Abstract
The aim of this work was to develop the composition of a medium for the cultivation of six microbial strains forming a deodorizing consortium: Pseudomonas fluorescens, Enterococcus faecium, Bacillus subtilis, Bacillus megaterium, Leuconostoc mesenteroides and Lactobacillus plantarum. The study focused on the optimization of a highly efficient culture medium composed of readily available components of plant origin to maximize microbial biomass yields, and to create a less expensive alternative to the commercial Tryptic Soy Broth medium (TSB). After preliminary efficiency screening of all tested media components, we selected four substrates for further optimization—soy protein concentrate (SPC), glucose or sucrose, and phosphate salts. The final concentrations of all components were fine-tuned using the Taguchi design for experiments according to an L9 array. Taguchi optimization led to formulation of a culture medium, which was approximately 5 times cheaper than TSB (depending on the components used). Consequently, microbial biomass yields were improved by up to 15-fold (1564%), depending on the strain. The results obtained in the laboratory experiments were then confirmed in pilot- (42 L) and industrial- (300 L) scale fermentation. Our results show that this method of using a parallel culture microbioreactor with the Taguchi approach can be recommended for optimization of culture media based on substrates of plant origin.
1. Introduction
A significant increase in worldwide poultry and poultry product consumption has been observed. This has led to intensification of livestock production and increasing amounts of waste, mainly poultry manure, which can affect human health and the quality of animal life due to the generation of volatile odorous compounds. There are many ways to reduce odour from poultry farms, inter alia, biological, chemical and physical methods. Among biological methods, biopreparations are of great interest. They are the only methods that do not require specialized use of installations and equipment. Biopreparations: (1) are safe and easy in practical use; (2) do not corrode metals or destroy ceramics and plastics; (3) prevent excessive microorganism development, (4) contribute to the restoration/ reconstruction of natural microflora; (5) bind some heavy metals; and (6) contribute to manure hygienisation.
In our previous research, we selected six bacterial strains for deodorization of poultry manure: Pseudomonas fluorescens, Enterococcus faecium, Bacillus subtilis, Bacillus megaterium, Leuconostoc mesenteroides and Lactobacillus plantarum, on the basis of their ability to reduce the content of volatile odorous compounds from poultry manure and the antagonistic interactions between the strains [1,2]. These strains were then applied to produce a novel biopreparation which consisted of two parts: a perlite–bentonite mixture (20:80 by weight) used as a mineral sorbent, and a 20% addition (by weight) of active microorganisms in the form of spray-dried microcapsules. The preparation showed great effectiveness in the removal of volatile odorous compounds, especially ammonia, for which concentration was reduced in the exhaust air by more than 90% during 2 days of deodorization. The other nitrogenous odorants—indole and pyridine—were removed by 78% and 59%, respectively. The average reduction rate of hydrogen sulphide by the biopreparation was 60%, whereas dimethyl sulphide and dimethyl disulfide were removed with around 70% efficiency. The preparation also revealed high activity against hydrocarbons, aldehydes and phenols. It is worth mentioning that the biopreparation was stable, as no decrease of its microbial stability was reported with at least 5 months of storage at both cool (4 °C) and room (22 °C) temperatures [3].
