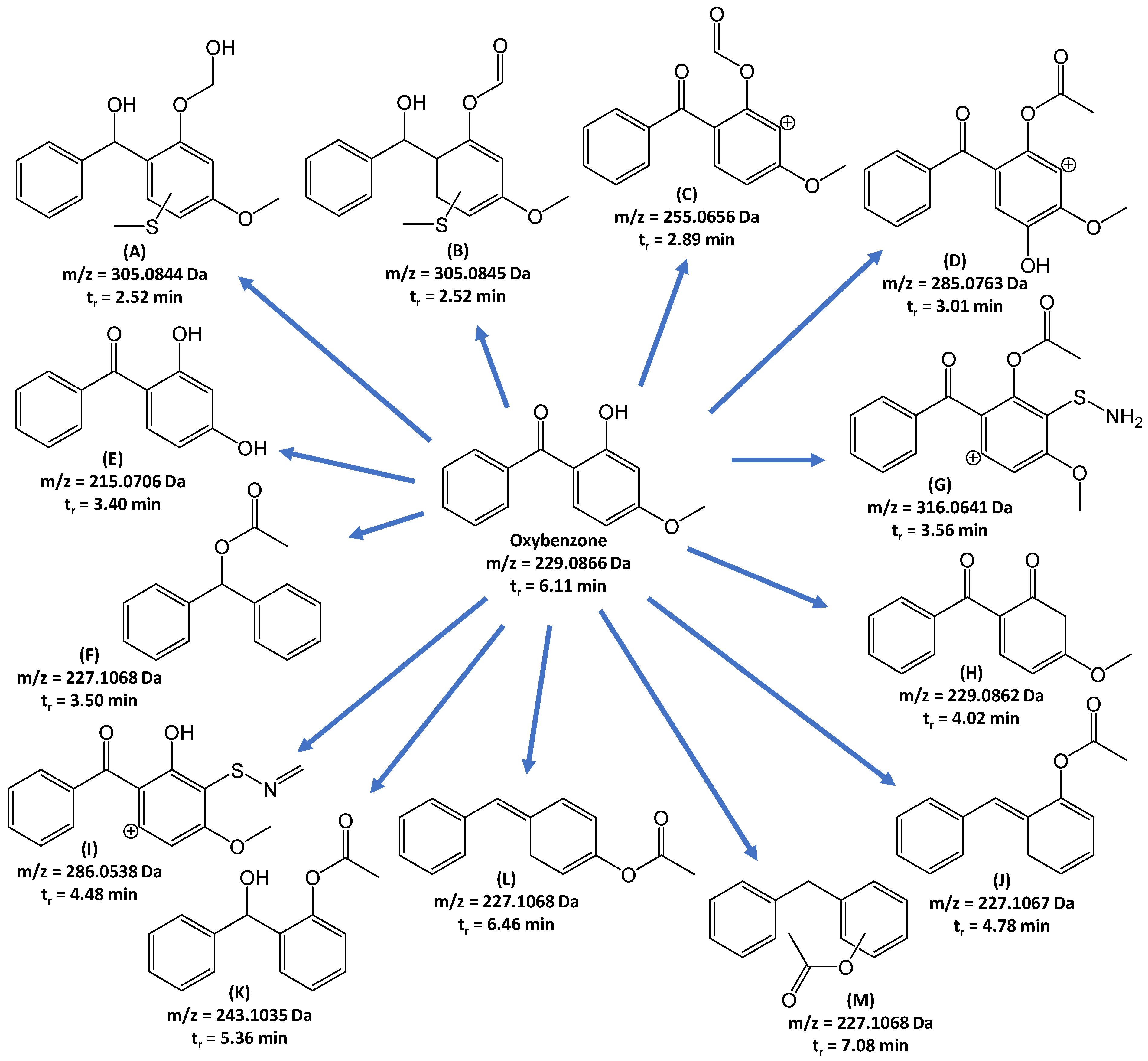
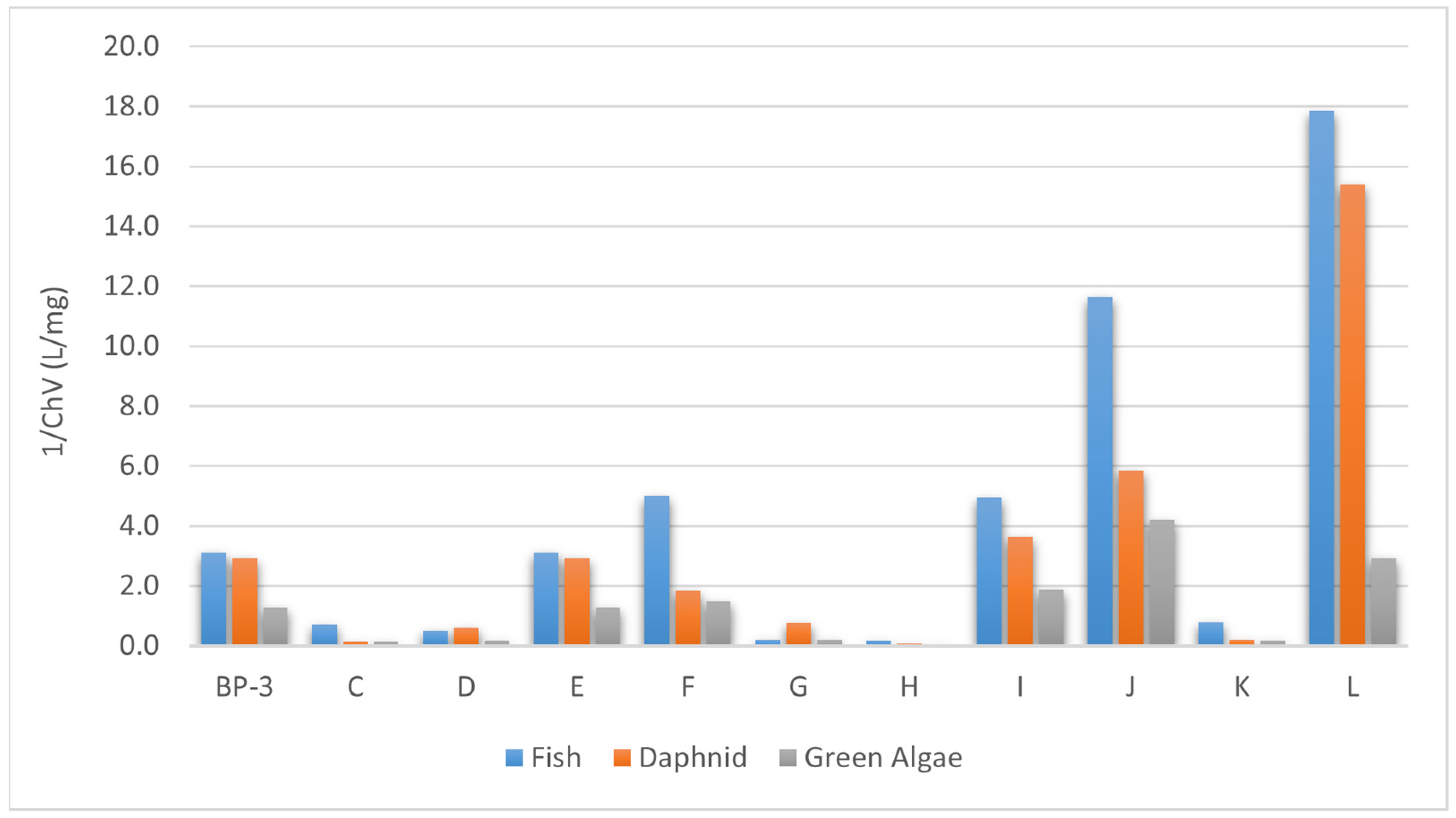
3.2. Identification of Biodegradation Products of Oxybenzone and Toxicity Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cooper, A.; Shenkiryk, A.; Chin, H.; Morris, M.; Mehndiratta, L.; Roundtree, K.; Tafuri, T.; Slade, J.H. Photoinitiated degradation kinetics of the organic UV filter oxybenzone in solutions and aerosols: Impacts of salt, photosensitizers, and the medium. ACS EST Air 2024, 1, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.; Lupuliasa, D.; Adam-Dima, I.; Nitulescu, G.M. Ultraviolet filters for cosmetic applications. Cosmetics 2023, 10, 101. [Google Scholar] [CrossRef]
- Mao, F.; He, Y.; Gin, K.Y.-H. Occurrence and fate of benzophenone-type UV filters in aquatic environments: A review. Environ. Sci. Water Res. Technol. 2019, 5, 209–223. [Google Scholar] [CrossRef]
- Downs, C.A.; Diaz-Cruz, M.S.; White, W.T.; Rice, M.; Jim, L.; Punihaole, C.; Dant, M.; Gautam, K.; Woodley, C.M.; Walsh, K.O.; et al. Beach showers as sources of contamination for sunscreen pollution in marine protected areas and areas of intensive beach tourism in Hawaii, USA. J. Hazard. Mater. 2022, 438, 129546. [Google Scholar] [CrossRef]
- ECHA. Substance Infocard—Oxybenzone. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.004.575 (accessed on 20 December 2024).
