Chitosan-Based Nanocarriers for Nose to Brain Delivery
Abstract
:1. Introduction
2. Chitosan Properties and Biomedical Application
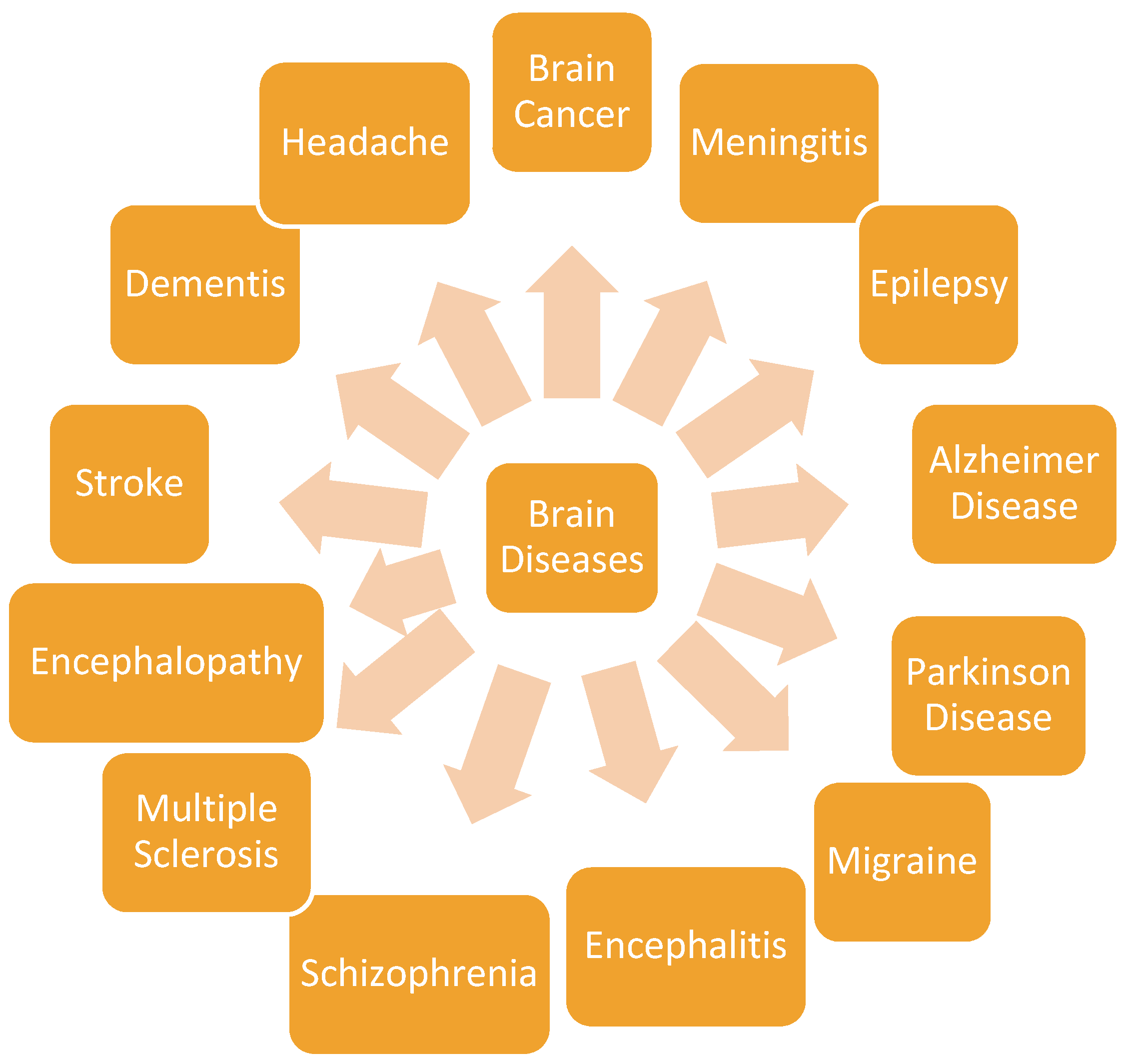
3. Brain Diseases
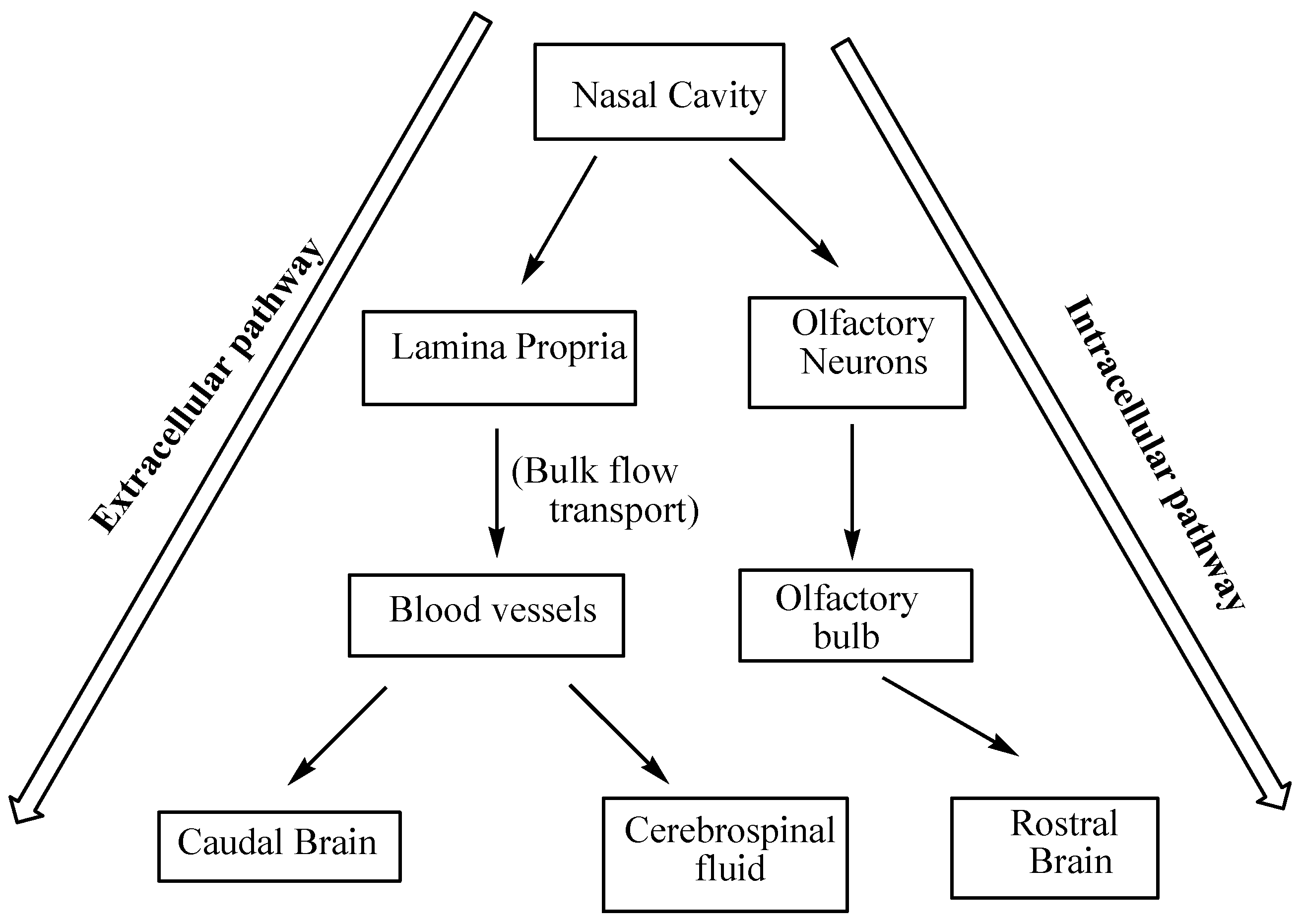
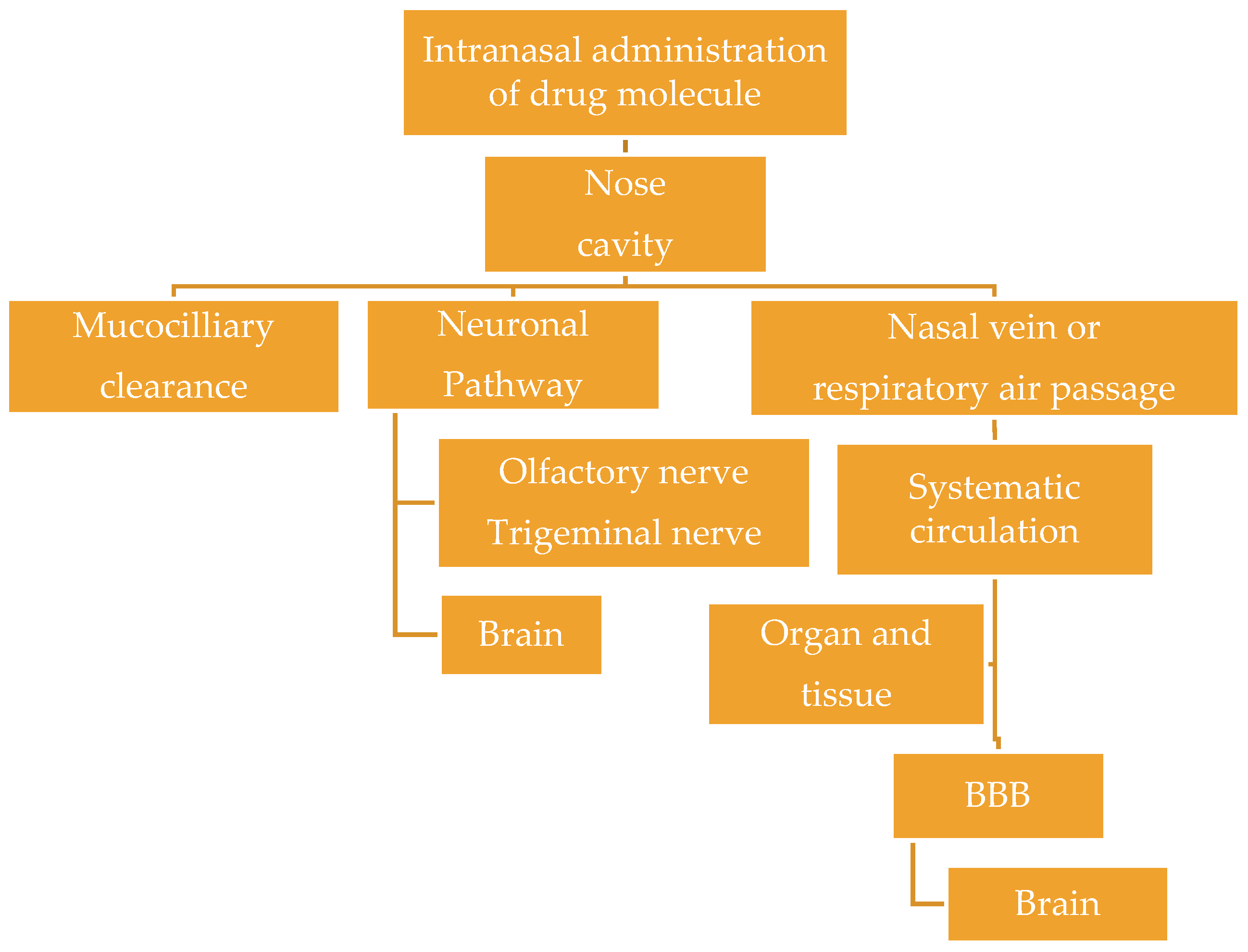
4. Brain Targeting
5. Chitosan Nanocarriers in Brain Targeting
5.1. Nanoparticles (NPs)
5.2. In Situ Gel
5.3. Emulsions
5.4. Liposomes
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alazne, D.; Antonia, A.; Enrique, H.; Blanca, S.-M.; Goñi-de-Cerio, F. Central nervous system diseases and the role of the blood-brain barrier in their treatment. Neurosci. Discov. 2013, 1, 3. [Google Scholar]
- Chin-Chan, M.; Navarro-Yepes, J.; Quintanilla-Vega, B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front. Cell. Neurosci. 2015, 9, 124. [Google Scholar] [CrossRef]
- Feigin, V.L. Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897. [Google Scholar] [CrossRef]
- Meng, Z.; Wei, T.; Yan, Z.; Omid, C.F.; Bingyang, S. Nanotechnology-based strategies for siRNA brain delivery for disease therapy. Trends Biotechnol. 2018, 36, 562–575. [Google Scholar]
- Prabaharan, M.; Mano, J.F. Chitosan-based particles as controlled drug delivery systems. Drug Deliv. 2005, 12, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A. In Situ-Based Gels for Nose to Brain Delivery for the Treatment of Neurological Diseases. Pharmaceutics 2018, 10, 40. [Google Scholar] [CrossRef]
- Sejin, S.; Do Won, H.; Kaushik, S.; Ji Hoon, J.; Tae, G.P.; Dong, S.L.; Won, J.K. RVG peptide tethered bioreducible polyethylenimine for gene delivery to brain. J. Control. Release 2011, 155, 18–25. [Google Scholar]
- Gaurav, T.; Ruchi, T.; Birendra, S.; Bhati, L.; Pandey, S.; Pandey, P.; Saurabh, K.B. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [Green Version]
