A Numerical Investigation of Enhanced Microfluidic Immunoassay by Multiple-Frequency Alternating-Current Electrothermal Convection
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Device Geometry
2.2. Theory of MET in Electrically Conductive Fluid
2.2.1. Quasi-Electrostatics
2.2.2. Heat Transfer
2.2.3. Electrothermal Convection
2.2.4. Mass Transfer of Antigen and Immune Response Enhancement
2.3. Numerical Simulation
2.3.1. Dual Frequency AC Electric Fields in a Hybrid SW and TW Excitation
2.3.2. Thermal Diffusion in the Fluidic Device
2.3.3. Electrokinetic Fluid Motion Driven by MET
2.3.4. Transient Immune Response
3. Results and Discussion
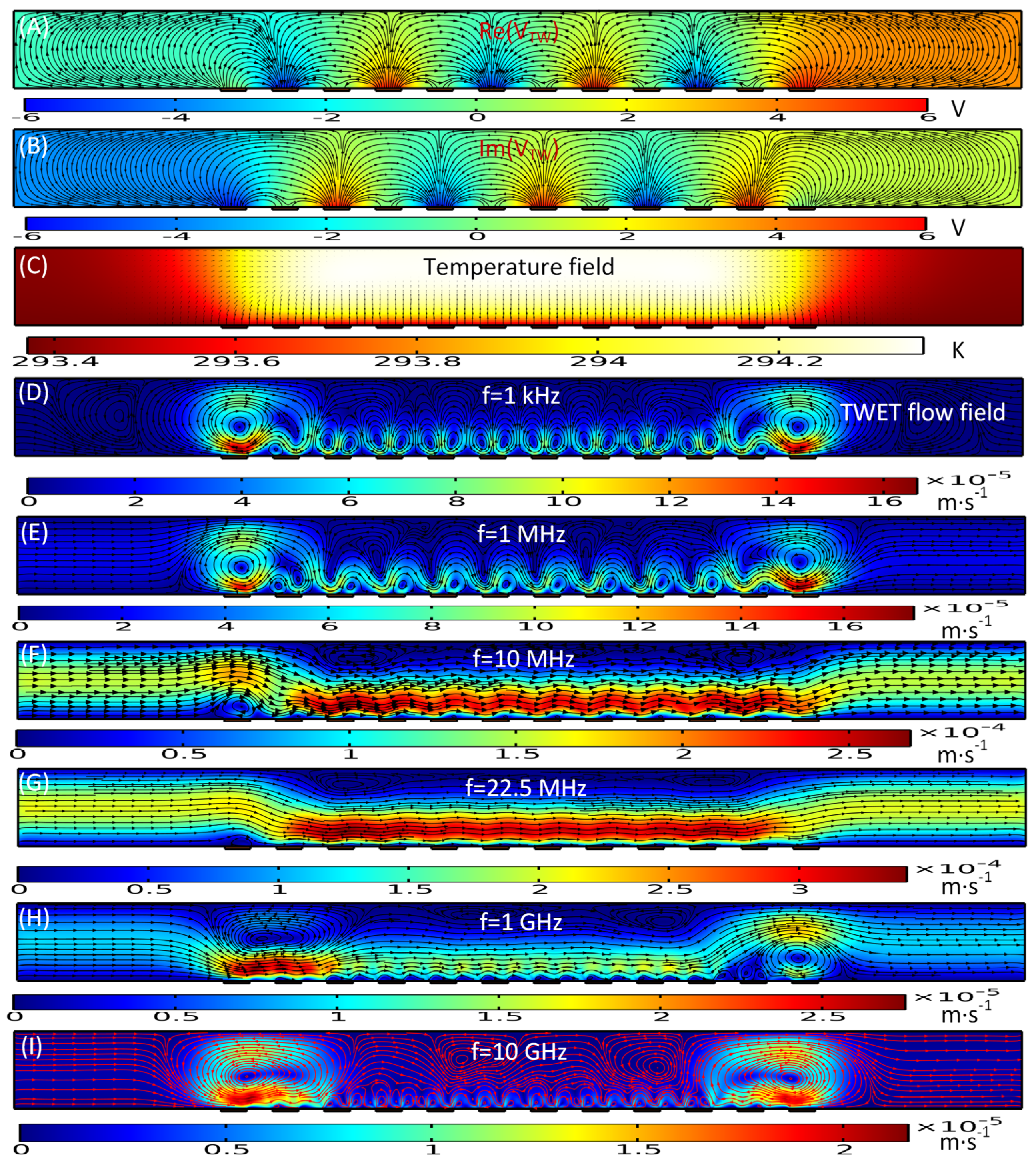
3.1. Numerical Characterization of Electrothermal Induced Convection
3.1.1. Standing-Wave Electrothermal Fluid Motion
3.1.2. Traveling-Wave Induced Electrothermal Flow
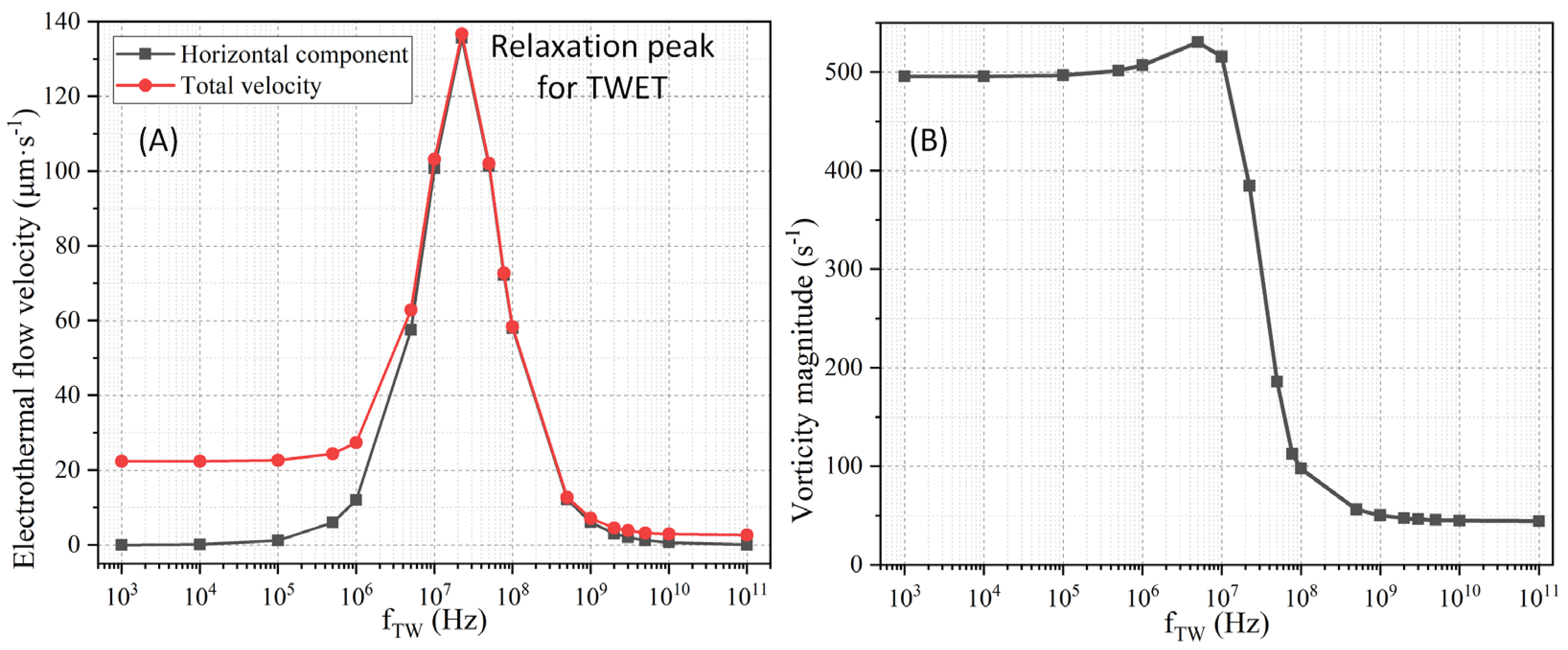
3.1.3. Multiple-Frequency Electrothermal Induced Convection
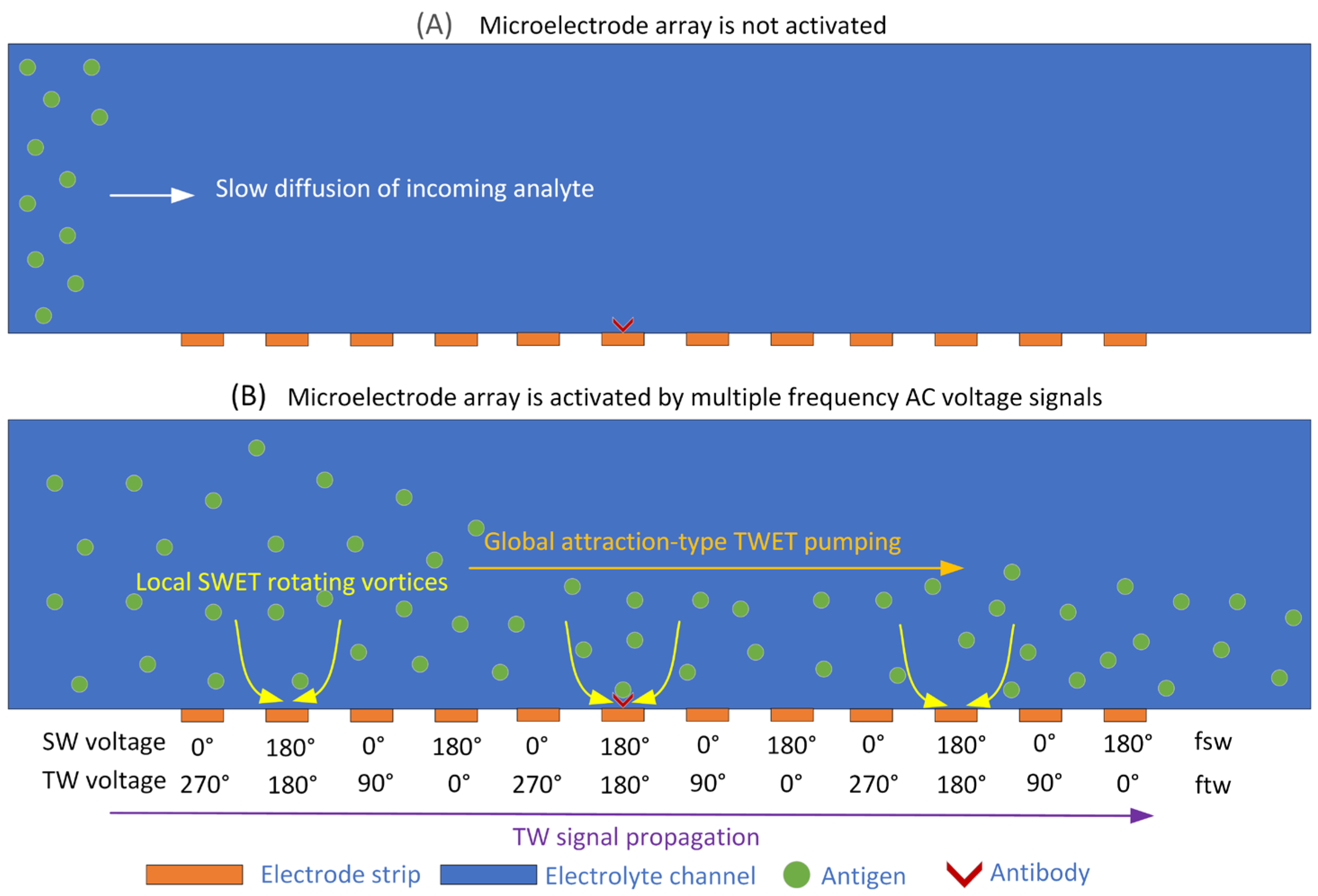
3.2. Enhanced Microfluidic Immunoassay by Applying MET
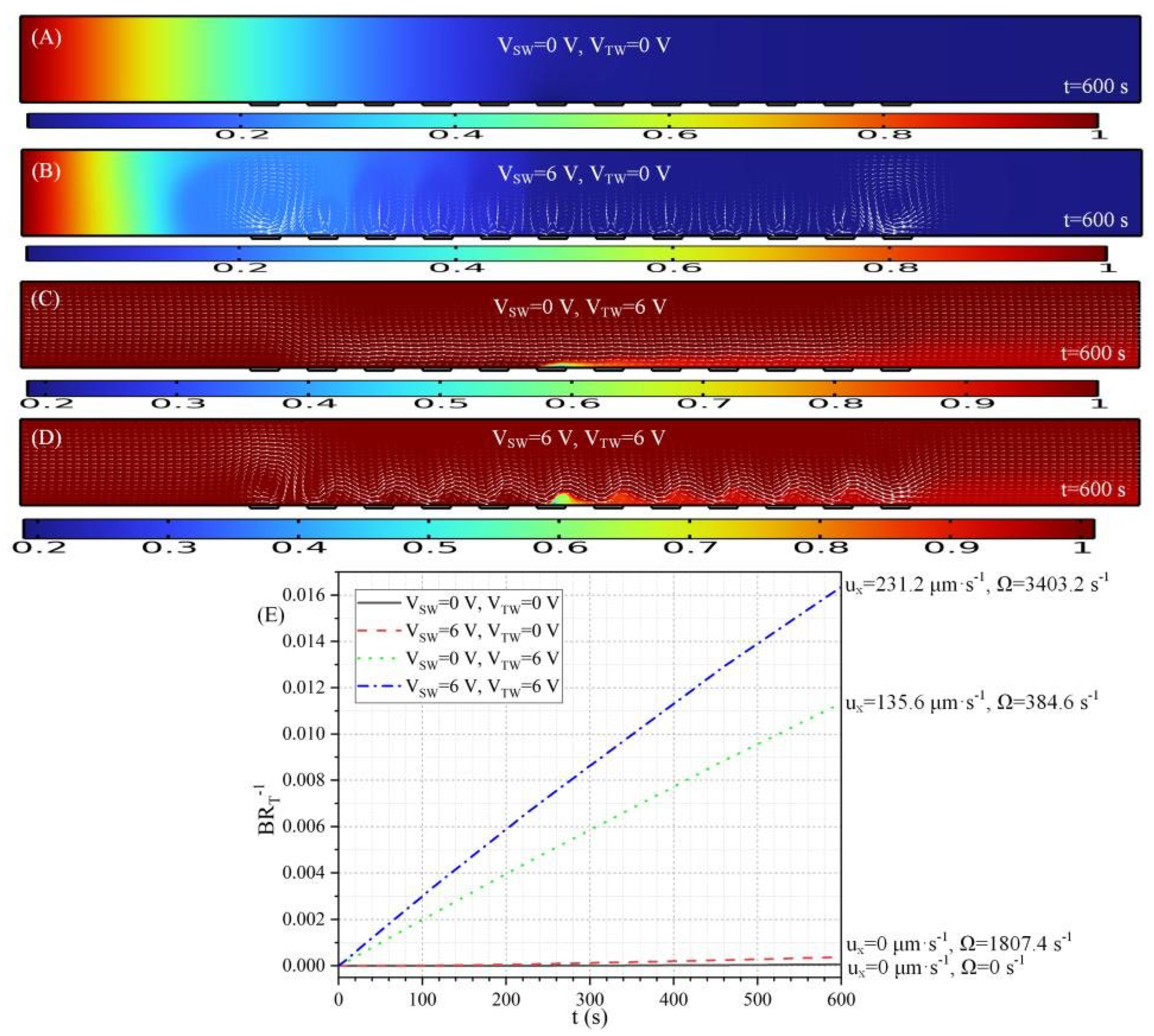
3.2.1. Transient Immune Response Under MET
3.2.2. Frequency Effect on Binding Reaction Efficiency
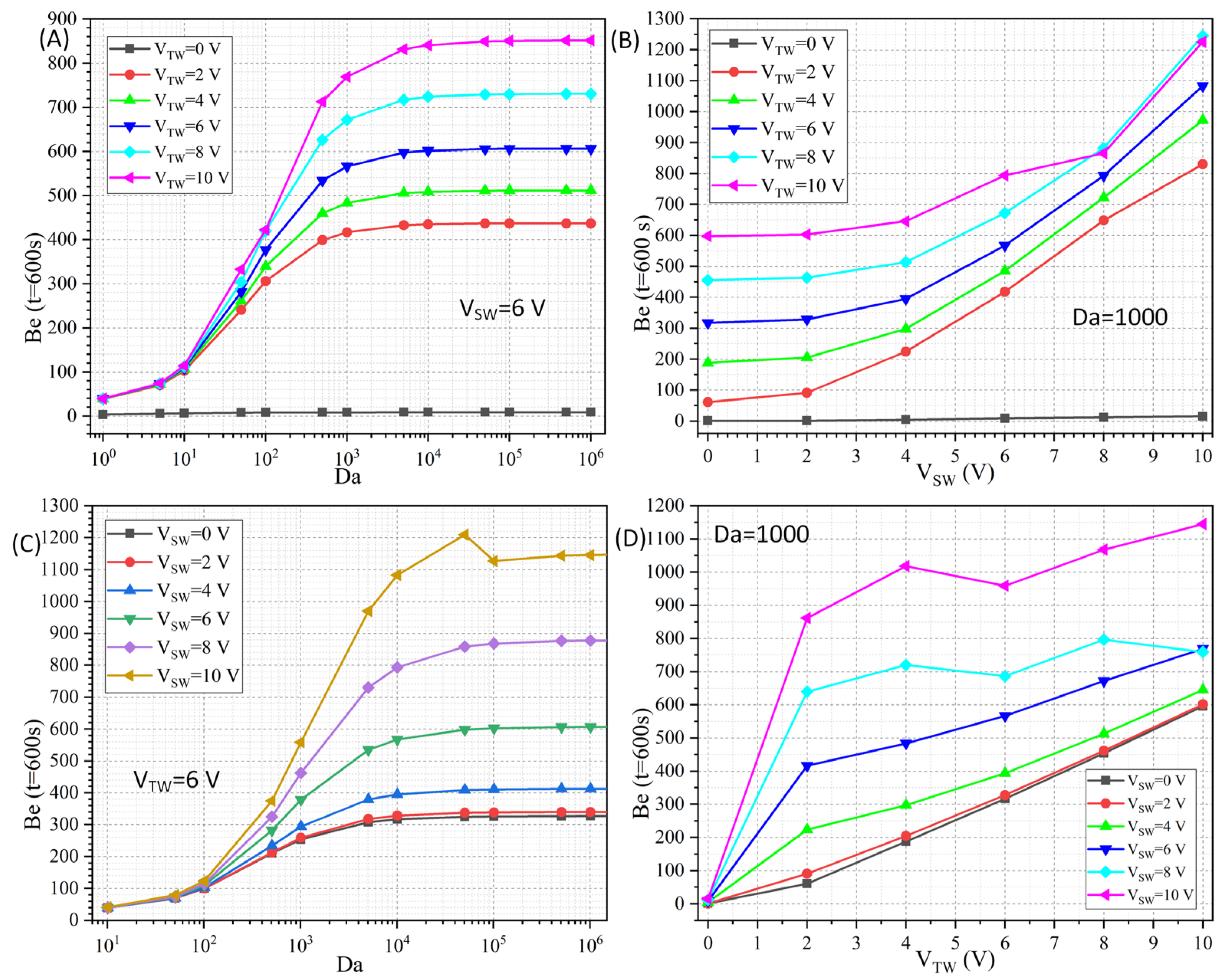