The efficiency of biotechnological processes involving microorganism cultivation depends on many factors, among which the composition of culture medium is of great importance. Microorganisms, depending on their nutritional requirements, need access to different sources of carbon and nitrogen, as well as vitamins. Both a shortage and excess of even one component may inhibit the growth and activity of the cells, and consequently decrease the process efficiency. Moreover, large-scale production forces the use of cheap waste materials. Due to the number of variables, optimization of media composition is a great challenge. Conventional methods, such as one-factor-at-time (OFAT), rely on matching the concentration of each substrate individually while maintaining a constant concentration of the remaining components. Such a solution, although still very popular, is time-consuming and generates significant costs. Synergistic and antagonistic effects that appear only in a certain range of concentrations of selected components, resulting in an increase or decrease of the process efficiency, respectively, are skipped. Consequently, attempts are being made to intuitively modify the parameters of the culture, however, this does not lead to achievement of the given goals [4,5]. A solution to the described problem may lie in the statistical methods of experimental design, which quickly and efficiently allow for determination of the appropriate values of all operating variables [6]. The most commonly used are: factorial design, response surface methodology (RMS), Plackett–Burmana and Box–Behnkena (BBD) design, central composite design (CCD), artificial neural networks (ANN) and Taguchi methods [6,7]. Factorial design and central composite plans have been used, among others, to establish optimal conditions for the biosynthesis of pyruvic acid by Torulopsis glabrata [8], arginine deaminase by Enterococcus faecium [9], as well as carboxymethyl-cellulase and pro tease by Bacillus subtilis [10]. Proliferation methods of probiotic microorganisms such as Lactobacillus rhamnosus [11] Bacillus coagulans [12], and Bifidobacterium longum [13] have also been developed. Methods by Plackett–Burman and Box–Behnken have been used to establish the optima composition of the medium for the production of succinic acid by Corynebacterium glutamicum [14], as well as the fungal biomass of Pleurotus spp., which has the ability to degrade lignin [15]. Artificial neural networks have been applied, among others, to determine the medium composition for the production of the lipopeptide iturin A by Bacillus subtilis, and vitamin C [16]. Both iturin A and vitamin C are obtained from a two-stage fermentation process operated with a mixture of microorganisms containing Bacillus subtilis and Ketogulonicigenium vulgare strains [17]. In recent years, a steady growth in interest has been observed in the optimization of the process parameters by mathematical methods. The Taguchi method, used in this study for instance, implies the existence of an ideal, infinitely regular output factor level, and simultaneously assumes that any deviation from the optima value of operating variables results in a squared loss of quality [18]. This method, used in practice for culture medium optimization, can easily determine how the biomass concentration or process efficiency is affected by each medium component at its various concentrations. It is confirmed in literature that data obtained with this methodology allow for taking action with the aim of selecting the optimal parameters of a culture. The Taguchi method has been applied to optimize conditions in the production of the enzymes protease, produced by Bacillus subtilis [19], and lactase, by Pleurotus ostreatus [20]. No investigations with the Taguchi method have been performed to optimize a plant-based medium used for the efficient production of microbial biomass. Hence, the aim of this work was to use the Taguchi approach to optimize the composition of the plant-based medium used for the cultivation of the biomass containing six bacterial strains, which are components of the deodorizing consortium. The scope of the research included optimization of the composition of the plant-based medium for the production of the above-mentioned biomass in a parallel-culture microbioreactor under laboratory conditions. The biomass yield thus obtained from the optimized medium was compared with the yield obtained from a commercial medium. Finally, the bacterial biomass yields achieved in laboratory conditions were confirmed in pilot and industrial-scale experiments.
2. Materials and Methods
2.1. Microorganisms
Six strains deposited in the Pure Culture Collection ŁOCK 105 (ITFM, LUT, Łódź, Poland) were used in the study: Pseudomonas fluorescens (ŁOCK 0961), Enterococcus faecium (ŁOCK 0965), Bacillus subtilis (ŁOCK 0962), Bacillus megaterium (ŁOCK 0963), Leuconostoc mesenteroides (ŁOCK 0964) and Lactobacillus plantarum (ŁOCK 0996), and were characterized as described by Gutarowska et al. [21]. The microbes were selected based on previous study determining their high ability to reduce volatile odorous compounds from poultry manure. These microbes are a part of the deodorizing consortium [3,21].