- Cui-Tao, L.; Ying-Zheng, Z.; Ho Lun, W.; Jun, C.; Lei, P.; Xin-Qiao, T. Current approaches to enhance CNS delivery of drugs across the brain barriers. Int. J. Nanomed. 2014, 9, 2241–2257. [Google Scholar] [Green Version]
- Bhakar, S.; Tian, F.; Stoeger, T.; Kreyling, W.; de la Fuente, J.M.; Grazú, V.; Borm, P.; Estrada, G.; Ntziachristos, V.; Razansky, D. Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: Perspectives on tracking and neuroimaging. Part. Fibre Toxicol. 2010, 7, 3. [Google Scholar] [CrossRef]
- Yasir, M.; Ayesha, T.; Faheem, A.S. Brain targeting Drug Delivery System: A Review. Int. J. Basic Med. Sci. Pharm. 2015, 5, 2049–4963. [Google Scholar]
- Upasana, S.; Pragya, N.B.; Swati, G. Polymeric Nanoparticles Drug Delivery to Brain: A Review. Int. J. Pharmacol. Pharm. Sci. 2015, 2, 60–69. [Google Scholar]
- Ravi, K.U. Drug Delivery Systems, CNS Protection, and the Blood Brain Barrier. BioMed Res. Int. 2014, 2014, 869269. [Google Scholar]
- Epilepsy. Available online: https://communitymedicine4asses.com/2017/02/11/who-updates-fact-sheet-on-epilepsy-10-february-2017/ (accessed on 28 October 2018).
- Alamri, Y.; MacAskill, M.; Anderson, T.; Benamer, H. Parkinson’s disease in the Gulf Countries: An updated review. Eur. Neurol. 2015, 74, 222–225. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Chinmayee, S.; Pankaj, G.; Tarun, K.M. Chitosan: A Promising Biopolymer in Drug Delivery Applications. J. Mol. Genet. Med. 2015, S4, 006. [Google Scholar]
- Sarvaiya, J.; Agrawal, Y.K. Chitosan as a suitable nanocarrier material for anti-Alzheimer drug delivery. Int. J. Biol. Macromol. 2015, 72, 454–465. [Google Scholar] [CrossRef]
- Divya, L.; Vijay, K.M. Preparation and Applications of Chitosan Nanoparticles: A Brief Review. J. Mater. Sci. 2016. [Google Scholar] [CrossRef]
- Rosa, T.; Corrado, S.; Luca, C.; Moreno, B.; Federica, B. Functionalization of PVC by chitosan addition: Compound stability and tensile properties. Compos. B Eng. 2018, 149, 240–247. [Google Scholar]