3.2.3. Cross Influence of Da Number and Voltage Amplitude on Binding Rate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Liu, F.; KC, P.; Zhang, G.; Zhe, J. Microfluidic magnetic bead assay for cell detection. Anal. Chem. 2016, 88, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J. A microfluidic-based dynamic microarray system with single-layer pneumatic valves for immobilization and selective retrieval of single microbeads. Microfluid. Nanofluid. 2014, 16, 623–633. [Google Scholar] [CrossRef]
- Grilli, S.; Miccio, L.; Gennari, O.; Coppola, S.; Vespini, V.; Battista, L.; Orlando, P.; Ferraro, P. Active accumulation of very diluted biomolecules by nano-dispensing for easy detection below the femtomolar range. Nat. Commun. 2014, 5, 5314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, H.; Xu, H. Recent progress in digital immunoassay: How to achieve ultrasensitive, multiplex and clinical accessible detection? Sens. Diagn. 2024, 3, 9–27. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, J.; Xiao, B.; Chen, A. Microfluidic-assisted integrated nucleic acid test strips for POCT. Talanta 2024, 267, 125150. [Google Scholar] [CrossRef]
- Chong, S.W.; Shen, Y.; Palomba, S.; Vigolo, D. Nanofluidic Lab-On-A-Chip Systems for Biosensing in Healthcare. Small 2025, 21, 2407478. [Google Scholar] [CrossRef]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic diagnostic technologies for global public health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef]