2.2. Media Components
Based on the previous study, among compounds of plant origin (e.g., molasses, beet pulp, apple and rape pomace, soy protein concentrate), four different media were selected for further optimization: (1) Soy protein concentrate (SPC) containing: proteins 45%; fat 1.8%; insoluble fibres 3–4%, lysine 2.65%; and methionine 0.55% (Agro Kocięba, Czarnocin, Poland); (2) glucose (G) (POCh, Gliwice, Poland); (3) sucrose (S) (POCh, Gliwice, Poland); and (4) phosphate salts KH2PO4 (POCh, Gliwice Poland). Components containing insoluble solid particles, e.g., soy protein concentrate, were centrifuged after dissolution (3200 g, 5 min), and the supernatant obtained in this operation was used for medium preparation. As a reference, commercial medium Tryptic Soy Broth—TSB (BTL, Łódź, Poland) and TSB at a higher concentration (3×) than the recommended concentration by the manufacturer were used. All media were sterilized at 121 °C, for 15 min prior to use.
2.3. Microbial Cultures in a BioLector Microbiooreactor System
One millilitre of each type of culture media was introduced to a 48-well plate in the Biolector microbioreactor (m2p Labs, Baesweiler, Germany), and inoculated with 100 µL of bacterial inoculate (108–109 colony forming units (CFU)/mL). Incubation was carried out at 30 °C with shaking (1000 rpm). The biomass growth as light dispersion was measured online every hour, using a built-in Biolector detector, until cultures reached stationary phase (maximal scatter light value (SL)). The corresponding media at appropriate concentrations without microorganisms served as reference samples. In addition, pH measurements were performed every hour for each culture.
2.4. Taguchi Array Design
Optimization of culture media composition was performed according to the Taguchi design of experiment methodology [22]. Standard orthogonal L9 (33) arrays were used to determine biomass production as scatter light values (SL) through a three-factor (X1–X3) three-level system (1,2,3), where L is a Latin square array and 9 is the number of experimental runs. The values of levels are: 2, 5 and 8% of glucose/sucrose; 2, 4 and 6% SPC and 0.2, 0.4, and 0.8% of KH2PO4. In our study, culture media were prepared based on the L9 array, which allowed for testing of two sets of three factors (set (1)—sucrose, set (2)—glucose), on three levels (concentrations), and in nine combinations. The design matrix of the nutrient concentrations and their levels are listed in Table 1. A total of 18 experiments (nine for glucose and nine for sucrose) were performed for each strain.

Table 1.
L9 array for the design of media composition for all bacterial strains.
2.5. Biomass Production
All tested strains were cultivated on the optimized medium in the reactors as follows: under laboratory conditions of 0.5 L (Multifors, INFORS HT, Bottmingen-Basel, Switzerland), 7 L (Labfors, INFORS HT, Bottmingen-Basel, Switzerland); 42 L (Techfors, INFORS HT, Bottmingen-Basel, Switzerland) for a pilot scale; and 300 L fermenters (OBRAM, Olsztyn, Polska) for industrial scale (JHJ Sp. z o.o., Gizałki, Poland). The culture medium (SPC 6%, G 5%, KH2PO4 0.2%) was inoculated with 10% of the bacterial strain culture in TSB (density 108–109 CFU/mL). The strains were cultivated for 24 h at 30 °C with shaking (80 rpm) and the pH was adjusted for L. plantarum and L. mesenteroides (pH = 5.5), P. fluorescens, B. subtilis, B. megaterium and E. faecium (pH = 6.5). At the end of cultivation, the samples were collected and streaked on agar plates with Tryptic Soy Agar (TSA; BTL, Warszawa, Poland) as a serial dilution. The plates were incubated at 30 °C for 24 h. At the end of incubation, colony forming units were counted. All cultures were performed in triplicate.
2.6. Statistical Methods
Statistical analyses were performed using STATISTICA 9.0 software (TIBCO Software Inc., Palo Alto, CA, USA). All results were evaluated using a one-way analysis of variance (ANOVA) at the 0.05 significance level. Fisher’s least significant difference (LSD) post hoc test (at 0.05 significance level) was used to verify statistical differences.