- Shakeel, A.; Saiqa, I. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [Green Version]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Hasbun, R. Meningitis. Available online: https://emedicine.medscape.com/article/232915-overview (accessed on 12 December 2018).
- Rajesh, Y.; Pal, I.; Banik, P.; Chakraborty, S.; Borkar, S.A.; Dey, G.; Mukherjee, A.; Mandal, M. Insights into molecular therapy of glioma: Current challenges and next generation blueprint. Acta Pharmacol. Sin. 2017, 38, 591–613. [Google Scholar] [CrossRef]
- Linda, L.B.; Joshana, G. Management of migraine headaches in a chronic pain patient: A case report. Ment. Health Clin. 2016, 6, 154–158. [Google Scholar]
- Saenger, A.K.; Christenson, R.H. Stroke biomarkers: Progress and challenges for diagnosis, prognosis, differentiation, and treatment. Clin. Chem. 2010, 56, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Common Stroke Medications Horrible Side Effects. Available online: https://www.cheatsheet.com/health-fitness/common-stroke-medications-horrible-side-effects.html/ (accessed on 26 February 2019).
- Wahab, A. Difficulties in treatment and management of epilepsy and challenges in new drug development. Pharmaceuticals 2010, 3, 2090–2110. [Google Scholar] [CrossRef] [PubMed]
- Remy, S.; Beck, H. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain 2005, 129, 18–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudduth, T.L.; Powell, D.K.; Smith, C.D.; Greenstein, A.; Wilcock, D.M. Induction of hyperhomocysteinemia models vascular dementia by induction of cerebral microhemorrhages and neuroinflammation. J. Cereb. Blood Flow Metab. 2013, 33, 708–715. [Google Scholar] [CrossRef] [PubMed]
- FDA-Approved Treatments for Alzheimer’s. Available online: https://www.alz.org/dementia/downloads/topicsheet_treatments.pdf (accessed on 14 February 2018).