- Hu, G.; Gao, Y.; Li, D. Modeling micropatterned antigen–antibody binding kinetics in a microfluidic chip. Biosens. Bioelectron. 2007, 22, 1403–1409. [Google Scholar] [CrossRef]
- Gao, Y.; Sherman, P.M.; Sun, Y.; Li, D. Multiplexed high-throughput electrokinetically-controlled immunoassay for the detection of specific bacterial antibodies in human serum. Anal. Chim. Acta 2008, 606, 98–107. [Google Scholar] [CrossRef]
- Hu, G.; Li, D. Multiscale phenomena in microfluidics and nanofluidics. Chem. Eng. Sci. 2007, 62, 3443–3454. [Google Scholar] [CrossRef]
- Ramos, A.; Morgan, H.; Green, N.G.; Castellanos, A. Ac electrokinetics: A review of forces in microelectrode structures. J. Phys. D Appl. Phys. 1998, 31, 2338. [Google Scholar] [CrossRef]
- Ramos, A. Electrokinetics and Electrohydrodynamics in Microsystems; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Ory, S.; Ehud, Y. The Taylor-Melcher leaky dielectric model as a macroscale electrokinetic description. J. Fluid Mech. 2015, 773, 1–33. [Google Scholar]
- Velev, O.D.; Bhatt, K.H. On-chip micromanipulation and assembly of colloidal particles by electric fields. Soft Matter 2006, 2, 738–750. [Google Scholar] [CrossRef] [PubMed]