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Division on Earth and Life Studies; Board on Health Sciences Policy; Board on Environmental Studies and Toxicology; Ocean Studies Board; Committee on Environmental Impact of Currently Marketed Sunscreens and Potential Human Impacts of Changes in Sunscreen Usage. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health; National Academies Press: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Burnett, M.E.; Wang, S.Q. Current sunscreen controversies: A critical review. Photodermatol. Photoimmunol. Photomed. 2011, 27, 58–67. [Google Scholar] [CrossRef]
- Raffa, R.B.; Pergolizzi, J.V., Jr.; Taylor, R., Jr.; Kitzen, J.M. NEMA Research Group. Sunscreen bans: Coral reefs and skin cancer. J. Clin. Pharm. Ther. 2019, 44, 134–139. [Google Scholar] [CrossRef]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar] [CrossRef]
- Downs, C.A.; Bishop, E.; Diaz-Cruz, M.S.; Haghshenas, S.A.; Stien, D.; Rodrigues, A.M.S.; Woodley, C.M.; Sunyer-Caldú, A.; Doust, S.N.; Espero, W.; et al. Oxybenzone contamination from sunscreen pollution and its ecological threat to Hanauma Bay, Oahu, Hawaii, U.S.A. Chemosphere 2022, 291, 132880. [Google Scholar] [CrossRef]
- Gao, L.; Li, L.Y.; Zheng, L.X.; Wu, H.J.; Tao, H.; Liu, D.C. Contamination Characteristics and Ecological Risk Assessment of Pharmaceuticals and Personal Care Products in Drains Flowing into the Yellow River of Ningxia. Huan Jing Ke Xue 2024, 45, 1468–1479. [Google Scholar] [CrossRef]
- Carve, M.; Singh, N.; Grist, S.; Shimeta, J.; Nugegoda, D. Toxicity of the organic UV filter oxybenzone to the brown macroalga Hormosira banksii and the green macroalga Ulva lactuca. Sci. Total Environ. 2025, 958, 177982. [Google Scholar] [CrossRef]
- Fivenson, D.; Sabzevari, N.; Qiblawi, S.; Blitz, J.; Norton, B.B.; Norton, S.A. Sunscreens: UV filters to protect us: Part 2-Increasing awareness of UV filters and their potential toxicities to us and our environment. Int. J. Womens Dermatol. 2020, 7, 45–69. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, R.; Zhu, Z.; Xiao, W.; Wang, J.; Zhao, W.; Li, Y. Benzophenone-3 exposure induced apoptosis via impairing mitochondrial function in human chondrocytes. Ecotoxicol. Environ. Saf. 2024, 287, 117286. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiao, X.; Zhou, C.; Zhang, Y.N.; Fu, Z.; Chen, J. Photochemical transformation of sunscreen agent benzophenone-3 and its metabolite in surface freshwater and seawater. Chemosphere 2016, 153, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Consumer Safety. Opinion on Benzophenone-3. (CAS No. 131-57-7, EC No. 205-031-5). 2021. Available online: https://health.ec.europa.eu/system/files/2022-08/sccs_o_247.pdf (accessed on 20 December 2024).