- Oertel, W.H. Recent advances in treating Parkinson’s disease. F1000Resarch 2017, 6, 620. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.; Wichmann, T.; Factor, S.A.; DeLong, M.R. Parkinson’s disease therapeutics: New developments and challenges since the introduction of levodopa. Neuropsychopharmacology 2012, 37, 213–246. [Google Scholar] [CrossRef]
- Patel, K.R.; Cherian, J.; Gohil, K.; Atkinson, D. Schizophrenia: Overview and treatment options. Pharm. Ther. 2014, 39, 638–645. [Google Scholar]
- FDA-approved drugs to treat schizophrenia. J. Psychosoc. Nurs. Ment. Health Serv. 2014, 52, 11–12. [CrossRef]
- Chunmeng, S.; Yang, D.; Li, Z.; Di, S.; Linlin, S.; Thomas, J.W.; Yan, S. Noninvasive nanoparticle strategies for brain tumor targeting. Nanomedicine 2017, 13, 2605–2621. [Google Scholar]
- Patrícia, C.P.; Adriana, O.S. Nanosystems in nose-to-brain drug delivery: A review of non-clinical brain targeting studies. J. Control. Release 2018, 270, 89–100. [Google Scholar]
- Abdur, R.K.; Mengrui, L.; Muhammad, W.K.; Guangxi, Z. Progress in brain targeting drug delivery system by nasal route. J. Control. Release 2017, 268, 364–389. [Google Scholar]
- Huile, G. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar]
- Selvaraj, K.; Gowthamarajan, K.; Karri, V.V.S.R. Nose to brain transport pathways an overview: Potential of nanostructured lipid carriers in nose to brain targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2088–2095. [Google Scholar] [CrossRef]
- Liu, X.F.; Fawcett, J.R.; Hanson, L.R.; Frey, W.H. The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J. Stroke Cerebrovasc. Dis. 2004, 13, 16–23. [Google Scholar] [CrossRef]
- Thorne, R.G.; Pronk, G.J.; Padmanabhan, V.; Frey Ii, W.H. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 2004, 127, 481–496. [Google Scholar] [CrossRef]
- Arora, P.; Sharma, S.; Garg, S. Permeability issues in nasal drug delivery. Drug Deliv. Today 2002, 7, 67–97. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Novel vasoconstrictor formulation to enhance intranasal targeting of neuropeptide therapeutics to the central nervous system. J. Pharmacol. Exp. Ther. 2009, 328, 312–320. [Google Scholar] [CrossRef]
- Alexander, A.; Saraf, S. Nose-to-brain drug delivery approach: A key to easily accessing the brain for the treatment of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 2102–2104. [Google Scholar]
- Crowe, T.P.; Greenlee, M.H.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Mackay-Sim, A.; St. John, J.; Schwob, J.E. Neurogenesis in the adult olfactory neuroepithelium. In Handbook of Olfaction and Gustation; Doty, R., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; pp. 232–265. [Google Scholar]
- Gomes, L.P.; Paschoalin, V.M.F.; Del Aguila, E.M. Chitosan Nanoparticles: Production, Physicochemical Characteristics, and Nutraceutical Applications. Rev. Virtual Quim. 2017, 9, 387–409. [Google Scholar] [CrossRef]
- Akbar, A.; Shakeel, A. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Optimization of brain-targeted chitosan nanoparticles of Rivastigmine for improved efficacy and safety. Int. J. Biol. Macromol. 2013, 59, 72–83. [Google Scholar] [CrossRef]
- Hattori, H. Effectiveness and limitation of newly approved drugs for Alzheimer’s disease. Psychiatria et neurologia Japonica 2013, 115, 22–31. [Google Scholar] [PubMed]
- Szeto, Y.Y.J.; Lewis, G.S.S. Current treatment options for Alzheimer’s disease and Parkinson’s disease dementia. Curr. Neuropharmacol. 2016, 14, 326–338. [Google Scholar] [CrossRef]
- Khoury, R.; Rajamanickam, J.; Grossberg, G.T. An update on the safety of current therapies for Alzheimer’s disease: Focus on rivastigmine. Ther. Adv. Drug Saf. 2018, 9, 171–178. [Google Scholar] [CrossRef]
- Adem, S.; Digdem Y, E.; Secil, C.T.; Utku, H.; Yesim, A.; Patrick, C.; Gunes, E.; Yilmaz, C. Evaluation of brain-targeted chitosan nanoparticles through blood-brain barrier cerebral microvessel endothelial cells. J. Microencapsul. 2017, 34, 659–666. [Google Scholar]
- Aktaş, Y.; Yemisci, M.; Andrieux, K.; Gürsoy, R.N.; Alonso, M.J.; Fernandez-Megia, E.; Novoa-Carballal, R.; Quinoa, E.; Riguera, R.; Sargon, M.F.; et al. Development and brain delivery of chitosan-PEG nanoparticles functionalized with the monoclonal antibody OX26. Bioconjug. Chem. 2005, 16, 1503–1511. [Google Scholar] [CrossRef]
- Alam, S.; Khan, Z.I.; Mustafa, G.; Kumar, M.; Islam, F.; Bhatnagar, A.; Ahmad, F.J. Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: A pharmacoscintigraphic study. Int. J. Nanomed. 2012, 7, 5705–5718. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Hu, Y.L.; Gao, J.Q. Brain Localization and Neurotoxicity Evaluation of Polysorbate 80-Modified Chitosan Nanoparticles in Rats. PLoS ONE 2015, 10, e0134722. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Hu, Y.; You, J.; Higashisaka, K.; Nagano, K.; Tsutsumi, Y.; Gao, J. Chitosan nanoparticles and their tween 80 modified counterparts disrupt the developmental profile of zebrafish embryos. Int. J. Pharm. 2016, 515, 644–656. [Google Scholar] [CrossRef]
- Porporatto, C.; Bianco, I.D.; Correa, S.G. Local and systemic activity of the polysaccharide chitosan at lymphoid tissues after oral administration. J. Leukoc. Biol. 2005, 78, 62–69. [Google Scholar] [CrossRef]
- Borchard, G.; Luessen, H.L.; deBoer, A.G.; Verhoef, J.C.; Lehr, C.M.; Junginger, H.E. The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. III effects of chitosanglutamate and carbomer on epithelial tight junctions in vitro. J. Control. Release 1996, 39, 131–138. [Google Scholar] [CrossRef]
- Fazil, M.; Md, S.; Haque, S.; Kumar, M.; Baboota, S.; Sahni, J.K.; Ali, J. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur. J. Pharm. Sci. 2012, 47, 6–15. [Google Scholar] [CrossRef]
- Ruby, J.; Pandey, V.P. Formulation, and evaluation of olanzapine loaded chitosan nanoparticles for nose to brain targeting an in vitro and ex vivo toxicity study. J. Appl. Pharm. Sci. 2016, 6, 34–40. [Google Scholar] [CrossRef]