3. Results and Discussion
The highest scatter light (SL) values observed in the laboratory culture microbioreactor parallels for each strain are shown in Table 2, whereas the final optimized concentrations of culture media components based on Taguchi design are depicted in Table 3.

Table 2.
Maximum scatter light values for bacterial strains at Taguchi’s experiment.

Table 3.
Final optimized concentrations of culture media components based on Taguchi design.
Statistical analyses allowed us to conclude that in most variants the highest scatter light values (SL) were reported for variant Glucose or Sucrose no. (6), which was statistically significant from the others. In comparison to the reference media (TSB and 3TSB) the values SL for media with SPC, glucose and sucrose were 3–10 times higher depending on the variant.
The results obtained in the first stage of the experiment clearly indicate the media, which are interesting for further optimization work.
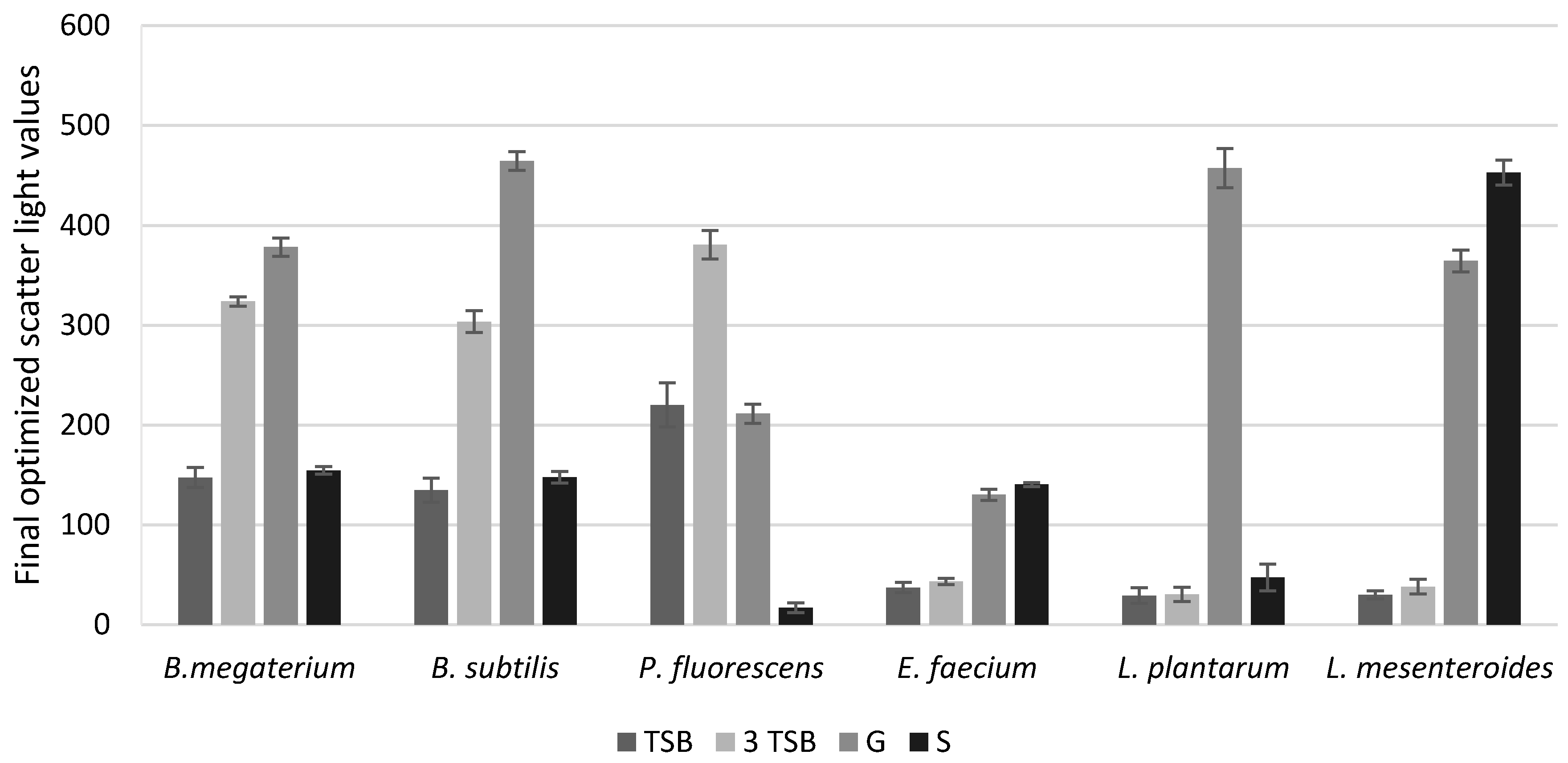
The maximum SL values obtained in the optimization experiment based on three variables (X1, X2, X3) set the level of optimal component concentration of the medium for each strain (Table 3). Bacterial cultures were made with the optimized medium. The commercial medium TSB, at the concentration recommended by the manufacturer, was used for control samples, and was three times higher than recommended (Figure 1).