- Calafat, A.M.; Wong, L.Y.; Ye, X.; Reidy, J.A.; Needham, L.L. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003–2004. Environ. Health Perspect. 2008, 116, 893–897. [Google Scholar] [CrossRef]
- Suh, S.; Pham, C.; Smith, J.; Mesinkovska, N.A. The banned sunscreen ingredients and their impact on human health: A systematic review. Int. J. Dermatol. 2020, 59, 1033–1042. [Google Scholar] [CrossRef]
- Wang, W.; Kannan, K. Mass loading and emission of benzophenone-3 (BP-3) and its derivatives in wastewater treatment plants in New York State, USA. Sci. Total Environ. 2017, 579, 1316–1322. [Google Scholar] [CrossRef]
- Yang, C.-W.; Tu, P.-H.; Tso, W.-Y.; Chang, B.-V. Removal of organic UV filters using enzymes in spent mushroom composts from fungicultures. Appl. Sci. 2021, 11, 3932. [Google Scholar] [CrossRef]
- Akhtar, N.; Mannan, M.A. Mycoremediation: Expunging environmental pollutants. Biotechnol. Rep. 2020, 26, e00452. [Google Scholar] [CrossRef]
- Zapaśnik, A.; Bryła, M.; Wojtczak, A.; Sokołowska, B. In vivo Effectiveness of Pleurotus ostreatus in degradation of toxic metabolites of filamentous fungi such as aflatoxin B1 and zearalenone. Metabolites 2025, 15, 20. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Kryczyk-Poprawa, A.; Sánchez-Hidalgo, A.; Baran, W.; Adamek, E.; Sułkowska-Ziaja, K.; Kała, K.; Muszyńska, B.; Opoka, W. The toxicological impact of the ultraviolet filter oxybenzone on antioxidant profiles in in vitro cultures of Lentinula edodes. Toxics 2025, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Chawla, H.M.; Mrig, S. Simultaneous quantitative estimation of oxybenzone and 2-ethylhexyl-4-methoxycinnamate in sunscreen formulations by second order derivative spectrophotometry. J. Anal. Chem. 2009, 64, 585–592. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim. Biophys. Acta. 2012, 1822, 784–793. [Google Scholar] [CrossRef]
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef]
- Taofiq, O.; Silva, A.R.; Costa, C.; Ferreira, I.; Nunes, J.; Prieto, M.A.; Simal-Gandara, J.; Barros, L.; Ferreira, I.C. Optimization of ergosterol extraction from Pleurotus mushrooms using response surface methodology. Food Funct. 2020, 11, 5887–5897. [Google Scholar] [CrossRef]
- Kikuchi, T. Ergostane-type steroids from mushrooms of Pleurotus genus. J. Nat. Med. 2024, 78, 123–132. [Google Scholar] [CrossRef]
- Herrmann, K.M.; Weaver, L.M. The shikimate pathway. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef]
- Vicidomini, C.; Palumbo, R.; Moccia, M.; Roviello, G.N. Oxidative processes and xenobiotic metabolism in plants: Mechanisms of defense and potential therapeutic implications. J. Xenobiot. 2024, 14, 1541–1569. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety. Opinion on Benzophenone-1. (CAS No. 131-56-6, EC No. 205-029-4). 2024. Available online: https://health.ec.europa.eu/document/download/1161e3b7-8e52-47d8-9153-54433fa0d7fe_en?filename=sccs_o_293.pdf (accessed on 20 December 2024).
- Minnesota Department of Health. Revised Compilation of USGS Water Quality Monitoring Data for Contaminants of Emerging Concern; Minnesota Department of Health: St. Paul, MN, USA, 2018.
| Component A | H2O (for LC-MS Chromasolv®; Fluka-Analytical, Buchs, Switzerland), with 0.01% HCOOH (98–100%, for LC-MS, LiChropur®; Sigma-Aldrich, St. Louis, MO, USA) |
| Component B | CH3CN (for LC-MS LiChrosolv®; Sigma-Aldrich, St. Louis, MO, USA) with 0.01% HCOOH |
| Gradient (min; % A) | 0.0–90, 6.0–40, 8.0–10, 8.5–10, 9.0–90, 10.0–90 |
| Flow rate | 0.300 mL min−1 |
| Sample volume | 0.5 and 1.0 µL |
| Column temperature | 35 °C |
| Source | ES+ |
| Scan time | 0.1 s |
| Start mass | 50.0 Da |
| End mass | 600.0 Da |
| Maximum mass error | 0.5 mDa |
| Operating mode | ms and ms/ms |