- Heres, S.; Kraemer, S.; Bergstrom, R.F.; Detke, H.C. Pharmacokinetics of olanzapine long-acting injection: The clinical perspective. Int. Clin. Psychopharmacol. 2014, 29, 299–312. [Google Scholar] [CrossRef]
- Katagiri, H.; Tohen, M.; McDonnell, D.P.; Fujikoshi, S.; Case, M.; Kanba, S.; Takahashi, M.; Gomez, J.C. Safety and efficacy of olanzapine in the long-term treatment of J apanese patients with bipolar I disorder, depression: An integrated analysis. Psychiatry Clin. Neurosci. 2014, 68, 498–505. [Google Scholar] [CrossRef]
- Mattiuz, E.; Franklin, R.; Gillespie, T.; Murphy, A.; Bernstein, J.; Chiu, A. Disposition and metabolism of olanzapine in mice, dogs, and rhesus monkeys. Drug Metab. Dispos. 1997, 25, 573–583. [Google Scholar]
- Bhavna, M.D.; Shadab, A.; Mushir, B.; Aseem, B.; Sanjula, S.; Jasjeet, K.; Ali, J. Design, Development, Optimization and Characterization of Donepezil Loaded Chitosan Nanoparticles for Brain Targeting to Treat Alzheimer’s Disease. Sci. Adv. Mater. 2014, 6, 720–735. [Google Scholar] [CrossRef]
- Casey, D.A.; Antimisiaris, D.; O’Brien, J. Drugs for Alzheimer’s disease: Are they effective? Pharm. Ther. 2010, 35, 208–211. [Google Scholar]
- Wilson, B.; Samanta, M.K.; Muthu, M.S.; Vinothapooshan, G. Design and evaluation of chitosan nanoparticles as novel drug carrier for the delivery of rivastigmine to treat Alzheimer’s disease. Ther. Deliv. 2011, 2, 599–609. [Google Scholar] [CrossRef]
- Fernandes, J.; Ghate, M.V.; Basu M, S.; Lewis, S.A. Amino acid conjugated chitosan nanoparticles for the brain targeting of a model dipeptidyl peptidase-4 inhibitor. Int. J. Pharm. 2018, 547, 563–571. [Google Scholar] [CrossRef]
- Caban, S.; Capan, Y.; Couvreur, P.; Dalkara, T. Preparation and characterization of biocompatible chitosan nanoparticles for targeted brain delivery of peptides. Methods Mol. Biol. 2012, 846, 321–332. [Google Scholar]
- Arezou, G.; Soleiman, M.; Fatemeh, T.; Farid, T. Synthesis and optimization of chitosan nanoparticles: Potential applications in nanomedicine and biomedical engineering. Caspian J. Intern. Med. 2014, 5, 156–161. [Google Scholar]
- Somasree, R.; Pratyusha, S.; Bibek, L.; Sabyasachi, M.; Uttam, K.B.; Amit, K.N. Polysorbate 80 coated crosslinked chitosan nanoparticles of ropinirole hydrochloride for brain targeting. J. Drug Deliv. Sci. Technol. 2018, 48, 21–29. [Google Scholar]
- Jain, S.; Jain, R. Design and evaluation of chitosan nanoparticles as novel drug carrier for the delivery of Galantamine to treat Alzheimer’s disease. Abstr. Parkinsonism Relat. Disord. 2018, 46, e47–e51. [Google Scholar] [CrossRef]
- Tolou-Ghamari, Z.; Zare, M.; Habibabadi, J.M. A quick review of carbamazepine pharmacokinetics in epilepsy from 1953 to 2012. J. Res. Med. Sci. 2013, 18, S18–S85. [Google Scholar]
- Liu, S.; Yang, S.; Ho, P.C. Intranasal administration of carbamazepine-loaded carboxymethyl chitosan nanoparticles for drug delivery to the brain. Asian J. Pharm. Sci. 2018, 13, 72–81. [Google Scholar] [CrossRef]
- Shevtsov, M.; Nikolaev, B.; Marchenko, Y.; Yakovleva, L.; Skvortsov, N.; Mazur, A.; Tolstoy, P.; Ryzhov, V.; Multhoff, G. Targeting experimental orthotopic glioblastoma with chitosan-based superparamagnetic iron oxide nanoparticles (CS-DX-SPIONs). Int. J. Nanomed. 2018, 13, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Singh, R.P.; Kumari, L.; Sharma, G.; Koch, B.; Rajesh, C.V.; Mehata, A.K.; Singh, S.; Pandey, B.L.; Muthu, M.S. TPGS-chitosan cross-linked targeted nanoparticles for effective brain cancer therapy. Mater. Sci. Eng. C 2017, 74, 167–176. [Google Scholar] [CrossRef]
- Gelperina, S.; Maksimenko, O.; Khalansky, A.; Vanchugova, L.; Shipulo, E.; Abbasova, K.; Berdiev, R.; Wohlfart, S.; Chepurnova, N.; Kreuter, J. Drug delivery to the brain using surfactant-coated poly(lactide-co-glycolide) nanoparticles: Influence of the formulation parameters. Eur. J. Pharm. Biopharm. 2010, 74, 157–163. [Google Scholar] [CrossRef]
- Trapani, A.; Denora, N.; Iacobellis, G.; Sitterberg, J.; Bakowsky, U.; Kissel, T. Methotrexate-loaded chitosan-and glycolchitosan-based nanoparticles: A promising strategy for the administration of the anticancer drug to brain tumors. AAPS Pharmscitech. 2011, 12, 1302–1311. [Google Scholar] [CrossRef]
- Beduneau, A.; Saulnier, P.; Benoit, J.P. Active targeting of brain tumors using nanocarriers. Biomaterials 2007, 28, 4947–4967. [Google Scholar] [CrossRef]
- Bhaskar, K.R.; Vaijayanthi, V.; Raj, S.B.; Mohanambal, E.; Charulatha, R.; Madhusudan, Y.R. Nanoparticles for brain targeting. Ind. J. Novel Drug Deliv. 2011, 3, 91–97. [Google Scholar]
- Chacko, B.J.; Palanisam, S.; Gowrishankar, N.L.; Honeypriya, J.; Sumathy, A. Effect of Surfactant Coating on Brain Targeting Polymeric Nanoparticles; a Review. Ind. J. Pharm. Sci. 2018, 80, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Xie, C.; Wang, H.; Hu, Y. Specific role of polysorbate 80 coating on the targeting of nanoparticles to the brain. Biomaterials 2004, 25, 3065–3071. [Google Scholar] [CrossRef]
- Kreuter, J. Nanoparticulate systems for brain delivery of drugs. Adv. Drug Deliv. Rev. 2012, 64, 213–222. [Google Scholar] [CrossRef]
- Geldenhuys, W.J.; Allen, D.D. The blood–brain barrier choline transporter. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 95–99. [Google Scholar] [CrossRef]
- Puente, P.; Fettig, N.; Luderer M, J.; Jin, A.; Shah, S.; Muz, B.; Kapoor, V.; Goddu, S.M.; Salama, N.N.; Tsien, C.; et al. Injectable Hydrogels for Localized Chemotherapy and Radiotherapy in Brain Tumors. J. Pharm. Sci. 2018, 107, 922–933. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Q.; Lu, X.; Zhou, H. In situ forming hydrogels based on chitosan for drug delivery and tissue regeneration. Asian J. Pharm. Sci. 2016, 11, 673–683. [Google Scholar] [CrossRef] [Green Version]
- Guo-Chung, D.; Che-Yung, K.; Subramaniam, S.; Jiong-Yao, Z.; Savitha, S.; Hwan-You, C.; Feng-Huei, L. A potent inhibition of oxidative stress induced gene expression in neural cells by sustained ferulic acid release from chitosan based hydrogel. Mater. Sci. Eng. 2015, 49, 691–699. [Google Scholar]