Figure 1.
Final scatter light values for standard media and media optimized on the basis of Tauguchi experiments. TSB—reference/control medium in the concentration recommended by the manufacturer; 3TSB—reference medium in concentration three times higher than what is recommended by the manufacturer; G—variant of the optimization of the medium containing glucose; S—variant of the optimization of the medium containing sucrose.
In the case of the glucose variant with B. megaterium culture, the scatter light value was more than two times higher than that of the reference medium. For B. subtilis, the glucose optimization variant was more than three times higher than the reference TSB. For L. plantarum and L. mesenteroides, the differences in scatter light values in glucose-optimized media were 12- and 15-times higher than for the reference medium, respectively. For the E. faecium strain, the best results were obtained with the glucose variant, about 3.5 times higher than for the commercial medium. The commercial medium TSB was completely useless for E. faecium, L. plantarum, and L. mesenteroides. P. fluorescens was the only strain which showed good growth with the commercial medium at a concentration three times higher than that recommended by the manufacturer. However, in this case, a very long adaptation phase which extended cultivation was observed.
It can therefore be concluded that the medium based on glucose is the best solution for all tested strains.
Hwang et al. [22] performed the optimization experiments using the Taguchi approach and achieved a 107% greater yield in Lactobacillus bacteria density. Taguchi method was also used to optimize the cultures of Bacillus and Pseudomonas, for biosynthesis of the enzymes proteinase and xylanase, and for the removal of mercury from the environment. This method was found to improve the required parameters by over 10%. By comparison, in our study, the microbial biomass yields were increased by up to 15-fold, depending on the strain. Regarding cost minimization, Gu et al. [23] conducted research on the use of Taguchi approach for the optimization of medium with the lowest price. They have reported a decrease in the costs of media from US$4/L for commercial medium to US$1.16/L for medium developed during optimization. Likewise, the cost analysis of media in our study showed that the cost of medium developed under the optimization is 5 times lower compared to the commercial medium, considering that both offer similar biomass densities.
The last stage of our research was the comparison between the biomass yield obtained during the cultivation on the optimized medium in different volume cultures: from 0.5 L to 7 L on a laboratory scale to 42 L on a pilot scale, and 300 L on an industrial scale.
Regarding the scale-up, the highest number of microorganisms was observed in the 0.5 L fermenters (2.16 × 109 CFU/mL–1.78 × 1010 CFU/mL, depending on the strain) (Table 4), but there were no statistical differences observed in the microorganism number for three strains (B. megaterium, E. faecium and L. mesenteroides). For the other strains (B. subtilis, P. fluorescens and L. plantarum) the analysis showed statistically significant differences only between the cultures of 0.5 L and 300 L. Likewise, other authors performing scale-up experiments did not report any significant changes in the process efficiency e.g., synthesis of 1,3-propanediol by Clostridium butyricum [24] or bioflocculant synthesis by Halomonans sp. [25].