- López-Vizcaíno, R.; Navarro, V.; Yustres, Á. Two-Dimensional Modelling Approach for Electrokinetic Water Transport in Unsaturated Kaolinite. Appl. Sci. 2022, 13, 519. [Google Scholar] [CrossRef]
- Tripathi, D.; Narla, V.K.; Aboelkassem, Y. Electrokinetic membrane pumping flow model in a microchannel. Phys. Fluids 2020, 32, 082004. [Google Scholar] [CrossRef]
- Chen, L.; Lee, S.; Choo, J.; Lee, E.K. Continuous dynamic flow micropumps for microfluid manipulation. J. Micromech. Microeng. 2007, 18, 013001. [Google Scholar] [CrossRef]
- González, A.; Ramos, A.; Green, N.G.; Castellanos, A.; Morgan, H. Fluid flow induced by nonuniform ac electric fields in electrolytes on microelectrodes. II. A linear double-layer analysis. Phys. Rev. E 2000, 61, 4019. [Google Scholar] [CrossRef]
- Liu, W.; Tao, Y.; Chen, Y.; Ge, Z.; Chen, J.; Li, Y. Developing an Active Microfluidic Pump and Mixer Driven by AC Field-Effect-Mediated Induced-Charge Electro-Osmosis of Metal–Dielectric Janus Micropillars: Physical Perspective and Simulation Analysis. Appl. Sci. 2023, 13, 8253. [Google Scholar] [CrossRef]
- Salari, A.; Navi, M.; Dalton, C. A novel alternating current multiple array electrothermal micropump for lab-on-a-chip applications. Biomicrofluidics 2015, 9, 014113. [Google Scholar] [CrossRef]