| Collision energy | 0–25 eV |
| Reference compound | Leucine Enkephalin single point (ms) |
| Software | MassLynx v4.1 |
| Analyzed Compounds | Mycelium from In Vitro Cultures | Mycelium from In Vitro Cultures with BP-3 |
|---|---|---|
| Indole compounds | ||
| L-Tryptophan | – | 8.47 ± 0.34 * |
| 6-metylo-D,L-tryptophan | 1.52 ± 0.06 | 4.56 ± 0.55 * |
| Phenolic compounds | ||
| p-Hydroxybenzoic acid | – | 5.90 ± 0.08 * |
| Sterols | ||
| Ergosterol | 0.15 ± 0.03 | 8.88 ± 0.11 * |
| Other organic compounds | ||
| Lovastatin | – | – |
| Ergothioneine | 3.96 ± 0.17 | 1.58 ± 0.11 * |
| L-Phenylalanine | 54.03 ± 4.3 | 176.33 ± 5.85 * |
| Retention Time (tr) (min) | Symbol in Figure | Summary Formula [M+H+] | Daughter Ions [m/z]; Formula |
|---|---|---|---|
| 2.52 | A | C16H17O4S | 287.0738; C16H15O3S 269.0633; C16H13O2S 241.0684; C15H13OS |
| 2.65 | B | C16H17O4S | 255.0478; C15H11O2S 227.052; C14H11OS 211.0576; C14H11S 209; C14H9S |
| 2.89 | C | C15H11O4 | 237.0552; C15H9O3 227.0707; C14H11O3 199.0758; C13H11O2 181.0650; C13H9O 137.0239; C7H5O3 |
| 3.01 | D | C16H13O5 | 270.0524; C15H10O5 229.0863; C14H13O3 |
| 3.40 | E | C13H11O3 | 137.0235; C7H5O3 105.0342; C7H5O |
| 3.50 | F | C15H15O2 | 91.0548; C7H7 |
| 3.56 | G | C16H14NO4S | 274.0538; C14H12NO3S 259.0305; C13H9NO3S 237.0094; C10H7NO4S 105.0336; C7H5O |
| 4.02 | H | C14H13O3 | 151.0400; C8H7O3 105.0345; C7H5O |
| 4.48 | I | C15H12NO3S | 208.0072; C9H6NO3S 180.0120; C8H6NO2S 152.0164; C7H6NOS |
| 4.78 | J | C15H15O2 | 91.0548; C7H7 |
| 5.36 | K | C15H15O3 | 165.0551; C9H9O3 105.0345; C7H5O |
| 6.11 | Oxybenzone | C14H13O3 | 151.0400; C8H7O3 105.0345; C7H5O |
| 6.46 | L | C15H15O2 | 151.0399; C8H7O3 91.0548; C7H7 |
| 7.08 | M | C15H15O2 | 151.0399; C8H7O3 91.0548; C7H7 |
| Compound | Mutagenic | Tumorigenic | Irritant | Reproductive Effects |
|---|---|---|---|---|
| Oxybenzone | known to be mutagenic | known to be tumorigenic | – | high-risk fragment |
| Product A | medium-risk fragment | – | – | – |
| Product B | – | – | medium-risk fragment | – |
| Product C | – | – | medium-risk fragment | – |
| Product D | – | – | – | – |
| Product E | high-risk fragment | medium-risk fragment | high-risk fragment | medium-risk fragment |
| Product F | – | – | medium-risk fragment | – |
| Product G | – | – | medium-risk fragment | – |
| Product H | – | – | – | – |
| Product I | medium-risk fragment | – | – | – |
| Product K | – | – | – | – |
| Product L | high-risk fragment | – | medium-risk fragment | high-risk fragment |
| Product M | – | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryczyk-Poprawa, A.; Baran, W.; Sułkowska-Ziaja, K.; Kała, K.; Adamek, E.; Król, M.; Sánchez-Hidalgo, A.; Muszyńska, B. Bioremediation of Persistent Organic Pollutant—Oxybenzone with Pleurotus djamor. Appl. Sci. 2025, 15, 4707. https://doi.org/10.3390/app15094707
Kryczyk-Poprawa A, Baran W, Sułkowska-Ziaja K, Kała K, Adamek E, Król M, Sánchez-Hidalgo A, Muszyńska B. Bioremediation of Persistent Organic Pollutant—Oxybenzone with Pleurotus djamor. Applied Sciences. 2025; 15(9):4707. https://doi.org/10.3390/app15094707
Chicago/Turabian StyleKryczyk-Poprawa, Agata, Wojciech Baran, Katarzyna Sułkowska-Ziaja, Katarzyna Kała, Ewa Adamek, Małgorzata Król, Adrián Sánchez-Hidalgo, and Bożena Muszyńska. 2025. "Bioremediation of Persistent Organic Pollutant—Oxybenzone with Pleurotus djamor" Applied Sciences 15, no. 9: 4707. https://doi.org/10.3390/app15094707
APA StyleKryczyk-Poprawa, A., Baran, W., Sułkowska-Ziaja, K., Kała, K., Adamek, E., Król, M., Sánchez-Hidalgo, A., & Muszyńska, B. (2025). Bioremediation of Persistent Organic Pollutant—Oxybenzone with Pleurotus djamor. Applied Sciences, 15(9), 4707. https://doi.org/10.3390/app15094707