Table 4.
The number of microorganisms (colony forming units, CFU/mL) during scaling up.
This study allowed for developing culture medium with a composition based on components of plant origin, for the production of biomass containing six bacterial strains. These six bacterial strains are constituents of a biopreparation that is applied for deodorization of livestock premises. Furthermore, similar biomass yields were achieved in both laboratory, pilot- and industrial-scale experiments.
Acknowledgments
This research was financially supported by the National Centre for Research and Development grant No. PBS2/B8/14/2014 “Innovative deodorizing biopreparation for poultry production premises”. The authors would like to thank JHJ Sp. z o. o., especially Mr Hieronim Burchardt for the possibility to conduct research on an industrial scale.
Author Contributions
Krzysztof Makowski planned, supervised and conducted the experiments, collected data and wrote the manuscript, Jakub Bielnicki conducted the experiments and collected data, Alicja Tarazewicz, Marta Maroszyńska and Szymon Powałowski conducted the experiments, Katarzyna Matusiak, Sebastian Borowski, Martyna Leszczewicz and Beata Gutarowska wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gutarowska, B.; Borowski, S.; Durka, K.; Korczyński, M.; Kołacz, R. Screening of microorganisms capable to remove odorous compounds from poultry manure. Przem. Chem. 2009, 5, 2–7. (In Polish) [Google Scholar]
- Matusiak, K.; Oleksy, M.; Borowski, S.; Nowak, A.; Korczyński, M.; Dobrzański, Z.; Gutarowska, B. The use of Yucca schidigera and microbial preparation for poultry manure deodorization and hygienization. J. Environ. Manag. 2016, 170, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Borowski, S.; Matusiak, K.; Powałowski, S.; Pielech-Przybylska, K.; Makowski, K.; Nowak, A.; Rosowski, M.; Komorowski, P.; Gutarowska, B. A novel microbial-mineral preparation for the removal of offensive odors from poultry manure. Int. Biodeter. Biodegr. 2017, 119, 299–308. [Google Scholar] [CrossRef]
- Czitrom, V. One factor at a time versus designed experiments. Am. Stat. Assoc. 1999, 53, 126–131. [Google Scholar] [CrossRef]
- Wahid, Z.; Nadir, N. Improvement of one factor at a time through design of experiments. World Appl. Sci. J. 2013, 21, 56–61. [Google Scholar]
- Connors, N.C. Culture medium optimization and scale-up for microbial fermentations. In Handbook of Industrial Cell Culture, 1st ed.; Vinci, V.A., Parekh, S.R., Eds.; Humana Press: New Delhi, India, 2003; pp. 171–193. ISBN 978-1-59259-346-0. [Google Scholar]
- Panda, B.P.; Ali, M.; Javed, S. Fermentation process optimization. Res. J. Microbiol. 2007, 2, 201–208. [Google Scholar]
- Jian, Z.; Nian-fa, G. Application of response surface methodology in medium optimization for pyruvic acid production of Torulopsis glabrata TP19 in batch fermentation. J. Zhejiang. Univ. Sci. B 2007, 8, 98–104. [Google Scholar]
- Kaur, B.; Kaur, R. Application of response surface methodology for optimizing arginine deiminase production medium for Enterococcus faecium sp. GR7. Sci. World J. 2013, 2013, 892587. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, P.; Arun, A.; Al-Dhabi, N.A.; Gnana, S.; Vincent, P.; Arasu, M.V.; Choi, K.C. Novel Bacillus subtilis IND19 cell factory for the simultaneous production of carboxy methyl cellulase and protease using cow dung substrate in solid-substrate fermentation. Biotechnol. Biofuels 2016, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Polak-Berecka, M.; Waśko, A.; Kordowska-Wiater, M.; Podleśny, M.; Targoński, Z.; Kubik-Komar, A. Optimization of medium composition for enhancing growth of Lactobacillus rhamnosus PEN using response surface methodology. Polish J. Microbiol. 2010, 59, 113–118. [Google Scholar]