- Li, Y.; Rodrigues, J.; Tomas, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Huang, L. Feasibility of chitosan-alginate (Chi-Alg) hydrogel used as scaffold for neural tissue engineering: Apilot study in vitro. Biotechnol. Biotechnol. Equip. 2017, 31, 766–773. [Google Scholar] [CrossRef]
- Giovanna, R.; Andrea, S.; Elena, P.P.; Paolo, G.; Marta, R.; Elisabetta, G. Composite Chitosan/Alginate Hydrogel for Controlled Release of Deferoxamine: A System to Potentially Treat Iron Dysregulation Diseases. Carbohydr. Polym. 2016, 136, 1338–1347. [Google Scholar]
- Atta, S.; Khaliqa, S.; Islam, A.; Javeria, I.; Jamil, T.; Athar, M.M.; Shafiq, M.I.; Ghaffar, A. Injectable biopolymer based hydrogels for drug delivery applications. Int. J. Biol. Macromol. 2015, 80, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Lia, Z.; Shimd, H.; Chob, M.O.; Chob, I.S.; Leef, J.H.; Sun-Woong, K.; Kwond, B.; Huhb, K.M. Thermo-sensitive injectable glycol chitosan-based hydrogel for treatment of degenerative disc disease. Carbohydr. Polym. 2018, 184, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhao, X.; Liang, X.; Mac, P.X.; Guo, B. Injectable hydrogel based on quaternized chitosan, gelatin and dopamine as localized drug delivery system to treat Parkinson’s disease. Int. J. Biol. Macromol. 2017, 105, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Abouhussein, D.M.; Khattab, A.; Bayoumi, N.A.; Mahmoud, A.F.; Sakr, T.M. Brain targeted rivastigmine mucoadhesive thermosensitive in situ gel: Optimization, in vitro evaluation, radiolabeling, in vivo pharmacokinetics and biodistribution. J. Drug Deliv. Sci. Technol. 2018, 43, 129–140. [Google Scholar] [CrossRef]
- Ravi, P.R.; Aditya, N.; Patil, N.S.; Cherian, L. Nasal in-situ gels for delivery of rasagiline mesylate: Improvement in bioavailability and brain localization. J. Drug Deliv. 2015, 22, 903–910. [Google Scholar] [CrossRef]
- Sandhya, G.; Saahil, A. In-situ nasal gel of levodopa for brain targeting using chitosan-thioglycolic acid conjugate and musk ketone by efflux transport modulation. World J. Pharm. Pharm. Sci. 2015, 4, 25. [Google Scholar]
- Khan, S.; Patil, K.; Bobade, N.; Yeole, P.; Gaikwad, R. Formulation of intranasal mucoadhesive temperature-mediated in situ gel containing ropinirole and evaluation of brain targeting efficiency in rats. J. Drug Target. 2010, 18, 223–234. [Google Scholar] [CrossRef]
- Sharma, S.; Lohan, S.; Murthy, R.S. Formulation and characterization of intranasal mucoadhesive nanoparticulates and thermo-reversible gel of levodopa for brain delivery. Drug Dev. Ind. Pharm. 2014, 40, 869–878. [Google Scholar] [CrossRef]
- Naik, A.; Naik, H. Formulation and evaluation of thermosensitive biogels for nose to brain delivery of doxepin. Biomed Res. Int. 2014, 2014, 847547. [Google Scholar] [CrossRef]
- Ahmad, E.; Feng, Y.; Qi, J.; Fan, W.; Ma, Y.; He, H.; Xia, F.; Dong, X.; Zhao, W.; Lu, Y.; et al. Evidence of nose-to-brain delivery of nanoemulsions: Cargoes but not vehicles. Nanoscale 2017, 9, 1174–1183. [Google Scholar] [CrossRef]
- Mahajan, H.S.; Mahajan, M.S.; Nerkar, P.P.; Agrawal, A. Nanoemulsion-based intranasal drug delivery system of saquinavir mesylate for brain targeting. Drug Deliv. 2014, 21, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Misra, A.; Mishra, A.K.; Mishra, P.; Pathak, K. Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J. Drug Target. 2008, 16, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Fachel, F.N.; Medeiros-Neves, B.; Dal Prá, M.; Schuh, R.S.; Veras, K.S.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Braganhol, E.; Teixeira, H.F. Box-Behnken design optimization of mucoadhesive chitosan-coated nanoemulsions for rosmarinic acid nasal delivery—In vitro studies. Carbohydr. Polym. 2018, 199, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G.; Baboota, S.; Ahuja, A.; Ali, J. Formulation development of chitosan coated intra nasal ropinirole nanoemulsion for better management option of Parkinson: An in vitro ex vivo evaluation. Curr. Nanosci. 2012, 8, 348–360. [Google Scholar] [CrossRef]
- Bshara, H.; Osman, R.; Mansour, S.; El-Shamy, A.E. Chitosan and cyclodextrin in intranasal microemulsion for improved brain buspirone hydrochloride pharmacokinetics in rats. Carbohydr. Polym. 2014, 99, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Puskás, I.; Pápai, K.; Salvador, E.; Roewer, N.; Förster, C.; Broscheit, J.A. Sevoflurane-sulfobutylether-β-cyclodextrin complex: Preparation, characterization, cellular toxicity, molecular modeling and blood-brain barrier transport studies. Molecules 2015, 20, 10264–10279. [Google Scholar] [CrossRef] [PubMed]
- Calias, P. 2-Hydroxypropyl-β-cyclodextrins and the Blood-Brain Barrier: Considerations for Niemann-Pick Disease Type C1. Curr. Pharm. Des. 2017, 23, 6231–6238. [Google Scholar] [CrossRef] [PubMed]
- Abdou, E.M.; Kandil, S.M.; El Miniawy, H.M. Brain targeting efficiency of antimigraine drug loaded mucoadhesive intranasal nanoemulsion. Int. J. Pharm. 2017, 529, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Figueiró, F.; de Fraga, D.A.; Teixeira, H.F.; Battastini, A.M.; Koester, L.S. Kaempferol-loaded mucoadhesive nanoemulsion for intranasal administration reduces glioma growth in vitro. Int. J. Pharm. 2018, 543, 214–223. [Google Scholar] [CrossRef]
- Pathak, R.; Dash, R.P.; Misra, M.; Nivsarkar, M. Role of mucoadhesive polymers in enhancing delivery of nimodipine microemulsion to brain via intranasal route. Acta Pharm. Sin. B 2014, 4, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Vieira, D.B.; Gamarra, L.F. Getting into the brain: Liposome-based strategies for effective drug delivery across the blood–brain barrier. Int. J. Nanomed. 2016, 11, 5381–5414. [Google Scholar] [CrossRef]