- Liu, W.; Ren, Y.; Shao, J.; Jiang, H.; Ding, Y. A theoretical and numerical investigation of travelling wave induction microfluidic pumping in a temperature gradient. J. Phys. D Appl. Phys. 2014, 47, 075501. [Google Scholar] [CrossRef]
- Gunda, N.S.K.; Bhattacharjee, S.; Mitra, S.K. Study on the use of dielectrophoresis and electrothermal forces to produce on-chip micromixers and microconcentrators. Biomicrofluidics 2012, 6, 034118. [Google Scholar]
- Melcher, J.R.; Firebaugh, M.S. Traveling-Wave Bulk Electroconvection Induced across a Temperature Gradient. Phys. Fluids 1967, 10, 1178–1185. [Google Scholar] [CrossRef]
- Perch-Nielsen, I.R.; Green, N.G.; Wolff, A. Numerical simulation of travelling wave induced electrothermal fluid flow. J. Phys. D Appl. Phys. 2004, 37, 2323. [Google Scholar] [CrossRef]
- Lian, M.; Islam, N.; Wu, J. AC electrothermal manipulation of conductive fluids and particles for lab-chip applications. IET Nanobiotechnol. 2007, 1, 36–43. [Google Scholar] [CrossRef]
- González, A.; Ramos, A.; Morgan, H.; Green, N.G.; Castellanos, A. Electrothermal flows generated by alternating and rotating electric fields in microsystems. J. Fluid Mech. 2006, 564, 415–433. [Google Scholar] [CrossRef]
- Park, S.; Koklu, M.; BeskoK, A. Particle trapping in high-conductivity media with electrothermally enhanced negative dielectrophoresis. Anal. Chem. 2009, 81, 2303–2310. [Google Scholar] [CrossRef]