- Sen, R.; Babu, K.S. Modeling and optimization of the process conditions for biomass production and sporulation of a probiotic culture. Process Biochem. 2005, 40, 2531–2538. [Google Scholar] [CrossRef]
- Kiviharju, K.; Leisola, M.; Eerikäinen, T. Optimization of a Bifidobacterium longum production process. J. Biotechnol. 2005, 117, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Jong-Min, J.; Thangamani, R.; Song, E.; Lee, H.; Lee, H.; Yang, Y. Media optimization of Corynebacterium glutamicum for succinate production under oxygen-deprived condition. J. Microbiol. Biotechnol. 2013, 23, 211–217. [Google Scholar]
- Sarria-Alfonso, V.; Sánchez-Sierra, J.; Aguirre-Morales, M.; Gutiérrez-Rojas, I.; Moreno-Sarmiento, N.; Poutou-Piñales, R.A. Culture media statistical optimization for biomass production of a ligninolytic fungus for future rice straw degradation. Indian J. Microbiol. 2013, 53, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zhong, J.; Yang, J.; Ren, Y.; Xu, T.; Xiao, S.; Zhou, J.; Tan, H. The artificial neural network approach based on uniform design to optimize the fed-batch fermentation condition: Application to the production of iturin A. Microb. Cell Fact. 2014, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, M.; Yu, X.; Zhang, Y.; Lyu, S. Optimization of medium composition for two-step fermentation of vitamin C based on artificial neural network–genetic algorithm techniques. Biotechnol. Biotechnol. Equip. 2015, 29, 1128–1134. [Google Scholar] [CrossRef]
- Velazco, E.E.; Bendell, A.; Disney, J.; Pridmore, W.A. Taguchi methods: Applications in world industry. Interfaces (Providence) 1991, 21, 99–101. [Google Scholar]
- Venil, C.K.; Lakshmanaperumalsamy, P. Taguchi experimental design for medium optimization for enhanced protease production by Bacillus subtilis HB04. E-J. Sci. Technol. 2001, 4, 1–10. [Google Scholar]
- Prasad, K.K.; Mohan, S.V.; Rao, R.S.; Pati, B.R.; Sarma, P.N. Laccase production by Pleurotus ostreatus 1804: Optimization of submerged culture conditions by Taguchi DOE methodology. Biochem. Eng. J. 2005, 24, 17–26. [Google Scholar] [CrossRef]
- Gutarowska, B.; Matusiak, K.; Borowski, S.; Rajkowska, A.; Brycki, B. Removal of odorous compounds from poultry manure by microorganisms on perlite—Bentonite carrier. J. Environ. Manag. 2014, 141, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.F.; Chang, J.H.; Houng, J.Y.; Tsai, C.C.; Lin, C.K.; Tsen, H.Y. Optimization of medium composition for improving biomass production of Lactobacillus plantarum Pi06 using the Taguchi array design and the Box-Behnken method. Biotechnol. Bioprocess Eng. 2012, 17, 827–834. [Google Scholar] [CrossRef]
- Gu, W.; Chen, S.W.; Chen, G.P.; Ji, Z.X. Enhacement of Haemophilus parasuis serovar 5 yields by medium optimization. Lett. Appl. Microbiol. 2015, 61, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska-Powałowska, D.; Białas, W. Scale-up of anaerobic 1,3-propanediol production by Clostridium butyricum DSP1 from crude glycerol. BMC Microbiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhen, Q.; Qiu, N.; Liu, Z.; Wang, B.; Shao, Z.; Yu, Z. Medium optimization for the production of a novel bioflocculant from Halomonas sp. V3a’ using response surface methodology. Bioresour. Technol. 2009, 100, 5922–5927. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).