- Rasoulianboroujeni, M.; Kupgan, G.; Moghadam, F.; Tahriri, M.; Boughdachi, A.; Khoshkenar, P.; Ambrose, J.J.; Kiaie, N.; Vashaee, D.; Ramsey, J.D.; et al. Development of a DNA-liposome complex for gene delivery applications. Mater. Sci. Eng. C 2017, 75, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Salade, L.; Wauthoz, N.; Deleu, M.; Vermeersch, M.; De Vriese, C.; Amighi, K.; Goole, J. Development of coated liposomes loaded with ghrelin for nose-to-brain delivery for the treatment of cachexia. Int. J. Nanomed. 2017, 12, 8531. [Google Scholar] [CrossRef] [PubMed]
- Akamizu, T.; Takaya, K.; Irako, T.; Hosoda, H.; Teramukai, S.; Matsuyama, A.; Tada, H.; Miura, K.; Shimizu, A.; Fukushima, M.; et al. Pharmacokinetics, safety, and endocrine and appetite effects of ghrelin administration in young healthy subjects. Eur. J. Endocrinol. 2004, 150, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Salade, L.; Wauthoz, N.; Vermeersch, M.; Amighi, K.; Goole, J. Chitosan-coated liposome dry-powder formulations loaded with ghrelin for nose-to-brain delivery. Eur. J. Pharm. Biopharm. 2018, 129, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Qiang, F.; Shin, H.J.; Lee, B.J.; Han, H.K. Enhanced systemic exposure of fexofenadine via the intranasal administration of chitosan-coated liposome. Int. J. Pharm. 2012, 430, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, J.; Sun, M.; Guo, C.; Yu, A.; Cao, F.; Zhao, L.; Tan, Q.; Zhai, G. N-trimethyl chitosan chloride-coated liposomes for the oral delivery of curcumin. J. Liposome Res. 2012, 22, 100–109. [Google Scholar] [CrossRef] [PubMed]

| Drugs | Composition | Physicochemical Properties | Biological Studies Outcome | References |
|---|---|---|---|---|
| Rivastigmine | Chitosan | Particle size below 200 nm | The uptake of the coated nanoparticles by the Sertoli cells was low and revealed reduced toxicity. A significant reversal of scopolamine-induced amnesia was achieved using the drug loaded coated nanoparticles. | [51] |
| Chitosan, TfRmAb | Particle sizes of 274 nm and 284 nm, Polydispersity index of 0.45 ± 0.13 and 0.48 ± 0.13, respectively. Zeta potential of 29.7 ± 1.7 and 34.4 ± 2.1 mV. | The conjugation with TfRmAb influenced good brain uptake | [55] | |
| Interleukin-1 receptor antagonist FMK | Chitosan, poly(ethylene glycol), OX26 monoclonal antibody | Good stability | Good brain uptake, brain tumor reduction, and behavioral recover in vivo. | [56] |
| Thymoquinone | Chitosan, sodium tripolyphosphate | Particle size of 172–281 nm, Zeta potential of 24.5–30.3 mV, polydispersity index of 0.13–0.24. | Sustained drug concentration in the brain. Prolonged contact time of the drug-loaded NPs with the nasal mucosa. | [57] |
| A fluorescence marker, rhodamine B isothiocyanate (RBITC). | Tween 80, chitosan | The particle size and zeta-potential of the NPs were 251 ± 15 nm and 26.5 ± 4.2 mV, respectively | Good NPs uptake into the brain frontal cortex and cerebellum followed by a decrease in the concentration of the NPs in the two aforementioned regions of the brain over time. Absence of significant oxidative stress damage. A significant reduction in the GFAP expression which was dose-dependent. | [58] |
| Rivastigmine | Chitosan | The higher drug transport efficiency (355%) and direct transport percentage (71.80%) | The concentration of the drug in the plasma after intranasal administration was low. The brain blood ratio was also low after intranasal administration of the drug-loaded NPs. | [62] |
| Olanzapine | Chitosan | The mean particle size, polydispersity index, and zeta potential was 183.1 nm, 0.122, +52.1 mV, respectively. The entrapment efficiency and drug loading were found to be 72% and 26%. | In vitro drug release profile of the NPs was an initial burst release followed by a sustained release mechanism. The toxicity of the formulation on RPMI 2650 human nasal epithelial cell line by MTT assay revealed low toxicity when compared to the free drug. Ex vivo studies on excised goat nasal mucosa further indicated the non-toxic nature of the NPs. | [63] |
| Donepezil | Chitosan | 100–200 nm particle size. | Intranasal administration of the drug-loaded NPs in rats resulted in a high percentage of radioactivity per gram in the brain when compared to the donepezil solution. | [67] |
| Rivastigmine | Chitosan, polysorbate 80 | Particle size of 47 nm | A biphasic drug release was significant. Coating the nanoparticles with 1% polysorbate 80 influenced the uptake of nanoparticles in different organs. | [69] |
| Saxagliptin | Chitosan, valine | Good stability | Good stability of the nanoparticles in the plasma with a release of only 2.5 ng/mL of the drug which is less than the Cmax of the free drug (51 ng/mL). The (AUC0-t) of the drug from the NPs was over 3.42 times lower than the free drug. | [70] |
| Ropinirole | Chitosan | Good stability | In vitro drug release was an initial burst release followed by a sustained drug release mechanism over a period of 10 h. A high drug concentration in the brain with low drug concentrations in vital organs such as the liver, kidney, and spleen after 1 h intravenous administration of the drug-loaded coated NPs when compared to the uncoated drug-loaded NPs and free drug was significant in vivo. | [73] |
| Galantamine | Chitosan, polysorbate 80 | Particle size of 62 nm | High drug release occurred in vitro and a high concentration of galantamine was observed in the brain in vivo. | [74] |
| Carbamazepine | Chitosan | Particle size of 219 nm with 35% drug loading and 80% entrapment efficiency | High drug concentration in the brain. | [76] |
| Chitosan, dextran | Particle size of 55 nm | Improved internalization of the NPs in the C6 glioma cell line in vitro. Retained magnetic properties of the NPs after internalization into the cell. | [77] | |
| Docetaxel | D-α-tocopherol polyethylene glycol 1000 succinate, chitosan, transferrin | Particle size of 130–300 nm | The in vitro cytotoxic effect of the formulation on C6 glioma cell lines was lower when compared to Docel™. The nanoparticles with transferrin exhibited a high AUC with a prolonged circulation in blood when compared to Docel™. | [78] |