- Liu, W.; Ren, Y.; Tao, Y.; Zhou, Z.; Wu, Q.; Xue, R.; Yao, B. Multiple frequency electrothermal induced flow: Theory and microfluidic applications. J. Phys. D Appl. Phys. 2020, 53, 175304. [Google Scholar] [CrossRef]
- Iverson, B.D.; Cremaschi, L.; Garimella, S.V. Effects of discrete-electrode configuration on traveling-wave electrohydrodynamic pumping. Microfluid. Nanofluid. 2009, 6, 221–230. [Google Scholar] [CrossRef]
- Gimsa, J.; Stubbe, M.; Gimsa, U. A short tutorial contribution to impedance and AC-electrokinetic characterization and manipulation of cells and media: Are electric methods more versatile than acoustic and laser methods? J. Electr. Bioimpedance 2014, 5, 74–91. [Google Scholar] [CrossRef]
- Castellanos, A.; Ramos, A.; Gonzalez, A.; Green, N.G.; Morgan, H. Electrohydrodynamics and dielectrophoresis in microsystems: Scaling laws. J. Phys. D Appl. Phys. 2003, 36, 2584. [Google Scholar] [CrossRef]
- Green, N.G.; Ramos, A.; Gonzalez, A.; Castellanos, A.; Morgan, H. Electrothermally induced fluid flow on microelectrodes. J. Electrost. 2001, 53, 71–87. [Google Scholar] [CrossRef]
- Green, N.G.; Ramos, A.; González, A.; Castellanos, A.; Morgan, H. Electric field induced fluid flow on microelectrodes: The effect of illumination. J. Phys. D Appl. Phys. 2000, 33, L13. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Huang, S.; Wang, S.; Zhou, X.; Shi, Y.; Zhou, X.; Gong, X.; Tao, Y.; Liu, W. A Numerical Investigation of Enhanced Microfluidic Immunoassay by Multiple-Frequency Alternating-Current Electrothermal Convection. Appl. Sci. 2025, 15, 4748. https://doi.org/10.3390/app15094748
Wu Q, Huang S, Wang S, Zhou X, Shi Y, Zhou X, Gong X, Tao Y, Liu W. A Numerical Investigation of Enhanced Microfluidic Immunoassay by Multiple-Frequency Alternating-Current Electrothermal Convection. Applied Sciences. 2025; 15(9):4748. https://doi.org/10.3390/app15094748
Chicago/Turabian StyleWu, Qisheng, Shaohua Huang, Shenghai Wang, Xiying Zhou, Yuxuan Shi, Xiwei Zhou, Xianwu Gong, Ye Tao, and Weiyu Liu. 2025. "A Numerical Investigation of Enhanced Microfluidic Immunoassay by Multiple-Frequency Alternating-Current Electrothermal Convection" Applied Sciences 15, no. 9: 4748. https://doi.org/10.3390/app15094748
APA StyleWu, Q., Huang, S., Wang, S., Zhou, X., Shi, Y., Zhou, X., Gong, X., Tao, Y., & Liu, W. (2025). A Numerical Investigation of Enhanced Microfluidic Immunoassay by Multiple-Frequency Alternating-Current Electrothermal Convection. Applied Sciences, 15(9), 4748. https://doi.org/10.3390/app15094748