| Methotrexate | Chitosan, Tween 80, Poly(lactide-co-glycolide) | Particle size range of 177–408 nm. | The NPs were cytotoxic against C6 glioma cells line and were able to overcome MDCKII-MDR1 cell barrier. | [79] |
| Drug | Composition | Physicochemical Properties | Biological Outcome | References |
|---|---|---|---|---|
| Rivastigime | HPMC, Carbopol 934, NaCMC, Chitosan, pluronic F127 | Low viscosity and gelation capability | High drug concentration in the brain. | [96] |
| Rasagiline mesylate | Poloxamer 407, carbopol 934 P, poloxamer 188, and chitosan | Good mucoadhesive properties | Non-toxic and non-irritant to the rat nasal mucosa. High drug concentration in rat brain tissue. | [97] |
| Levodopa | Chitosan, ketone musk | The optimized thiolated chitosan NPs showed 223 nm particle size, 0.296 PDI and +27.91mV zeta potential. | The concentration of thiolated chitosan nanoparticles in the brain was high when compared to the free drug. The addition of musk ketone to the gel enhanced the concentration of the drug uptake in the brain by increasing the sensitivity of the nasal cavity, thereby inhibiting the efflux of levodopa through P-glycoprotein efflux pump in the brain. | [98] |
| Ropinirole | Chitosan, HPMC | Improved drug bioavailability. | A 90% high brain transport and a drug targeting index greater than 1. | [99] |
| Levodopa | Chitosan, Pluronic PF127 | Good gelation capability. | The release profile followed Hixson-Crowell model. A high percentage of levodopa in the brain when compared to the drug solution in the saline. | [100] |
| Doxepin | Chitosan, PEG | Good gelation and mucoadhesive properties | In vivo studies showed an enhanced increase in activity count and a reduction in the immobility time, indicating good antidepressant activity. | [101] |
| Drug | Composition | Physicochemical Properties | Biological Outcome | References |
|---|---|---|---|---|
| Rosmarinic acid | 0.1% chitosan final concentration (w/v), 8.5% oil phase (w/v), and 3:10 lecithin to oil phase ratio (w/w) | High mucoadhesive property. | The extended permeation time was also significant with a high drug penetration via the porcine nasal mucosa. The nanoemulsion did not induce a cytotoxic effect on the MRC-5 cell lines, human lung fibroblast cells. | [105] |
| Ropinirole | Chitosan | Globule size of 58.61, polydispersity (0.201), and viscosity (31.42 MPa). | Ex vivo study revealed drug transportation in the different parts of the Wister rat brain. | [106] |
| Buspirone hydrochloride | Chitosan aspartate and hydroxypropyl-β-cyclodextrin | The high viscosity. The size of the microemulsion droplets was less than the size of the axons in the filia olfactoria | Increased brain drug targeting by a 7-fold after intranasal administration when compared to the intranasal drug solution in vivo. | [107] |
| Zolmitriptan | Chitosan | A high permeation coefficient on the nasal mucosa and small globule size | Extended nasal clearance time and contact time. | [110] |
| Kaempferol | Chitosan | Low polydispersity index. High free energy and large surface area of the nanosized droplets. Low viscosity | Extended residence time in the nasal cavity with a delay in the drug release to the mucosal surface. High permeation of the drug from the formulation. Increased cytotoxic effect of the formulation against glioma cells. | [111] |
| Nimodipine | Pluronic F 127, Carbopol 934 P, Pluronic F 68, chitosan, sodium alginate and sodium CMC, Capmul MCM, Labrasol and Transcutol P | The particle size of 250 nm and zeta potential value of −15 mV. | Nasal uptake of nimodipine from the microemulsion formulation followed a sustained release with maximal plasma concentration achieved over a period of 6 h. In vivo pharmacokinetic studies in rats showed a high drug uptake in the brain | [112] |
| Drug | Composition | Physicochemical Properties | Biological Outcome | References |
|---|---|---|---|---|
| Ghrelin | Chitosan | Particle size range of 146.9 ± 2.7 to 194 ± 6.1 nm, for uncoated and coated liposomes, respectively. The potential in the range of 0.3 ± 1.2 mV to 6 ± 0.4 mV | Increased mucoadhesion and extended residence time of the formulation in the nasal cavity, and hence enhanced the brain uptake | [116] |
| Ghrelin | Chitosan | Particles size of 195–263 nm, zeta potential range of +5–+9 Mv, | In vitro study of the formulation from a USB aerosol on artificial nasal cavity indicated that the total recovered drug was 23% in the nasal valves section, 25% in the turbinates section, 0% in the rhinopharynx and filter sections, and 52% in the olfactory region | [118] |
| Fexofenadine | Chitosan | Particle size of 359 nm. Narrow size distribution. | 3-fold higher adsorption of mucin in the liposomes coated with chitosan. Extended time in the nasal cavity when compared to the uncoated liposomes. Improved bioavailability of fexofenadine. Reduced mucociliary clearance and increased drug retention in the nasal cavity. | [119] |
| Curcumin | Chitosan, soybean phosphotidylcholine, cholestrol, and D-α-tocopheryl polyethylene glycol 1000 succinate | Particle size range of 221–656 nm with zeta potential in the range of −9.63–+15.64 mV. | The pharmacokinetic parameters and bioavailability of the coated liposomes was (Cmax = 46 μg/L, t1/2 = 12 h, AUC = 417 μg/L·h) when compared to the uncoated liposomes which was (Cmax = 32 μg/L, t1/2 = 9.8 h, AUC = 264 μg/L·h). | [120] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aderibigbe, B.A.; Naki, T. Chitosan-Based Nanocarriers for Nose to Brain Delivery. Appl. Sci. 2019, 9, 2219. https://doi.org/10.3390/app9112219
Aderibigbe BA, Naki T. Chitosan-Based Nanocarriers for Nose to Brain Delivery. Applied Sciences. 2019; 9(11):2219. https://doi.org/10.3390/app9112219
Chicago/Turabian StyleAderibigbe, Blessing Atim, and Tobeka Naki. 2019. "Chitosan-Based Nanocarriers for Nose to Brain Delivery" Applied Sciences 9, no. 11: 2219. https://doi.org/10.3390/app9112219





